Abstract
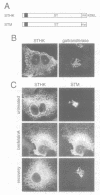
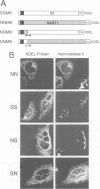
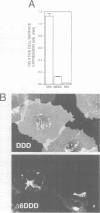
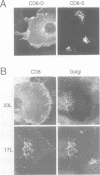
The single transmembrane domains (TMDs) of the resident glycosylation enzymes of the Golgi apparatus are involved in preventing these proteins moving beyond the Golgi. It has been proposed that either the TMDs associate, resulting in the formation of large oligomers of Golgi enzymes, or that they mediate the lateral segregation of the enzymes between lipid microdomains. Evidence for either type of interaction has been sought by examining the retention of sialyltransferase (ST), an enzyme of the mammalian trans Golgi. No evidence could be obtained for specific interactions or 'kin recognition' between ST and other proteins of the trans Golgi. Moreover, it is shown that the previously described kin recognition between enzymes of the medial Golgi involves the lumenal portions of these proteins rather than their TMDs. To investigate further the role of the ST TMD, the effects on Golgi retention of various alterations in the TMD were examined. The addition or removal of residues showed that the efficiency of retention of ST is related to TMD length. Moreover, when a type I plasma membrane protein was expressed with a synthetic TMD of 23 leucines it appeared on the cell surface, but when the TMD was shortened to 17 leucines accumulation in the Golgi was observed. These observations are more consistent with lipid-based sorting of ST TMD, but they also allow for reconciliation with the kin recognition model which appears to act on sequences outside of the TMD.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Patel S. The Golgi sorting domain of coronavirus E1 protein. J Cell Sci. 1991 Apr;98(Pt 4):567–575. doi: 10.1242/jcs.98.4.567. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Farquhar M. G. Beyond bulk flow. Trends Cell Biol. 1995 Jan;5(1):16–19. doi: 10.1016/s0962-8924(00)88928-x. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Keller D. S. ATP-coupled transport of vesicular stomatitis virus G protein. Functional boundaries of secretory compartments. J Biol Chem. 1986 Nov 5;261(31):14690–14696. [PubMed] [Google Scholar]
- Banfield D. K., Lewis M. J., Rabouille C., Warren G., Pelham H. R. Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J Cell Biol. 1994 Oct;127(2):357–371. doi: 10.1083/jcb.127.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K., Wraight C., Stanley K. K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993 May;12(5):2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S., Munro S. Cholesterol and the Golgi apparatus. Science. 1993 Sep 3;261(5126):1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
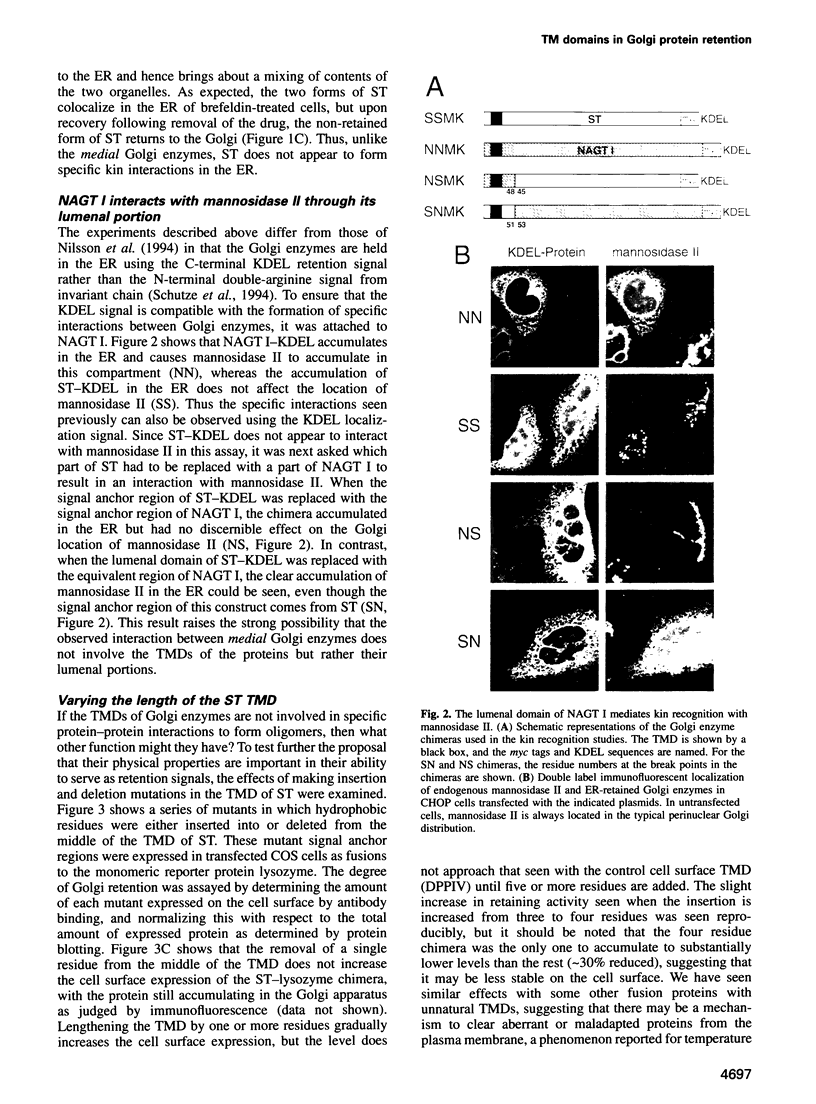
