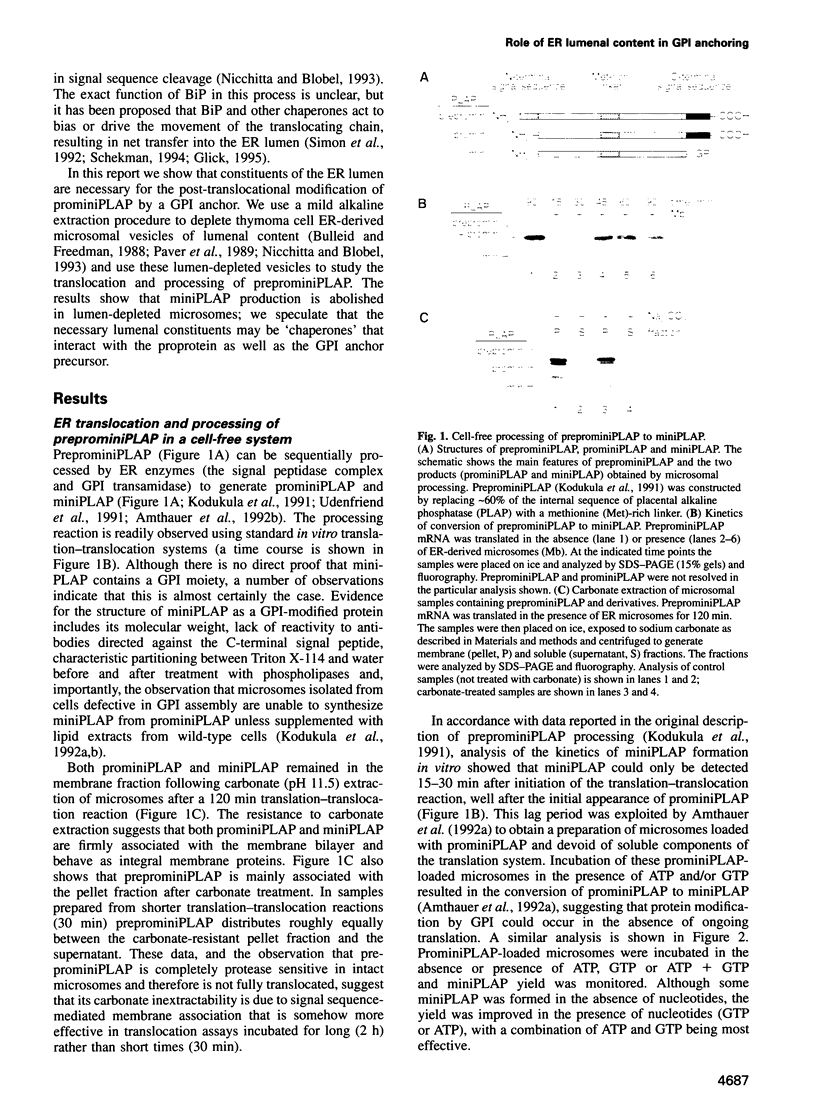
Abstract
Transfer of a glycosylphosphatidylinositol (GPI) anchor to proteins carrying a C-terminal GPI-directing signal sequence occurs after protein translocation across the endoplasmic reticulum (ER). We describe the translocation and GPI modification of a model protein, preprominiPLAP, in ER microsomes depleted of lumenal content by high pH washing. In untreated microsomes preprominiPLAP was processed to prominiPLAP and GPI-anchored miniPLAP. Both products were fully translocated, since they resisted proteinase K treatment of the microsomes, and both behaved as membrane proteins by the carbonate extraction criterion. Microsomes depleted of lumenal content were able to translocate and process preprominiPLAP to give protease-protected prominiPLAP, but were unable to convert prominiPLAP to miniPLAP. Loss of GPI anchoring capacity occurred with a wash of pH > 9.5. If the alkaline wash was performed after formation of prominiPLAP conversion to miniPLAP was relatively unimpaired. The results indicate that constituents of the ER lumen, possibly chaperones interacting with the proprotein and/or the GPI anchor precursor, are required in the initial steps of GPI anchoring.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amthauer R., Kodukula K., Brink L., Udenfriend S. Phosphatidylinositol-glycan (PI-G)-anchored membrane proteins: requirement of ATP and GTP for translation-independent COOH-terminal processing. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6124–6128. doi: 10.1073/pnas.89.13.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthauer R., Kodukula K., Gerber L., Udenfriend S. Evidence that the putative COOH-terminal signal transamidase involved in glycosylphosphatidylinositol protein synthesis is present in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3973–3977. doi: 10.1073/pnas.90.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthauer R., Kodukula K., Udenfriend S. Placental alkaline phosphatase: a model for studying COOH-terminal processing of phosphatidylinositol-glycan-anchored membrane proteins. Clin Chem. 1992 Dec;38(12):2510–2516. [PubMed] [Google Scholar]
- Arar C., Carpentier V., Le Caer J. P., Monsigny M., Legrand A., Roche A. C. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995 Feb 24;270(8):3551–3553. doi: 10.1074/jbc.270.8.3551. [DOI] [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988 Oct 13;335(6191):649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Clairmont C. A., De Maio A., Hirschberg C. B. Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94. J Biol Chem. 1992 Feb 25;267(6):3983–3990. [PubMed] [Google Scholar]
- Doering T. L., Masterson W. J., Englund P. T., Hart G. W. Biosynthesis of the glycosyl phosphatidylinositol membrane anchor of the trypanosome variant surface glycoprotein. Origin of the non-acetylated glucosamine. J Biol Chem. 1989 Jul 5;264(19):11168–11173. [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Parton R. G., Kellner R., Etzold T., Simons K. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J. 1994 Apr 1;13(7):1729–1740. doi: 10.1002/j.1460-2075.1994.tb06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gilmore R. Protein translocation across the endoplasmic reticulum: a tunnel with toll booths at entry and exit. Cell. 1993 Nov 19;75(4):589–592. doi: 10.1016/0092-8674(93)90476-7. [DOI] [PubMed] [Google Scholar]
- Glick B. S. Can Hsp70 proteins act as force-generating motors? Cell. 1995 Jan 13;80(1):11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marquardt T., Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992 Aug;2(8):227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Kodukula K., Cines D., Amthauer R., Gerber L., Udenfriend S. Biosynthesis of phosphatidylinositol-glycan (PI-G)-anchored membrane proteins in cell-free systems: cleavage of the nascent protein and addition of the PI-G moiety depend on the size of the COOH-terminal signal peptide. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1350–1353. doi: 10.1073/pnas.89.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodukula K., Micanovic R., Gerber L., Tamburrini M., Brink L., Udenfriend S. Biosynthesis of phosphatidylinositol glycan-anchored membrane proteins. Design of a simple protein substrate to characterize the enzyme that cleaves the COOH-terminal signal peptide. J Biol Chem. 1991 Mar 5;266(7):4464–4470. [PubMed] [Google Scholar]
