Abstract
Objectives
The objective of this study is to evaluate whether a patient navigation (PN) program is effective in reducing delay in diagnostic resolution among medically underserved patients with colorectal cancer (CRC) related abnormalities in Tampa Bay, Florida.
Methods
This study involved 10 primary care clinics randomized either to receive navigation or serve as controls (5 clinics per arm). Each clinic identified all subjects with colorectal-related abnormalities in the year prior to the clinic beginning participation in the Moffitt Patient Navigation Research Program. Patients with CRC related abnormalities were navigated from time of a colorectal abnormality to diagnostic resolution. Control patients received usual care, and outcome information was obtained from medical record abstraction. Using a frailty Cox proportional hazard model, we examined the length of time between colorectal abnormality and definitive diagnosis.
Results
193 patients were eligible for the study because of a colorectal cancer related abnormality (75 navigated; 118 control). Analysis of PN effect by two time periods of resolution (0-4 months and > 4 months) showed a lagged effect of PN. The adjusted time-varying PN effect on diagnostic resolution compared to control was marginally significant (adjusted Hazard Ratio, aHR=1.15, 95% CI: 1.02-1.29) after controlling for insurance status. The predicted aHR at 4 months was 1.2, but showed no significant effect until 12 months.
Conclusions
For patients having an abnormal symptom of CRC, PN appeared to have a positive effect over time and sped diagnostic resolution after 4 months. However, the small sample size limits drawing a definitive conclusion regarding the positive PN effect.
Keywords: patient navigation, colorectal cancer, time-to-diagnostic resolution, cluster randomized trial
Introduction
Detection and treatment of colorectal cancer (CRC) in its early stage improves long term survival. The length of the diagnostic interval, from the first symptom to a definitive diagnosis, has been shown to significantly reduce mortality in CRC cancer patients [1-3]. However, the burden of CRC is consistently heavier for the medically underserved population facing substantial barriers to obtaining cancer care, experiencing more late-stage diagnosis [4, 5] and the highest CRC mortality rates [6].
Patient navigation (PN) is an intervention model that centers on reducing barriers to cancer diagnostic and treatment care [5]. Recent studies have found that PN is a promising strategy to promote better adherence to recommended diagnostic care or more timely receipt of diagnostic care following an abnormal symptom of CRC [7, 8]. However, other studies had methodological limitations that precluded drawing firm conclusions about PN, such as a lack of concurrent control group and randomization including small sample sizes [9-13]. Recently, some PN programs reported a significant positive PN effect on combined cancer sites including CRC, breast, or cervical cancers on time to resolution and receipt of definitive diagnosis within a follow-up period [14, 15]. We are aware of only one study that shows PN shortens the time to diagnostic resolution and significantly increases the proportion of patients' adherent through diagnostic resolution of CRC screening abnormalities [16].
The Moffitt Patient Navigator Research Program (PNRP) was a cluster randomized trial to evaluate the efficacy of PN in improving timeliness of diagnostic resolution of cancer related abnormalities among a vulnerable, medically underserved population of racial and ethnic minorities and farm workers in Tampa Bay, Florida.[5] We have previously reported that PN did not have a significant effect on median time to, diagnostic resolution in multivariable analysis of the overall sample including both breast and colorectal cancer types [15]. Further, we also reported that PN did not speed diagnostic resolution during the initial three months of follow up for breast cancer patients but started to reduce time to diagnostic resolution and showed a significant effect after 4.7 months [17]. As clinical care of persons with breast and colorectal abnormalities is different, and patients may face different barriers to care, it is important to understand if the effectiveness of PN differs in these two cancer conditions.
The objective of this study is to evaluate the effect of PN on reducing the time to definitive diagnostic resolution for CRC-related abnormalities among medically underserved populations in Tampa, Florida through the Moffitt PNRP study.
Methods
Moffitt PNRP
Moffitt PNRP was conducted using a cluster randomized design with clinics randomized to either intervention (PN) group or control (usual care) group. Five health care organizations that provide primary care through a total of 12 clinics agreed to participate in the study. Because clinics within each health care organization were relatively homogeneous, randomization was stratified by health care organizations. Seven clinics were randomly assigned PN, and five clinics served as controls.
