Abstract
Objective:
Assess the prevalence and impact of pain in children with Charcot-Marie-Tooth (CMT) disease.
Methods:
In this prospective cross-sectional study on children with CMT disease seen at study sites of the Inherited Neuropathy Consortium, we collected standardized assessments of pain (Wong-Baker FACES Pain Rating Scale) from 176 patients (140 children aged 8–18 years, and 36 children aged 2–7 years through parent proxies), along with standardized clinical assessments and quality-of-life (QOL) outcomes. We then developed a series of multivariate regression models to determine whether standardized measures of neuropathy severity, functional impact, or structural changes to the feet explained the observed pain scores.
Results:
The mean score on the Wong-Baker FACES Pain Rating Scale was 2 (range 0–5). Increased pain strongly correlated with worse QOL scores but not with more severe neuropathy. Independent determinants of increased pain in children with CMT disease included measures of ankle inflexibility.
Conclusion:
Pain is present in children with CMT disease and negatively affects QOL. Pain scores do not positively correlate with neuropathy severity but do correlate in limited univariate analyses with measures of ankle inflexibility. Further studies to elucidate the mechanisms of pain may help identify treatments that can reduce pain and improve QOL in patients with CMT disease.
There are currently no approved therapies to improve the neuropathy in Charcot-Marie-Tooth (CMT) disease, one of the most common inherited neuromuscular disorders.1,2 Understanding what, apart from the neuropathy, affects the patient with CMT disease may help in determining alternative ways of improving patients’ lives even as we await definitive cures. We have shown that health-related quality of life (QOL) is significantly reduced in pediatric CMT disease.3 Our follow-up study identified several determinants of reduced QOL4 but did not prospectively assess pain measurements in these children. A significant proportion of adults with CMT disease report pain5–9; however, there is no consensus on whether that pain is due to nerve damage or to the relentless structural changes that may occur in the hands and feet of patients with CMT disease. Assessing pain in children with CMT disease, who have less structural changes than adults, may thus offer insight not only into the physiology of the pain but also into the contributors of reduced QOL in children with this disease. Our study objective was to prospectively assess the prevalence and impact of pain in pediatric CMT disease. We hypothesized that pain would negatively affect children with CMT disease and that the etiology of the pain would be structural changes in the feet, as opposed to neuropathy severity.
METHODS
Participants.
We collected prospective cross-sectional data on children with CMT disease seen through the multicenter natural history study of the Inherited Neuropathy Consortium (U54-NS065712) from 2010 through 2013. Pain data were prospectively collected as contributors of reduced QOL in children with CMT disease. We recruited children aged 2 to 18 years with genetically confirmed CMT disease, or a confirmed test in a first- or second-degree relative with a consistent clinical phenotype and confirmatory electrophysiologic testing in the child, through participating sites of the Inherited Neuropathy Consortium in the United States, United Kingdom, and Australia.
Standard protocol approvals, registrations, and patient consents.
Institutional ethics review boards approved the protocol at each participating institution. We obtained informed consent from participants' guardians as well as assent in older children.
Standardized assessments.
Demographic data collected included age, sex, race, and ethnicity. The Wong-Baker FACES Pain Rating Scale10 measured child self-reported and parent-reported (proxy) pain scores. This validated scale has scores ranging from 0 to 5 associated with faces ranging from smiling to crying; the descriptors below each face and score are as follows: 0 = “no hurt” (widely smiling face), 1 = “hurts little bit” (slightly smiling face), 2 = “hurts little more” (neutral face), 3 = “hurts even more” (slightly sad face), 4 = “hurts whole lot” (very sad face), and 5 = “hurts worst” (crying face). The instruction provided with the scale was to rate the average pain the person had experienced over the past year. We measured QOL through the Child Health Questionnaire (CHQ), a validated generic measure of health status in children.11–13 Clinical measures included sensory information (pinprick and vibration), the standardized 6-Minute Walk Test, the CMT Neuropathy Score version 2 (CMTNSv2) (a validated composite measure including historical, clinical, and electrophysiologic data, shown to correlate with disability),14,15 and the validated Foot Posture Index,16 a clinician-assessed measure of foot position.
Statistical analysis.
We calculated descriptive statistics to characterize the study sample and determine the overall prevalence of pain using Stata/IC 11 (StataCorp, College Station, TX) and SAS version 9.3 (SAS Institute Inc., Cary, NC). We calculated Pearson correlation coefficients (α = 0.05) to examine associations between pain reported by child or parent proxy and 1) CMTNSv2—a measure of neuropathy severity, and 2) CHQ and the standardized 6-Minute Walk Test—measures of functional impact. Finally, we entered the individual components of the Foot Posture Index as well as the total score into a univariate model (simple linear regression) and multivariate model (multiple linear regression) to explore associations between ankle/foot structural deformity and child-reported pain in pediatric CMT disease.
