Abstract
Large-scale epidemiological surveillance of dengue in the field and dengue patient management require simple methods for sample collection, storage, and transportation as well as effective diagnostic tools. We evaluated the kinetics of three biological markers of dengue infection—non-structural protein 1 (NS1) antigen, immunoglobulin M (IgM), and IgA—in sequential capillary blood samples collected from fingertips of confirmed dengue patients. The overall sensitivities and specificities of the tests were 96% and 100%, respectively, for NS1, 58.1% and 100%, respectively, for IgM, and 33% and 100%, respectively, for IgA. During the acute phase of the disease, NS1 was the best marker of dengue infection, with a sensitivity of 98.7%, whereas from day 5, all three markers exhibited relevant levels of sensitivity. This first descriptive study of the kinetics of biological markers of dengue in capillary blood samples confirms the usefulness of this biological compartment for dengue diagnosis and argues for its exploitation in community-level and remote settings.
Introduction
Dengue fever is an arboviral disease that is a public health priority in most tropical countries.1 The disease is caused by infection with one of four serotypes of dengue virus (DENV), a member of the Flaviviridae family. Considered to be a re-emerging disease by the World Health Organization, it affects up to 100 million people each year, with about 500,000 cases of severe illness and around 30,000 deaths.2,3 However, the true impact of dengue on health is probably underestimated, because there is no specific clinical presentation for dengue infection; also, there are insufficient or no specialized laboratories in most endemic regions. In the absence of specific therapy, the management of dengue cases mainly relies on early detection of infected patients and symptomatic treatment of the most severe cases. However, the tools currently available for dengue diagnosis have shortcomings and limitations. First, a combination of different techniques has to be used for dengue diagnosis, and the most appropriate combination of techniques varies according to the phase of the disease. During the acute phase, from day 0 to day 4 (day 0 being the first day of the onset of the symptoms), direct methods are preferred, regardless of the DENV serotype: viral RNA detection by reverse transcription polymerase chain reaction (RT-PCR), virus isolation, and/or detection of non-structural protein 1 (NS1).4–9 From day 5 on (early convalescent phase), it is recommended to use serological tests that can detect dengue virus-specific immunoglobulin M (IgM) and IgA.10–13 Second, a venous blood sample is required, and such samples may be difficult to collect, store, and transport to specialized diagnostic laboratories, particularly for children and patients living in remote areas. Therefore, other alternatives for dengue diagnosis based on non-invasive sample collection combined with sensitive, specific, rapid, and cost-effective detection tools are required to identify and manage patients suspected of dengue; such tools would also be useful for field studies in endemic regions.14–17 Against this background, we previously showed that capillary blood samples collected from the fingertip by pricking and absorbed onto filter papers could be a promising alternative to venous puncture for dengue diagnosis and epidemiological studies.14,18 In particular, we showed that this sampling approach allowed the detection of the viral genome, the NS1 antigen, and dengue-specific IgM antibodies without cold chain constraints.18 In the present descriptive study, we assessed testing for NS1 antigen and IgM and IgA antibodies in capillary blood samples from dengue patients collected sequentially over the course of the disease. Through this study, we show that this biological compartment may be very useful for dengue diagnosis, notably during the period from day 4 to day 7 of the disease, during which time many patient present clinical complications. We also confirm that the combined use of these three biological markers reinforces the diagnosis of dengue infection.
Materials and Methods
Ethics statement.
Patients from the People's Hospital 115, Ho Chi Minh City, Vietnam and healthy individuals were included in the cohort according to a detailed protocol that was approved by the Ethics Committee of the People's Hospital 115. Conforming to this protocol, dengue-positive patients and healthy individuals were included after oral informed consent was obtained. The inclusion of dengue-positive patients in the study was notified in personal medical files. The committee did not ask for a specific form (oral/written) for the consent. We favored an oral agreement, notified in each medical file, because few of the patients can read French or English.
Patients and study design.
From July to October of 2007, a prospective longitudinal study was carried out in the People's Hospital 115, Ho Chi Minh City, Vietnam. In total, 26 dengue-positive patients ages 16–45 years old (range = 24.9 years old) were enrolled after obtaining oral informed consent (parent/guardian consent was obtain for minors). All patients included presented with a sudden onset of continuous high fever lasting less than 3 days that was associated with intense headache, arthralgia, or myalgia but not with rhinitis or other clinically obvious alternative symptoms. The first day of fever was defined as day 0 of the disease.