- Burke J., Pettitt J. M., Humphris D., Gleeson P. A. Medial-Golgi retention of N-acetylglucosaminyltransferase I. Contribution from all domains of the enzyme. J Biol Chem. 1994 Apr 22;269(16):12049–12059. [PubMed] [Google Scholar]
- Celis A., Madsen P., Nielsen H. V., Rasmussen H. H., Thiessen H., Lauridsen J. B., van Deurs B., Celis J. E. Human proteins IEF 58 and 57a are associated with the Golgi apparatus. FEBS Lett. 1988 Jan 18;227(1):14–20. doi: 10.1016/0014-5793(88)81404-2. [DOI] [PubMed] [Google Scholar]
- Chapman R. E., Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994 May 15;13(10):2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. E., Munro S. The functioning of the yeast Golgi apparatus requires an ER protein encoded by ANP1, a member of a new family of genes affecting the secretory pathway. EMBO J. 1994 Oct 17;13(20):4896–4907. doi: 10.1002/j.1460-2075.1994.tb06817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley K. J., Lee E. U., Paulson J. C. The signal anchor and stem regions of the beta-galactoside alpha 2,6-sialyltransferase may each act to localize the enzyme to the Golgi apparatus. J Biol Chem. 1992 Apr 15;267(11):7784–7793. [PubMed] [Google Scholar]
- Conibear E., Pearse B. M. A chimera of the cytoplasmic tail of the mannose 6-phosphate/IGF-II receptor and lysozyme localizes to the TGN rather than prelysosomes where the bulk of the endogenous receptor is found. J Cell Sci. 1994 Apr;107(Pt 4):923–932. doi: 10.1242/jcs.107.4.923. [DOI] [PubMed] [Google Scholar]
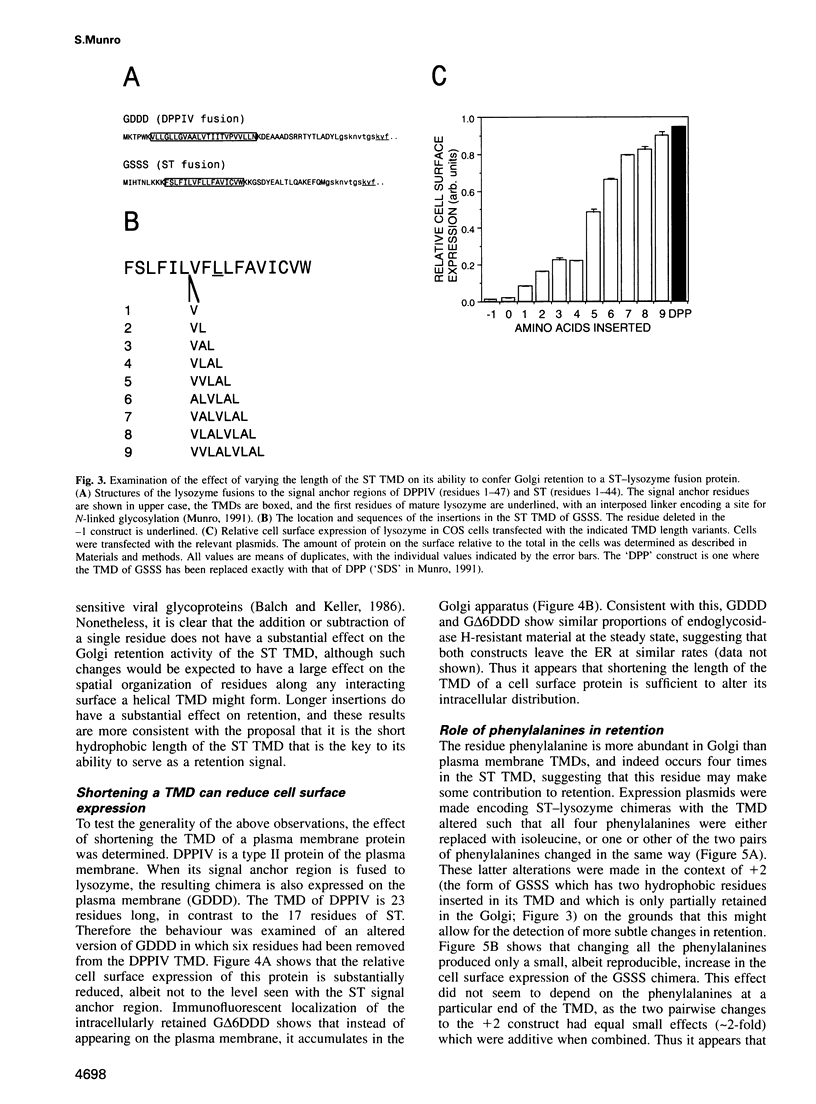
- Coxey R. A., Pentchev P. G., Campbell G., Blanchette-Mackie E. J. Differential accumulation of cholesterol in Golgi compartments of normal and Niemann-Pick type C fibroblasts incubated with LDL: a cytochemical freeze-fracture study. J Lipid Res. 1993 Jul;34(7):1165–1176. [PubMed] [Google Scholar]
- Dahdal R. Y., Colley K. J. Specific sequences in the signal anchor of the beta-galactoside alpha-2,6-sialyltransferase are not essential for Golgi localization. Membrane flanking sequences may specify Golgi retention. J Biol Chem. 1993 Dec 15;268(35):26310–26319. [PubMed] [Google Scholar]
- Delahunty M. D., Stafford F. J., Yuan L. C., Shaz D., Bonifacino J. S. Uncleaved signals for glycosylphosphatidylinositol anchoring cause retention of precursor proteins in the endoplasmic reticulum. J Biol Chem. 1993 Jun 5;268(16):12017–12027. [PubMed] [Google Scholar]
- Fiedler K., Simons K. The role of N-glycans in the secretory pathway. Cell. 1995 May 5;81(3):309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. C., Moran P., Li W., Keller G. A., Caras I. W. Retention and degradation of proteins containing an uncleaved glycosylphosphatidylinositol signal. J Biol Chem. 1994 Apr 8;269(14):10830–10837. [PubMed] [Google Scholar]