RESULTS
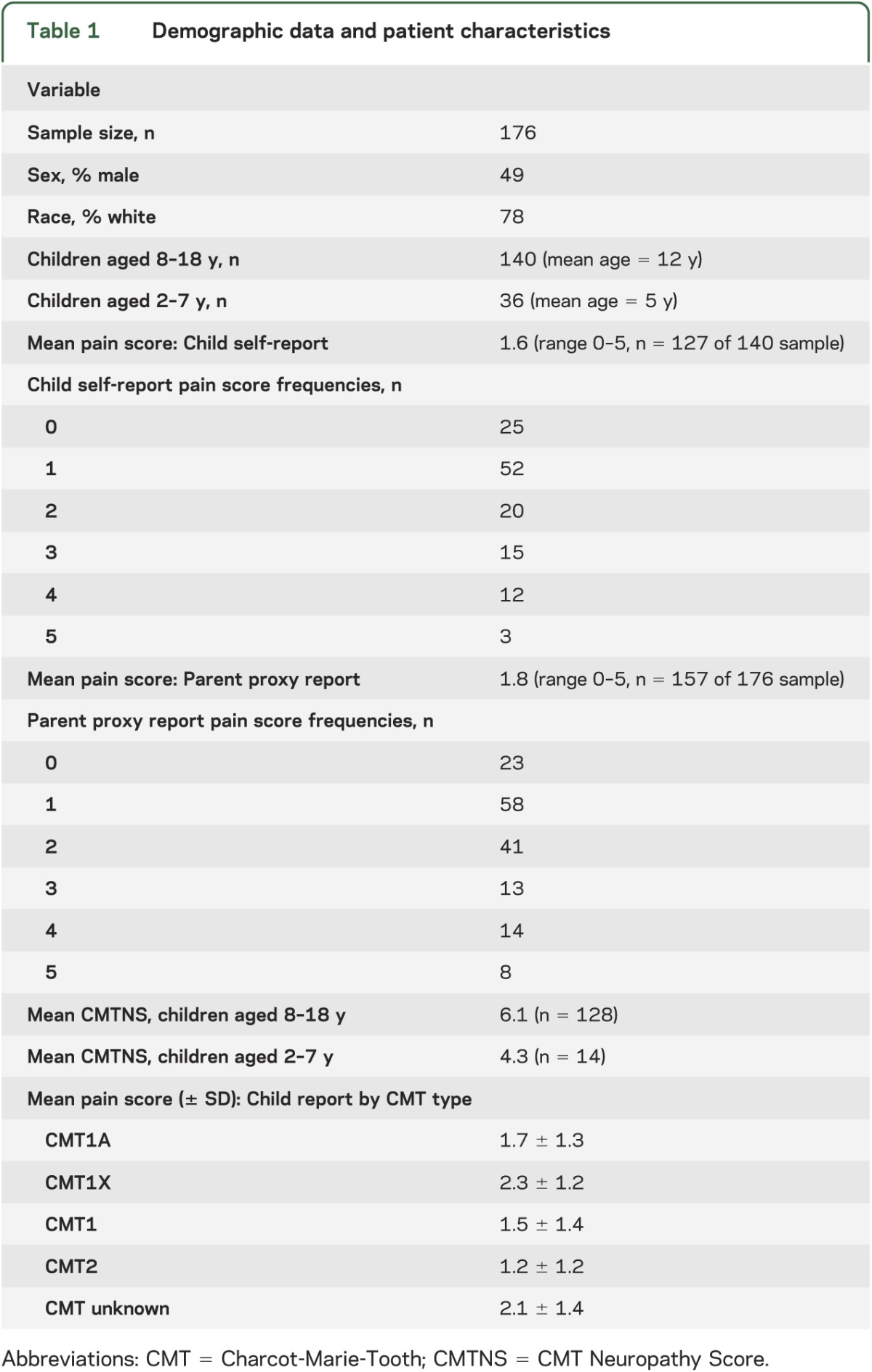
We analyzed data on 176 children assessed through the Inherited Neuropathy Consortium sites. The mean pain score was 2, which correlates to the descriptor “hurts little more” on the 0 to 5 Wong-Baker FACES Pain Rating Scale (parent proxy mean pain score was 1.8 and child self-reported mean pain score was 1.6; we rounded up to 2 because the original scale is not sensitive to the tenth decimal range). The prevalence of pain, as assessed by scores greater than 0, was 80% by child direct report and 85% by parent proxy report. The pain frequencies and demographic data are summarized in table 1.