Participant Population and Study Sample
The populations served by the primary care clinics in this study were mostly Hispanic, African-American, and White, which is similar to the demographic characteristics of the Tampa Bay region [18]. Patients with a CRC-related clinical abnormality (rectal, bleeding, abnormal digital rectal examination), or abnormality detected during screening (e.g. abnormal fecal occult blood test, abnormal screening sigmoidoscopy) that required additional diagnostic imaging or referral to a specialist for further evaluation were eligible to participate in the study. Although newly diagnosed cancer patients were eligible for the Moffitt PNRP, these patients were not the focus of this analysis and were excluded from the sample. Patients were excluded if they were cognitively impaired, less than 18 years old, diagnosed with a previous cancer within the past five years (excluding non-melanoma skin cancer), currently undergoing cancer treatment, or had previously received patient navigation.
Participant Identification and Recruitment
Participants were enrolled between 3/11/2006 and 12/15/2009. Eligible patients were identified through several methods including referral coordinators, identification/referral from clinical staff, and computer searches of patients' relevant clinical diagnostic codes. At clinics randomized to navigation, once participants were identified and a written referral was provided by the patient's health care provider, a patient navigator contacted the patient and obtained informed consent for the study during an in-person visit. Patient navigators assessed barriers to care and matched resources where possible to overcome these barriers. Moffitt PNRP employed a lay patient navigation model in which four navigators were selected by investigators based on knowledge of community and experience with the health care system [19]. Patient navigators received annual standardized training through the PNRP [20], as well as local training, and were supervised by a registered nurse. Participation of control patients was limited to medical chart review for which informed consent was waived by the USF Institutional Review Board which approved and oversaw this study. Patients of clinics randomized to the control condition were provided usual medical care which included referral to specialty services for follow up of the colorectal cancer related abnormality.
Chart abstractions were performed to collect information on the initial CRC related abnormality, health services received and definitive diagnosis if available, patient socio-demographic characteristics and other health-related information (e.g. comorbidity, family history of cancer). Charts were abstracted approximately every six months until definitive diagnosis could be verified or until the end of the study's follow up. The last chart reviews were performed in August 2010.
Primary Study Outcome
The outcome for this study was length of time (in months) between the CRC related abnormality date and the definitive diagnosis or last follow-up date. Definitive diagnosis for CRC was defined as having determined the presence or absence of cancer from endoscopic examination colonoscopy/sigmoidoscopy (with or without biopsy), additional imaging (barium enema, CT colonography), or other diagnostic tests at the point in which a medical specialist determined no further immediate evaluation was required.
Analytic Sample Sizes
After the Moffitt PNRP began, there were 230 patients with CRC related abnormalities who received care at clinics allocated either to receive PN (n=104) or to receive usual care (n=126). Thirty-two patients (n=32) were found to be ineligible after chart reviews: patients who had cancer related abnormalities that occurred before the start date of the project, patients who were referred for problems not meeting eligibility criteria (e.g. upper gastrointestinal bleeding, routine colon polyp surveillance), patients who had no abnormality in their medical record that met eligibility criteria, and patients who were found to have a disqualifying condition on chart review (e.g. prior cancer). We also excluded patients who had both breast and colorectal abnormalities simultaneously (n=5). Therefore, 193 participants (75 patients from 5 navigated clinics and 118 from 5 control clinics) were analyzed for this study.
Statistical Analysis
Demographic and clinical variables at baseline were compared between the navigated, and control groups, using the generalized linear mixed effects models in which the clinic, was treated as a random effect to account for the cluster randomized trial of the study, design.
Survival analysis approaches were used for the time-to-resolution outcome (T1) to assess the PN effect. Participants who had not achieved definitive diagnostic resolution were censored at the time of last medical record abstraction. The median resolution time and resolution rate with their 95% confidence intervals were summarized, using the Kaplan Meier method. Further, the PN effect on T1 was investigated separately by stratifying two time periods: 0 - 4 months and >4 months after the implementation of PN. All study participants were included in the 0-4 month period analysis. Patients who resolved or were lost to follow-up after 4 months were censored at 4 months. For PN effect of > 4 months, patients who resolved or were lost to follow-up on or before 4 months were excluded.