Serum samples were collected (by intravenous puncture) from each patient on day 2 of their disease, and they were used to confirm dengue infection by RT-PCR or virus isolation.4,5 Among 26 dengue-positive patients, 8 patients had DENV-1, 7 patients had DENV-2, 6 patients had DENV-3, 1 patient had DENV-4, and 4 patients had DENV-1 and DENV-4 coinfections (Table 1 ). Three drops of about 20 μL capillary blood were collected daily (from day 2 until the end of hospitalization) from a finger of each patient and independently absorbed onto filter paper (Whatman 3M-Schleicher and Schuell, Germany). One of the triplicate samples was used for each of the following tests: NS1 detection by Platelia Dengue NS1 Ag Capture Enzyme-Linked Immunosorbent Assay (ELISA; Bio-Rad Laboratories, Marnes-La-Coquette, France); specific dengue IgM and IgA antibodies detection using IgM and IgA Antibody Capture (MAC and AAC) ELISA; and dengue IgG antibodies detection by indirect ELISA. In total, 129 sequential capillary samples were collected between day 2 and day 7 of the disease. These sequential capillary samples included 41 sequential samples from DENV-1 patients, 34 sequential samples from DENV-2 patients, 30 sequential samples from DENV-3 patients, 5 sequential samples from DENV-4 patients, and 19 sequential samples from coinfected DENV-1 and -4 patients (Table 1). According to the information provided by the patients, 77 samples were collected during the acute phase of the disease (from day 2 to day 4), and 52 samples were collected during the early convalescent phase (day 5 and later) (Table 1). These samples were stored at −80°C for 1 year at the Institut Pasteur, Ho Chi Minh City, Vietnam. They were then sent at room temperature to the Institut Pasteur de la Guyane, where they were stored at −80°C until testing.
Table 1.
NS1-positive capillary blood samples by dengue serotype during the course of disease in 26 confirmed dengue patients
| Days after onset | DENV-1 | DENV-2 | DENV-3 | DENV-4 | DENV-1 and -4 | Total (%) |
|---|---|---|---|---|---|---|
| Day 2 | 8/8 | 7/7 | 6/6 | 1/1 | 4/4 | 100 (26/26) |
| Day 3 | 8/8 | 6/6 | 6/6 | 1/1 | 4/4 | 100 (25/25) |
| Day 4 | 8/8 | 6/7 | 6/6 | 1/1 | 4/4 | 96.1 (25/26) |
| Sensitivity during the acute phase | 24/24 | 19/20 | 18/18 | 3/3 | 12/12 | 98.7 (76/77) |
| Day 5 | 8/8 | 6/6 | 5/6 | 1/1 | 4/4 | 96.0 (24/25) |
| Day 6 | 6/7 | 5/6 | 4/4 | 0/1 | 2/2 | 85.0 (17/20) |
| Day 7 | 2/2 | 2/2 | 2/2 | – | 1/1 | 100.0 (7/7) |
| Sensitivity during the convalescent phase | 16/17 | 13/14 | 11/12 | 1/2 | 7/7 | 92.3 (48/52) |
| Sensitivity/DENV serotype (95% CI) | 40/41 (92.8–100) | 32/34 (86.2–100) | 29/30 (90.2–100) | 4/5 (44.9–100) | 19/19 (85.4–100) | 96 (124/129; 92.8–99.5) |
The specificity of the biological markers in capillary blood samples was assessed using samples from 17 healthy individuals ages 24–49 years old (range = 35.6 years old). To exclude possible asymptomatic DENV infection, RT-PCR, NS1, and specific dengue IgM assays were performed from each patient's venous sample collected at inclusion in the study. In parallel to this intravenous puncture, three drops of capillary blood were collected daily from each individual for 6 days. In total, 102 capillary blood samples were analyzed using the Platelia Dengue, MAC and AAC ELISA for detection of NS1 antigen, and dengue IgM and IgA antibodies tests.
Laboratory analyses.