- Heffernan M., Dennis J. W. Polyoma and hamster papovavirus large T antigen-mediated replication of expression shuttle vectors in Chinese hamster ovary cells. Nucleic Acids Res. 1991 Jan 11;19(1):85–92. doi: 10.1093/nar/19.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobman T. C., Woodward L., Farquhar M. G. Targeting of a heterodimeric membrane protein complex to the Golgi: rubella virus E2 glycoprotein contains a transmembrane Golgi retention signal. Mol Biol Cell. 1995 Jan;6(1):7–20. doi: 10.1091/mbc.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
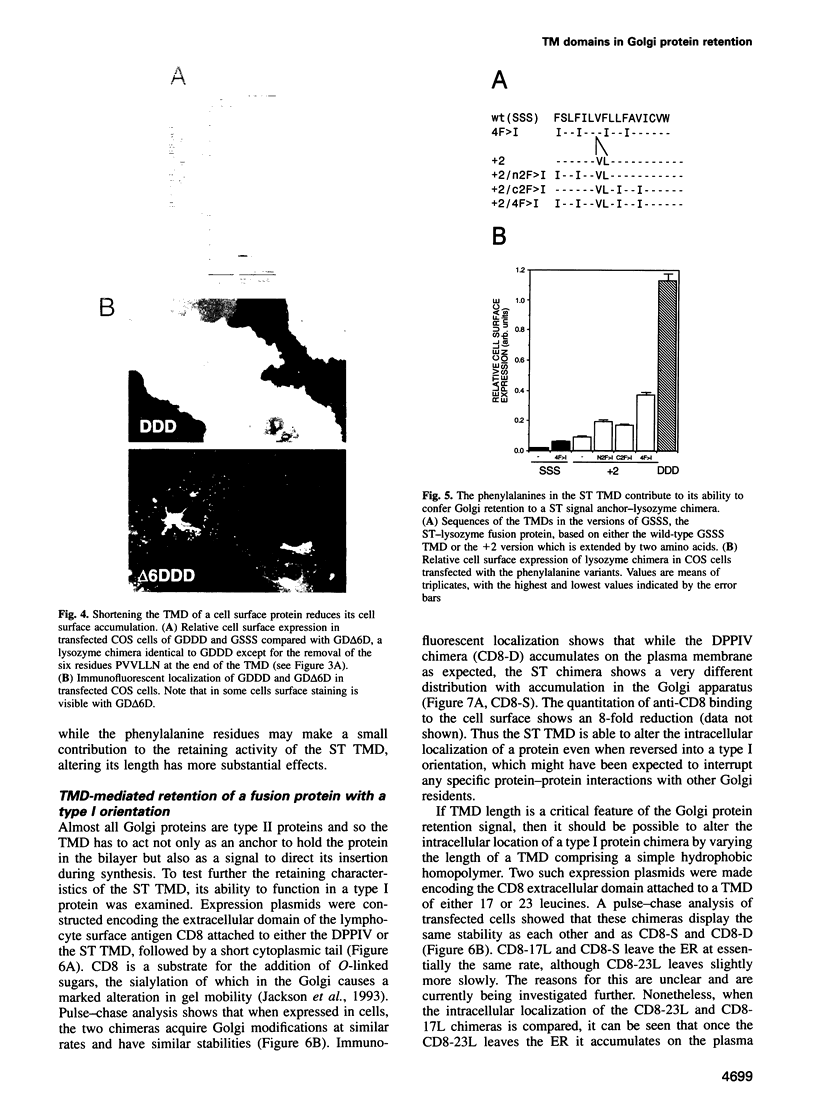
- Humphrey J. S., Peters P. J., Yuan L. C., Bonifacino J. S. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993 Mar;120(5):1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Retrieval of transmembrane proteins to the endoplasmic reticulum. J Cell Biol. 1993 Apr;121(2):317–333. doi: 10.1083/jcb.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R., Berger E. G. The molecular and cell biology of glycosyltransferases. Biochim Biophys Acta. 1993 Dec 21;1154(3-4):283–325. doi: 10.1016/0304-4157(93)90003-7. [DOI] [PubMed] [Google Scholar]
- Kumar R., Yang J., Larsen R. D., Stanley P. Cloning and expression of N-acetylglucosaminyltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9948–9952. doi: 10.1073/pnas.87.24.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Locker J. K., Opstelten D. J., Ericsson M., Horzinek M. C., Rottier P. J. Oligomerization of a trans-Golgi/trans-Golgi network retained protein occurs in the Golgi complex and may be part of its retention. J Biol Chem. 1995 Apr 14;270(15):8815–8821. doi: 10.1074/jbc.270.15.8815. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Banting G. Eukaryotic membrane traffic: retrieval and retention mechanisms to achieve organelle residence. Trends Biochem Sci. 1993 Oct;18(10):395–398. doi: 10.1016/0968-0004(93)90097-7. [DOI] [PubMed] [Google Scholar]
- Machamer C. E. Targeting and retention of Golgi membrane proteins. Curr Opin Cell Biol. 1993 Aug;5(4):606–612. doi: 10.1016/0955-0674(93)90129-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masibay A. S., Balaji P. V., Boeggeman E. E., Qasba P. K. Mutational analysis of the Golgi retention signal of bovine beta-1,4-galactosyltransferase. J Biol Chem. 1993 May 5;268(13):9908–9916. [PubMed] [Google Scholar]
- Molloy S. S., Thomas L., VanSlyke J. K., Stenberg P. E., Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994 Jan 1;13(1):18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K. W., Touster O., Robbins P. W. Novel purification of the catalytic domain of Golgi alpha-mannosidase II. Characterization and comparison with the intact enzyme. J Biol Chem. 1991 Sep 5;266(25):16876–16885. [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Models of lipid-protein interactions in membranes. Annu Rev Biophys Biomol Struct. 1993;22:145–171. doi: 10.1146/annurev.bb.22.060193.001045. [DOI] [PubMed] [Google Scholar]
