Abstract
Objective:
To evaluate the risks of continuously administered IV anesthetic drugs (IVADs) on the outcome of adult patients with status epilepticus (SE).
Methods:
All intensive care unit patients with SE from 2005 to 2011 at a tertiary academic medical care center were included. Relative risks were calculated for the primary outcome measures of seizure control, Glasgow Outcome Scale score at discharge, and death. Poisson regression models were used to control for possible confounders and to assess effect modification.
Results:
Of 171 patients, 37% were treated with IVADs. Mortality was 18%. Patients with anesthetic drugs had more infections during SE (43% vs 11%; p < 0.0001) and a 2.9-fold relative risk for death (2.88; 95% confidence interval 1.45–5.73), independent of possible confounders (i.e., duration and severity of SE, nonanesthetic third-line antiepileptic drugs, and critical medical conditions) and without significant effect modification by different grades of SE severity and etiologies. As IVADs were used after first- and second-line drugs failed, there was a correlation between treatment-refractory SE and the use of IVADs, leading to insignificant results regarding the risk of IVADs and outcome after additional adjustment for refractory SE.
Conclusion:
Our findings heighten awareness regarding adverse effects of IVADs. Randomized controlled trials are needed to further clarify the association of IVADs with outcome in patients with SE.
Classification of evidence:
This study provides Class III evidence that patients with SE receiving IVADs have a higher proportion of infection and an increased risk of death as compared to patients not receiving IVADs.
In 10%–40% of patients with status epilepticus (SE), ictal activity cannot be controlled with first-line (benzodiazepines) and second-line antiepileptic drugs (AEDs) such as phenytoin, valproate, or levetiracetam, resulting in a mortality of ∼40%.1
Most opinion leaders recommend IV anesthetic drugs (IVADs)—thiopental, midazolam, propofol, and high-dose phenobarbital—for refractory SE to induce total seizure suppression, an EEG burst-suppression pattern,2 or an isoelectric EEG.3 The Neurocritical Care Society (NCS) outlines the role of IVADs, but notes the lack of supporting data,4 while the European Federation of Neurological Societies (EFNS) points to the need for further studies.5 Aside from a few systematic reviews,6–8 a retrospective analysis of 126 patients with mainly convulsive SE provided perturbing results, as the administration of anesthetics was associated with poor outcome.9 However, interpretation of this study is impeded by the lack of adjustment for possible confounders.
Randomized trials regarding risks and benefits of IVADs are lacking and not registered according to the US NIH (www.clinicaltrials.org), mirroring ethical restrictions of assigning or excluding patients with treatment-refractory SE from IVADs, with the inherent risk of sustained SE. These limitations add to the drawback of already small sample sizes, resulting in insufficient statistical power.
In response to the unacknowledged appeal of the EFNS and the NCS, we evaluated the relative risks of continuously administered IVADs on outcomes of adult SE patients independent of confounders.
METHODS
Primary research question and classification of level of evidence.
The primary research question was to assess the relative risks of continuously administered IVADs on outcomes of adult SE patients. Our study provides Class III evidence.
Setting and design.
This observational cohort study was performed in the intensive care units (ICUs) at the University Hospital Basel, Switzerland, a tertiary academic medical care center with >4,000 ICU admissions per year. All ICUs requested consults from the same team of neurologists regarding diagnosis and treatment of SE patients and followed the same treatment algorithm. We adhered to the STROBE guidelines for reporting observational studies (www.strobe-statement.org).
Standard protocol approvals, registrations, and patient consents.
The study was approved by the local ethics committee according to the declaration of Helsinki and patient consent was waived.
Patients and data collection.
