Abstract
Objectives:
Using high-resolution structural MRI, we endeavored to study the relationships among APOE ε4, hippocampal subfield and stratal anatomy, and episodic memory.
Methods:
Using a cross-sectional design, we studied 11 patients with Alzheimer disease dementia, 14 patients with amnestic mild cognitive impairment, and 14 age-matched healthy controls with no group differences in APOE ε4 carrier status. Each subject underwent ultra-high-field 7.0-tesla MRI targeted to the hippocampus and neuropsychological assessment.
Results:
We found a selective, dose-dependent association of APOE ε4 with greater thinning of the CA1 apical neuropil, or stratum radiatum/stratum lacunosum-moleculare (CA1-SRLM), a hippocampal subregion known to exhibit early vulnerability to neurofibrillary pathology in Alzheimer disease. The relationship between the ε4 allele and CA1-SRLM thinning persisted after controlling for dementia severity, and the size of other hippocampal subfields and the entorhinal cortex did not differ by APOE ε4 carrier status. Carriers also exhibited worse episodic memory function but similar performance in other cognitive domains compared with noncarriers. In a statistical mediation analysis, we found support for the hypothesis that CA1-SRLM thinning may link the APOE ε4 allele to its phenotypic effects on memory.
Conclusions:
The APOE ε4 allele segregated dose-dependently and selectively with CA1-SRLM thinning and worse episodic memory performance in a pool of older subjects across a cognitive spectrum. These findings highlight a possible role for this gene in influencing a critical hippocampal subregion and an associated symptomatic manifestation.
ApoE influences the metabolism of β-amyloid, the major peptide constituent of amyloid plaques in Alzheimer disease (AD).1 The ApoE4 isoform confers an increased risk of sporadic late-onset AD dementia.2,3
Carriers of the APOE ε4 allele who have AD dementia or its clinical precursor, amnestic mild cognitive impairment (aMCI), exhibit greater hippocampal volume loss than noncarriers in some studies,4–9 although this finding is controversial.10–12 Perhaps these divergent findings arise from disproportionate effects of the ε4 allele on individual hippocampal subfields that are not always evident in global volumetric analyses.
Specific effects of the APOE ε4 allele on hippocampal subfields are of interest. AD-related neuropathology itself is subfield- and even strata-selective, affecting the CA1 subfield, including specifically the stratum radiatum/stratum lacunosum-moleculare (CA1-SRLM), before affecting the remainder of the hippocampus.13–19 Postmortem measurements of CA1-SRLM synapse loss and atrophy correlated with episodic memory performance before death.18 Given the central importance of the hippocampus in episodic memory function, it is notable that the ε4 allele associates with a more amnestic phenotype of AD.20
Perhaps the APOE ε4 allele, CA1-SRLM atrophy, and episodic memory dysfunction interrelate. In prior work, we used high-resolution 7.0-tesla MRI to show that CA1-SRLM atrophy distinguished patients with mild AD dementia from age-matched controls21 and correlated closely with memory performance.22 Here, we investigated the effect of APOE ε4 carrier status on hippocampal subfield and stratal size and memory dysfunction, with the central hypothesis that ε4 would associate with greater CA1-SRLM atrophy and worse episodic memory performance.
METHODS
Standard protocol approvals, registrations, and patient consents.
All study procedures were approved by the Stanford Institutional Review Board. Written informed consent was obtained from all subjects in this study.
Subjects.
Subjects with mild AD dementia and aMCI were recruited from the Stanford Center for Memory Disorders, an outpatient subspecialty clinic and clinical research unit. Inclusion criteria included either 1) a diagnosis of probable AD dementia (amnestic presentation) according to the National Institute on Aging–Alzheimer's Association (NIA-AA) criteria23 and a Clinical Dementia Rating (CDR) score of 0.5 or 1, or 2) a diagnosis of MCI according to the NIA-AA criteria,24 a score of 1.5 SDs below age-adjusted normative means on at least one test of episodic memory (see neuropsychological assessment section below), and a CDR score of less than 1. Healthy older controls (OCs) were recruited from the community, were selected to have a similar average age as enrolled patients, and were required to have normal neuropsychological performance and CDR of 0. All subjects were required to have sufficient English language skills to participate in the neuropsychological assessment. Exclusion criteria included any contraindication to MRI, a history of stroke or other structural brain abnormality, or the presence of any neurologic or medical condition other than AD that could interfere with normal cognition.