Shared frailty Cox proportional hazards models were used to estimate the PN effect. We included the following covariates as potential confounders in the models: race-ethnicity (Hispanic, non-Hispanic), language (English, non-English), insurance (some form of health insurance, uninsured), and marital status (married, not-married). The multivariable Cox models included the clinic variable as a normally distributed random effect using a shared frailty to adjust for the study design. . We tested the proportional hazard assumption using graphical and numerical methods [21]. Variables that violated the proportional hazard assumption were dealt with by adding an interaction term of the variable with time to the multivariable Cox model. The hazard ratio of time-varying PN effect at a certain time point was estimated using a linear combination of the main effect of PN and the interaction term between PN and T1 and, tested the statistical significance using a Wald test. All tests were two-sided and considered as significant at 0.05 level. SAS software version 9.3 was used for the analyses.
Results
Demographic and Socio-Economic Characteristics
Most patients were eligible because of rectal bleeding (in patients age 30 or older with referral to specialist; 68.9%) or positive fecal occult blood testing (FOBT) with referral to specialist (23.3%). Most participants were female (68.9%), non-Hispanic (56.7%), English speakers (59.8%), had health insurance coverage (64.8%), had at least a high school education (50.5%), had household incomes of less than $20,000 per year (84.7%), and were not married (59.8%). The mean age of the participants was 52 (Standard Deviation [SD]: 13, range: 24-88) years old. Despite the apparent lack of comparability between navigated and control patients for some variables (e.g., having health insurance: 48% vs. 75% for PN and controls, respectively), there were no statistically significant differences between the two groups at 5% significant level after adjusting for the intra-cluster correlation within the same clinics (Table 1).
Table 1. Demographic and Social Economic Characteristic of Patients by Intervention Groups at Baseline (n=193).
| Variables: Levels | Control Group N=118 | Patient Navigation Group N=75 |
|---|---|---|
| Age at diagnosis in years Mean [STD] (min.-max) | 52.9 (24-88) | 49.7 (24-77) |
| Gender | ||
| Female | 76 (64.4%) | 57 (76.0%) |
| Male | 42 (35.6%) | 18 (24.0%) |
| Race-ethnicity | ||
| Black non Hispanic | 25 (23.8%) | 13 (17.8%) |
| White non Hispanic | 37 (35.2%) | 19 (26.0%) |
| Hispanic/Latino | 36 (31.3%) | 41 (56.2%) |
| Mixed/Other non Hispanic | 7 (6.7%) | 0 (0.0%) |
| Ethnicity | ||
| Not Hispanic/Latino | 69 (56.7%) | 32 (43.8%) |
| Hispanic/Latino | 36 (34.3%) | 41 (56.2%) |
| Language | ||
| English | 77 (67.5%) | 36 (48%) |
| Non-English | 37 (32.5%) | 39 (52%) |
| Marital Status | ||
| Married | 37 (34.6%) | 35 (48.6%) |
| Non-Married | 70 (65.4%) | 37 (51.4%) |
| Education | ||
| 8th grade or less | 9 (22.0%) | 28 (48.3%) |
| Some high school | 7 (17.1%) | 5 (8.6%) |
| High school diploma (including equivalency) | 17 (41.5%) | 17 (29.3%) |
| Some college/vocational training after high School, Associate degree, or College graduate | 8 (19.5%) | 8 (13.8%) |
| Income | ||
| Less than $10,000 | 21 (46.7%) | 19 (35.8%) |
| $10,000 to $19,999 | 16 (35.6%) | 27 (50.9%) |
| $20,000 to $29,999 | 6 (13.3%) | 6 (11.3%) |
| $30,000 or more | 2 (4.4%) | 1 (1.9%) |
| Employment | ||
| Employed full time | 17 (21.5%) | 20 (30.8%) |
| Not employed full time | 62 (78.5%) | 45 (69.2%) |
| Health Insurance Status | ||
| Any Coverage | 89 (75.4%) | 36 (48.0%) |
| No health insurance coverage | 29 (24.6%) | 39 (52.0%) |
| Insurance Type among Covered | ||
| Private insurance | 11 (12.5%) | 3 (8.3%) |
| Medicare (no private) | 17 (19.3%) | 8 (22.8%) |
| Medicaid (no private or Medicare) | 21 (23.9%) | 5 (13.9%) |
| Other government insurance (no private, Medicare, or Medicaid) | 39 (44.3%) | 28 (55.6%) |
| Family history of CRC | ||
| No | 112 (94.9%) | 73 (97.3%) |
| Yes | 6 (5.1%) | 2 (2.7%) |
| Charlson Comorbidity Index score | ||
| 0 | 79 (66.9%) | 54 (72.0%) |
| 1 | 23 (19.5%) | 15 (20.0%) |
| 2+ | 16 (13.6%) | 6 (8.0%) |
Note that the sample size varies because of missing responses in several of the variables.