Clinical and electroencephalographic data of all consecutive adult patients with SE from January 2005 to January 2011 in the medical, cardiac, and surgical ICUs derived from a prospectively established SE database.10,11 Demographics, level of consciousness at SE onset, SE duration and etiology, worst seizure type at presentation, additional critical medical conditions, duration of mechanical ventilation, use of vasopressors during SE, infections, and ICU and hospital stay were assessed from digital databases. Furthermore, death and Glasgow Outcome Scale score (GOS) at discharge, as well as time from SE onset to follow-up after discharge, were noted. The diagnosis criteria for infections during SE are given in table e-1 on the Neurology® Web site at www.neurology.org. During the study period, antiepileptic therapy was standardized as follows: initial treatment with first-line AEDs (IV benzodiazepines administered as bolus), followed by second-line AEDs (including phenytoin, levetiracetam, and valproic acid) if SE persisted, and further escalated with nonanesthetic third-line AED treatment (including lacosamide, topiramate, pregabalin, carbamazepine, and oxcarbazepine), with or without IVADs (including continuous infusions of midazolam, propofol, and barbiturates) if second-line AEDs failed. In our institution, midazolam or propofol were administered for seizure suppression before barbiturates were considered. Barbiturates were used to induce an isoelectric EEG.12 We assessed the order of AEDs and IVADs. Patients with missing data were excluded, as were post–cardiac arrest patients with SE from hypoxic-ischemic encephalopathy, as these patients receive IVADs during therapeutic hypothermia and this SE type is considered a different entity because of its largely irreversible brain damage and poor outcome.13
Status epilepticus: Definition and severity.
According to the guidelines for evaluation and management of SE, we defined SE as clinical and EEG evidence of seizures that lasted at least 5 minutes or as a series of epileptic seizures that were present without complete intervening clinical recovery.4,14 We defined SE duration as the period from SE diagnosis to SE cessation and seizure control if there were no clinical manifestations or ictal activity on EEG.
In order to assess and adjust analyses for SE severity, we graded SE with the independently validated Status Epilepticus Severity Score (STESS), a scoring system that reliably grades SE severity and prediction of death.15–17 We categorized the following integral components of STESS as proposed elsewhere15,16: worst seizure types at presentation (simple partial, complex partial, and absence seizures = 0 points; generalized convulsive seizures = 1 point; and nonconvulsive status epilepticus in coma = 2 points), history of prior seizures (present = 0 points; absent = 1 point), age ≥65 years (2 points), and level of consciousness at SE onset (stuporous/comatose = 1 point).
Primary and secondary outcomes.
Primary outcomes included seizure control, GOS, and death. GOS was dichotomized as favorable (GOS 1–3) and unfavorable outcome (GOS >3).
Duration of ICU and hospital stay were defined as secondary outcomes.
Statistics.
Patients were categorized into 2 groups: with and without treatment with IVADs. χ2 and Fisher exact test (where appropriate) were used for comparisons of proportions. For continuous variables, the Shapiro-Wilk test was used to distinguish between normal and abnormal distributions. Normally distributed variables were analyzed by using the Student t test and non-normally-distributed variables by using the Mann-Whitney U test.
Relative risks of death were estimated by Poisson regression with robust error variance,18 and were adjusted for potential confounders including SE duration and severity (graded by STESS including age, prior history of seizures, worst seizure type, and level of consciousness at SE onset), as well as receipt of nonanesthetic third-line AEDs and additional critical medical conditions, all differing significantly in the univariable comparison of patients with and without IVADs. Additional adjustment for age was not performed to avoid overadjustment, as age is an integral component of STESS. Subsequently, an additional multivariable analysis was performed with adjustment for SE refractory to first- and second-line AEDs. Interaction terms were fit to the regression models evaluating the associations between IVADs and death by variables of STESS, and by etiologies according to the guidelines of the International League Against Epilepsy to evaluate effect modification.19 The Pearson and deviance goodness-of-fit tests were performed to assess the fit of the data to a Poisson distribution in the final regression models.
Two-sided p values ≤0.05 were considered significant. Statistical analysis was performed with STATA version 12.0 (Stata Corp., College Station, TX).
RESULTS
Between January 2005 and January 2011, 213 consecutive adult patients were identified with SE. Patients with hypoxic-ischemic encephalopathy (36; 17%) or missing data (6; 3%) were excluded. Of the remaining 171 patients, 63 (37%) were treated with continuously administered IVADs. Demographics, clinical characteristics, and their univariable comparisons are presented in table 1. Detailed information regarding SE etiologies is outlined in table e-2. Severity of SE and all integral variables of STESS differed between patients with and without IVADs.
