Abstract
Polydimethylsiloxane (PDMS) is a commonly used polymer in the fabrication of microfluidic devices due to such features as transparency, gas permeability, and ease of patterning with soft lithography. The surface characteristics of PDMS can also be easily changed with oxygen or low pressure air converting it from a hydrophobic to a hydrophilic state. As part of such a transformation, surface methyl groups are removed and replaced with hydroxyl groups making the exposed surface to resemble silica, a gas impermeable substance. We have utilized Platinum(II)-tetrakis(pentaflourophenyl)porphyrin immobilized within a thin (~1.5 um thick) polystyrene matrix as an oxygen sensor, Stern-Volmer relationship, and Fick's Law of simple diffusion to measure the effects of PDMS composition, treatment, and storage on oxygen diffusion through PDMS. Results show that freshly oxidized PDMS showed a significantly smaller diffusion coefficient, indicating that the SiO2 layer formed on the PDMS surface created an impeding barrier. This barrier disappeared after a three-day storage in air, but remained significant for up to three weeks if PDMS was maintained in contact with water. Additionally, higher density PDMS formulation (5:1 ratio) showed similar diffusion characteristics as normal (10:1 ratio) formulation, but showed 60% smaller diffusion coefficient after plasma treatment that never recovered to pre-treatment levels even after a three-week storage in air. Understanding how plasma surface treatments contribute to oxygen diffusion will be useful in exploiting the gas permeability of PDMS to establish defined normoxic and hypoxic oxygen conditions within microfluidic bioreactor systems.
Keywords: Pt-TPFP, PDMS, oxygen diffusion in PDMS, surface treatment, Stern-Volmer, oxygen sensing
INTRODUCTION
Polydimethylsiloxane (PDMS) is a commonly used polymer in the fabrication of microfluidic devices due to such features as transparency, gas permeability(M. Adler et al. 2010; M. Polinkovsky et al. 2009), and ease of patterning with soft lithography (D.C. Duffy et al. 1998; B.H. Jo et al. 3013; J.C. McDonald et al. 2000; J.C. McDonald and G.M. Whitesides 2002; M. Polinkovsky et al. 2009). Areas of applications for the microfluidic devices made with PDMS are quite broad ranging from electrophoresis (C.S. Effenhauser et al. 1997; J.A. Vickers, M.M. Caulum, and C.S. Henry 2006) to cell culture (Y. Gao et al. 2011; E. Leclerc, Y. Sakai, and T. Fujii 2004; T. Saito et al. 2006; P.C. Thomas, S.R. Raghavan, and S.P. Forry 2011) and long-term organotypic cultures (M.S. Kim, J.H. Yeon, and J.K. Park 2007; L. Liu et al. 2010; D.A. Markov et al. 2012; J.C. McDonald and G.M. Whitesides 2002; A. Prokop et al. 2004). One of the attractive features of PDMS is that its surface properties can be easily adjusted, i.e., exposure to oxygen plasma for short periods of time converts PDMS surface from its native hydrophobic state to hydrophilic, which with time reverts back to hydrophobic state (J.A. Vickers, M.M. Caulum, and C.S. Henry 2006). During such plasma treatment, surface methyl groups are removed and replaced with hydroxyl groups making the exposed surface resemble silica (K. Efimenko, W.E. Wallace, and J. Genzer 2002; E.P. Everaert, H.C. Van Der Mei, and H.J. Busscher 1996; J.L. Fritz and M.J. Owen 1995; H. Hillborg et al. 2000; M.J. Owen and P.J. Smith 1994). Such treatment is routinely used as part of the device assembly, where multiple plasma-treated PDMS layers are bonded to each other or glass substrate to form a complete device. Presence of silica on the surface of the microfluidic channels makes it highly hydrophilic, and thus easy to load with liquids, and enabling them to sustaining electroosmotic flows (EOFs) (X. Wang et al. 2009), which are important in electrokinetic pumping (D. Erickson, D. Sinton, and D. Li 2003) and electrophoresis applications. However, reproducibility of EOF in PDMS devices has been an issue for a long time (X. Ren et al. 2001). It was found that oxidized PDMS can support EOF for several hours before it starts to significantly deteriorate. This reduction in sustaining EOF is attributed to deterioration of the silica film formed on the microfluidic surface. Underwater storage of the freshly oxidized devices maintains hydrophilicity and extends application life for almost a week (G.B. Lee et al. 2005). Additionally, when PDMS is utilized for cell culture and drug testing platform applications there is a potential for loss of small molecules into the substrate (i.e., growth factors, hormones, small molecule inhibitors) thus altering agent concentrations. We have previously exploited oxidation of PDMS surfaces of our Thick Tissue Bioreactor (TTB) as a means to develop a silica barrier that reduced diffusion of small molecules into the PDMS (D.A. Markov et al. 2012), and were able to successfully sustain growth of long-term cell cultures within the TTB. The other implication of having silica along the perimeter of the microfluidic channel is that silica is typically a gas impermeable substance, thus potentially affecting gas exchange within the microfluidic environment. Currently we are working on the further development of microfluidic-based bioreactors for long-term cell culture with the possibility of delivering oxygen to cell culture areas through the PDMS. During our initial experiments we noticed that the time it took for oxygen to be delivered to the cell culture chamber varied significantly with age of our bioreactors (D.A. Markov et al. 2012). We hypothesized that oxygen plasma treatment during device assembly, consecutive aging of the surface, and different composition of PDMS were potential variables affecting gas diffusion through PDMS
To test this hypothesis, we have fabricated a series of PDMS membranes and measured diffusion of oxygen across them. Platinum(II)-tetrakis(pentaflourophenyl)porphyrin (Y. Amao, T. Miyashita, and I. Okura 2001; Y. Amao and I. Okura 1998; E.R. Carraway et al. 1991; G.E. Khalil et al. 2005; P.C. Thomas et al. 2009) immobilized within a thin polystyrene matrix spun on top of a standard glass slide was used as an oxygen sensor. The PDMS membranes were placed on top of this film and clamped with a Plexiglas lid containing a gas delivery channel. Using the Stern-Volmer relationship (E.R. Carraway et al. 1991; E.R. Carraway, J.N. Demas, and B.A. Degraff 1991) and Fick's Law of simple diffusion, the diffusivity of oxygen in PDMS was measured for the untreated, freshly treated (oxidized), and aged membranes. It is noted that since the plasma treatment affects only the surface it would be more appropriate to talk in terms of the effective diffusion coefficients.
Materials and methods
Experimental set up for measuring gas diffusion
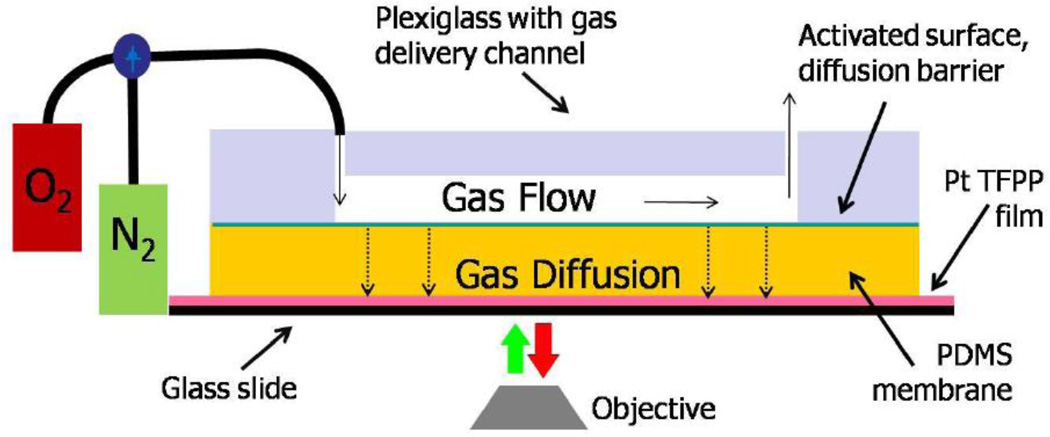
Experimental setup for the gas diffusion measurements is shown in Figure 1. It consists of an aluminum holder, a glass slide with the 1.5 µm thick oxygen sensitive film deposited on top of it, PDMS membrane to be tested and the polycarbonate lid with the gas delivery channel that is securely attached to the aluminum holder. The channel was 1 mm wide and 5 cm long. Both, oxygen and nitrogen containing E-size tanks were connected to the polycarbonate lid via three-way valve, that allowed for rapid gas switching. Before each experiment the whole system, including the membrane, was flushed with nitrogen for at least 25 min. This was experimentally determined time period to completely substitute present in the system oxygen with nitrogen
Figure 1. Measuring oxygen diffusion through PDMS.
Experimental set up for diffusion testing.
