Abstract
Objective:
To investigate the associations among β-amyloid (Aβ), cortical thickness, and episodic memory in a cohort of cognitively normal to mildly impaired individuals at increased risk of vascular disease.
Methods:
In 67 subjects specifically recruited to span a continuum of cognitive function and vascular risk, we measured brain Aβ deposition using [11C] Pittsburgh compound B–PET imaging and cortical thickness using MRI. Episodic memory was tested using a standardized composite score of verbal memory, and vascular risk was quantified using the Framingham Coronary Risk Profile index.
Results:
Increased Aβ was associated with cortical thinning, notably in frontoparietal regions. This relationship was strongest in persons with high Aβ deposition. Increased Aβ was also associated with lower episodic memory performance. Cortical thickness was found to mediate the relationship between Aβ and memory performance. While age had a marginal effect on these associations, the relationship between Aβ and cortical thickness was eliminated after controlling for vascular risk except when examined in only Pittsburgh compound B–positive subjects, in whom Aβ remained associated with thinner cortex in precuneus and occipital lobe. In addition, only the precuneus was found to mediate the relationship between Aβ and memory after controlling for vascular risk.
Conclusion:
These results suggest strong links among Aβ, cortical thickness, and memory. They highlight that, in individuals without dementia, vascular risk also contributes to cortical thickness and influences the relationships among Aβ, cortical thickness, and memory.
The amyloid hypothesis of Alzheimer disease (AD) suggests that β-amyloid (Aβ) initiates a cascade of pathologic events that eventually lead to dementia. While much evidence argues in favor of this hypothesis, some fails to support it.1 In addition, epidemiologic data indicate that vascular risk factors increase the incidence of a clinical diagnosis of AD2; however, little is known regarding the effects of vascular risk factors on the Aβ cascade.
The hypothetical model of dynamic biomarkers of the AD pathology cascade3 provides an interesting framework to test the amyloid hypothesis and investigate the influence of vascular factors. The model proposes a temporal ordering of AD biomarkers, which may reflect an underlying pathophysiologic sequence: CSF Aβ42 and amyloid PET changes are detectable first, followed by CSF tau, hypometabolism on [18F]-fluorodeoxyglucose–PET, atrophy on MRI, and finally cognitive deficits. The temporal ordering suggests that the relationship between Aβ and cognitive impairment is less proximate and thus should be less evident than the relationship between neurodegenerative biomarkers and cognitive impairment. It also proposes that Aβ accumulation reaches a plateau before the clinical diagnosis of AD, which implies that no (or a mild) relationship should be detectable past this point between Aβ and the other biomarkers. At the present time, little is known or hypothesized about effects of vascular risk on this temporal cascade. The goal of this study was to assess the relationships among Aβ, neurodegeneration, and episodic memory in a sample predominantly without dementia, enriched for vascular risk factors. We hypothesized that Aβ would be associated with thinner cortex and that thinner cortex would in turn be associated with episodic memory deficits.
METHODS
Participants.
This study included 67 participants aged 70 years or older who underwent Pittsburgh compound B (PIB)-PET scanning, 3-tesla MRI scanning, and cognitive testing. Participants were from the Aging Brain Project, a multisite program recruiting older adults with cognitive ability ranging from normal to mild impairment, many at increased risk of arteriosclerotic cardiovascular disease. Subjects were selected to represent a spectrum of vascular diseases, using the Framingham Coronary Risk Profile (FCRP), and had normal to mildly impaired cognition. Participants with severe or unstable medical illness, neurologic or major psychiatric conditions other than AD or vascular dementia, alcohol or substance abuse, significant head injury, or moderate to severe dementia were excluded as were 11 subjects with large cortical strokes. Clinical Dementia Rating scale scores were 0 (n = 35), 0.5 (n = 31), and 1 (n = 1). Times between PIB-PET, MRI, and cognitive testing averaged approximately 3 months (range, 0–15).
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from participants or their legal representatives under protocols approved by the Institutional Review Boards at all participating institutions.
Neuropsychological testing.