Detection of NS1 antigen in capillary blood samples using the Platelia Dengue NS1 Ag test.
A drop of blood was collected daily onto blotting paper from a finger prick from each confirmed dengue patient and healthy individual. These capillary blood samples were used for NS1 detection by the Platelia Dengue NS1 Ag Capture ELISA Kit (Bio-Rad Laboratories, Marnes-La-Coquette, France). Filter papers carrying one entire drop of capillary blood (about 20 μL whole blood) were cut and placed individually into sterile tubes containing 150 μL kit dilution buffer. In addition to the controls provided by the kit, we included two NS1-positive and -negative sera as internal controls: 10 μL these sera were absorbed onto filter paper and processed in the same way as capillary blood samples. After 30 minutes of incubation at room temperature, an aliquot of 100 μL each sample was incubated with 100 μL diluted conjugate for 90 minutes. Subsequent steps were as recommended by the manufacturer.7,18 The sample/cutoff ratio was calculated for each sample. Ratios of < 1.0 and > 1.0 were designated as negative and positive, respectively.
Detection of specific IgM and IgA in capillary blood samples.
In total, 129 sequential capillary blood samples from 26 confirmed dengue cases and 102 capillary samples from dengue-negative patients were tested for dengue-specific IgM and IgA antibodies by MAC and AAC ELISA using an in-house protocol similar to the protocol described in the work by Talarmin and others.11 Each filter paper carrying a capillary blood sample (20 μL) was cut entirely into strips and placed in sterile tubes. They were incubated for 2 hours in 600 μL 1× phosphate-buffered saline (PBS) containing 0.5% Tween 20 and 5% non-fat dried milk (PBS-T NDM). Aliquots of 100 μL each eluate were used as serum in the MAC and AAC ELISA tests.11 Briefly, each well of the flat-bottomed microplates (Maxisorp; Nunc) was coated with 100 μL either goat anti-human IgM diluted 1:500 in PBS or goat anti-human IgA diluted 1:250 in PBS (Sigma Laboratories, L'Isle d'Abeau Chesnes, France). The microplates were incubated for 1 hour at 37°C and washed with PBS-T. Each of 231 capillary blood sample eluates was dispensed at 100 μL per well in duplicate. Six negative and two positive whole-blood samples were included on each plate as controls. The plates were then incubated for 1 hour at 37°C. The plates were washed with PBS-T, and 100 μL (16 hemagglutination units) tetravalent dengue virus antigen or an uninfected control sample diluted in PBS-T NDM were dispensed into one of a pair of wells. These antigens were obtained from suckling mouse brain preparations. The plates were incubated at 4°C overnight and washed with PBS-T, and bound antigens were detected with anti-dengue virus mouse ascitic fluid prepared in our laboratory and diluted 1:10,000 in PBS-T NDM. Peroxidase-conjugated goat anti-mouse IgG (Sigma Laboratories) diluted 1:1,000 in PBS-T NDM was then added, and tetramethylbenzidine (TMB; Sigma) was used as the substrate. After 30 minutes, the reaction was stopped by the addition of 0.5 N sulfuric acid to each well, and optical density (OD) at 450 nm was read in an ELISA reader. The results for MAC ELISA were calculated by dividing the absorbance of the antigen-containing well by the absorbance of the infected control well for each capillary blood sample. A ratio of three or more was considered positive.
For AAC ELISA, the mean ± SD OD values for the negative controls were determined. A result was scored negative when the OD value was less than the mean value for the negative controls plus 2 SDs, and it was scored positive when the OD value was above 3 SDs.
Detection of specific dengue IgG in capillary blood samples of confirmed dengue patients.
