Abstract
Objective:
To determine whether extracranial-intracranial (EC-IC) bypass can improve cognition over 2 years compared to best medical therapy alone in patients with symptomatic internal carotid artery (ICA) occlusion and increased oxygen extraction fraction (OEF) on PET.
Methods:
Patients underwent 15O PET and were randomized if OEF ratio was >1.13 on the occluded side. Using blinded baseline and 2-year cognitive assessments, age-adjusted composite z scores were generated from subtests sensitive to right/left hemisphere plus global cognitive functioning. Multiple regression predicted 2-year cognitive change.
Results:
Eighty-nine patients were enrolled; 41 had increased OEF and were randomized. Two died, 2 were lost to follow-up, and 2 refused 2-year testing. Of the 35 remaining, 6 had ipsilateral stroke or death, leaving 13 surgical and 16 medical patients. Controlling for age, education, and depression, there was no difference in 2-year cognitive change between the medical and surgical arms (95% confidence interval −0.5 to 0.5, p = 0.9). In post hoc analysis of 26 patients with no stroke in the follow-up period, cognitive improvement was associated with less impaired PET OEF at baseline (p = 0.045).
Conclusion:
Cognitive improvement following bypass surgery was not superior to medical therapy among patients with recently symptomatic carotid occlusion and increased OEF. Among those with no recurrent stroke, less hemodynamic impairment at baseline was associated with greater cognitive gain in both groups. Reversing cognitive impairment in hemodynamic failure remains an open challenge.
Classification of evidence:
This study provides Class II evidence that for patients with symptomatic ICA occlusion and increased OEF on PET, EC-IC bypass compared to no bypass does not improve cognitive function after 2 years.
Cognitive impairment in carotid artery disease is thought to be due in part to cerebrovascular hemodynamic failure independent of infarction,1–4 making it a potentially reversible cause of dementia. Evidence of reversibility has been supported by case reports and small noncontrolled studies,5 but the treatment of cognitive impairment in this population has never been tested in a randomized controlled trial. The Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON), an ancillary study of the Carotid Occlusion Surgery Study (COSS), was a National Institute of Neurological Disorders and Stroke–sponsored, phase III, multicenter, randomized, controlled, open-label (blinded outcome) clinical trial that ran from November 2004 to June 2012. RECON tested the hypothesis that extracranial-intracranial (EC-IC) surgical bypass plus best medical therapy could improve or preserve cognitive function at 2 years in patients with symptomatic internal carotid artery (ICA) occlusion and stage II hemodynamic failure (increased oxygen extraction fraction [OEF]) compared with best medical therapy alone. Although the parent study, COSS, failed to show a difference in rate of subsequent stroke and death between the 2 treatment arms6 and was terminated early for futility, thus discontinuing RECON enrollment, RECON completed the 2-year follow-up visits for the already randomized RECON patients. We recently reported that cognitive impairment in our RECON patients at baseline was independently correlated with increased OEF on PET,4 supporting the hemodynamic hypothesis of cognitive impairment upon which the RECON intervention trial was based. We report the main results of the RECON trial after the 2-year follow-ups were completed.
METHODS
Research question.
Can EC-IC bypass, when added to best medical therapy, improve cognitive function after 2 years in patients with symptomatic ICA occlusion and increased OEF on PET, compared with best medical therapy alone? Class of evidence assigned was Class II.
Subjects.
Patients were enrolled into RECON between 2005 and 2010 at academic medical centers if they met the following inclusion criteria: 1) age 18 to 85, 2) complete ICA occlusion (confirmed by catheter angiography prior to randomization), 3) hemispheric TIA or minor stroke in the territory of the carotid occlusion within 120 days prior to enrollment, 4) Barthel Index ≥12/20 at the time of enrollment (after the qualifying event), 5) education level >4 years, 5) no prior diagnosis of dementia. Nonatherosclerotic causes of carotid occlusion were excluded based on all available clinical data. TIA was defined clinically as having no residual neurologic deficit after 24 hours. MRI was not available in all patients and so was not used in the definition of TIA. Once enrolled, patients were then eligible for randomization if they had asymmetrically increased OEF by PET, with an OEF ratio >1.13 on the side of occlusion, indicating stage II hemodynamic failure. Those who had ≤1.13 OEF ratio were not randomized and went no further in the study. Those randomized to the surgical arm also underwent PET at 30 days after surgery. The PET threshold of 1.13 was determined by the St. Louis Carotid Occlusion Study, which examined thresholds of OEF associated with a higher rate of subsequent stroke.7 A copy of the full protocol is available upon request.
Randomization and masking.
The 1:1 randomization was performed as part of the parent clinical trial (COSS) using permuted blocks with stratification for clinical site using the SAS uniform random number generator (RANUNI). Cognitive assessments were done by testers blinded to the treatment arm as described below.
Standard protocol approvals, registrations, and patient consents.
All patients signed informed consent. The study was approved by the Columbia University Institutional Review Board. ClinicalTrials.gov identifier: NCT00390481.