- Mayor S., Menon A. K., Cross G. A. Transfer of glycosyl-phosphatidylinositol membrane anchors to polypeptide acceptors in a cell-free system. J Cell Biol. 1991 Jul;114(1):61–71. doi: 10.1083/jcb.114.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Ferguson M. A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993 Sep 1;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Eppinger M., Mayor S., Schwarz R. T. Phosphatidylethanolamine is the donor of the terminal phosphoethanolamine group in trypanosome glycosylphosphatidylinositols. EMBO J. 1993 May;12(5):1907–1914. doi: 10.1002/j.1460-2075.1993.tb05839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon A. K., Mayor S., Schwarz R. T. Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: involvement of mannosyl-phosphoryldolichol as the mannose donor. EMBO J. 1990 Dec;9(13):4249–4258. doi: 10.1002/j.1460-2075.1990.tb07873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensa-Wilmot K., LeBowitz J. H., Chang K. P., al-Qahtani A., McGwire B. S., Tucker S., Morris J. C. A glycosylphosphatidylinositol (GPI)-negative phenotype produced in Leishmania major by GPI phospholipase C from Trypanosoma brucei: topography of two GPI pathways. J Cell Biol. 1994 Mar;124(6):935–947. doi: 10.1083/jcb.124.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran P., Raab H., Kohr W. J., Caras I. W. Glycophospholipid membrane anchor attachment. Molecular analysis of the cleavage/attachment site. J Biol Chem. 1991 Jan 15;266(2):1250–1257. [PubMed] [Google Scholar]
- Nicchitta C. V., Blobel G. Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell. 1993 Jun 4;73(5):989–998. doi: 10.1016/0092-8674(93)90276-v. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J. Bidirectional movement of a nascent polypeptide across microsomal membranes reveals requirements for vectorial translocation of proteins. Cell. 1992 Oct 2;71(1):87–96. doi: 10.1016/0092-8674(92)90268-h. [DOI] [PubMed] [Google Scholar]
- Paver J. L., Hawkins H. C., Freedman R. B. Preparation and characterization of dog pancreas microsomal membranes specifically depleted of protein disulphide-isomerase. Biochem J. 1989 Feb 1;257(3):657–663. doi: 10.1042/bj2570657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Schekman R. Translocation gets a push. Cell. 1994 Sep 23;78(6):911–913. doi: 10.1016/0092-8674(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Peskin C. S., Oster G. F. What drives the translocation of proteins? Proc Natl Acad Sci U S A. 1992 May 1;89(9):3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens V. L. Regulation of glycosylphosphatidylinositol biosynthesis by GTP. Stimulation of N-acetylglucosamine-phosphatidylinositol deacetylation. J Biol Chem. 1993 May 5;268(13):9718–9724. [PubMed] [Google Scholar]
- Udenfriend S., Kodukula K. Prediction of omega site in nascent precursor of glycosylphosphatidylinositol protein. Methods Enzymol. 1995;250:571–582. doi: 10.1016/0076-6879(95)50098-7. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Micanovic R., Kodukula K. Structural requirements of a nascent protein for processing to a PI-G anchored form: studies in intact cells and cell-free systems. Cell Biol Int Rep. 1991 Sep;15(9):739–759. doi: 10.1016/0309-1651(91)90030-m. [DOI] [PubMed] [Google Scholar]
- Vidugiriene J., Menon A. K. Early lipid intermediates in glycosyl-phosphatidylinositol anchor assembly are synthesized in the ER and located in the cytoplasmic leaflet of the ER membrane bilayer. J Cell Biol. 1993 Jun;121(5):987–996. doi: 10.1083/jcb.121.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidugiriene J., Menon A. K. The GPI anchor of cell-surface proteins is synthesized on the cytoplasmic face of the endoplasmic reticulum. J Cell Biol. 1994 Oct;127(2):333–341. doi: 10.1083/jcb.127.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. P., Misra L. M., Rose M. D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990 Jun;110(6):1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]