Membrane fabrication and treatments
Standard 10:1 and 5:1 (for higher degree of cross-linking) PDMS pre-polymer to curing agent ratios were used for membrane fabrication. Curing was done over night at 65 °C in a leveled oven. Membranes less than 200 µm thick were fabricated using a spin coater. The detailed procedure and the relationship between the membrane thickness and spin speed are shown in the supplemental materials (see Supplemental Figure S1). Thick membranes were fabricated using volumetric dispense method, where a known volume of PDMS was deposited into a carefully leveled custom made flat-bottom container with known surface area, thus controlling thickness of the resulting thin film. Membrane thicknesses were measured on Olympus BX-41 upright microscope equipped with the measuring reticule in multiple places across the membrane to ensure uniformity. Declared membrane thickness was an average of six measurements. Plasma treatments were done in a low pressure air-filled plasma oven (PDC-32G, Harrick Co.) for 30 seconds on “High” power setting (according to manufacturer specifications 18W of power being delivered to the RF coils at that setting). The diffusion measurements were performed immediately after plasma treatments and then membranes were stored either in air or submerged under water (MilliQ 18 MΩ, pH = 7) at RT for 21 days with additional measurements performed at 3 and 7 days.
Oxygen sensitive film
Platinum(II)-tetrakis(pentaflourophenyl)porphyrin (Pt-TPFP) immobilized within a thin (~1.5 um thick) polystyrene matrix spun on top of a standard microscope glass slide was used as an oxygen sensor. To promote film adhesion and uniformity, the glass slide was first coated with 1% solution of polyvinyl alcohol. Then a 10% w:v solution of polystyrene in toluene containing Pt-TPFP in 1:100 w:w to PS was spun onto the slide. Fluorescence images were taken with Zeiss microscope Axiovert 25 equipped with QColor 5 cooled ccd camera, and Tx Red fluorescence filter set (Chroma, Inc, part # 410004). Prior to each experiment, the oxygen sensitive film performance was calibrated with 0%, 21%, and 100% oxygen (Y. Rharbi et. al. 1999 and M. Adler et. al. 2010) and Stern-Volmer relationship was used to determine quenching constant Ksv as described below (see supplemental materials Figure S2 and S3). Typical emission spectra of the fabricated film for 0% and 21% oxygen are shown in Figure S5.
Data collection and analysis
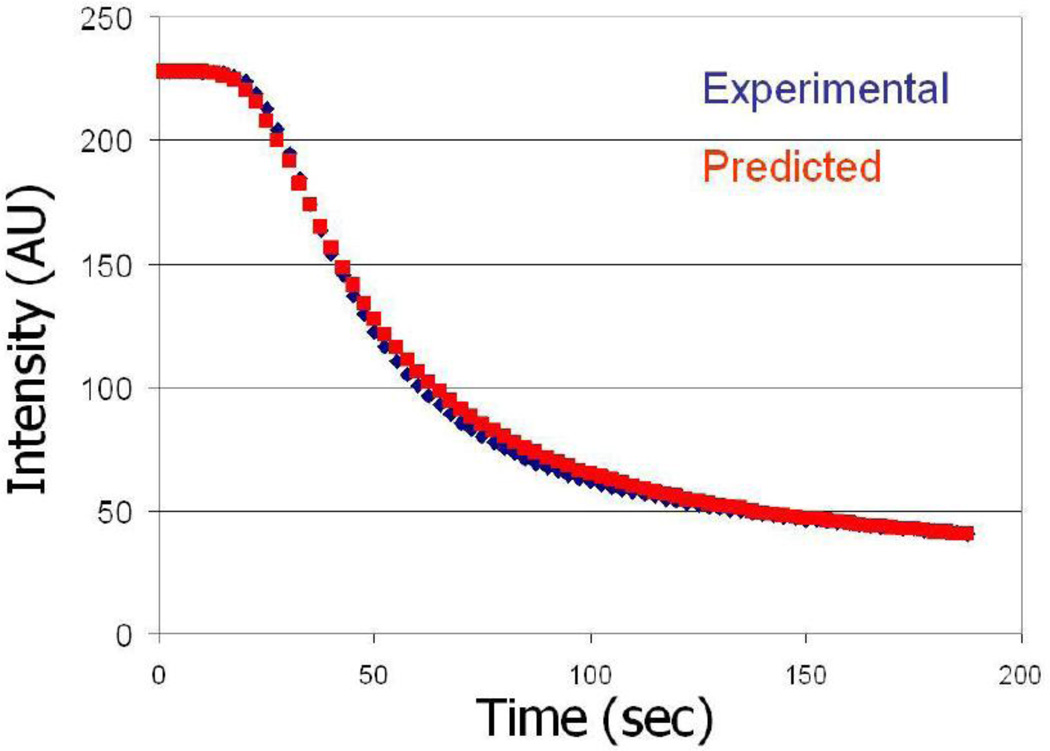
Fluorescent images of the oxygen sensitive films were captured with QColor 5 cooled ccd camera in black and white mode at 2.5 second intervals with QCapture software and stored on the computer. ImageJ was used to extract mean intensity over a small area within the channel from each of images. Changes in the fluorescence due to oxygen diffusion as function of time across a representative 1 mm thick membrane was plotted in Figure 2. Experimental data were then fitted in Mathematica 7.0 to the non-linear equation: , where Ix is the observed intensity as a function of time, I0 is the initial intensity in the absence of O2, Ksv is the Stern-Volmer quenching constant, Cs is the constant O2 concentration at the diffusion interface, x is the membrane thickness, t is time, and D is the diffusion coefficient. This equation is the