Neurocognitive testing.
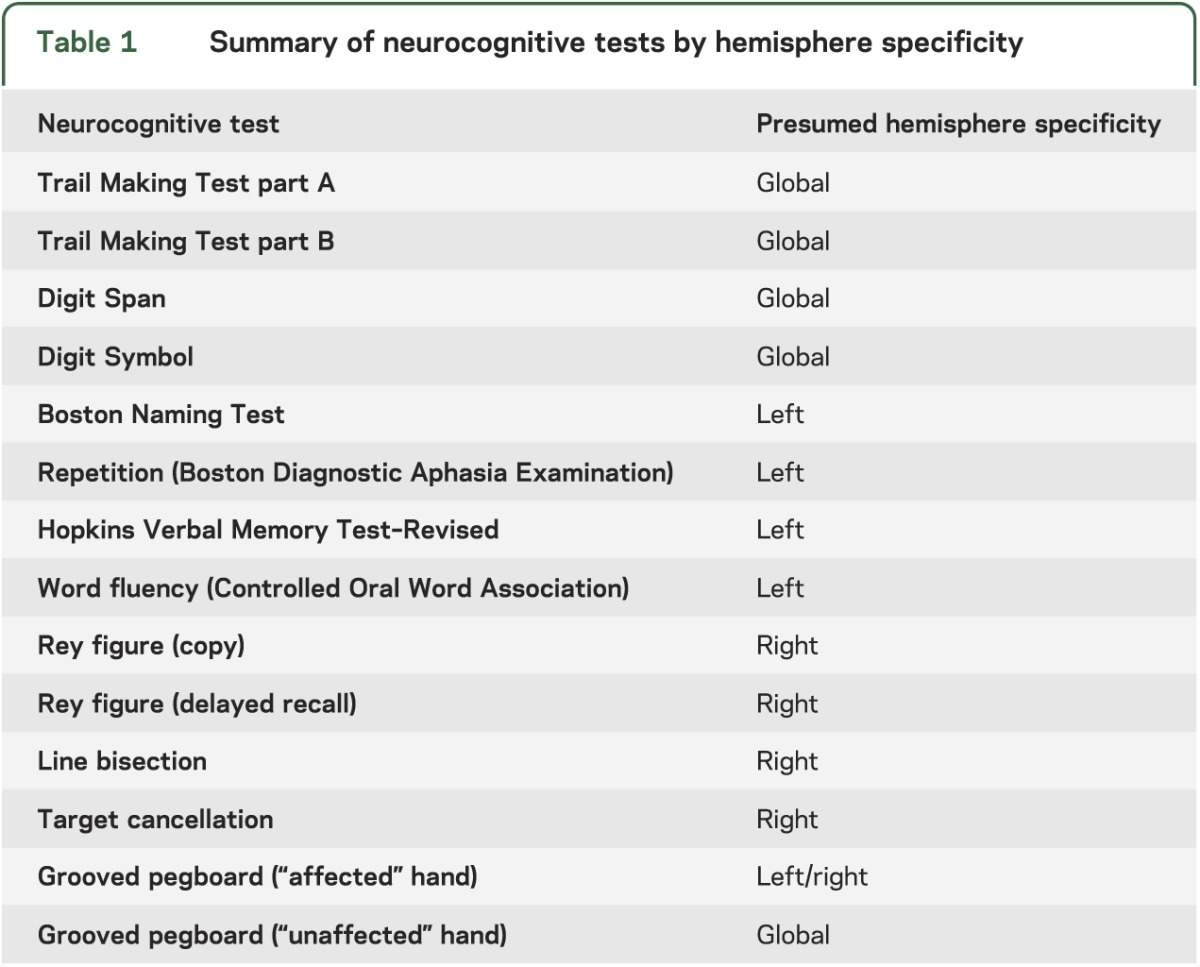
At baseline and at 2 years, patients underwent a 1-hour neurocognitive battery consisting of 14 standardized neuropsychological tests, administered by a neuropsychologist or trained technician blinded to the patient's treatment arm. The battery was designed to assess left hemisphere function, right hemisphere function, and global function. Published age-adjusted normative data were available for all tests.8–17 All patients were administered all 14 neurocognitive tests. The order of test administration remained constant, accommodating the delays required for recall intervals and minimizing stimulus interference in memory tasks. The test battery is summarized in table 1. Patients also completed the Center for Epidemiological Studies–Depression (CES-D) scale.
Table 1.