Table 1.
Demographics and clinical characteristics of patients with and without continuous IV anesthetic drugs

Treatment.
The mean number of nonanesthetic AEDs did not differ between patients with and without IVADs (table 2). IVADs were used in 63 patients refractory to first- and second-line AEDs. Among patients with IVADs, 29 had midazolam only, 22 midazolam followed by propofol, and 12 received barbiturates after midazolam. In patients with IVADs, a higher proportion had to be intubated (with IVADs 57/63, 90%, without IVADs 25/108, 25%, p < 0.0001). However, there was no difference in the mean duration of mechanical ventilation between both groups (12.3 days ± 11.5 with and 12.6 days ± 37.1 without IVADs; p = 0.956).
Table 2.
Antiepileptic treatment in patients with and without continuous IV anesthetic drugs

A higher proportion of patients with IVADs had infections that were diagnosed during SE (27/63, 43% with and 12/108, 11% without IVADs; p < 0.0001). Most infections were respiratory tract infections (25/27). Severe hypotension requiring vasopressors during SE was more frequent in patients with IVADs (11/63, 16% with and 2/108, 1.9% without IVADs). Eight patients received noradrenalin, 3 adrenalin, and 2 dobutamine.
Outcome.
Seizure control in patients with and without IVADs during SE was similar (table 3). Of the 141 survivors, follow-up after discharge was available in 136 patients who were all still alive (96.5% of survivors); median time from SE onset to follow-up was 392 days (interquartile range 23.5–2058). A higher proportion of patients with IVADs died. Use of IVADs was associated with longer ICU and hospital stays, unfavorable outcome, and death in the univariable analysis (table 3). Among the 19 nonsurvivors treated with IVADs, 5 died during SE, 11 most likely from infections developing during SE, 2 from multiorgan failure, and in 1 patient the cause of death could not be determined. In the 11 nonsurvivors without IVADs, 3 died during SE, 3 from infections, and 5 from progression of the underlying pathologic condition (i.e., brain tumors, cerebral ischemia, or hemorrhage).
Table 3.
Primary and secondary outcomes in patients with and without continuous IV anesthetic drugs

As SE duration and severity, etiology, as well as critical medical conditions may have played an important role in the decisions regarding administration of IVADs (and may have substantially influenced course and outcome), a multivariable approach was applied for primary outcome measures (table 4). After adjusting for SE duration, critical medical conditions, SE severity (graded by STESS), and receipt of nonanesthetic third-line AEDs, variables differing between the 2 groups (table 1), the relative risk of death was associated with IVADs. Regarding the number of IVADs, every additional drug was associated with an incremental relative risk of 1.6 for death in the multivariable analysis (table 4).
Table 4.
Crude and adjusted relative risks for primary outcomes according to antiepileptic treatment

The administration of different combinations of IVADs during SE and their associations with death revealed the strongest associations for midazolam followed by barbiturates or propofol. As IVADs were only used after first- and second-line AEDs failed, there was a strong correlation between treatment-refractory SE and the use of IVADs, leading to insignificant results regarding the risk of IVADs and outcome after additional adjustment for refractory SE.
The Pearson and deviance goodness-of-fit tests revealed insignificant p values, indicating adequate model-fit (data not shown).
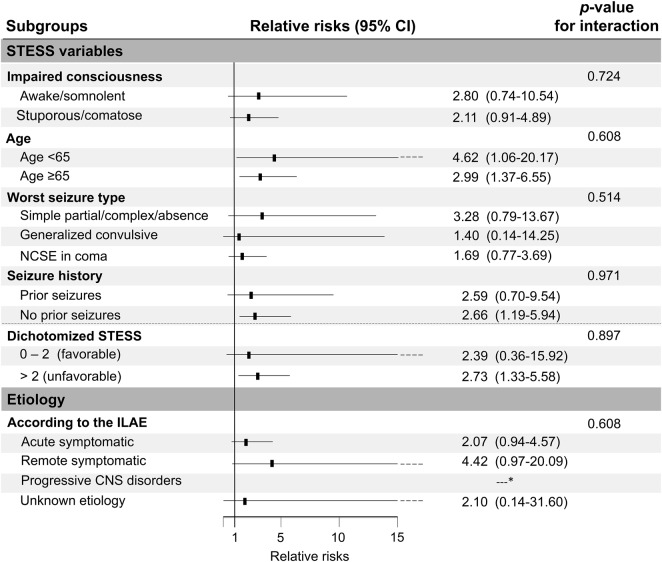
Effect modification.