In parallel to dengue IgM and IgA detection in the sequential capillary blood samples, past exposure to dengue virus was determined for the positive dengue patients. The third drop from each of 26 samples collected on day 2 of the disease was used to determine the dengue IgG response using a test adapted from a previously described indirect IgG ELISA.19 Briefly, for each of 26 patient samples, one well (Polysorp; Nunc) was coated with 100 μL dengue antigen (dilution 1:2,000 in PBS), and one well was coated with 100 μL normal mouse brain antigen (dilution 1:2,000 in PBS). After overnight incubation at 4°C and washing, 100-μL aliquots of each capillary blood sample eluate were added to the wells as described above. Six negative and two positive reference serum samples were included as controls. The plates were then incubated for 1 hour at 37°C. Finally, bound IgG was detected by the addition of 100 μL/well horseradish peroxidase-conjugated goat anti-human IgG (Jackson Immunoresearch Laboratories, West Grove, PA) diluted 1:2,000 in PBS-T NDM. After 1 hour at 37°C, the plates were washed, and TMB was added as substrate. After 15 minutes at room temperature, the absorbance at 650 nm (A650) was read with a microplate reader, and the ΔA650 was calculated (ΔA650 = ΔA650 Den Ag – ΔA650 control Ag). To validate the test, the ΔA650 of the positive control had to be ≥ 0.5, and the ΔA650 of the negative control had to be ≤ 0.2. The means (±SDs) of ΔA650 values for the negative controls were then determined. A result was considered negative when the ΔA650 values were less than the mean value for the negative control plus 2 SDs, indeterminate when the ΔA650 values were between 2 and 3 SDs, and positive when the ΔA650 values were more than 3 SDs above the mean negative control value.
Statistical analyses.
The sensitivities and specificities of each biological marker in capillary blood samples were determined using 2 × 2 tables. The performances of each assay were evaluated by comparing results obtained from capillary and venous samples.
Results
During a prospective longitudinal study in Vietnam in 2007, we evaluated the time course of detection of three biological markers of dengue infection—NS1 antigen and dengue IgM and IgA antibodies—in dried capillary blood samples collected from 26 confirmed dengue patients. Dengue diagnosis was confirmed for each patient by RT-PCR or virus isolation from venous samples collected on day 2 of the disease. For each confirmed dengue patient, capillary blood samples were collected sequentially from day 2 to day 7 (at the latest) and dried on sterile filter papers. For medical reasons, blood was not collected on day 3 from one patient infected by DENV-2. This patient left the hospital on day 5 of the disease (Table 1). Because specific IgGs to dengue antigens were detected for each of 26 capillary blood samples collected on day 2 of the disease, we concluded that all infected patients presented with secondary infection.20
In total, 102 capillary blood samples from 17 healthy individuals were used to determine the specificity of the biological markers of dengue infection in the capillary compartment. These individuals were enrolled in the study after exclusion of possible asymptomatic dengue infection by negative NS1, RT-PCR, and dengue antibodies detection from venous samples collected on the first day of inclusion.
Of 129 dried capillary blood samples, 124 samples scored positive for NS1 with the Platelia Dengue NS1 Ag Capture ELISA Kit (Table 1). Thus, the overall sensitivity of NS1 detection in dried capillary blood samples was 96% (124 of 129; 95% confidence interval [95% CI] = 93–99%). As expected, NS1 was more frequently detected during the acute phase of the disease (from day 2 to day 4), during which time the sensitivity was 98.7% (76 /77; 95% CI = 96–100%). Nonetheless, the sensitivity was only slightly lower during the early convalescent phase of disease (from day 5 to day 7), when it reached 92.3% (48/52; 95% CI = 85–100%) (Table 1). On day 5 of the disease, the level of NS1 antigen detection was still high, because 24 of 25 (96%) capillary blood samples scored positive. Excluding the DENV-4 serotype, which was detected in one patient and four coinfected patients, there was no significant difference in NS1 sensitivity (P < 0.05) between the three other viral serotypes. It is noteworthy that all sequential capillary blood samples collected from 17 healthy patients scored negative for NS1.