Summary of neurocognitive tests by hemisphere specificity
Because hemodynamic failure in the hemisphere ipsilateral to the symptomatic carotid occlusion was hypothesized to affect predominantly functions associated with that hemisphere, as well as global cognitive function that would also depend on the integrity of that hemisphere, composite scores were generated for each patient based on the hemisphere of interest. We first transformed the raw test scores for each patient into z scores for each test in the battery,18 derived from the published norms. We then calculated a composite z score19,20 based on the average z score for the appropriate set of tests for each patient (sum of the relevant z scores divided by the number of tests included). For patients with left carotid occlusion, “left hemisphere” and “global” test scores were used; for patients with right carotid occlusion, “right hemisphere” and “global” test scores were used. To test the assumptions of our hypothesis that hemisphere-specific plus global composite scores were associated with cognitive impairment in this patient population, we also performed a principal components analysis (PCA)21 of baseline neurocognitive test scores of all enrolled patients to derive a data-driven composite score that would explain the greatest proportion of variance in baseline cognitive function.
Statistical analysis.
Patients who reached a COSS endpoint (ipsilateral stroke or death) during the 2-year follow-up period were censored from analysis. A multivariable linear regression was performed, with change in composite neurocognitive z score over 2 years as the dependent variable. The primary independent variable of interest was treatment arm (surgical vs medical). A power calculation based on definitions of small, moderate, and large effect size22 determined that we would need a total of >30 patients randomized to identify a large effect size of 0.8 SD for the primary outcome. Age, education level (≤8th grade, 9–12th grade [high school], ≥13 years [some college]) and depression (as measured by the CES-D at 2 years) were entered into the model as covariates in a prespecified primary regression analysis. A post hoc univariate regression was also performed to identify all variables that had a significant association with cognitive z score change, with an adjustment for baseline score. Covariates with p < 0.05 in the univariate analyses were added one at a time in a forward variable selection process; only covariates with p < 0.05 after adjusting for other factors in the final multivariable model were retained. The same univariate analyses were also performed using the PCA-derived composite neurocognitive z score as the dependent variable.
RESULTS
Eighty-nine patients (27 women, mean age 57 ± 9.3) from 19 RECON centers were enrolled in the study. Of those, 41 had PET OEF ratio >1.13 and were randomized to the surgical (n = 19) vs medical (n = 22) treatment arm. Two died (medical arm), 2 were lost to follow-up (surgical arm), and 2 refused 2-year testing (medical arm). Of the 35 remaining patients, 6 had COSS endpoints (ipsilateral stroke or death: 4 in the surgical group, 2 in the medical group), leaving 13 in the surgical group and 16 in the medical group for full analysis (figure 1).
Figure 1. Study design flow diagram.
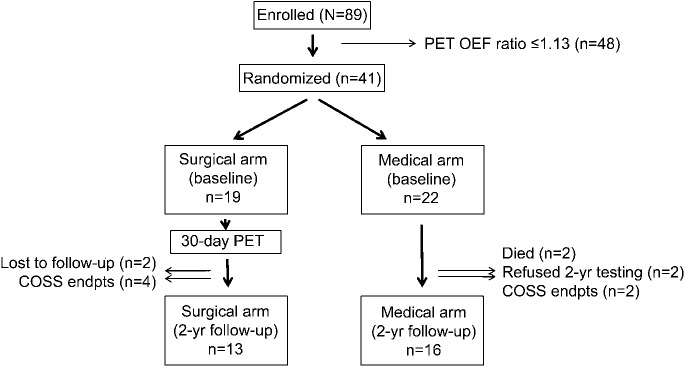
COSS = Carotid Occlusion Surgery Study; endpts = endpoints; OEF = oxygen extraction fraction.
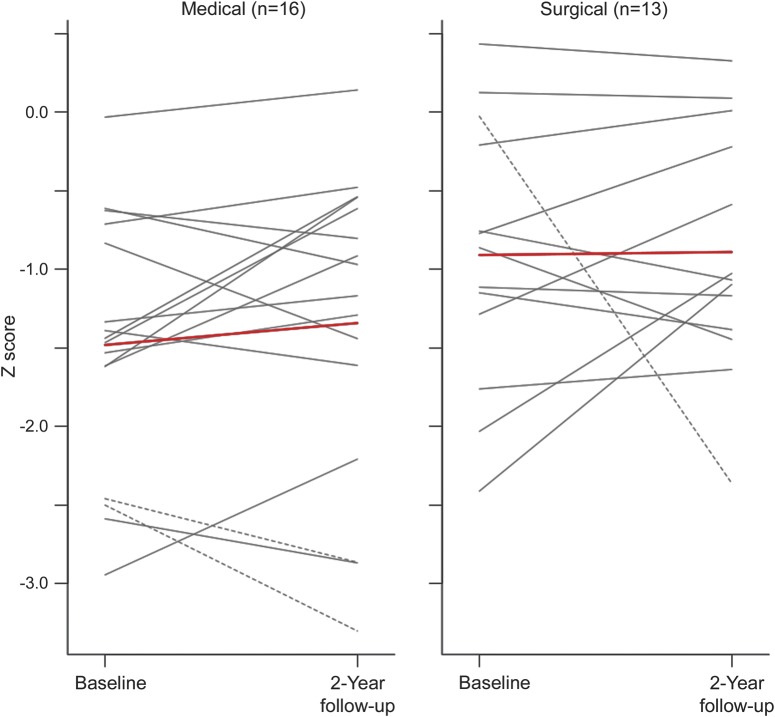
Table 2 shows baseline characteristics for the 29 patients in the analysis cohort. There were no differences in demographic, clinical, or radiologic characteristics between the groups. The average neurocognitive composite z score at baseline across all patients was 1.2 SD below the age- and education-adjusted mean (range −3.7 to −0.3); there was no difference in cognitive scores between groups at baseline. Although no patients had a diagnosis of dementia at time of enrollment, nearly all patients had scores below the means for their age, indicating mild cognitive impairment in this group. No workup for dementia was pursued since the patients were functioning normally prior to enrollment in the study. Controlling for age, education, depression, and baseline composite neurocognitive z score as prespecified for the study, there was no difference in change in composite z score between the surgical and medical groups over the 2-year follow-up period (point estimate 0.02, 95% confidence interval [CI] −0.50 to 0.54, p = 0.93; table 3). Of note, the point estimate (mean) was nearly 0. Individual cognitive change scores are illustrated in figure 2. Three patients—2 in the nonsurgical arm and 1 in the surgical arm—had contralateral strokes during the 2-year period, represented by dashed lines in the figure. They were included as per our protocol in the primary analysis.