Reported Barriers
Navigated patients reported on average 4.6 (range 0-13) barriers to needed care. Most common barriers identified were: Insurance related issue (60%), fear about any aspect of medical care or their health (47%), financial problems (44%), and language barriers (41%). Navigation was performed with in person visits or by phone as necessary. Navigators averaged 12 total encounters (including family members of the patient), with an average of 11 encounters directly with the patient.
Characteristics of the Time to Diagnostic Resolution of the CRC Abnormality
Fifty two PN patients (69%) and 79 controls (67%) reached a definitive diagnosis with the median follow-up time of 10 months (range 14 days - 59.6 months) and 6 months (range 1 day - 37 months), respectively. The test required to reach diagnostic resolution was almost always colonoscopy, either with biopsy (29%) or without biopsy (59.5%).
Although the median time between the CRC abnormality and diagnostic resolution was longer for the PN group (4.4 months; 95% Confidence Interval [95% CI]: 3.4-6.5 months) compared to the control group (3.1 months; 95% CI: 2.4-6.4 months), this difference was not statistically significant. Figure 1A shows the cumulative diagnostic resolution rate over time by PN group, and the two curves crossed around four months. Prior to four months the control group appeared to have quicker diagnostic resolution than the PN group, but beyond four months, those receiving PN seemed more likely to, achieve diagnostic resolution in less time (Figure 1B and 1C).
Figure 1.
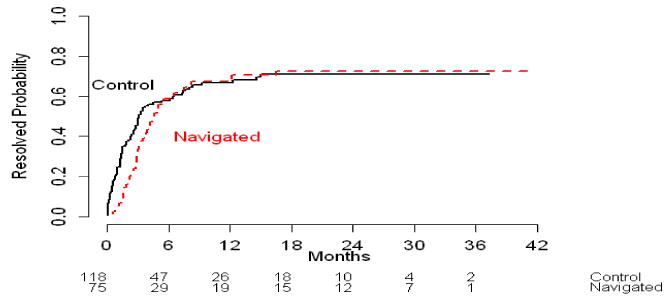
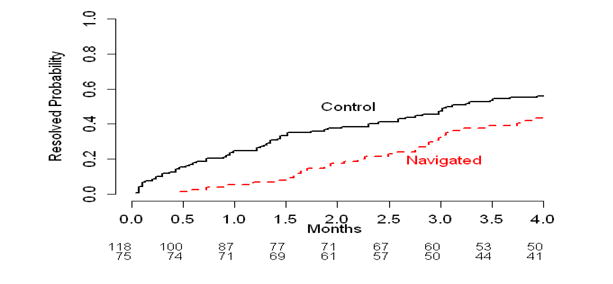
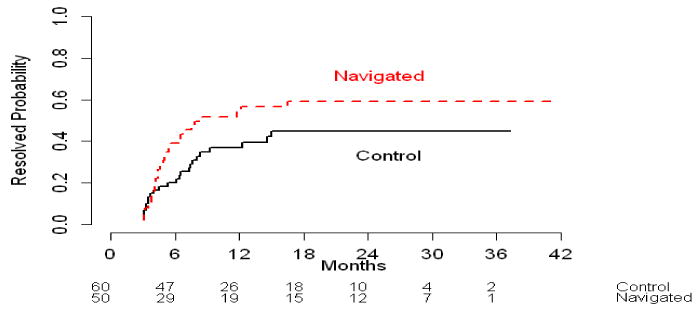
Cumulative resolution rate (%) by intervention arms for across overall time period (1A) and by time period (1B and 1C).