Increased risk of death in patients receiving IVADs was similar across the predefined subgroups (figure). Patients with simple partial, complex partial, or absence seizures had a trend towards higher relative risks for death from IVADs than patients with convulsive SE and NCSE.
Figure. Effect modification of the use of IV anesthetic drugs by clinical variables.
CI = confidence interval; ILAE = International League Against Epilepsy; NCSE = nonconvulsive status epilepticus; STESS = Status Epilepticus Severity Score. *Only 3 cases.
Sensitivity analyses.
As the median SE duration differed between patients dying during SE (2.5 days, range 0.5–15) and surviving (1 day, range 0.5–43) (p = 0.032), sensitivity analyses were performed by multivariable regression models after exclusion of patients dying during SE. The associations detected in table 4 remained significant, except for the association of IVADs with GOS 1–3, for which the p value changed from 0.041 to 0.060.
DISCUSSION
The use of IVADs in SE was associated with an increased relative risk of death independent of possible confounders and without significant changes in the risk by different grades of SE severity and different SE etiologies. This study represents an investigation adjusting for critical medical conditions, duration, severity, and refractoriness of SE by using a prospectively validated scoring system (STESS), and evaluating for possible effect modification.
SE was controlled in 90% of patients with IVADs, a result similar to a study of midazolam infusion for treatment of refractory SE with seizure control in 60%–82%.6,20,21 The proportion of infections in patients with IVADs, however, was higher (43%) than in a study of the use of propofol in refractory SE (33%).22 This result needs to be interpreted with caution, as aspiration and consecutive respiratory tract infections may also be caused by insufficient swallowing and impaired cough reflex in patients with an altered level of consciousness due to SE. However, in a retrospective study of 63 refractory SE episodes, duration of drug-induced coma, arrhythmias requiring intervention, and pneumonia were associated with poor outcome.23 Regarding different IVADs, the association with death was strongest with midazolam followed by barbiturates or propofol in our study—results that are underscored by a study of patients with mainly convulsive SE.9 In a multicenter study, coma induction in SE patients was associated with death in the univariable analysis but lost significance in the multivariable model.16 However, the proportion of patients with coma induction was small (25%, 39/154), possibly impeding the statistical power, and the proportion of survivors in the coma induction group was lower (54%) than in the group without (65%).
In our cohort, the relation between the use of IVADs and death was not modified by different grades of SE severity, suggesting that the association of IVADs with an increased risk of death did not depend on to whom or when, but on the fact that IVADs were used. The question if earlier administration of IVADs (i.e., as second-line treatment) might be less problematic and more effective in certain SE types24 remains unresolved.
Several hazards may contribute to poor outcome, including cardiotoxicity with phenobarbital and pentobarbital, severe hypotension from thiopental,25 or hepatotoxicity, metabolic acidosis, rhabdomyolysis, and cardiac failure seen in the propofol infusion syndrome.26 Further, infections affect one-third of patients in some studies.22 In a randomized trial regarding effectiveness of midazolam, propofol, and pentobarbital in refractory SE, mortality was still 48% independent of drug or treatment intensity, and with a higher proportion of severe hypotension in patients on pentobarbital,8 a frequent side effect in SE patients treated with IVADs.27 A higher proportion of infections and the use of vasopressors indicating severe hypotension were identified as possible mediators for poor outcome in our cohort.