Table 2.
Baseline characteristics: Medical vs surgical groups
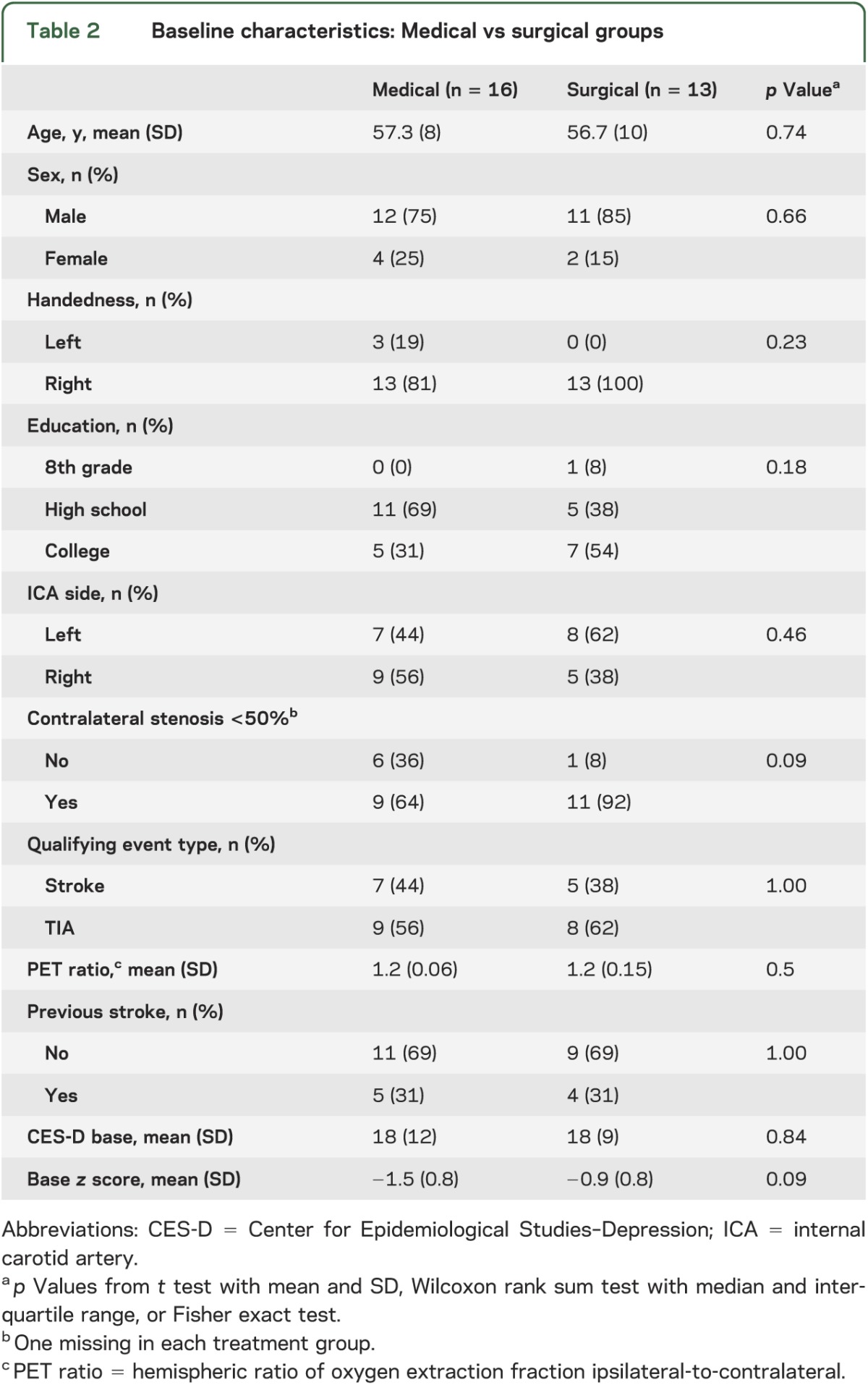
Table 3.