Study procedures.
Each subject provided a blood sample for genetic analysis (stored at −80°C) and underwent a clinical evaluation that included a history, physical examination, and neurologic examination by a physician at the Stanford Center for Memory Disorders. With an identified study partner (usually a spouse), each subject then underwent a functional assessment that included the CDR and the Functional Assessment Questionnaire. A neuropsychological evaluation was completed (see below), and the faculty of the center, including neurologists and neuropsychologists, then discussed each potential subject in a consensus conference to determine a research diagnosis. Each eligible subject underwent 7.0-tesla MRI (see below). For each subject, imaging and neuropsychological assessment were acquired within an average window of 48 days (range 0–96).
Neuropsychological assessment.
Standardized neuropsychological tests were administered by a trained psychometrist who was blinded to the imaging data and was not involved in any other aspect of the study. The battery covered episodic memory, working memory, global intellectual function, language, visuospatial function, and executive function as previously described.22 Episodic memory was assessed using the Hopkins Verbal Learning Test–Revised,25 the Brief Visuospatial Memory Test–Revised,26 and the Logical Memory subtest of the Wechsler Memory Scale, third edition.27 From these 3 tests, we derived composite metrics of immediate free recall memory, delayed free recall memory, and delayed recognition memory as described previously.22
APOE genotyping.
Genomic DNA was extracted from frozen whole blood using the Gentra Puregene Blood Kit (Qiagen, Valencia, CA). APOE genotyping was performed using PCR restriction fragment length polymorphism analysis. Genomic DNA was amplified by PCR with the forward primer 5′-TAA GCT TGG CAC GGC TGT CCA AGG A and reverse primer 5′-ACA GAA TTC GCC CCG GCC TGG TAC AC to yield a 244-bp fragment that spans both APOE polymorphic sites. In the PCR, 100 to 200 ng of DNA was added to 25 μL of reaction mixture containing 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 2 mM MgCl2, 500 nM of each primer, 0.2 mM dNTPs (deoxyribonucleotide triphosphates), 10% dimethyl sulfoxide, and 1 U of Taq polymerase (Applied Biosystems, Foster City, CA). PCR reactions were subjected to 40 cycles in a thermal cycler (MJ Research, Waltham, MA) with 30 seconds of denaturing at 94°C, 30 seconds of annealing at 66°C, and 90 seconds of extension at 70°C. Amplified DNA (20 μL) was digested simultaneously with 4 U of AflIII and 3 U of HaeII (New England Biolabs, Ipswich, MA) for 2 hours at 37°C, then analyzed on a 3% agarose gel.
Image acquisition.
Subjects were scanned on a 7T GE Signa HDx whole-body MRI scanner (GE Healthcare, General Electric Company, Waukesha, WI) using a 32-channel radiofrequency receive head coil contained within a quadrature transmit coil (Nova Medical, Inc., Wilmington, MA). The subject's head was stabilized by packing foam between the temples and the inner surface of the receive coil to minimize motion during the scan, and a plethysmograph was placed on a finger on the right hand to monitor peripheral pulse. Imaging was targeted to the medial temporal lobes (MTLs), with acquisition of oblique coronal images oriented perpendicular to the longitudinal axis of the hippocampus, using a T2-weighted fast spin echo sequence: effective echo time 49 milliseconds; repetition time approximately 5 to 6 seconds (cardiac gated, with the R-R interval set for each subject according to their average heart rate, so as to achieve a repetition time within this range). Acquired voxel size was 0.22 × 0.22 × 1.5 mm3, and images were interpolated on the scanner by zero filling k-space to a 1,024 × 1,024 matrix to yield a reconstructed voxel size of 0.166 × 0.166 × 1.5 mm3.
Image analysis.