Participants underwent neuropsychological testing with a uniform protocol previously described.4 The Mini-Mental State Examination5 was used as a standard clinical measure of global cognition. Verbal episodic memory was measured with a composite scale that was created using item response theory methods that have been previously described.4 The composite scale was psychometrically matched with linear measurement properties using item response theory.6 The scale incorporates items from the Memory Assessment Scale list learning task7,8 and was transformed to have a mean of 100 and SD of 15.
Vascular risk.
We used the FCRP to measure vascular risk. This index represents a weighted sum of smoking, diabetes, hypertension, and low- and high-density lipoprotein cholesterol.9 It was designed to predict the 10-year risk of coronary heart disease. In the present study, based on FCRP scores, 33 subjects (49.3%) had a 10-year risk lower that 10%, 11 subjects (16.4%) had a 10-year risk between 10% and 20%, and 23 subjects (34.3%) had a 10-year risk higher than 20%. Our sample had more individuals with high cardiovascular risk compared with the US population surveyed from 1988 to 1994, in which 34.5% of subjects aged 70 to 80 years had risk below 10%, 51.5% between 10% and 20%, and only 14% more than 20%.10
Imaging data acquisition.
Pittsburgh compound B.
PIB was synthesized at Lawrence Berkeley National Laboratory using a previously published protocol.11 All PIB-PET images were acquired using a Siemens ECAT EXACT HR PET scanner (Siemens Medical Systems, Erlangen, Germany) in 3-dimensional acquisition mode. Dynamic acquisition frames (35 frames total) were obtained over 90 minutes after the injection of 10 to 15 mCi of [11C] PIB.
Structural MRI.
We acquired structural MRI scans on 3-tesla systems, using sequences that had been harmonized by implementing the Alzheimer's Disease Neuroimaging Initiative (ADNI) scan parameters at each MRI site, then scanning the ADNI phantom to test for signal-to-noise ratio. Images included a T1-weighted volumetric magnetization-prepared rapid-acquisition gradient echo scan (repetition time [TR] = 2,500, echo time [TE] = 2.98, inversion time [TI] = 1,100 milliseconds, 1 × 1 × 1 mm3 isotropic resolution) and a fluid-attenuated inversion recovery scan (TR = 8,800, TE = 495, TI = 2,360 milliseconds, with 1 × 1 × 2 mm3 resolution or TR = 5,000, TE = 430, TI = 1,700 milliseconds, with 1 × 1 × 2 mm3 resolution). Sixty participants were scanned at University of California, Davis on a Siemens Magnetom Trio system with an 8-channel head coil. Seven participants were scanned at University of California, San Francisco on a Siemens Magnetom TrioTim system with a 12-channel head coil. No frontal, parietal (precuneus), temporal, or occipital cortical thickness difference was found between the scanners (data not shown).
Imaging data processing.
Pittsburgh compound B.
We preprocessed PIB-PET data using the Statistical Parametric Mapping version 8 software package (http://www.fil.ion.ucl.ac.uk/spm). Regions of interest (ROIs), used to define the cerebellar reference region and to guide PIB-PET processing, were created with the FreeSurfer version 5.1 software package (http://surfer.nmr.mgh.harvard.edu). Frames 6 through 35 as well as the sum of frames 1 to 5 were realigned to the middle frame (17th frame). Realigned frames 1 to 23 (corresponding to the first 20 minutes of acquisition) were then averaged. This average volume was used to transform the MRI (and cerebellar reference region) to the native PET space. PIB distribution volume ratio images were calculated using Logan graphical analysis with PIB frames corresponding to 35 to 90 minutes postinjection and a gray matter masked cerebellar reference region.12,13
We created a global PIB index representing overall cortical Aβ deposition by averaging the mean distribution volume ratio value from frontal (cortical regions anterior to the precentral gyrus), temporal (middle and superior temporal regions), parietal (supramarginal gyrus, inferior/superior parietal lobules, and precuneus), and posterior cingulate ROIs. These ROIs, as well as the ones used for cortical thickness, were created in each subject's native space using the Desikan-Killiany atlas and the semiautomated FreeSurfer processing stream.14 Subjects with a PIB index value of 1.16 and higher were classified as having abnormal Aβ deposition (PIB+) and those below this cutoff were considered PIB−. This cutoff was validated in an independent sample using a similar processing technique.15
Structural MRI.