combination of the solution to Fick’s 2nd law and the Stern-Volmer relationship. Its derivation is shown in supplemental materials (Figure S2). From this fit, the effective diffusion constant D was determined. Each of these experiments was repeated for three different membranes of the same thickness. Figure 2 shows both theoretically predicted and experimentally measured changes in fluorescent intensity due to oxygen diffusing through 1 mm thick untreated PDMS membrane. For long-term observations of the evolution of hypoxic and hyperoxic conditions within our bioreactor we have used b/w CGE-B013-U camera (Mightex Systems, Toronto, Canada) in external trigger mode that was triggered by a “synch” signal from the VCM-D1 Uniblitz shutter driver (Vincent Associates, Rochester, NY).
Figure 2.
Comparison of observed and predicted fluorescent intensity quenching of Pt-TPFP due to oxygen diffusion for 1 mm thick non-treated PDMS membrane. Experimental measures were performed as described in Methods. The predicted quenching was plotted using actual Stern-Volmer equation (see data analysis section) with experimentally measured values for I0, Ksv and D.
Experimental set up for testing oxygen level delivery into cell chamber of TTB
The previously reported TTB was modified to be compatible with the standard 96-well format and amenable to imaging with such high throughput microscopes as Opera QEHS. All cell culture chambers and fluidic and gas input / output ports were located on nine × nine mm grid. Interdigitated blind gas delivery channels (1.5 mm wide) were connected to main gas supply channels (3 mm wide) running the full length of the reactor and extended inward past the cell culture chambers such that they were at equal distances from each adjacent chamber. The assembled reactor was placed on top of the 2”×3” slide coated with oxygen sensitive film as described above. Oxygen and nitrogen containing E-size tanks were connected via tygon tubing that was directly inserted into PDMS and included a three-way valve that allowed for gas switching. Only a smaller portion, 5 rows × 6 columns, of the multi-well reactor was used for these preliminary experiments. Before each experiment, the whole system was equilibrated with ambient room conditions for at least 10 h and then either nitrogen was first allowed to diffuse into the cell chamber and then replaced with oxygen or vice versa, oxygen was delivered first and then substituted with nitrogen.
COMSOL modeling
A simplified 2D model of the multi- well TTB reactor containing gas delivery channels was created in COMSOL Multiphysics environment to confirm time evolution of the oxygen gradient into the cell culture chamber. For the oxygen diffusion coefficient in air we have used the value of Dair = 2.1×10−5 m2/s (J.R. Welty et. al. 2001), while for diffusion in PDMS we have used the experimentally determined (as described above and within Results section) coefficient of DPDMS = 3.25×10−9 m2/s. For ease of visualization and comparison with experimental results, modeled changes in O2 were then converted into fluorescence decay signal using Stern-Volmer equation (Figure S2). For the specific polystyrene film with Pt-TPFP used to analyze oxygen delivery into cell chambers of the TTB, the Ksv coefficient was experimentally determined to be 0.088 (R2 = 0.99).