IVADs are widely used in the treatment of SE despite known risks of adverse effects. Conversely, there are few case series and systematic reviews, and no Class I evidence supporting their use.6–8 Systematic reviews conclude that coma induction with barbiturates effectively terminates seizures, but delays the recovery from coma and prolongs mechanical ventilation and intensive care.6–8 One small multicenter trial showed wide confidence intervals for thiopental and propofol regarding seizure control, suggesting that the study was underpowered, or that the drugs largely vary in efficacy.28 In our study, barbiturates were the only IVADs used to induce an isoelectric EEG. Hence it remains questionable whether barbiturates have similar relative risks for death when used for seizure control without isoelectric EEG.
The risks regarding the use of IVADs in SE in our study raise great concern. There is growing uncertainty regarding the benefits of these drugs in SE vs their potential harm. Should patients be treated with rapid intubation and high-dose IVADs, or managed less urgently, even when delayed seizure control might incur neuronal damage? This decision is complex.29 Simplified “one-fits-all” management algorithms are not tenable, as morbidity and mortality vary considerably among different SE etiologies. The question whether SE in general damages the brain remains unresolved. While animal models support neuronal damage, there are insufficient data in humans.30,31 Most clinical studies emphasize that SE etiology remains the main determinant of outcome.32,33 Hence the main therapeutic goals should include developing strategies tailored to SE etiology and worst seizure type at onset, and consequently avoiding therapeutic harm from adverse effects. New generations of broad-spectrum AEDs with fewer adverse effects were not associated with increased risk of death in our cohort and are promising alternatives that may reduce the use of IVADs (e.g., topiramate34 or lacosamide35). In a study of refractory SE, seizures were terminated in 71% of patients within 72 hours and in 9% within 24 hours after administration of topiramate.34 However, SE termination could not be linked to topiramate, as most patients received additional nonanesthetic AEDs and IVADs. While the effect of topiramate on outcome without IVADs remains unclear, the independent association between lacosamide and favorable short-term outcome in patients with refractory SE was detected with no differences in the use of IVADs in patients with and without lacosamide.35
The strengths of this study are the large cohort, the use of relative risks to avoid an overestimation of associations in contrast to odds ratios,36 and adjustments for duration, refractoriness, and severity of SE, and finally for additional critical medical conditions to exclude possible confounding by indication.
The limitations of this study include the retrospective observational design in a single tertiary care center, which may limit generalizability. Therefore, analysis could only provide associations, and inference regarding causality cannot be drawn. In patients who developed SE prior to hospital admission, SE duration may be underestimated. However, it is unlikely that this possible inaccuracy influenced our results regarding the effect of IVADs, as this shortcoming would affect the entire cohort and not a specific subgroup. Furthermore, time from SE onset to SE diagnosis remains a critical parameter if “out-of-hospital” SE onset is unwitnessed, even in prospective studies. Due to the collinearity between SE refractory to first- and second-line AEDs and the use of IVADs, no significant results regarding the risk of IVADs and outcome were detected after additional adjustment for refractory SE. Many authors require failure of 2 AEDs before deeming SE refractory, producing unacceptable delays in using definitive therapies. In the Veterans Affairs trial, there was an unacceptably small likelihood that a second conventional agent would succeed,37 leading to the suggestion that failure of any additional drug should constitute refractory SE.38 These conflicting definitions of treatment-refractory SE and the fact that it dichotomizes patients into only 2 different SE severity groups are critical. We therefore argue that adjusting for SE duration and STESS including several important determinants of SE severity (i.e., age, seizure type, level of consciousness at presentation, and seizure history) more likely reflects true associations in our cohort. Although IVADs followed second-line AEDs and were accompanied by third-line therapy, IVAD administration was not strictly standardized. Clinicians possibly selected patients for IVADs who had seemingly more refractory or severe SE, a bias that cannot be excluded completely. Furthermore, additional potential confounding factors may have not been captured in the medical records and hence not been accounted for in the multivariable model.
The correlation between dose and duration of IVAD administration and outcome could not be analyzed, as they were influenced by the individual EEG responses and not by the actual serum levels. Furthermore, individual IVAD doses were adapted according to interacting comedication and individual factors such as induced liver enzymes. Hence, administered doses did not reflect the serum levels in many patients.