We measured cortical thickness (mm) with FreeSurfer version 5.1. ROIs were defined by averaging weighted left and right frontal, parietal, temporal, occipital, and precuneus ROIs included in the Desikan-Killiany atlas.14 Images were visually examined to ensure segmentation accuracy, and regions with poor image quality or misregistration were excluded from statistical analysis. We excluded the frontal ROI for 3 subjects, the parietal ROI for 2 subjects, the precuneus ROI for 2 subjects, the temporal ROI for 4 subjects, and the occipital ROI for 3 subjects.
Vascular brain injury.
A neurologist identified infarcts (>3 mm) on T1-weighted images, and white matter hyperintensities were quantified on fluid-attenuated inversion recovery images using a semiautomated procedure previously described elsewhere.16,17 Intrarater and interrater reliability for this method is high and these data have been published previously.18
Statistical analysis.
Analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY). Differences between groups for baseline analyses were conducted using 2-tailed Student t tests or Pearson χ2 tests. Linear regressions were used to test the relationship between global cortical Aβ accumulation and cortical thickness in ROIs. Three regression models (all older adults, PIB+ and PIB−) were run for each ROI (frontal, parietal, precuneus, temporal, and occipital). Mediation analyses were then conducted among all older adults using path analyses. Mediation exists when a predictor affects a dependent variable indirectly through a third variable (the mediator).19 Looking at figure 1, mediation would be represented by significant indirect (paths a*b) effects. Indirect effects were examined using a 95% bootstrapped bias-corrected confidence interval (BCI)19 established via bootstrapping with 5,000 bootstrap samples.20 The regression and the path analyses models were first conducted without covariates in order not to remove variance associated with Aβ.21 They were repeated, adjusted for age and for FCRP (in separate models), to test whether these factors influenced our findings. These adjustments were done using the standardized residuals after regressing each variable included in each model on age or FCRP. A p value <0.05 was considered significant.
Figure 1. Cortical thickness mediates the effect of Aβ on episodic memory.

Path analyses showing that cortical thickness in the frontal (A), parietal (B), and precuneus (C) mediates the effect of Aβ on episodic memory. Full lines indicate the significant direct and indirect (mediation) effects while dotted lines represent the nonsignificant direct effects. Standardized regression coefficients are presented; a = the association between Aβ and cortical thickness, b = the association between cortical thickness and memory adjusted for the effect of Aβ on memory, c' = the association between Aβ and memory adjusting for cortical thickness, and c = the direct association between Aβ and memory. Standardized regression coefficients are also presented for the mediation (a*b) effects. *p < 0.05. Aβ = β-amyloid; BCI = bias-corrected confidence interval (bootstrapped).
RESULTS
Participant characteristics.
Demographic, clinical, and MRI characteristics are presented in table 1. PIB+ and PIB− participants did not differ on age, level of education, or APOE ε4 status. There were trends for more men and more cognitively impaired persons (Clinical Dementia Rating score) in the PIB+ group compared with the PIB− group. PIB+ subjects had increased vascular risk (FCRP score) compared with PIB− subjects. PIB+ subjects performed worse on the episodic memory scale. A trend was found for thinner frontal cortex in PIB+ subjects compared with PIB− subjects.
Table 1.
Participant characteristics by PIB status

Relationship between Aβ and cortical thickness.
The following results are first presented without covariates, then controlling for age, and finally controlling for vascular risk (FCRP score). The analyses adjusted for FCRP are not adjusted for age. In this cohort, age was marginally associated with Aβ (β = 0.22, standard error = 0.004, p < 0.08) and vascular risk was associated with Aβ (β = 0.35, standard error = 0.004, p < 0.01).