Univariate analysis of all independent variables for the 26 patients with no ipsilateral or contralateral stroke
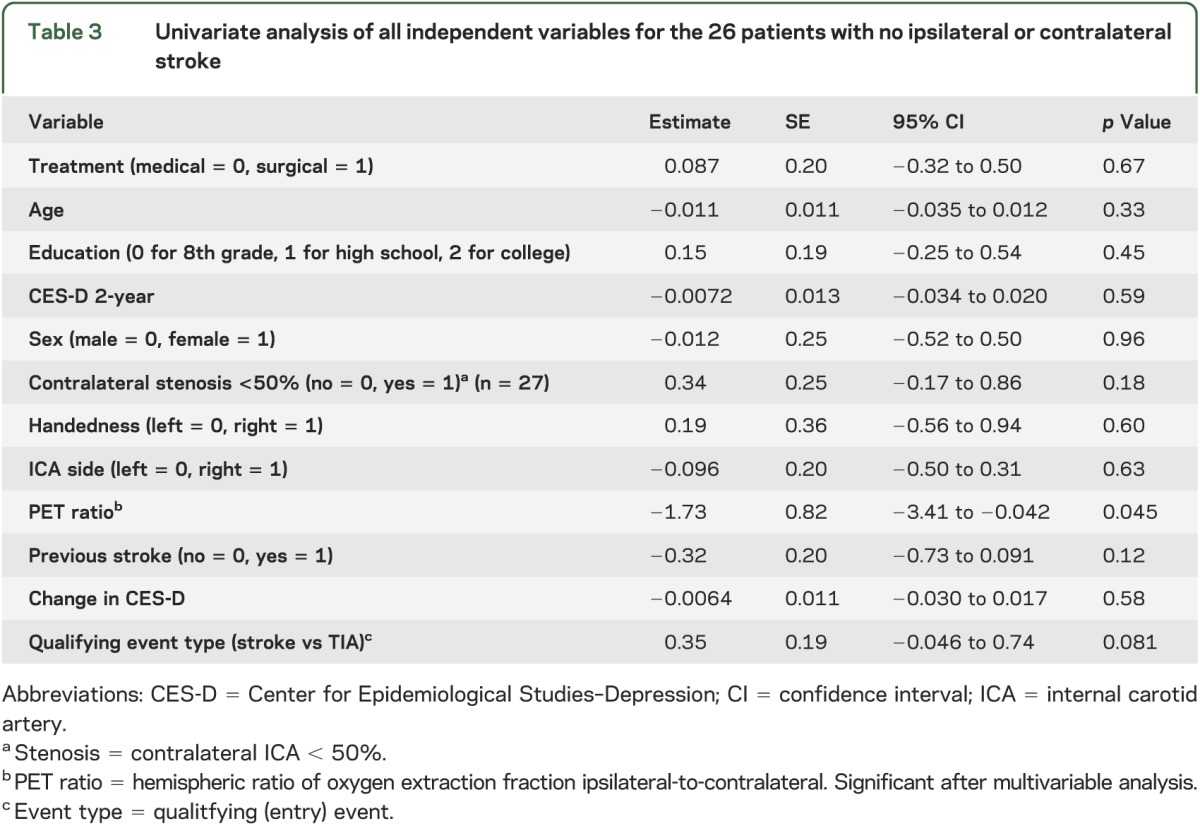
Figure 2. Individual composite cognitive change scores by treatment group.
Dashed lines indicate patients with contralateral stroke during the follow-up period. Red line represents mean change score for each group.
The OEF PET ratio in the surgical group had a statistically significant decrease from an average of 1.24 at baseline to 1.14 at 30 days postoperatively (t = 2.8, p = 0.013). Only 3 of the 13 surgical patients had 30-day PET OEF ratios ≤1.13, the COSS threshold for randomization into the study, and none of the 13 achieved OEF ratio <1.067, the upper limit of normal for the COSS cohort overall. The patency rate for the bypass was 98% at the 30-day postoperative point and 96% at the last follow-up in the COSS cohort, based on low-resistance, high-flow signal by Doppler.23
We also performed a post hoc regression analysis of all collected variables against cognitive change among all patients, taking as our dataset only patients who had no stroke (ipsilateral or contralateral) in the 2-year follow-up period. We focused on this set of patients to minimize the potential cognitive effects of a new stroke. The only variable that was associated with greater improvement in 2-year composite neurocognitive z score was a less impaired PET ratio at baseline, which remained significant in the multiple regression analysis (t = −1.73, p = 0.045, 95% CI = −3.41 to −0.042; see table 3). When the alternative, PCA-derived composite neurocognitive z score was used in the univariate analysis, PET ratio was significantly associated with cognitive change, as well as qualifying event type: TIA, indicating good concordance with our primary composite test grouping. It is possible that individual test battery components may have correlated with some of our independent variables including the treatment arm, but this analysis was not prespecified and will be explored separately.