Results
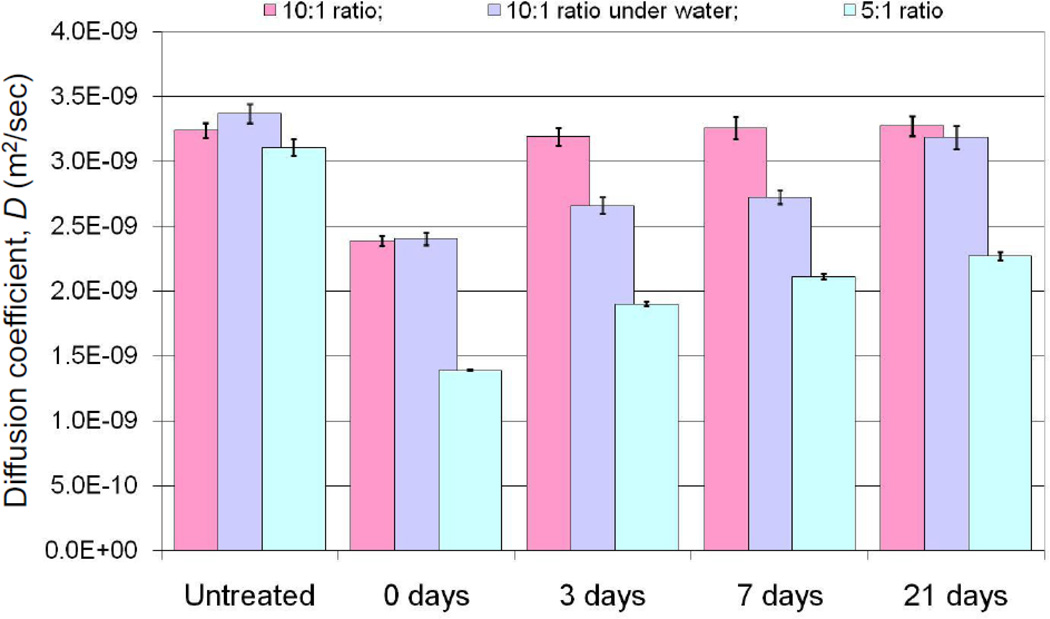
The diffusion experiments were repeated with the same membranes prior to exposing them to plasma treatment, immediately after plasma treatment, then after three-day, one-week, and three-week aging periods in the presence of air or water submerged for both regular (10:1) and highly cross-linked (5:1) PDMS. The presence of an oxidized surface should affect oxygen partition into PDMS and consequently the apparent diffusion coefficient across the membrane. It was also expected that with time the SiO2 layer would gradually disappear due to diffusional exchange of the polymer chains of the surface with those in the bulk, with the potential of restoring the diffusion levels close to those prior to the initial plasma treatment. The diffusion coefficients D obtained from our experiment conditions are plotted in Figure 3. In comparing oxygen diffusivity of untreated naïve PDMS membranes versus the same membranes following plasma treatment, it is clear that the plasma treatment does create a diffusional barrier significantly reducing amount of oxygen partitioning into PDMS and diffusing across. These observations were consistent with our prior results where we noted that Rhodimine partition into PDMS was impaired with oxidized surface treatment of PDMS (D.A. Markov et al. 2012).
Figure 3. Experimentally determined oxygen diffusivity through 1 mm thick PDMS membranes.
as a function of surface properties and storage conditions. Note: 1) normal and high density PDMS have almost identical O2 diffusivities in native state; 2) initial plasma treatment significantly decreases O2 diffusivity, in some cases by more that 50%; 3) original diffusivity values are fully restored in 3 days for normal PDMS stored in air, while high-density PDMS does not recover in 3 weeks under the same storage conditions; storage under water (normal operating conditions for a bioreactor) delays recovery by 3 weeks. Error bars represent the 95% confidence interval based on 3 independent measurements with 3 different membranes.
The values for diffusion coefficients of PDMS treated membranes are also restored over time, which we expected as studies have shown that the plasma-induced SiO2 surface layer disappears with time, restoring the hydrophobic nature of the surface. Interestingly, we did not anticipate the complete restoration of the diffusive properties. Previous literature reports have shown that the hydrophobicity of the surface is restored (G.B. Lee et al. 2005) only to 70% of its original value (determined by contact angle measurements), suggesting that some of the SiO2 layer still remains on the surface. However, this residual layer appears to have no effect on oxygen diffusing into PDMS. As it can be noted from Figure 3 after a three-day aging period D was completely restored to the pretreatment levels in the case of standard 10:1 cross-linked PDMS maintained in the presence of air. Submerging PDMS following plasma treatment delayed the restoration of diffusivity; however, by day 21 the effective diffusion, while statistically significantly below the control untreated diffusion values (p < 0.04), was approaching untreated levels and was restored to 94.6% of the original values. In the case of a highly cross-linked PDMS, the surface is not restored even after a 21-day waiting period; furthermore, the values for D remain well below initial values, suggesting that the surface restoration of plasma treated highly-crosslinked PDMS is highly impeded. The fact that this is an effect primarily due to changes of the surface properties is evident from the diffusivity comparison of the results for the native, untreated PDMS at 10:1 and 5:1 rations. It appears that the diffusion coefficients D in both cases are very similar to each other (statistically, with p=0.28, they are indistinguishable ) indicating that the increased cross-linking of the PDMS polymer chains (which results in a stiffer material) does not extensively affect the oxygen partition and diffusion through PDMS.