Table 1.
Demographic data and patient characteristics
To assess the impact of pain in children with CMT disease, we generated a Pearson correlation coefficient matrix to examine associations between pain and patient-reported and clinical standardized outcomes (table 2). Increasing pain scores strongly correlated with lower (i.e., worse) QOL physical and psychosocial CHQ composite scores. Standardized 6-Minute Walk Test did not correlate significantly with pain. Pain scores also did not correlate with neuropathy severity, as assessed by the CMTNSv2: as the CMTNS increased, pain scores decreased (figure).
Table 2.
Pain correlates in children with CMT diseasea
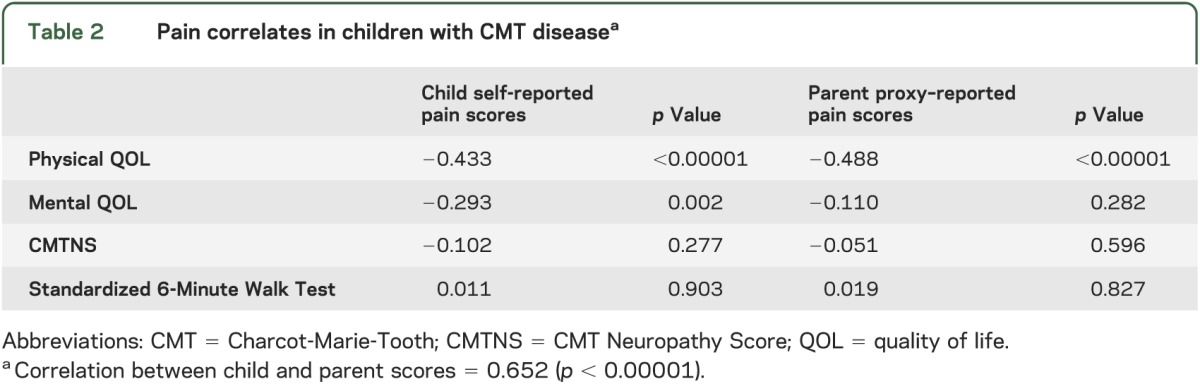
Figure. Scatterplot of neuropathy severity and child-reported pain scores.
The portion to the right of the red line indicates moderate neuropathy; as Charcot-Marie-Tooth Neuropathy Scores (CMTNS) increase, the pain scores decrease.
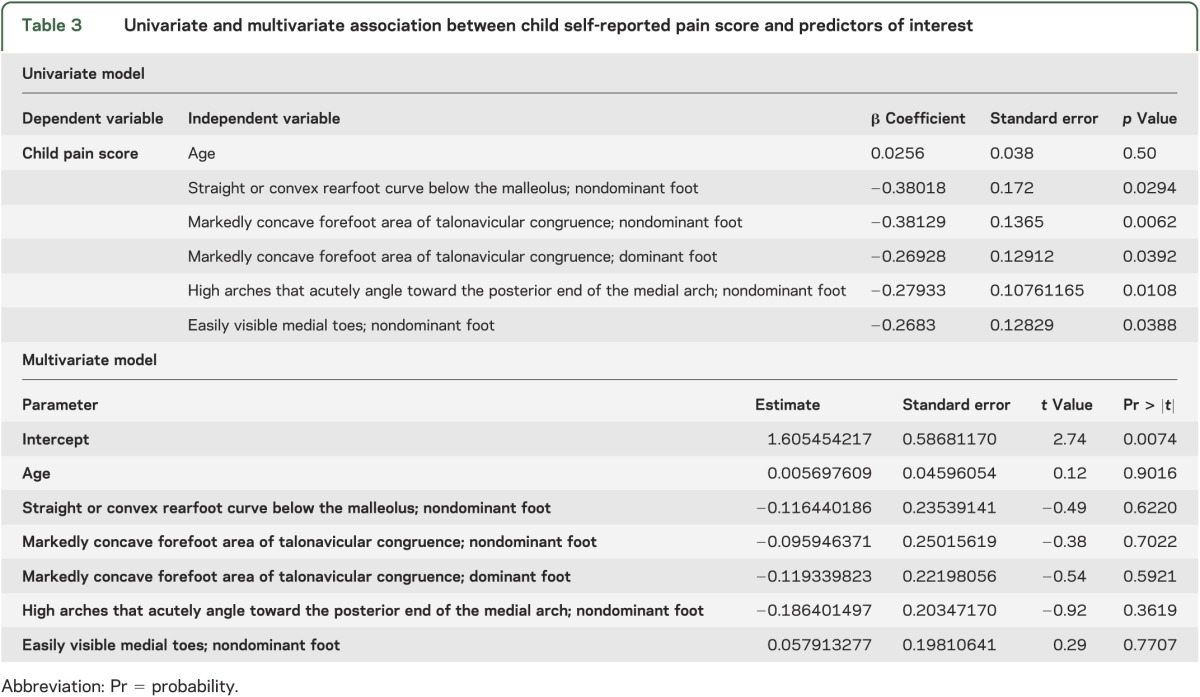
In univariate regression models, individual components of the ankle/foot index, including features of a supinated foot (straight or convex rearfoot curve below the malleolus, markedly concave forefoot area of talonavicular congruence, high arches that acutely angle toward the posterior end of the medial arch, and easily visible medial toes), correlated with increased pain scores, especially on the nondominant foot. However, in multivariate regression models with child self-reported pain scores, the associations were not significant (table 3).