DISCUSSION
Our main finding for this phase III randomized controlled trial was that there was no evidence to support superiority of EC-IC bypass surgery plus best medical therapy in preserving or improving cognition over medical therapy alone in patients with recently symptomatic carotid artery occlusion and stage II hemodynamic failure. There was a range of cognitive change scores over the 2-year follow-up period, but the change did not depend on which treatment was received.
The hemodynamic hypothesis that formed the premise for this trial was supported most compellingly by studies in which cognitive impairment was found in the absence of frank stroke. An early study reported cognitive impairment in 39 cases with cerebral or retinal TIAs but no stroke on MRI.24 No hemodynamic measurements were made in that study. A more recent study showed that, among 333 patients with asymptomatic high-grade carotid stenosis, cognitive impairment was present compared to those without carotid stenosis, and was associated with impaired cerebrovascular reactivity as measured by transcranial Doppler breath-holding index.3 Finally, in a baseline analysis of our own RECON patients, among 71 with complete clinical data and adequate PET imaging, we showed that increased OEF ratio >1.13 was independently associated with cognitive impairment in the subset of patients with TIA as qualifying event.4 Taken together, these results suggest that the substrate upon which the surgical intervention was intended to work was present, supporting the notion that reversing the hemodynamic failure could potentially reverse cognitive impairment. An alternative hypothesis would be that the cognitive impairment was due to hemodynamically induced, fixed injury that would not be amenable to reversal by improvement in hemodynamics.
Revascularization surgery has been reported to improve cognitive impairment in carotid artery stenosis and occlusion, although reported results have been variable and appear only in nonrandomized studies.25–28 Many early studies included nonstandardized measurement of cognition, varying clinical states among patients (stroke, TIA, asymptomatic), and no comparison group. Two reports did include quantitative blood flow measurements. First, in a single case of bilateral ICA occlusions presenting with subacute onset of severe behavioral and cognitive decline, neuropsychological improvement was demonstrated after EC-IC bypass that was associated with significant increases in cerebral blood flow (CBF) and metabolism measured by quantitative CBF and PET.2 Second, in a larger case series, 25 patients with unilateral carotid occlusion and poor neuropsychological performance underwent EC-IC bypass, and were shown to have cognitive improvement associated with increased CBF, improved cerebral vasomotor reactivity, and improved OEF as measured by PET.5
The cognitive outcomes in RECON were based on randomization against a control arm, which had never been done previously. There were limitations to the study, however. The first was the study's small sample size, resulting from early termination of the parent trial. Although the final number of subjects for RECON met our prestudy power calculation for detecting a large effect size (a difference of 0.8 SD in composite cognitive change score between treatment arms), it is possible that greater numbers of patients may have demonstrated a small or moderate superiority of one treatment arm or the other, although with the point estimate near zero this would have been unlikely. The 95% CI for cognitive change as shown in table 3 was −0.5 to 0.5 SD, the limits of a moderate effect size.22 A second limitation was the presence of other variables that could have affected cognitive performance at the time of testing, including use of sedative or anticholinergic medications, medical illnesses, or sleep deprivation. We know that prevalence of sedatives was low (2 of 29 in the fully evaluated cohort), and there was no change from baseline to 2-year follow-up, so there would be low likelihood of bias. In addition, stroke itself might have affected cognitive function. In our cohort, 9 patients had previous strokes and 12 had stroke as qualifying event. Leukoaraiosis was not measured. Because the stroke numbers were equally distributed between treatment arms, however, the randomization process should have mitigated any bias that might have been present with respect to our treatment outcomes. Furthermore, previous stroke was not a predictor for cognitive decline, either in the current cohort (table 3) or in our previously published article on baseline correlates of cognitive dysfunction, in which OEF but not previous strokes correlated with (mild) cognitive impairment.4 A third limitation may have been the relatively modest reduction in OEF ratio achieved by the bypass in our RECON cohort. Despite an outstanding surgical result in terms of graft patency, and a statistically significant improvement in OEF, only 3 of the 13 patients in the surgical arm had postoperative OEF ratio ≤1.13, the PET threshold for study randomization, and none of them achieved OEF ratio <1.067, the upper limit of normal for the overall COSS cohort. Thus, our primary hypothesis regarding cognitive gains following hemodynamic improvement may not have been adequately tested. While the improvement in OEF from EC-IC bypass in the entire COSS surgical group was sufficient to reduce the subsequent risk of ipsilateral stroke in those who did not have stroke as a surgical complication,6 the threshold for cognitive improvement might be greater. In an addition, hemodynamics may have improved over time in the medical arm,29 perhaps due to gradual improvement in collateral flow. Finally, our findings may be related to the aggressive medical management in both arms, including blood pressure control and administration of statins. Notably, neither the nonsurgical nor the surgical group in our cohort had cognitive deterioration over 2 years despite entering the study with some cognitive dysfunction. Despite recent concerns that statins may worsen cognitive function,30,31 we found no evidence for this in our study. Rather, statins32–34 as well as blood pressure management35–37 appear to positively affect cognitive status and have known hemodynamic effects.38–40 Two recent surgical trials, RECON's parent trial COSS and the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) study, failed to show superiority of an intervention in part due to better than expected outcomes in the medical arm. Although the outcomes in these 2 trials were recurrent stroke, it is plausible that medical therapy plays an important role in treatment of cognitive impairment.