We visualized images with the OsiriX 3.9 software package28 (http://www.osirix-viewer.com/) and with FSL-View, a component of the FSL 4.1 software package (http://www.fmrib.ox.ac.uk/fsl/). We previously described the landmarks used for subfield identification and the methods used to derive subfield metrics.22 In brief, on slices through the hippocampal body (starting from the first slice posterior to the uncus to the last slice through the colliculi), we determined CA1-SRLM and CA1 stratum pyramidale (CA1-SP) widths using a semiautomated edge-detection algorithm that takes as its input a manually drawn line through the middle of the long axis of the laminar area; we previously showed that this method correlates strongly with manual measurements.22 In the same slices, we traced the circumference of the entire hippocampus and of the combined dentate gyrus and CA3 subfields (DG/CA3; we combined these 2 areas, because no physical boundary appears at this imaging resolution) and determined their cross-sectional areas. We traced the entorhinal cortex (ERC) manually on a block of slices from the hippocampal head to the last slice that includes the uncus. For each subfield, we averaged raw metrics across slices to yield 1 value per side per subject, and then averaged the 2 sides to yield a single subfield metric per subject. Because of the large slice thickness, skip between slices, and incomplete coverage of the hippocampus, all necessary to achieve a very high in-plane resolution and signal-to-noise, we constrain our analyses to 1- or 2-dimensional metrics, rather than volumes; for example, we report average hippocampal cross-sectional area rather than hippocampal volume. In our analyses, we do not control for head size, because there is not a strong a priori reason to suspect that hippocampal laminar widths should vary with intracranial volume, and we found in prior work that there was no correlation between head size and CA1-SRLM or CA1-SP width in patients with mild AD dementia or healthy controls.21 Also, our imaging sequence included a slab of images covering the hippocampus but not the entire brain, thus not permitting a direct measurement of total intracranial volume.
Statistics.
All statistical tests were performed using SPSS 21.0 (IBM Corp., Armonk, NY). Individual statistical comparisons are described in the results section, and 2-tailed p values with a threshold of 0.05 were used as the threshold for significance. The mediation analysis was performed using the method of Preacher and Hayes,29 and the significance of the indirect pathway was assessed by running 10,000 bootstrap samples for estimation of the bias-corrected bootstrap confidence interval.
RESULTS
Subjects included 14 OCs, 14 patients with aMCI, and 11 patients with AD dementia (table 1). Prevalence of the APOE ε4 allele was not statistically different between groups, whether analyzed according to binary carrier status or by rates of homo- or heterozygosity (table 1). There was no relationship between APOE ε4 carrier status and sex (p = 0.40, Pearson χ2), age (p = 0.63, t test), or education (p = 0.41, t test). There were 4 ε2 carriers in our sample; all 4 had an ε2/ε3 genotype, and of these 4, one was an OC and 3 were patients with aMCI.
Table 1.
Subjects

We examined the effect of the APOE ε4 allele on MTL structural metrics and memory performance using 2 × 3 analyses of variance (ANOVAs) with APOE ε4 carrier status and diagnostic group as factors (table 2). There was a main effect of APOE ε4 carrier status on hippocampal size and CA1-SRLM width, with carriers exhibiting a thinner CA1-SRLM. There was no main effect of APOE ε4 carrier status on CA1-SP width, and trends were apparent for ERC width and DG/CA3 area. For CA1-SP and ERC, there were interactions between carrier status and diagnosis, such that carriers but not noncarriers exhibited thinner structures with increasing diagnostic severity.
Table 2.
Effects of APOE ε4 carrier status on MTL structures and memory performance

There was a main effect of APOE ε4 carrier status on delayed free recall and delayed recognition composite scores, with carriers exhibiting worse performance (table 2). There was no main effect of APOE ε4 carrier status on the immediate recall composite score or on Verbal or Performance IQ, Digit Span, figure copy performance, confrontation naming, category fluency, or Trails B performance (p > 0.05 in all cases), implying that the effect on delayed episodic memory was specific. There were interactions between carrier status and diagnosis on all 3 memory composite scores, such that carriers exhibited greater score declines with increasing diagnostic severity than noncarriers.
To test the dose-dependence of the APOE ε4 allele on MTL structural metrics and memory performance, 1-way ANOVAs were performed on pooled data after adjusting for diagnostic group membership, using APOE ε4 allele dose as the factor (figure). Among the structural metrics, there were effects of APOE ε4 allele dose on hippocampal size and CA1-SRLM width, but not other structures (figure). Among memory subtests, there were effects of APOE ε4 allele dose on the 2 delayed memory composite scores, but not on immediate recall (figure).