Table 3.
Univariate and multivariate association between child self-reported pain score and predictors of interest
DISCUSSION
This study provides evidence that children with CMT disease report mild to moderate levels of pain, which negatively affects their physical and psychosocial QOL. Worsening severity of neuropathy does not result in increasing pain scores, suggesting that the physiology of the pain in CMT disease is not due to the nerve damage alone. Alternatively, we found that structural changes, particularly in the nondominant foot, showed significant association with pain in the univariate regression models. This association did not hold in more stringent multivariate regression models, suggesting that the measures we used—components of the foot position index—may be proxies of other, more direct measures of structural deformities in the feet.
If mediated by structural changes, the nociceptive pain that originates in pediatric CMT disease could significantly worsen by adulthood due to progressive damage to the joints; rehabilitative interventions would, theoretically, reduce this nociceptive burden. However, if the pain in adult CMT disease is more neuropathic or central, there are evidence-based guidelines for targeted therapies that have shown effectiveness (e.g., neuropathic: exercise, tricyclic antidepressants, anticonvulsants17; central: exercise and cognitive behavioral therapy,18 serotonin-norepinephrine reuptake inhibitors).19 Characterizing the pain as nociceptive, neuropathic, or structural can therefore help identify targeted, evidence-based treatments to meaningfully improve the QOL of patients with CMT disease.
Chronic pain in children can be difficult to assess and can be influenced by recall bias as well as the cognitive development of the child and other behavioral issues.20,21 While these biases cannot be discounted, we believe the strong correlation between the parent proxy report average pain scores and direct child-report average pain scores supports the validity of our obtained scores. The 6-Minute Walk Test did not correlate with pain in our study; the test is a measure of endurance and muscle fatigue, which may not correlate with pain in the CMT population. Planned prospective studies on the associations among the 6-Minute Walk Test, specific pain mechanisms, and gait analyses may provide more information on the impact of pain on daily functioning. Finally, generalization of these results to a nonstudy population of patients with CMT disease must be done with caution because the characteristics of our study patients may differ from general patients.
We urgently need to identify therapeutic interventions that can improve the daily functioning of patients with inherited neuropathies. This need is especially evident in children with CMT disease because the long-term impact of changes occurring during critical developmental stages may result in high adulthood disease burden. We plan to build on these data by multimodal phenotype characterization of the pain in adults with CMT disease, as musculoskeletal, neuropathic, or central. These results would allow a more targeted, evidence-based approach to treatment, to reduce pain and improve the QOL of children, and in the long term, adults with CMT disease.
ACKNOWLEDGMENT
The authors are grateful for the assistance of site coinvestigators Richard Finkel, Sabrina Yum (Children's Hospital of Philadelphia, PA), David Herrmann (University of Rochester, NY), Mary Reilly, Francesco Muntoni (UCL Institute of Child Health & Great Ormond Street Hospital, London, UK), Joshua Burns (Children's Hospital at Westmead, University of Sydney, Australia) and other study personnel of the Inherited Neuropathy Consortium. The authors thank the patients and their families for their participation in the study.