Given the evidence from prior studies that cerebrovascular hemodynamic status is a determinant of cognitive function, it is reasonable to include cognitive outcomes in future trials of carotid revascularization, and to pursue other reperfusion interventions with greater hemodynamic efficacy and lower morbidity. Further research is needed to determine thresholds of cerebral hemodynamic failure that permit or promote cognitive improvement. For the present, however, it appears that medical therapy, including the consistent use of statins and antihypertensives, may help preserve cognition in this patient population; there is no evidence that surgical intervention with EC-IC bypass is better than medical therapy.
Supplementary Material
GLOSSARY
- CBF
cerebral blood flow
- CES-D
Center for Epidemiological Studies–Depression
- CI
confidence interval
- COSS
Carotid Occlusion Surgery Study
- EC-IC
extracranial-intracranial
- ICA
internal carotid artery
- OEF
oxygen extraction fraction
- PCA
principal components analysis
- RECON
Randomized Evaluation of Carotid Occlusion and Neurocognition
Editorial, page 738
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
R.S. Marshall: study conceptualization and design, data review, manuscript writing. J.R. Festa: manuscript editing, neuropsychological battery design. Y.K. Cheung: manuscript editing, statistical design and analysis. M.A. Pavol: manuscript review and revision, neuropsychological battery review. C.P. Derdeyn: PET data analysis, manuscript review and revision. W.R. Clarke: study design, data analysis, manuscript review and revision. T.O. Videen: PET study design and analysis, manuscript review and revision. R.L. Grubb: neurosurgical design input, manuscript review and revision. K. Slane: data preparation and analysis, manuscript review. W.J. Powers: study conceptualization, manuscript review and revision. R.M. Lazar: study design, conceptualization, neuropsychological battery design, manuscript review and revision.
STUDY FUNDING
Supported by NINDS NS048212 (Dr. Marshall), NINDS NS42167 (Dr. Powers), and 5U01NS041895 (Dr. Clarke).
DISCLOSURE
R. Marshall is supported by the National Institute of Neurological Disorders and Stroke NS048212. J. Festa is supported by the National Institute of Neurological Disorders and Stroke NS048212. Y. Cheung is supported by the National Institute of Neurological Disorders and Stroke NS048212. M. Pavol is supported by the National Institute of Neurological Disorders and Stroke NS048212. C. Derdeyn is supported by the National Institute of Neurological Disorders and Stroke NS048212 and NS42167. W. Clarke is supported by the National Institute of Neurological Disorders and Stroke 5U01NS041895. T. Videen is supported by the National Institute of Neurological Disorders and Stroke NS42167. R. Grubb is supported by the National Institute of Neurological Disorders and Stroke NS42167. K. Slane is supported by the National Institute of Neurological Disorders and Stroke NS048212. W. Powers is supported by the National Institute of Neurological Disorders and Stroke NS42167. R. Lazar is supported by the National Institute of Neurological Disorders and Stroke NS048212. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009;72:1062–1068 [DOI] [PubMed] [Google Scholar]
- 2.Tatemichi TK, Desmond DW, Prohovnik I, Eidelberg D. Dementia associated with bilateral carotid occlusions: neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J Neurol Neurosurg Psychiatry 1995;58:633–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balucani C, Viticchi G, Falsetti L, Silvestrini M. Cerebral hemodynamics and cognitive performance in bilateral asymptomatic carotid stenosis. Neurology 2012;79:1788–1795 [DOI] [PubMed] [Google Scholar]
- 4.Marshall RS, Festa JR, Cheung YK, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the RECON trial. Neurology 2012;78:250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasoh M, Ogasawara K, Kuroda K, et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol 2003;59:455–460; discussion 460–453 [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 2011;306:1983–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–1060 [DOI] [PubMed] [Google Scholar]
- 8.Lezak MD Howiezon DB, Loring DW, eds. Neuropsychological Assessment, 4th ed Oxford: Oxford University Press; 2004 [Google Scholar]
- 9.Meyers JE, Meyers K. The Meyers Scoring System for the Rey Complex Figure and the Recognition Trial: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1995 [Google Scholar]
- 10.Brandt J, Benedict RHB. Hopkins Verbal Learning Test: revised. Odessa, FL: Psychological Assessment Resources; 2001 [Google Scholar]
- 11.Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP. Distinct syndromes of hemineglect. Arch Neurol 1992;49:1187–1194 [DOI] [PubMed] [Google Scholar]