- Munro S. A comparison of the transmembrane domains of Golgi and plasma membrane proteins. Biochem Soc Trans. 1995 Aug;23(3):527–530. doi: 10.1042/bst0230527. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991 Dec;10(12):3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Hoe M. H., Slusarewicz P., Rabouille C., Watson R., Hunte F., Watzele G., Berger E. G., Warren G. Kin recognition between medial Golgi enzymes in HeLa cells. EMBO J. 1994 Feb 1;13(3):562–574. doi: 10.1002/j.1460-2075.1994.tb06294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Lucocq J. M., Mackay D., Warren G. The membrane spanning domain of beta-1,4-galactosyltransferase specifies trans Golgi localization. EMBO J. 1991 Dec;10(12):3567–3575. doi: 10.1002/j.1460-2075.1991.tb04923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Slusarewicz P., Hoe M. H., Warren G. Kin recognition. A model for the retention of Golgi enzymes. FEBS Lett. 1993 Sep 6;330(1):1–4. doi: 10.1016/0014-5793(93)80906-b. [DOI] [PubMed] [Google Scholar]
- Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. Heterogeneous distribution of filipin--cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Munro S. Sorting of membrane proteins in the secretory pathway. Cell. 1993 Nov 19;75(4):603–605. doi: 10.1016/0092-8674(93)90479-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S., Rabouille C., Luzio J. P., Nilsson T., Warren G. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J Cell Biol. 1994 Apr;125(2):253–268. doi: 10.1083/jcb.125.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Hui N., Hunte F., Kieckbusch R., Berger E. G., Warren G., Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995 Apr;108(Pt 4):1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Reaves B., Horn M., Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993 Jan;4(1):93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Weinstein J., Paulson J. C., Greenwell P., Watkins W. M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986 Oct 25;261(30):14307–14312. [PubMed] [Google Scholar]
- Russo R. N., Shaper N. L., Taatjes D. J., Shaper J. H. Beta 1,4-galactosyltransferase: a short NH2-terminal fragment that includes the cytoplasmic and transmembrane domain is sufficient for Golgi retention. J Biol Chem. 1992 May 5;267(13):9241–9247. [PubMed] [Google Scholar]
- Sandelius A. S., Penel C., Auderset G., Brightman A., Millard M., Morré D. J. Isolation of highly purified fractions of plasma membrane and tonoplast from the same homogenate of soybean hypocotyls by free-flow electrophoresis. Plant Physiol. 1986 May;81(1):177–185. doi: 10.1104/pp.81.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram M. B., Thompson T. E. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 1990 Nov 27;29(47):10676–10684. doi: 10.1021/bi00499a015. [DOI] [PubMed] [Google Scholar]