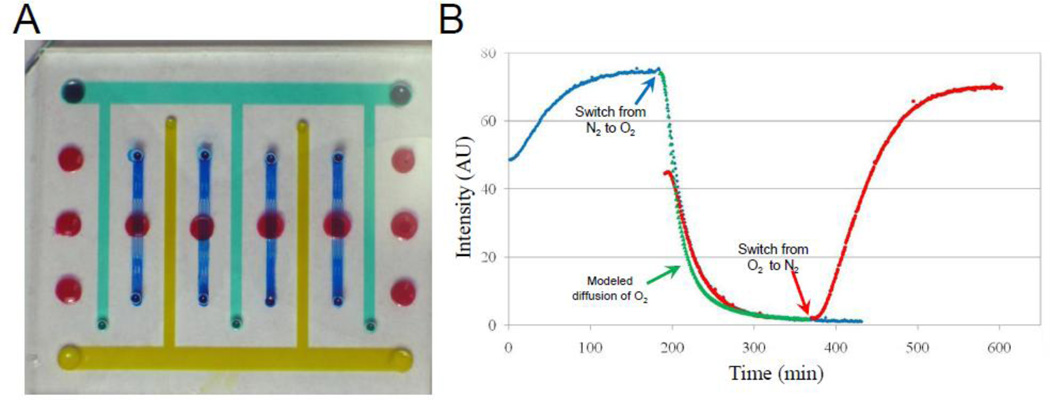
We tested the utility of these parameters in predicting oxygen delivery into our bioreactor system. Expanding the design of the thick tissue bioreactor (TTB) described in reference (D.A. Markov et al. 2012) to include gas supply channels (Figure 4 A) we have tested the delivery of both nitrogen and oxygen (varying from hypoxic to hyperoxic conditions) to multiple cell culture chambers (indicated in red in Figure 4A). Cell chambers are located on a nine mm center-to-center grid with single-ended gas supply channels in between. Figure 4B shows changes in fluorescence intensity of the oxygen sensitive film measured at one minute intervals as nitrogen or oxygen gases diffuse through PDMS into the chambers. The blue curve (diamond markers) depicts the transition from ambient (0 min) to 0% oxygen that is then switched to 100 % oxygen at a182 minute; while the red curve (circle markers) shows the reverse order, with oxygen delivery going from ambient to 100% oxygen and then switching to 0% oxygen). For ease of visualization and comparison and the red curve was shifted on the graph by three hours to overlap with corresponding nitrogen-to-oxygen transition from previous experiments. Green triangles show results of the COMSOL modeling of oxygen diffusion using the Stern-Volmer equation (Figure S2) and the diffusivity value calculated in Figure 3 for naïve PDMS. This delivery scheme appears to be stable, reproducible, and highly reversible.
Figure 4. Establishing different oxygen concentrations within TTB cell culture chambers.
A) A photograph of the modified TTB cartridge with gas supply channels. Red – culture chambers, blue – nutrient delivery / waste removal network, yellow / green – gas supply channels. B) Changes in fluorescent intensity of the Pt-TPFP film due to diffusion / removal of oxygen monitored at the center of the cell culture chamber in real-time. Blue diamonds: transition from ambient -> nitrogen -> oxygen. Red circles: transition from ambient -> oxygen -> nitrogen. Green triangle: diffusion of oxygen simulated by COMSOL modeling using experimentally obtained D = 3.25×10−9 m2/sec from Figure 3 and Stern-Volmer equation (Fig. S2).