This study provides Class III evidence that patients with SE receiving IVADs have a higher proportion of infections and an increased risk of death compared with patients not receiving IVADs. Randomized trials are needed to further clarify the association of IVADs with outcome.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Sarah Tschudin-Sutter, MD, MSc (Johns Hopkins Bloomberg School of Public Health), for statistical work.
GLOSSARY
- AED
antiepileptic drug
- EFNS
European Federation of Neurological Societies
- GOS
Glasgow Outcome Scale
- ICU
intensive care unit
- IVAD
IV anesthetic drug
- NCS
Neurocritical Care Society
- SE
status epilepticus
- STESS
Status Epilepticus Severity Score
Footnotes
Editorial, page 650
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Raoul Sutter (Johns Hopkins University School of Medicine and University Hospital Basel) planned the work, acquired, analyzed, and interpreted the data, and wrote the manuscript. Dr. Stephan Rüegg (University Hospital Basel) participated in the study design and coordination and revised the manuscript. Dr. Stephan Marsch (University Hospital Basel), Dr. Peter Fuhr (University Hospital Basel), and Dr. Peter W. Kaplan (Johns Hopkins Bayview Medical Center) analyzed and interpreted the data, revised the manuscript, and substantially contributed to the inaugural draft. All authors approved the final submitted version.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
R. Sutter is supported by the Research Fund of the University Basel, the Scientific Society Basel, and the Gottfried Julia Bangerter-Rhyner Foundation. He has held stock from Novartis and Roche since 2005. S. Marsch reports no disclosures. P. Fuhr is supported by the Swiss National Science Foundation, the Swiss Parkinson's Disease Society, the Gossweiler Foundation, the Botnar Foundation, the Scientific Society Basel, the Novartis Foundation, Novartis, and Roche. He received honoraria from serving on the advisory boards of UCB, Abbott, and Roche. P. Kaplan has provided unsponsored grand rounds; published books on EEG, status epilepticus, and epilepsy receiving honoraria; and is on Qatar Research Foundation grant on continuous EEG monitoring in status epilepticus. S. Rüegg received unconditional research grants from UCB. He received honoraria from serving on the scientific advisory boards of Desitin, Eisai, GSK, and UCB; travel grants from GSK, Janssen-Cilag, and UCB;’ and speaker fees from UCB and from serving as a consultant for Eisai, GlaxoSmithKline, Janssen-Cilag, Pfizer, Novartis, and UCB. He does not hold any stock in any pharmaceutical industries or manufacturers of medical devices. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia 2010;51:251–256 [DOI] [PubMed] [Google Scholar]
- 2.Holtkamp M, Masuhr F, Harms L, Einhaupl KM, Meierkord H, Buchheim K. The management of refractory generalised convulsive and complex partial status epilepticus in three European countries: a survey among epileptologists and critical care neurologists. J Neurol Neurosurg Psychiatry 2003;74:1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan PW. Nonconvulsive status epilepticus. Neurology 2003;61:1035–1036 [DOI] [PubMed] [Google Scholar]
- 4.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23 [DOI] [PubMed] [Google Scholar]
- 5.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol 2010;17:348–355 [DOI] [PubMed] [Google Scholar]
- 6.Parviainen I, Kalviainen R, Ruokonen E. Propofol and barbiturates for the anesthesia of refractory convulsive status epilepticus: pros and cons. Neurol Res 2007;29:667–671 [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar H, Bindra A, Singh GP, Kalaivani M. Propofol versus thiopental sodium for the treatment of refractory status epilepticus. Cochrane Database Syst Rev 2012;8:CD009202. [DOI] [PubMed] [Google Scholar]
- 8.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43:146–153 [DOI] [PubMed] [Google Scholar]
- 9.Kowalski RG, Ziai WC, Rees RN, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med 2012;40:2677–2684 [DOI] [PubMed] [Google Scholar]
- 10.Sutter R, Tschudin-Sutter S, Grize L, et al. Associations between infections and clinical outcome parameters in status epilepticus: a retrospective 5-year cohort study. Epilepsia 2012;53:1489–1497 [DOI] [PubMed] [Google Scholar]