Figure. Dose-dependent effects of the APOE ε4 allele on MTL structure and memory performance.
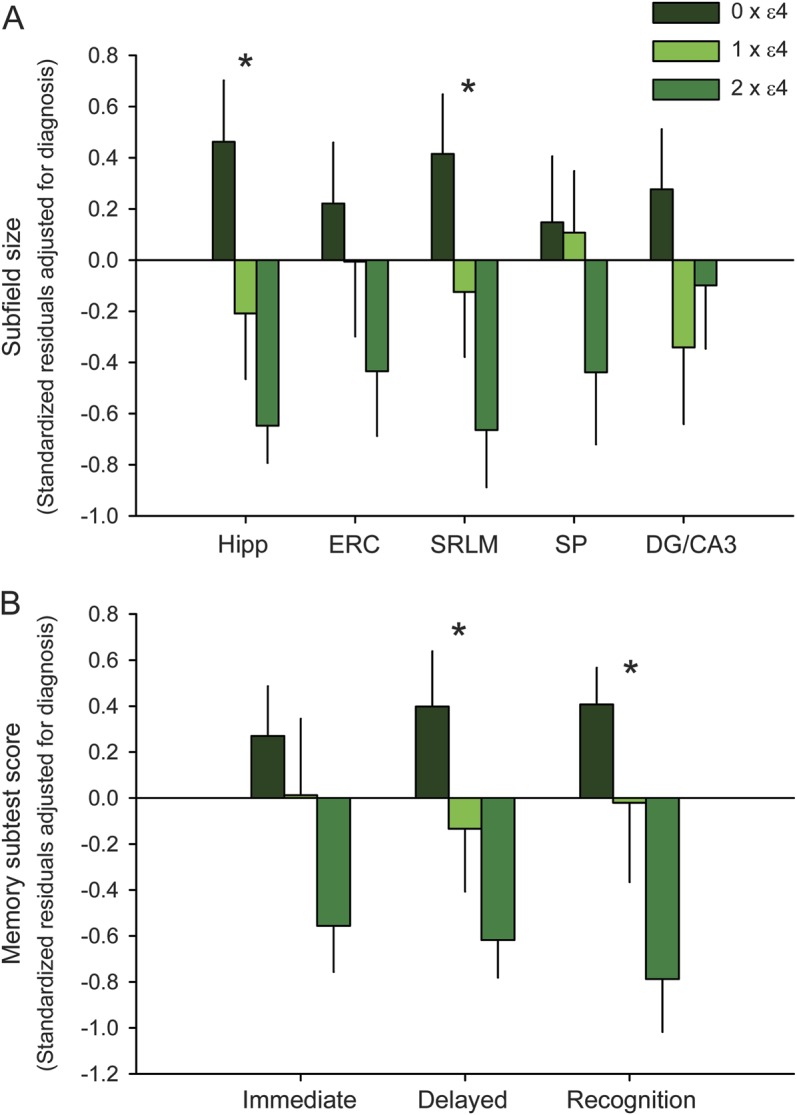
(A) Relative subfield sizes are expressed as z-transformed residuals after adjustment for diagnostic group membership (control, aMCI, or AD dementia), illustrated according to the number of APOE ε4 alleles (0, 1, or 2). Anatomical metrics include the cross-sectional area of the overall hippocampus; widths of the ERC, CA1-SRLM, and CA1-SP; and cross-sectional area of the DG/CA3. Error bars represent standard error. *p < 0.05 for the main effect of ε4 dose on subfield size (1-way ANOVA). (B) Relative memory subtest scores are expressed as z-transformed residuals after adjustment for diagnostic group membership (control, aMCI, or AD dementia), illustrated according to the number of APOE ε4 alleles. See the subjects section under methods for an explanation of the immediate, delayed, and recognition composite metrics. Error bars represent standard error. *p < 0.05 for the main effect of ε4 dose on composite subtest metric (1-way ANOVA). AD = Alzheimer disease; aMCI = amnestic mild cognitive impairment; ANOVA = analysis of variance; DG/CA3 = dentate gyrus and CA3; ERC = entorhinal cortex; Hipp = hippocampus; MTL = medial temporal lobe; SP = stratum pyramidale; SRLM = stratum radiatum/lacunosum-moleculare.