When the relationship between Aβ and cortical ROIs was assessed across all older adults, increased Aβ was related to thinner frontal, parietal, and precuneus cortices (table 2). When these analyses were adjusted for age, the association with the precuneus was still significant but the association between Aβ and thickness in the frontal and the parietal cortices became marginal (p < 0.08). No association between Aβ and cortical thickness reached significance when controlling for FCRP.
Table 2.
Relationship between β-amyloid and cortical thickness within the whole sample and within the PIB+ subgroup

When the analyses were restricted to PIB+ subjects (figure 2, table 2), Aβ was negatively associated with frontal, parietal, precuneus, and occipital cortical thickness, but not with temporal thickness. Similar results were found when controlling for age. Only the associations with the precuneus and the occipital cortices were significant when controlling for FCRP. As expected, no association was found between Aβ and cortical thickness in PIB− subjects regardless of the covariates included in the models.
Figure 2. Association between Aβ and cortical thickness in PIB+ and PIB− subjects.

Linear regression analyses assessing the association between global Aβ accumulation and frontal (A), parietal (B), and precuneus (C) cortical thickness in PIB+ and PIB− cases. Green dots represent PIB+ subjects while black dots represent PIB− subjects. *p < 0.05. Aβ = β-amyloid; PIB = Pittsburgh compound B.
Relationships among Aβ, cortical thickness, and memory.
The following results are first presented with no covariates. Then, the analyses were adjusted for age and FCRP by using the standardized residuals after regressing each variable in the model on age or FCRP. This was done to diminish the number of variables included in the analyses. These analyses were performed within the whole sample.
Direct association between Aβ and memory.
Increased Aβ was associated with lower episodic memory (figure 1). This result is consistent with the group difference found between PIB+ and PIB− (table 1). Neither age nor FCRP was associated with memory performance; therefore, results were similar when controlling for these factors.
Direct association between cortical thickness and memory.
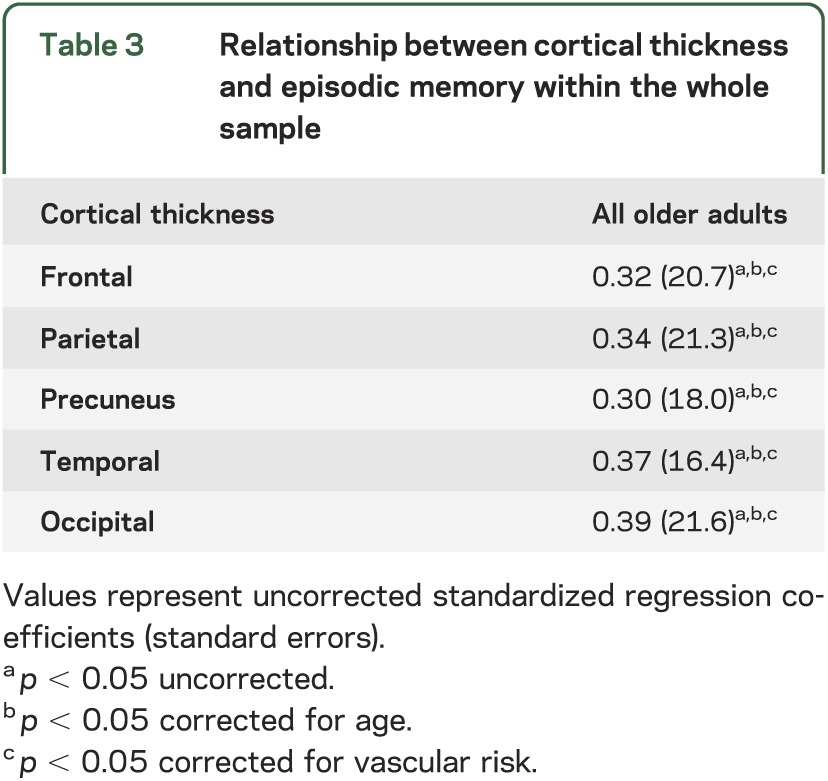
Thinner frontal, parietal, precuneus, temporal, and occipital regions were all associated with lower memory performance (table 3). Results were unchanged when controlling for age or FCRP.
Table 3.
Relationship between cortical thickness and episodic memory within the whole sample
Mediating effect of cortical thickness on memory.