GLOSSARY
- CHQ
Child Health Questionnaire
- CMT
Charcot-Marie-Tooth
- CMTNSv2
Charcot-Marie-Tooth Neuropathy Score version 2
- QOL
quality of life
AUTHOR CONTRIBUTIONS
Dr. Ramchandren: study concept, interpretation, and manuscript preparation. Dr. Jaiswal: data analysis, manuscript revision. Dr. Feldman: interpretation, manuscript revision. Dr. Shy: data acquisition, interpretation, manuscript revision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
S. Ramchandren is funded by the NIH (NINDS K23-NS072279). M. Jaiswal reports no disclosures. E. Feldman is funded by the A. Alfred Taubman Medical Research Institute. M. Shy is funded by NIH (NINDS/ORD U54-NS065712), the Charcot-Marie-Tooth Association, and the Muscular Dystrophy Association. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth disease. Clin Genet 1974;6:98–118 [DOI] [PubMed] [Google Scholar]
- 2.Martyn CN, Hughes RAC. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry 1997;62:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramchandren S, Shy ME, Finkel RS. Quality of life in children with CMT type1A. Lancet Neurol 2009;8:880–881 [DOI] [PubMed] [Google Scholar]
- 4.Burns J, Ramchandren S, Ryan MM, Shy M, Ouvrier RA. Determinants of reduced health-related quality of life in pediatric inherited neuropathies. Neurology 2010;75:726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribiere C, Bernardin M, Sacconi S, et al. Pain assessment in Charcot-Marie-Tooth (CMT) disease. Ann Phys Rehabil Med 2012;55:160–173 [DOI] [PubMed] [Google Scholar]
- 6.Skaribas IM, Washburn SN. Successful treatment of Charcot-Marie-Tooth chronic pain with spinal cord stimulation: a case study. Neuromodulation 2010;13:224–228 [DOI] [PubMed] [Google Scholar]
- 7.Padua L, Cavallaro T, Pareyson D, Quattrone A, Vita G, Schenone A; Italian CMT QoL Study Group Charcot-Marie-Tooth and pain: correlations with neurophysiological, clinical, and disability findings. Neurol Sci 2008;29:193–194 [DOI] [PubMed] [Google Scholar]
- 8.Pazzaglia C, Vollono C, Ferraro D, et al. Mechanisms of neuropathic pain in patients with Charcot-Marie-Tooth 1 A: a laser-evoked potential study. Pain 2010;149:379–385 [DOI] [PubMed] [Google Scholar]
- 9.Carter GT, Jensen MP, Galer BS, et al. Neuropathic pain in Charcot-Marie-Tooth disease. Arch Phys Med Rehabil 1998;79:1560–1564 [DOI] [PubMed] [Google Scholar]
- 10.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs 1988;14:9–17 [PubMed] [Google Scholar]
- 11.Landgraf JM, Abetz L, Ware JE. The Child Health Questionnaire (CHQ) User's Manual. Boston: HealthAct; 1999 [Google Scholar]
- 12.Waters E, Salmon L, Wake M. The parent-form Child Health Questionnaire in Australia: comparison of reliability, validity, structure, and norms. J Pediatr Psychol 2000;25:381–391 [DOI] [PubMed] [Google Scholar]
- 13.Rentz AM, Matza LS, Secnik K, Swensen A, Revicki DA. Psychometric validation of the Child Health Questionnaire (CHQ) in a sample of children and adolescents with attention-deficit/hyperactivity disorder. Qual Life Res 2005;14:719–734 [DOI] [PubMed] [Google Scholar]
- 14.Murphy SM, Herrmann DN, McDermott MP, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst 2011;16:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shy ME, Blake J, Krajewski K, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005;64:1209–1214 [DOI] [PubMed] [Google Scholar]
- 16.Keenan AM, Redmond AC, Horton M, Conaghan PG, Tennant A. The Foot Posture Index: Rasch analysis of a novel, foot-specific outcome measure. Arch Phys Med Rehabil 2007;88:88–93 [DOI] [PubMed] [Google Scholar]
- 17.Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011;3:345–352.e21 [DOI] [PubMed] [Google Scholar]
- 18.Lami MJ, Martínez MP, Sánchez AI. Systematic review of psychological treatment in fibromyalgia. Curr Pain Headache Rep 2013;17:345. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry 2009;31:206–219 [DOI] [PubMed] [Google Scholar]
- 20.Gorin A, Stone A. Recall bias and cognitive errors in retrospective self-reports: a call for momentary assessments. In: Baum A, Revenson T, Singer J, editors. Handbook of Health Psychology. Mahwah, NJ: Lawrence Erlbaum Associates; 2001:405–413 [Google Scholar]
- 21.von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children's memory for pain: overview and implications for practice. J Pain 2004;5:241–249 [DOI] [PubMed] [Google Scholar]