- Schimmöller F., Singer-Krüger B., Schröder S., Krüger U., Barlowe C., Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO J. 1995 Apr 3;14(7):1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze M. P., Peterson P. A., Jackson M. R. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994 Apr 1;13(7):1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Hauri H. P., Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J Cell Biol. 1994 Jul;126(1):25–39. doi: 10.1083/jcb.126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwientek T., Lorenz C., Ernst J. F. Golgi localization in yeast is mediated by the membrane anchor region of rat liver sialyltransferase. J Biol Chem. 1995 Mar 10;270(10):5483–5489. doi: 10.1074/jbc.270.10.5483. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Sweet D. J., Pelham H. R. The Saccharomyces cerevisiae SEC20 gene encodes a membrane glycoprotein which is sorted by the HDEL retrieval system. EMBO J. 1992 Feb;11(2):423–432. doi: 10.1002/j.1460-2075.1992.tb05071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. L., Wong S. H., Low S. H., Hong W. Retention of a type II surface membrane protein in the endoplasmic reticulum by the Lys-Asp-Glu-Leu sequence. J Biol Chem. 1992 Apr 5;267(10):7072–7076. [PubMed] [Google Scholar]
- Teasdale R. D., Matheson F., Gleeson P. A. Post-translational modifications distinguish cell surface from Golgi-retained beta 1,4 galactosyltransferase molecules. Golgi localization involves active retention. Glycobiology. 1994 Dec;4(6):917–928. doi: 10.1093/glycob/4.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A., Hendricks L., Moremen K. W., Tulsiani D. R., Touster O., Farquhar M. G. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J Cell Biol. 1993 Jul;122(1):39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. Primary structure of beta-galactoside alpha 2,6-sialyltransferase. Conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J Biol Chem. 1987 Dec 25;262(36):17735–17743. [PubMed] [Google Scholar]
- Weisz O. A., Swift A. M., Machamer C. E. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J Cell Biol. 1993 Sep;122(6):1185–1196. doi: 10.1083/jcb.122.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. H., Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993 Oct 25;268(30):22853–22862. [PubMed] [Google Scholar]
- Wong S. H., Low S. H., Hong W. The 17-residue transmembrane domain of beta-galactoside alpha 2,6-sialyltransferase is sufficient for Golgi retention. J Cell Biol. 1992 Apr;117(2):245–258. doi: 10.1083/jcb.117.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]