APOE ε4 carriers had lower scores than noncarriers on CDR–Sum of Boxes (p < 0.001), Mini-Mental State Examination (p = 0.005), and Functional Activities Questionnaire (p < 0.001) (2 × 3 ANOVAs using ε4 carrier status and diagnostic group as factors were each significant, at p < 0.001). This could reflect a higher proportional representation of carriers among the cognitively impaired groups (aMCI and AD dementia) compared with controls, even though these differential rates of carrier status did not reach statistical significance on their own (table 1). In any case, the influence of APOE ε4 carrier status on CA1-SRLM width was preserved in linear regressions that used CDR–Sum of Boxes, Mini-Mental State Examination, or Functional Activities Questionnaire scores as covariates (p < 0.05 for ANOVAs and for the main effect of ε4 carrier status), indicating that the relationship between the ε4 allele and CA1-SRLM width was not driven by genotype-associated differences in dementia severity.
To study the predictors of delayed recall memory performance among the subjects in this study, we performed a hierarchical linear regression (table 3). After accounting for diagnosis, education, and sex, adding APOE genotype to the model accounted for additional variance (see also table 2 and the figure). Adding CA1-SRLM width to the model accounted for yet more variance and reduced the contribution of APOE genotype. To test the hypothesis that CA1-SRLM thinning mediates the relationship between APOE ε4 carrier status and episodic memory impairment, we ran a statistical mediation model, using diagnosis-adjusted delayed recall composite score as the dependent variable, APOE ε4 carrier status as the independent variable, and diagnosis-adjusted CA1-SRLM width as the proposed mediator. The indirect pathway was significant (coefficient −0.45, bias-corrected bootstrap 95% confidence interval −1.00 to −0.095), supporting our hypothesis.
Table 3.
Influence of APOE ε4 and CA1-SRLM on delayed recall performance

DISCUSSION
We found that APOE ε4 carriers exhibited a thinner CA1-SRLM than noncarriers. We observed this effect in a pool of older adults that included healthy controls and patients with aMCI and AD dementia, controlling for diagnostic group membership. Carriers also demonstrated greater episodic memory dysfunction, and we found support for the hypothesis that CA1-SRLM thinning mediates the effect of the ε4 allele on memory performance. The effects of APOE ε4 on both memory and CA1-SRLM width were dose-dependent.
CA1-SRLM is an important hippocampal crossroads. This neuropil layer contains dendrites from CA1 principal neurons, axons from ERC and CA3, and the synapses between them. These synapses are among the most plastic in the brain and contribute to learning and memory; they are also among the first victims in AD, as tau pathology, synapse loss, and involutional change are evident in the CA1-SRLM at an early pathologic stage.13–19 CA1-SRLM atrophy is observable in vivo in patients with mild AD dementia21 and correlates with memory performance.22 The present study extends these prior observations by demonstrating a specific association of the APOE ε4 allele with CA1-SRLM thinning, even after controlling for differences in dementia severity. Overall hippocampal size was also affected, but among the individual MTL subregions measured, CA1-SRLM was unique in its relationship with APOE genotype (table 2 and figure); however, we observed trends for ERC and DG/CA3 (p < 0.1; table 2), suggesting the possibility that with larger sample sizes, significant effects of the ε4 allele could emerge for those areas, too. Notably, there was no main effect of APOE genotype on the thickness of CA1-SP—the layer adjacent to CA1-SRLM containing the associated principal neuron cell bodies. There were interactions between diagnosis and carrier status for both CA1-SP and ERC, and our data (table 2) suggest that the ε4 allele may be associated with greater thinning of these structures in symptomatic subjects, particularly in the context of AD dementia.