- 11.Sutter R, Tschudin-Sutter S, Grize L, Widmer AF, Marsch S, Ruegg S. Acute phase proteins and white blood cell levels for prediction of infectious complications in status epilepticus. Crit Care 2011;15:R274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroeger D, Amzica F. Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci 2007;27:10597–10607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 2007;69:255–260 [DOI] [PubMed] [Google Scholar]
- 14.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia 1999;40:120–122 [DOI] [PubMed] [Google Scholar]
- 15.Rossetti AO, Logroscino G, Bromfield EB. A clinical score for prognosis of status epilepticus in adults. Neurology 2006;66:1736–1738 [DOI] [PubMed] [Google Scholar]
- 16.Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–1566 [DOI] [PubMed] [Google Scholar]
- 17.Sutter R, Kaplan PW, Rüegg S. Independent external validation of the status epilepticus severity score. Crit Care Med 2013:27:321–329 [DOI] [PubMed] [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 19.Commission on Epidemiology and Prognosis of the International League Against Epilepsy. Guidelines for epidemiologic studies on epilepsy. Epilepsia 1993;34:592–596 [DOI] [PubMed] [Google Scholar]
- 20.Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology 2001;57:1036–1042 [DOI] [PubMed] [Google Scholar]
- 21.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210 [DOI] [PubMed] [Google Scholar]
- 22.Power KN, Flaatten H, Gilhus NE, Engelsen BA. Propofol treatment in adult refractory status epilepticus. Mortality risk and outcome. Epilepsy Res 2011;94:53–60 [DOI] [PubMed] [Google Scholar]
- 23.Hocker SE, Britton JW, Mandrekar JN, Wijdicks EF, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol 2013;70:72–77 [DOI] [PubMed] [Google Scholar]
- 24.Bleck TP. Status epilepticus and the use of continuous EEG monitoring in the intensive care unit. Continuum 2012;18:560–578 [DOI] [PubMed] [Google Scholar]
- 25.Etsten B, Li TH. Hemodynamic changes during thiopental anesthesia in humans: cardiac output, stroke volume, total peripheral resistance, and intrathoracic blood volume. J Clin Invest 1955;34:500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med 2009;37:3024–3030 [DOI] [PubMed] [Google Scholar]
- 27.Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol 2005;62:1698–1702 [DOI] [PubMed] [Google Scholar]
- 28.Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care 2011;14:4–10 [DOI] [PubMed] [Google Scholar]
- 29.Ferguson M, Bianchi MT, Sutter R, et al. Calculating the risk benefit equation for aggressive treatment of non-convulsive status epilepticus. Neurocrit Care 2013;18:216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scantlebury MH, Heida JG, Hasson HJ, et al. Age-dependent consequences of status epilepticus: animal models. Epilepsia 2007;48(suppl 2):75–82 [DOI] [PubMed] [Google Scholar]
- 31.Young GB. Status epilepticus and brain damage: pathology and pathophysiology. Adv Neurol 2006;97:217–220 [PubMed] [Google Scholar]
- 32.Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry 2006;77:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokic DV, Jankovic SM, Vojvodic NM, Ristic AJ. Etiology of a short-term mortality in the group of 750 patients with 920 episodes of status epilepticus within a period of 10 years (1988-1997). Seizure 2009;18:215–219 [DOI] [PubMed] [Google Scholar]
- 34.Hottinger A, Sutter R, Marsch S, Ruegg S. Topiramate as an adjunctive treatment in patients with refractory status epilepticus: an observational cohort study. CNS Drugs 2012;26:761–772 [DOI] [PubMed] [Google Scholar]
- 35.Sutter R, Marsch S, Rüegg S. Safety and efficacy of intravenous lacosamide for adjunctive treatment of refractory status epilepticus: a comparative cohort study. CNS Drugs 2013;27:321–329 [DOI] [PubMed] [Google Scholar]
- 36.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ 1998;316:989–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–798 [DOI] [PubMed] [Google Scholar]
- 38.Bleck TP. Refractory status epilepticus. Curr Opin Crit Care 2005;11:117–120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.