Conclusions
Plasma treatment of PDMS creates a SiO2 layer on the surface that significantly impedes partition and diffusion of oxygen through PDMS; however, for the case of standard formulation 10:1 crosslinked PDMS, the original observed diffusion rate of oxygen is completely restored if treated PDMS is stored in air for a 3 day period. PDMS storage under water slows down this surface restoration process. Use of much stiffer PDMS formulation showed no effect on oxygen penetration and diffusion in naïve, un-treated state, while hydrophobic recovery was significantly impeded. In vivo, tumor behavior is modified by the surrounding chemical environment such as the state of oxygenation (R.A. Gatenby et al. 2007; R.A. Gatenby and R.J. Gillies 2008; K. Lundgren, C. Holm, and G. Landberg 2007). The microenvironment of advanced tumors is frequently hypoxic, and the state of oxygenation (i.e., hypoxic versus normoxic) profoundly impacts anti-tumor drug efficacy (R. Cairns, I. Papandreou, and N. Denko 2006; O. Tredan et al. 2007; Y. Zhang et al. 2007). Understanding how surface treatments contribute to oxygen diffusion will be useful in exploiting the gas permeability of PDMS to establish defined normoxic and hypoxic oxygen conditions within microfluidic bioreactor systems. These studies provide an insight into time constrains associated with oxygen delivery and removal from the cell culture chambers as function TTB age and fabrication materials.
Supplementary Material
after curing at 65°C for 4 hours. Spinner: Laurell WS-400-6NPP; conditions: 2 sec spread cycle at 600 rpm, 30 sec at designated speed. Note: These parameters are given as a reference. The end-user would have to adjust them to accommodate their actual setup and equipment. Time course of fluorescence quenching for thin membranes is shown in Figure S4.
The Ksv coefficient from the Stern-Volmer relationship is found as a slope of the calibration curve.
in the presence of atmospheric oxygen (Ex = 540 +/−2.5 nm) visualized with Fluorolog-3 FL3-111 Spectrophotofluorometer.
ACKNOWLEDGMENTS
This research was supported by the Department of Defense Breast Cancer Research Program (DOD BCRP) grants W81XWH-09-1-0444 and W81XWH-10-1-0157 and NIH/NCI R21 CA126728-01 to L.J.M., by 1UH2TR000491-01, by the Vanderbilt Institute for Integrative Biosystems Research and Education (VIIBRE) and through the Searle Systems Biology and Bioengineering Undergraduate Research Experience (Searle SyBBURE) to E.M.L. We would like to thank VINSE for use of Fluorolog-3 FL3-111 Spectrophotofluorometer, L. Smith for help with establishing conditions for Figure S5, and J.P. Wikswo and P.C. Samson for useful discussions.
ABBREVIATIONS
- EOF
electroosmotic flow
- PDMS
polydimethylsiloxane
- Pt-TPFP
Platinum(II)-tetrakis(pentaflourophenyl)porphyrin
- TTB
thick tissue bioreactor
Reference List
- Adler M, Polinkovsky M, Gutierrez E, Groisman A. Lab on A Chip. 2010;10:388. doi: 10.1039/b920401f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amao Y, Miyashita T, Okura I. Journal of Fluorine Chemistry. 2001;107:101. [Google Scholar]
- Amao Y, Okura I. Tanpakushitsu Kakusan Koso. 1998;43:1492. [PubMed] [Google Scholar]
- Cairns R, Papandreou I, Denko N. Mol.Cancer Res. 2006;4:61. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- Carraway ER, Demas JN, Degraff BA. Analytical Chemistry. 1991;63:332. [Google Scholar]
- Carraway ER, Demas JN, Degraff BA, Bacon JR. Analytical Chemistry. 1991;63:337. [Google Scholar]
- Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Anal.Chem. 1998;70:4974. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- Effenhauser CS, Bruin GJ, Paulus A, Ehrat M. Anal.Chem. 1997;69:3451. doi: 10.1021/ac9703919. [DOI] [PubMed] [Google Scholar]
- Efimenko K, Wallace WE, Genzer J. J Colloid Interface Sci. 2002;254:306. doi: 10.1006/jcis.2002.8594. [DOI] [PubMed] [Google Scholar]
- Erickson D, Sinton D, Li D. Lab Chip. 2003;3:141. doi: 10.1039/b306158b. [DOI] [PubMed] [Google Scholar]
- Everaert EP, Van Der Mei HC, Busscher HJ. Journal of Adhesion Science and Technology. 1996;10:351. [Google Scholar]