We tested the mediating (indirect) effects of cortical thickness on the relationship between Aβ and memory with path analyses. Figure 1 shows that thickness within the frontal, parietal, and precuneus cortices all mediate the effect of global cortical Aβ on memory. On an exploratory basis, we also tested whether temporal or occipital thickness mediated the relationship between Aβ and memory. Even though the direct association between Aβ and temporal thickness did not reach significance (p = 0.13), temporal thickness was found to mediate the effect of Aβ on memory (β = −0.06; BCI: −0.17, −0.004). Results were similar when adjusting for age except for the temporal lobe where the mediation model was no longer significant. When adjusting for FCRP, only the mediation model including the precuneus was significant (β = −0.05; BCI: −0.15, −0.001). Indeed, when controlling for FCRP, the parietal, frontal, and temporal cortices no longer mediated the association between Aβ and memory.
DISCUSSION
We found that increased Aβ deposition was clearly associated with thinner cortex in persons with higher Aβ levels. This relationship was seen throughout the cortex, except in the temporal lobe, providing strong evidence that Aβ is a major factor associated with neuronal integrity in aging and highlighting its extensive effects. In addition, while many studies characterize individuals as “positive” or “negative” for fibrillar Aβ in the brain, these results indicate that the amount of Aβ is important in addition to its presence or absence (figure 2). These findings are consistent with previous studies suggesting that Aβ deposition is associated with cortical thinning in aging.22,23 They further show that the inclusion of PIB− subjects in analyses assessing the association between Aβ on other biomarkers might diminish the strength of the results.
While controlling for age in these diverse analyses had only a small effect on the results, controlling for vascular risk (using the FCRP score) had a more dramatic effect. Indeed, when controlling for vascular risk, only the precuneus and the occipital cortices were negatively associated with Aβ deposition in PIB+ subjects and no association reached significance when the analyses were conducted in the total sample. It is important to be careful when interpreting these results. Because Aβ and vascular risk share common variance,24,25 controlling for vascular risk probably diminishes the effect of amyloid that we are trying to capture. Therefore, these results do not imply that Aβ has no effect on cortical thickness, but rather that both factors probably affect cortical thickness via similar mechanisms and that the effect of vascular risk is stronger than the effect of Aβ in individuals without dementia. Vascular risk factors are well known to have a widespread effect on cortical thickness,26,27 which usually begin in early life.28 This might explain why they overcome the effect of Aβ in individuals without dementia. Compared with vascular pathology, AD pathology might therefore be associated with more severe neurodegeneration only in patients with dementia.29 The relative effects of age and vascular risk in this study must also be interpreted with caution because this sample was specifically chosen for diversity in vascular disease but not age.
Interestingly, even though vascular risk had an effect on cortical thickness in this study, only Aβ was associated with worse verbal memory performance. In addition to their effect on cortical thickness, these 2 pathologies are known to impair neuronal networks. Aβ for instance has a major effect on the default mode network,30,31 a network intrinsically affected in AD32 and involved in successful episodic memory.31 The precuneus, a region found to be affected by Aβ in PIB+ subjects when controlling for vascular risk in this study, is a major cortical hub of this network.33 Vascular risk in turn is predominantly associated with dysfunction of the frontal-striatal system, a system essential for attention and executive functions where dysfunction may lead to more subtle memory impairment.34 Together, these findings suggest that both factors may increase AD risk via cortical thinning, but in different brain regions and neural systems.
Our study provides evidence that cortical thickness in frontal and parietal (precuneus) lobes mediates the relationships between Aβ and memory (figure 1). Indeed, Aβ was found to influence cortical thickness, which in turn influenced memory. Furthermore, the association between Aβ and memory was no longer significant when accounting for cortical thickness. When these analyses were controlled for vascular risk, only the precuneus was found to mediate the association between Aβ and memory. Other factors such as tau pathology have also been suggested to mediate the relationship between Aβ and memory,35 possibly as an additional factor influencing cortical thickness and network integrity. Together, our findings suggest that cortical thickness has a major role in producing memory loss by mediating Aβ effects in precuneus, while in other brain regions it seems likely that a combination of vascular factors and Aβ may be important.