Figure 1A: All patients across overall time period
Figure 1B: Patients who resolved prior to 4 months
Figure 1C: Patients who resolved beyond 4 months
Multivariable Analysis of PN Effect on Time to Diagnostic Resolution of the CRC, Abnormality
An initial multivariable Cox model showed that the PN effect on the resolution rate, varied with time, while the other four covariates satisfied the proportional hazard, assumption at the 5% significance level. The departure from non-proportionality of PN, effect was addressed by including an interaction term between the PN and time in the, final models.
Table 2 shows the results from two multivariable models with shared frailty. Model 1 included the main PN effect and its interaction term with time as well as the 4 covariates. Model 2 is a nested version of Model 1, determined by excluding nonsignificant covariates (Hispanic ethnicity, language, and marital status). Both models, included a significant interaction term between PN and time measured in months, (p=0.01 for Model 1 and p=0.03 for Model 2) to account for the time-varying PN effect.
Table 2. Multivariable analysis for time to diagnostic resolution among patients having colorectal cancer abnormal symptoms.
| Variables | Model 11) Adjusted Hazard Ratio (95% CI) |
Model 22) Adjusted Hazard Ratio (95% CI) |
|---|---|---|
|
| ||
| Patient Navigation (PN) vs. Control | 0.64 (0.35-1.19) | 0.62 (0.33-1.15) |
| PN × Time | 1.15 (1.00-1.31) | 1.15 (1.02-1.29) |
| Hispanic vs. Non-Hispanic | 1.07 (0.62-1.85) | - |
| English vs. Non-English | 1.05 (0.59-1.86) | - |
| Health Insurance vs. No Health Insurance | 1.88 (1.20-2.95) | 2.01 (1.33-3.04) |
| Married vs. Not-married | 0.77 (0.51-1.16) | - |
Model 1 includes PN and time-PN interaction as a time-varying effect as well as 4 covariates (Hispanic, Language, Insurance, and Marital status).
Model 2 excludes non-significant covariates (Hispanic, Language, and Marital status) from Model 1.
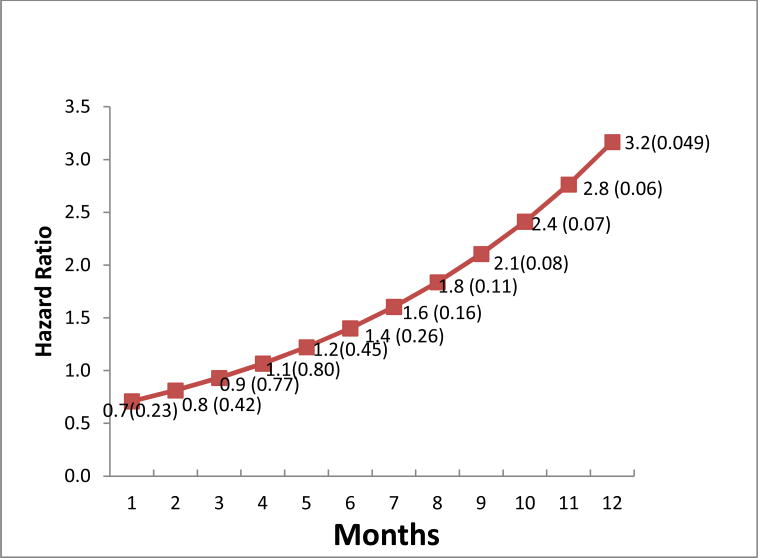
The main effect at month=0 showed no effect on diagnostic resolution comparing PN to control (adjusted Hazard Ratio [aHR] =0.64, 95% CI: 0.35-1.19 from Model 1 and aHR=0.62, 95% CI: 0.33-1.15 from Model 2). The adjusted time-varying PN effect was marginally significant after controlling those covariates with aHR=1.15, 95% CI: 1.02-1.29 from the Model 2. Among the 4 covariates, only having insurance had a statistically significant impact on the resolution rate (aHR=2.01) and remained in Model 2. We estimated the effect of PN for any given month from the equation, [−0.48 + 0.136 × Month] derived from Model 2, using the parameter estimates of the PN (−0.48) and interaction term (0.136). Figure 2 shows that the aHR of PN was less than 1, showing no positive effect (aHR > 1.0) on the hazard of diagnostic resolution up to 4 months of patient follow up. The PN effect was not statistically significant until 12 months after the CRC abnormality was detected (Figure 2).