Our finding that a subregion of CA1 is particularly sensitive to the effects of ApoE4 relates to one prior study showing, among patients with AD dementia, a differential effect of carrier status on a more macroscopic measure of CA1 size.30 Another group reported focal, longitudinal atrophy in the subiculum among healthy, older carriers.31 However, our findings are not compatible with those of another group, who found a differential effect on DG/CA3 size32,33; in those studies, hippocampal subfield volumetry was evaluated at 4.0 tesla, and it is not clear whether our different findings stem from a difference in technique, field strength, or subject characteristics. A focal effect of ApoE4 on hippocampal substructure could explain why effects on global hippocampal volume are subtler, observed in some studies4–9 but not others.10–12
A particular strength of our study is the finding of a possible symptomatic consequence of APOE ε4–associated CA1-SRLM thinning, which appeared to mediate the relationship between carrier status and the integrity of episodic memory. Mediation analyses are inherently limited and cannot prove causal relationships, but the model makes conceptual sense, given the critical role that CA1-SRLM synapses have in overall hippocampal circuit function and prior observations that CA1-SRLM atrophy correlates with episodic memory function in AD.18,22 Others have observed associations between the APOE ε4 allele and worse episodic memory function4 (cf. many subsequent studies, including some with contrary findings34). Although we found a main effect associating the ε4 allele with poorer memory across our pooled sample of subjects, there was an interaction between diagnosis and memory such that symptomatic patients, rather than healthy controls, appeared to drive this relationship (table 2).
There are several weaknesses in our study. We studied 39 subjects distributed among 3 diagnostic groups, leading to relatively small genotype-phenotype bins (table 1). Pooling subjects produced adequate power to drive our statistical analyses and allowed us to study the diagnosis-independent effects of APOE ε4 across a cognitive spectrum, but did not provide enough power to study any disproportionate effects of the allele within specific diagnostic categories. A second weakness is that this cross-sectional study design did not allow us to study different trajectories of CA1-SRLM atrophy or memory loss over time. Similarly, although we postulate that atrophy accounts for the finding of CA1-SRLM thinning in APOE ε4 carriers, our observations at a single time point cannot exclude a developmental mechanism or other explanation. Third, we did not have enough ε2 carriers in our sample to study the discrete effects of this allele, which confers a reduced risk for AD and could conceivably harbor its own unique associations with CA1-SRLM thinning or memory loss. Finally, a caveat shared with any similar study lacking postmortem histology is the possibility that ApoE carriers are more likely to harbor true AD pathology, and that disproportionate contamination of the noncarrier group with other disease processes may explain a reduced burden of CA1-SRLM atrophy and episodic memory impairment. Longitudinal study of larger groups of subjects is a goal for future research.
Taking advantage of the ability of ultra-high-field 7.0-tesla MRI to reveal details of hippocampal subfield and stratal anatomy in vivo, we report a selective association of the APOE ε4 allele with hippocampal CA1-SRLM thinning, a relationship that appeared to mediate worse episodic memory performance among carriers. This work highlights the CA1 apical neuropil as an area of potential interest for basic investigation into the biological effects of ApoE.
ACKNOWLEDGMENT
The authors thank Christina Wyss-Coray, RN, for assistance with subject recruitment and study coordination, and Maria Coburn for assistance with biospecimen cataloging and storage.
GLOSSARY
- AD
Alzheimer disease
- aMCI
amnestic mild cognitive impairment
- ANOVA
analysis of variance
- CA1-SP
CA1 stratum pyramidale
- CA1-SRLM
CA1 stratum radiatum/lacunosum-moleculare
- CDR
Clinical Dementia Rating
- DG/CA3
dentate gyrus and CA3
- ERC
entorhinal cortex
- MTL
medial temporal lobe
- NIA-AA
National Institute on Aging–Alzheimer's Association
- OC
older control
AUTHOR CONTRIBUTIONS
G.A.K. designed the study, analyzed and interpreted the data, and wrote the manuscript. D.B. analyzed and interpreted the data and revised the manuscript. J.C.S., J.D.B., M.C.F., and G.K.D. analyzed and interpreted the data. T.W.-C. and B.K.R. contributed to study design and revised the manuscript.
STUDY FUNDING
Supported by grants to G.A.K. from NIH-NIA (K23AG042858), American Federation for Aging Research, Alzheimer's Association (NIRG-11-205493), and the McKnight Endowment Fund for Neuroscience. Funding sources had no role in any aspect of study design, data collection, analysis, interpretation, or preparation of the manuscript.