In concordance with other studies,36,37 our results underscore that Aβ is not the only factor associated with cortical thinning in aging. This is particularly evident in figure 2, which shows that the variability in cortical thickness is similar between PIB+ and PIB−. Our results indicate that vascular factors account for part of this variability. Other factors such as neurofibrillary tangles,38 genetic factors,39 and brain reserve40 probably also explain part of this variability. Finally, we cannot exclude the possibility that some subjects included in the PIB− group were in the earliest stage of Aβ accumulation, particularly because ambiguous cases were included in this group.15
Both the sample size in this study and the differences in brain regions associated with Aβ accumulation were small. However, only subjects with similar data acquisition were included because cortical thickness assessments are highly influenced by methodologic differences in data acquisition. Furthermore, the present study has the advantage of being multimodal, which allows exploration of the interactions among several biomarkers.
These results indicate complex relationships among Aβ, vascular risk, cortical thinning, and memory. While Aβ is associated with cortical thinning and cognitive decline, it is not the only factor leading to cortical thinning in aging. In this cross-sectional study of individuals without dementia, the effect of Aβ on cortical thickness, but not on memory, seems to be overshadowed by vascular disease. These data argue that while Aβ can be a good target for clinical trials, controlling vascular risk might have a major effect on AD.
ACKNOWLEDGMENT
The authors thank H. St. Amant and A. Yi for help with data preparation and J. Vogel for helpful discussions throughout the project.
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- BCI
bias-corrected confidence interval
- FCRP
Framingham Coronary Risk Profile
- PIB
Pittsburgh compound B
- ROI
region of interest
- TE
echo time
- TI
inversion time
- TR
repetition time
AUTHOR CONTRIBUTIONS
Dr. Villeneuve: data analysis and data interpretation. Dr. Reed: study concept or design, critical revision of the manuscript for important intellectual content. Dr. Wirth: data analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Haase: data analysis, critical revision of the manuscript for important intellectual content. Ms. Madison: data analysis and programming. Ms. Ayakta: data analysis, critical revision of the manuscript for important intellectual content. Dr. Mack: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. DeCarli, Dr. Mungas, Dr. Chui, and Dr. Weiner: acquisition of data, study concept or design, critical revision of the manuscript for important intellectual content. Dr. Jagust: acquisition of data, study concept or design, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
Supported by grants P01 AG12435 (Chui), P30 AG10129 (DeCarli), and a CIHR postdoctoral fellowship to S. Villeneuve.
DISCLOSURE
S. Villeneuve, B. Reed, M. Wirth, C. Haase, C. Madison, N. Ayakta, W. Mack, D. Mungas, H. Chui, and C. DeCarli report no disclosures. M. Weiner reported that he was on the scientific advisory boards of Lilly, Araclon, Institut Català de Neurociències Aplicades, Gulf War Veterans Advisory Committee, and Biogen Idec in 2010 and Pfizer in 2011; that he consulted for AstraZeneca, Araclon, Medivation/Pfizer, Ipsen, TauRx Therapeutics, Bayer HealthCare, Biogen Idec, ExonHit Therapeutics, Servier, and Synarc in 2010 and Pfizer and Janssen in 2011; that he received funding for travel from NeuroVigil, CHRU Hôpital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–Alzheimer's Disease Neuroimaging Initiative (ADNI), Paris University, Institut Català de Neurociències Aplicades, University of New Mexico School of Medicine, Ipsen, and Clinical Trials on Alzhimer's Disease in 2010 and Pfizer, The Alzheimer's & Parkinson's Conference, Paul Sabatier University, Novartis, and Tohoku University in 2011; that he was on the editorial advisory board of Alzheimer's & Dementia and Magnetic Resonance Imaging; that he received honoraria from NeuroVigil and Institut Català de Neurociències Aplicades in 2010 and Pharmaceuticals and Medical Devices Agency/Japanese Ministry of Health, Labour and Welfare and Tohoku University in 2011; that he received commercial entities research support from Merck and Avid; that he received government entities research support from the US Department of Defense and the Department of Veterans Affairs; and that he has stock options in Synarc and Elan. The organizations contributing to the foundation for the NIH, and thus to the National Institute on Aging–funded ADNI, are Abbott, the Alzheimer's Association, the Alzheimer's Drug Discovery Foundation, the Anonymous Foundation, AstraZeneca, Bayer HealthCare, BioClinica (ADNI 2), Bristol-Myers Squibb, the Cure Alzheimer's Fund, Eisai, Elan, Gene Network Sciences, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Lilly, Medpace, Merck, Novartis, Pfizer, Roche, Schering Plough, Synarc, and Wyeth. W. Jagust has served as a consultant to Genentech Inc., Synarc, Janssen Alzheimer Immunotherapy, F. Hoffmann-La Roche, and Siemens, and his research has been supported by grants AG027859, AG027984, and AG024904 from the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353–356 [DOI] [PubMed] [Google Scholar]