Figure 2.
Estimated Adjusted Hazard Ratio of PN Effect (p-value) Over Time, using Model 2.
Discussion
This study examined whether PN was effective in reducing the time from identification of a CRC-related abnormality until diagnostic resolution of that abnormality. For patients having an abnormal symptom of CRC, PN appeared to have a positive effect over time. The PN effect varied with time and after approximately 4 months there was an increasing trend for navigation to increase timely diagnostic resolution of CRC. The effect of PN, however, was not statistically significant until 12 months of follow-up.
Previous research on patient navigation has largely targeted breast cancer. Studies that have addressed colon cancer have primarily focused on the role of PN in CRC screening [22]. Several studies have demonstrated that navigation type services, such as telephone care management and multifaceted intervention, can increase the likelihood that patients undergo CRC screening with either colonoscopy or FOBT [5, 8, 23, 24]. Additional studies, many of which are observational, suggest that PN can increase the likelihood that patients comply with colonoscopy once referred [1, 4, 9, 15, 16].
The goal of PN in the Tampa PNRP, however, may have been more difficult to achieve. In contrast to promoting CRC screening with either FOBT or colonoscopy, virtually all patients in the Tampa PNRP required diagnostic colonoscopy to evaluate clinical/screening abnormalities. Colonoscopy is clearly a more difficult test for patients to complete than FOBT as it is more invasive and costs more if a patient is uninsured or underinsured. In addition, colonoscopy resources were not readily available for uninsured patients in the Tampa PNRP, presenting a much more daunting navigation task compared to navigating patients having access to screening colonoscopy. Finally, because navigated patients required a referral from their primary care physician, it is possible that patients specifically referred to PN had greater barriers to care and fewer personal resources than the overall eligible population [15].
Overall, PN in the Tampa PNRP appeared to have a positive effect on reducing the definitive resolution time, although it did not emerge until after four months of follow up and did not reach statistical significance until 12 months. Other navigation trials have similarly reported that navigation had no effect early, but that effects began to emerge later in follow up [14, 17, 25, 26]. It is possible that navigation primarily benefits those persons at greatest risk of delayed diagnosis. Persons who do not reach diagnosis after considerable follow-up may have greater barriers to care and receive more benefit from navigation. However, we should note that the clinical significance of faster resolution rates after 12 months is unknown from this study.
This study had a number of limitations that should be considered when interpreting our findings. Our sample of patients with CRC abnormalities is a subset of, a larger trial [15]. As a result, the sample was relatively small and had limited power to detect modest effect sizes. We estimate, for example, that a sample of 193 observations achieves only 8% power at a 0.05 significance level to detect adjusted hazard ratios of 2.0. This study was conducted in community clinics serving medically underserved persons and results may differ among other populations and in other settings. Finally, the IRB required that patients receiving PN be referred from their providers and provide written informed consent which may have contributed to a lack of comparability between intervention and control populations.
In conclusion, PN appears to improve timeliness of diagnostic resolution primarily among patients having delayed diagnosis of CRC related abnormalities. Further research is needed to understand the effects of PN in patients with CRC related abnormalities and to better understand which patients are most likely to benefit. Although preliminary studies suggest that PN may be cost effective [27, 28], further research is also needed to understand how PN can be financed and integrated into our health care system.
Acknowledgments
The authors would like to thank the health care organizations, staff, navigators, and patients who contributed to the study and the study Community Advisory Board.
This study was funded by the National Cancer Institute (NCI), through its Center to Reduce Cancer Health Disparities, National Institutes of Health, Department of Health and Human Services (U01 CA 117281-01). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI. Dr. Wells' contribution to this study was also funded by a grant from NCI (R25 CA090314; Paul B. Jacobsen, Ph.D., Principal Investigator). Dr. Meade was also supported by NCI (U01 CA114627 and U54 CA153509).