DISCLOSURE
G. Kerchner received fees for consulting work unrelated to this research from Botamedi, Inc. and from Phloronol, Inc.; received royalties from McGraw-Hill; and is the site investigator for clinical trials sponsored by Genentech, Biogen Idec, and Merck. D. Berdnik, J. Shen, J. Bernstein, M. Fenesy, G. Deutsch, T. Wyss-Coray, and B. Rutt report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009;10:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology 1993;43:1467–1472 [DOI] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923 [DOI] [PubMed] [Google Scholar]
- 4.Lehtovirta M, Laakso MP, Soininen H, et al. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience 1995;67:65–72 [DOI] [PubMed] [Google Scholar]
- 5.Geroldi C, Pihlajamäki M, Laakso MP, et al. APOE-ε4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology 1999;53:1825–1832 [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto M, Yasuda M, Tanimukai S, et al. Apolipoprotein E ε4 and the pattern of regional brain atrophy in Alzheimer's disease. Neurology 2001;57:1461–1466 [DOI] [PubMed] [Google Scholar]
- 7.Troyer AK, Murphy KJ, Anderson ND, et al. Associative recognition in mild cognitive impairment: relationship to hippocampal volume and apolipoprotein E. Neuropsychologia 2012;50:3721–3728 [DOI] [PubMed] [Google Scholar]
- 8.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology 2004;63:1898–1901 [DOI] [PubMed] [Google Scholar]
- 9.Agosta F, Vossel KA, Miller BL, et al. Apolipoprotein E ε4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci USA 2009;106:2018–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morra JH, Tu Z, Apostolova LG, et al. Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Hum Brain Mapp 2009;30:2766–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Petersen RC, Xu YC, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer's disease. Ann Neurol 1998;43:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basso M, Gelernter J, Yang J, et al. Apolipoprotein E epsilon4 is associated with atrophy of the amygdala in Alzheimer's disease. Neurobiol Aging 2006;27:1416–1424 [DOI] [PubMed] [Google Scholar]
- 13.Braak E, Braak H. Alzheimer's disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon's horn. Acta Neuropathol 1997;93:323–325 [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady DR, Mufson EJ. Alz-50 immunoreactive neuropil differentiates hippocampal complex subfields in Alzheimer's disease. J Comp Neurol 1991;305:489–507 [DOI] [PubMed] [Google Scholar]
- 16.Lace G, Savva GM, Forster G, et al. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain 2009;132:1324–1334 [DOI] [PubMed] [Google Scholar]
- 17.Mizutani T, Kasahara M. Hippocampal atrophy secondary to entorhinal cortical degeneration in Alzheimer-type dementia. Neurosci Lett 1997;222:119–122 [DOI] [PubMed] [Google Scholar]
- 18.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007;68:1501–1508 [DOI] [PubMed] [Google Scholar]
- 19.Thal DR, Holzer M, Rub U, et al. Alzheimer-related tau-pathology in the perforant path target zone and in the hippocampal stratum oriens and radiatum correlates with onset and degree of dementia. Exp Neurol 2000;163:98–110 [DOI] [PubMed] [Google Scholar]
- 20.Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex 2007;43:835–845 [DOI] [PubMed] [Google Scholar]
- 21.Kerchner GA, Hess CP, Hammond-Rosenbluth KE, et al. Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology 2010;75:1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerchner GA, Deutsch GK, Zeineh M, Dougherty RF, Saranathan M, Rutt BK. Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer's disease. Neuroimage 2012;63:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 24.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991;5:125–142 [Google Scholar]
- 26.Benedict R. Brief Visuospatial Memory Test, revised, 2nd ed Odessa, FL: Psychological Assessment Resources; 1997 [Google Scholar]
- 27.Wechsler D. Wechsler Memory Scale, 3rd ed. San Antonio: The Psychological Corporation; 1997 [Google Scholar]
- 28.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 2004;17:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–891 [DOI] [PubMed] [Google Scholar]
- 30.Pievani M, Galluzzi S, Thompson PM, Rasser PE, Bonetti M, Frisoni GB. APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer's disease. Neuroimage 2011;55:909–919 [DOI] [PubMed] [Google Scholar]
- 31.Donix M, Burggren AC, Suthana NA, et al. Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE-4 polymorphism. Neuroimage 2010;53:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus 2009;19:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4 T. Neuroimage 2008;42:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small BJ, Graves AB, McEvoy CL, Crawford FC, Mullan M, Mortimer JA. Is APOE-ε4 a risk factor for cognitive impairment in normal aging? Neurology 2000;54:2082–2088 [DOI] [PubMed] [Google Scholar]