- 2.Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci 2012;322:141–147 [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013;12:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology 2003;17:380–392 [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 6.Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of item response theory. In: Measurement Methods for the Social Sciences Series. Thousand Oaks, CA: Sage Publications; 1991 [Google Scholar]
- 7.Williams JM. Memory Assessment Scales. Lutz, FL: Psychological Assessment Resources; 1991 [Google Scholar]
- 8.Glosser G, Goodglass H, Biber C. Assessing visual memory disorders. J Consult Clin Psychol 1989;1:82–91 [Google Scholar]
- 9.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Giles WH, Mokdad AH. The distribution of 10-Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 2004;43:1791–1796 [DOI] [PubMed] [Google Scholar]
- 11.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 2003;46:2740–2754 [DOI] [PubMed] [Google Scholar]
- 12.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834–840 [DOI] [PubMed] [Google Scholar]
- 13.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547 [DOI] [PubMed] [Google Scholar]
- 14.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980 [DOI] [PubMed] [Google Scholar]
- 15.Mormino EC, Brandel MG, Madison CM, et al. Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage 2012;59:1152–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr 1992;16:274–284 [DOI] [PubMed] [Google Scholar]
- 17.Yoshita M, Fletcher E, DeCarli C. Current concepts of analysis of cerebral white matter hyperintensities on magnetic resonance imaging. Top Magn Reson Imaging 2005;16:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510 [DOI] [PubMed] [Google Scholar]
- 19.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–891 [DOI] [PubMed] [Google Scholar]
- 20.Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [computer software, online], 2002. Available at: http://quantpsy.org. Accessed January 22, 2014
- 21.Mungas D, Reed BR, Farias ST, Decarli C. Age and education effects on relationships of cognitive test scores with brain structure in demographically diverse older persons. Psychol Aging 2009;24:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh H, Madison C, Villeneuve S, Markley C, Jagust WJ. Association of gray matter atrophy with age, beta-amyloid, and cognition in aging. Cereb Cortex Epub 2013. Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker JA, Hedden T, Carmasin J, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol 2011;69:1032–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging 2012;33:1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol 2013;70:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardenas VA, Reed B, Chao LL, et al. Associations among vascular risk factors, carotid atherosclerosis, and cortical volume and thickness in older adults. Stroke 2012;43:2865–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leritz EC, Salat DH, Williams VJ, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage 2011;54:2659–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maillard P, Seshadri S, Beiser A, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol 2012;11:1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 2011;70:774–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009;63:178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 2004;101:4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci 2009;29:1860–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 2004;44:195–208 [DOI] [PubMed] [Google Scholar]
- 35.Han SD, Gruhl J, Beckett L, et al. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer's disease. Brain Imaging Behav 2012;6:610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol 2012;71:765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci 2013;33:5553–5563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 2008;71:743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fennema-Notestine C, Panizzon MS, Thompson WR, et al. Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis 2011;26(suppl 3):49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Julkunen V, Paajanen T, et al. Education increases reserve against Alzheimer's disease: evidence from structural MRI analysis. Neuroradiology 2012;54:929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]