References
- 1.Tørring ML, et al. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. British journal of cancer. 2011;104(6):934–940. doi: 10.1038/bjc.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkin WS, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. The Lancet. 2010;375(9726):1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 3.Hewitson P, et al. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD001216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman HP. The Origin, Evolution, and Principles of Patient Navigation. Cancer Epidemiology Biomarkers & Prevention. 2012;21(10):1614–1617. doi: 10.1158/1055-9965.EPI-12-0982. [DOI] [PubMed] [Google Scholar]
- 5.Wells KJ, et al. Patient navigation: State of the art or is it science? Cancer. 2008;113(8):1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward E, et al. Association of insurance with cancer care utilization and outcomes. CA: A Cancer Journal for Clinicians. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 7.Percac-Lima S, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. Journal of general internal medicine. 2009;24(2):211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasser KE, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Archives of internal medicine. 2011;171(10):906. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl B, et al. Effect of a patient navigator program on the volume and quality of colonoscopy. Journal of clinical gastroenterology. 2011;45(5):e47. doi: 10.1097/MCG.0b013e3181f595c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie J, et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. Journal of the National Medical Association. 2008;100(3):278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen LA, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clinical Gastroenterology and Hepatology. 2008;6(4):443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Myers RE, et al. Tailored navigation in colorectal cancer screening. Medical care. 2008;46(9):S123–S131. doi: 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]
- 13.Nash D, et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. Journal of Urban Health. 2006;83(2):231–243. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paskett ED, et al. The Ohio Patient Navigation Research Program: Does the American Cancer Society Patient Navigation Model Improve Time to Resolution in Patients with Abnormal Screening Tests? Cancer Epidemiology Biomarkers & Prevention. 2012;21(10):1620–1628. doi: 10.1158/1055-9965.EPI-12-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells KJ, et al. A Cluster Randomized Trial Evaluating the Efficacy of Patient Navigation in Improving Quality of Diagnostic Care for Patients with Breast or Colorectal Cancer Abnormalities. Cancer Epidemiology Biomarkers & Prevention. 2012;21(10):1664–1672. doi: 10.1158/1055-9965.EPI-12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raich PC, et al. Patient Navigation Improves Cancer Diagnostic Resolution: An Individually Randomized Clinical Trial in an Underserved Population. Cancer Epidemiology Biomarkers & Prevention. 2012;21(10):1629–1638. doi: 10.1158/1055-9965.EPI-12-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, F W, Wells K, Meade CD, Calcano C, Roetzheim R. Patient Navigation and Time to Diagnostic Resolution: Results for a Cluster Randomized Trial Evaluating the, Efficacy of Patient Navigation among Patients with Breast Cancer Screening Abnormalities, Tampa, FL. PLoS One. 2013 doi: 10.1371/journal.pone.0074542. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Level PBP. US Census Bureau. State & County QuickFacts. Nevada. Data updated. 2007 Jul;:2005–2006. [Google Scholar]
- 19.Wells KJ, et al. Innovative approaches to reducing cancer health disparities. Journal of Cancer Education. 2011;26(4):649–657. doi: 10.1007/s13187-011-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calhoun EA, et al. A national patient navigator training program. Health Promotion Practice. 2010;11(2):205–215. doi: 10.1177/1524839908323521. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–572. [Google Scholar]
- 22.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. Journal of general internal medicine. 2012;27(8):1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich AJ, et al. Telephone care management to improve cancer screening among low-income women. Annals of Internal Medicine. 2006;144(8):563–571. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu SP, et al. Promoting culturally appropriate colorectal cancer screening through a health educator. Cancer. 2006;107(5):959–966. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 25.Warren-Mears V, et al. Impact of Patient Navigation on Cancer Diagnostic Resolution Among Northwest Tribal Communities. Journal of cancer education. 2013;28(1):109–118. doi: 10.1007/s13187-012-0436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battaglia TA, et al. Boston Patient Navigation Research Program: The Impact of Navigation on Time to Diagnostic Resolution after Abnormal Cancer Screening. Cancer Epidemiology Biomarkers & Prevention. 2012;21(10):1645–1654. doi: 10.1158/1055-9965.EPI-12-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jandorf L, et al. Cost analysis of a patient navigation system to increase screening colonoscopy adherence among urban minorities. Cancer. 2013;119(3):612–620. doi: 10.1002/cncr.27759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elkin EB, et al. The economic impact of a patient navigator program to increase screening colonoscopy. Cancer. 2012;118:5982–5988. doi: 10.1002/cncr.27595. [DOI] [PubMed] [Google Scholar]