- Fritz JL, Owen MJ. The Journal of Adhesion. 1995;54:33. [Google Scholar]
- Gao Y, Majumdar D, Jovanovic B, Shaifer C, Lin PC, Zijlstra A, Webb DJ, Li D. Biomed.Microdevices. 2011;13:539. doi: 10.1007/s10544-011-9523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Nat.Rev.Cancer. 2008;8:56. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ. Br.J Cancer. 2007;97:646. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillborg H, Ankner JF, Gedde UW, Smith GD, Yasuda HK, Wikstrom K. Polymer. 2000;41:6851. [Google Scholar]
- Jo BH, Van Lerberghe LM, Motsegood KM, Beebe DJ. Journal of Microelectromechanical Systems. 3013;9:76. [Google Scholar]
- Khalil GE, Chang A, Gouterman M, Callis JB, Dalton LR, Turro NJ, Jockusch S. Review of Scientific Instruments. 2005:76. [Google Scholar]
- Kim MS, Yeon JH, Park JK. Biomed.Microdevices. 2007;9:25. doi: 10.1007/s10544-006-9016-4. [DOI] [PubMed] [Google Scholar]
- Leclerc E, Sakai Y, Fujii T. Biotechnol.Prog. 2004;20:750. doi: 10.1021/bp0300568. [DOI] [PubMed] [Google Scholar]
- Lee GB, Lin CH, Lee KH, Lin YF. Electrophoresis. 2005;26:4616. doi: 10.1002/elps.200500382. [DOI] [PubMed] [Google Scholar]
- Liu L, Loutherback K, Liao D, Yeater D, Lambert G, Estevez-Torres A, Sturm JC, Getzenberg RH, Austin RH. Lab Chip. 2010;10:1807. doi: 10.1039/c003509b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K, Holm C, Landberg G. Cell Mol.Life Sci. 2007;64:3233. doi: 10.1007/s00018-007-7390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov DA, Lu JQ, Samson PC, Wikswo JP, McCawley LJ. Lab Chip. 2012;12:4560. doi: 10.1039/c2lc40304h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Electrophoresis. 2000;21:27. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Whitesides GM. Acc.Chem.Res. 2002;35:491. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Smith PJ. Journal of Adhesion Science and Technology. 1994;8:1063. [Google Scholar]
- Polinkovsky M, Gutierrez E, Levchenko A, Groisman A. Lab on A Chip. 2009;9:1073. doi: 10.1039/b816191g. [DOI] [PubMed] [Google Scholar]
- Prokop A, Prokop Z, Schaffer D, Kozlov E, Wikswo J, Cliffel D, Baudenbacher F. Biomed.Microdevices. 2004;6:325. doi: 10.1023/B:BMMD.0000048564.37800.d6. [DOI] [PubMed] [Google Scholar]
- Rharbi Y, Yekta A, Winnik MA. Anal. Chem. 1999;71:5045. [Google Scholar]
- Ren X, Bachman M, Sims C, Li GP, Allbritton N. J Chromatogr.B Biomed.Sci.Appl. 2001;762:117. doi: 10.1016/s0378-4347(01)00327-9. [DOI] [PubMed] [Google Scholar]
- Saito T, Wu CC, Shiku H, Yasukawa T, Yokoo M, Ito-Sasaki T, Abe H, Hoshi H, Matsue T. Analyst. 2006;131:1006. doi: 10.1039/b600080k. [DOI] [PubMed] [Google Scholar]
- Thomas PC, Halter M, Tona A, Raghavan SR, Plant AL, Forry SP. Anal.Chem. 2009;81:9239. doi: 10.1021/ac9013379. [DOI] [PubMed] [Google Scholar]
- Thomas PC, Raghavan SR, Forry SP. Anal.Chem. 2011;83:8821. doi: 10.1021/ac202300g. [DOI] [PubMed] [Google Scholar]
- Tredan O, Galmarini CM, Patel K, Tannock IF. J Natl.Cancer Inst. 2007;99:1441. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- Vickers JA, Caulum MM, Henry CS. Anal.Chem. 2006;78:7446. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- Wang X, Cheng C, Wang S, Liu S. Microfluid.Nanofluidics. 2009;6:145. doi: 10.1007/s10404-008-0399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty JR, Wicks CE, Wilson RE, Rorrer G. Fundamentals of Momentum, Heat, and Mass Transfer. 4th ed. Wiley; 2001. [Google Scholar]
- Zhang Y, Li M, Yao Q, Chen C. Med.Sci.Monit. 2007;13:RA175. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
after curing at 65°C for 4 hours. Spinner: Laurell WS-400-6NPP; conditions: 2 sec spread cycle at 600 rpm, 30 sec at designated speed. Note: These parameters are given as a reference. The end-user would have to adjust them to accommodate their actual setup and equipment. Time course of fluorescence quenching for thin membranes is shown in Figure S4.
The Ksv coefficient from the Stern-Volmer relationship is found as a slope of the calibration curve.
in the presence of atmospheric oxygen (Ex = 540 +/−2.5 nm) visualized with Fluorolog-3 FL3-111 Spectrophotofluorometer.