- 12.Mitrushina MBK, Razani J, D'Elia LF. Handbook of Normative Data for Neuropsychological Assessment. Oxford: Oxford University Press; 2005 [Google Scholar]
- 13.Goodglass H, Kaplan EF. Assessment of Aphasia and Related Disorders, 2nd ed Philadelphia: Lea & Febiger; 1983 [Google Scholar]
- 14.Zec RF, Burkett NR, Markwell SJ, Larsen DL. Normative data stratified for age, education, and gender on the Boston Naming Test. Clin Neuropsychol 2007;21:617–637 [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed San Antonio, TX: Harcourt Assessment; 1997 [Google Scholar]
- 16.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye hand coordination in adults: normative values for the finger tapping and grooved pegboard tests. Percept Mot Skills 1993;76:1219–1230 [DOI] [PubMed] [Google Scholar]
- 17.Tombaugh TNKJ, Reees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167–177 [PubMed] [Google Scholar]
- 18.Strauss E, Sherman EMS, Spreen O, eds. A Compendium of Neuropsychologcial Tests: Administration, Normas, and Commentary, 3rd ed New York: Oxford University Press; 2006 [Google Scholar]
- 19.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch Neurol 1999;56:1274–1279 [DOI] [PubMed] [Google Scholar]
- 20.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001;344:395–402 [DOI] [PubMed] [Google Scholar]
- 21.Tabachnick B, Fidell LS. Using Multivariate Statistics, 2nd ed New York: Harper Collins Publishers; 1989 [Google Scholar]
- 22.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1988 [Google Scholar]
- 23.Grubb RL, Jr, Powers WJ, Clarke WR, Videen TO, Adams HP, Jr, Derdeyn CP. Surgical results of the carotid occlusion surgery study. J Neurosurg 2013;118:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker FC, Klijn CJ, Jennekens-Schinkel A, van der Tweel I, Tulleken CA, Kappelle LJ. Cognitive impairment in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. J Neurol 2003;250:1340–1347 [DOI] [PubMed] [Google Scholar]
- 25.Drinkwater JE, Thompson SK, Lumley JS. Cerebral function before and after extra-intracranial carotid bypass. J Neurol Neurosurg Psychiatry 1984;47:1041–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen H, Hojer-Pedersen E, Gulliksen G, Haase J, Enevoldsen E. Reversible ischemic neurological deficit and minor strokes before and after EC/IC bypass surgery: a neuropsychological study. Acta Neurol Scand 1986;73:615–618 [DOI] [PubMed] [Google Scholar]
- 27.Lal BK, Younes M, Cruz G, Kapadia I, Jamil Z, Pappas PJ. Cognitive changes after surgery vs stenting for carotid artery stenosis. J Vasc Surg 2011;54:691–698 [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K, Ogasawara K, Kobayashi M, Kubo Y, Otawara Y, Ogawa A. Improvement and impairment in cognitive function after carotid endarterectomy: comparison of objective and subjective assessments. Neurol Med Chir 2012;52:154–160 [DOI] [PubMed] [Google Scholar]
- 29.Widder B, Kleiser B, Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke 1994;25:1963–1967 [DOI] [PubMed] [Google Scholar]
- 30.King DS, Wilburn AJ, Wofford MR, Harrell TK, Lindley BJ, Jones DW. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy 2003;23:1663–1667 [DOI] [PubMed] [Google Scholar]
- 31.Agostini JV, Tinetti ME, Han L, McAvay G, Foody JM, Concato J. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc 2007;55:420–425 [DOI] [PubMed] [Google Scholar]
- 32.Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 2008;71:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishi T, Sunagawa K. Combination therapy of atorvastatin and amlodipine inhibits sympathetic nervous system activation and improves cognitive function in hypertensive rats. Circ J 2012;76:1934–1941 [DOI] [PubMed] [Google Scholar]
- 34.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology 2005;65:1388–1394 [DOI] [PubMed] [Google Scholar]
- 35.Washida K, Ihara M, Nishio K, et al. Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator-activated receptor-gamma activation in mice with chronic cerebral hypoperfusion. Stroke 2010;41:1798–1806 [DOI] [PubMed] [Google Scholar]
- 36.Dregan A, Stewart R, Gulliford MC. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 2013;42:338–345 [DOI] [PubMed] [Google Scholar]
- 37.White WB, Wolfson L, Wakefield DB, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011;124:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab 2012;32:1973–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forteza A, Romano JG, Campo-Bustillo I, et al. High-dose atorvastatin enhances impaired cerebral vasomotor reactivity. J Stroke Cerebrovasc Dis 2012;21:487–492 [DOI] [PubMed] [Google Scholar]
- 40.Walters M, Muir S, Shah I, Lees K. Effect of perindopril on cerebral vasomotor reactivity in patients with lacunar infarction. Stroke 2004;35:1899–1902 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.