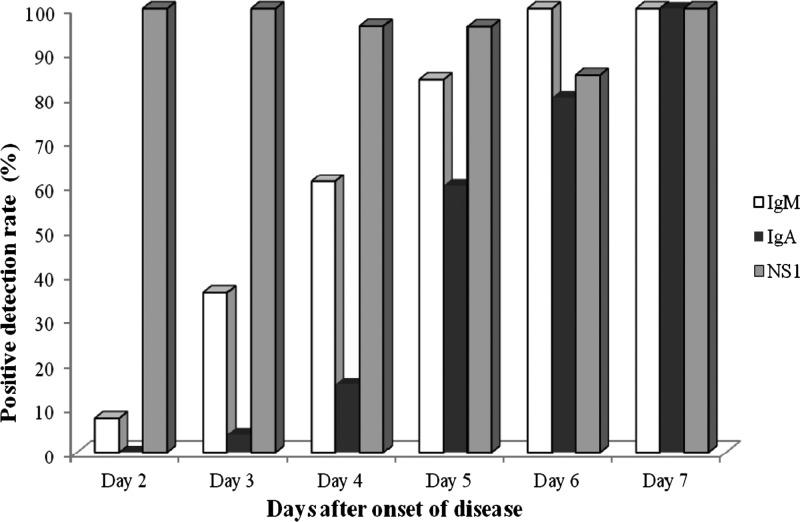
IgM and IgA antibodies were detected in 58.1% (75/129; 95% CI = 49.6–66.6%) and 33.3% (43/129; 95% CI = 25.2–41.5%) of 129 capillary blood samples, respectively (Table 2 ). As expected, IgA detection was slightly delayed compared with IgM detection, and both antibodies were less frequently detected during the acute phase of the disease. Indeed, only 35% (27/77; 95% CI = 24.4–45.7%) and 6.4% (5/77; 95% CI = 2.9–12%) of the samples collected between day 2 and day 4 scored positive for IgM and IgA, respectively (Table 2). During the early convalescent phase of the disease, IgM and IgA antibodies were detected in 92.3% (48/52; 95% CI = 85.1–99.5%) and 73.1% (38/52; 95% CI = 61–85.1%) of the samples (Table 2). Importantly, none of the patients was positive for IgA and negative for IgM throughout the course of the disease. Four DENV-1–infected patients consistently scored negative for dengue IgA antibodies until day 6 of the disease. However, these patients were positive for NS1 antigen, which was associated with the presence of IgM antibodies on day 5 (two cases) or day 6 (two cases) of the disease. In addition, based on the results obtained from 102 capillary blood samples of 17 dengue-negative patients, the specificity of the dengue IgM and IgA antibodies in the capillary compartment was 100%. Figure 1 illustrates the observed kinetics of these three biological markers of dengue infection during the course of disease.
Table 2.
Detection of IgM and IgA antibodies in capillary blood samples from 26 positive dengue patients during the course of the disease
| Days after onset of disease | Positive IgM | Positive IgA |
|---|---|---|
| Day 2 | 2/26; 7.7% (1.5–21) | 0/26; 0% (0–11) |
| Day 3 | 9/25; 36% (22.5–52.2) | 1/25; 4% (0.2–16.8) |
| Day 4 | 16/26; 61% (42.8–80.2) | 4/26; 15.4% (6–29.6) |
| Sensitivity in acute phase | 27/77; 35% (24.4–45.7) | 5/77; 6.4% (2.9–12) |
| Day 5 | 21/25; 84%(69.6–98.4) | 15/25; 60% (40.8–79.2) |
| Day 6 | 20/20; 100% (86.5–100) | 16/20; 80% (62.5–97.5) |
| Day 7 | 7/7; 100% (65.2–100) | 7/7; 100% (65.2–100) |
| Sensitivity in convalescent phase | 48/52; 92.3% (85.1–99.5) | 38/52; 73.1% (61–85.1) |
| Overall sensitivity | 75/129; 58.1% (49.6–66.7) | 43/129; 33.3% (25.2–41.5) |
Figure 1.
Kinetics of NS1 antigen and dengue IgM and IgA antibodies in capillary blood samples over the course of disease.
Between day 4 and day 7 of the disease, many patients present with clinical complications, and it is well-known that none of these biological markers are sensitive enough to allow dengue diagnosis from venous samples. Surprisingly, the sensitivity of NS1 testing from dried capillary samples collected between day 4 and day 7 ranged from 85% to 100% (mean = 94%; 73/78). More importantly, the combination of the results obtained with the three biological markers in capillary samples collected during this critical clinical phase of the disease allowed dengue to be diagnosed in 100% of the patients.
Discussion
The re-emergence of dengue fever worldwide and the concomitant increase in the severity of reported cases underline the need for sensitive and adapted tools for the diagnosis and management of suspected dengue patients. Such tools would also be valuable for surveillance and epidemiological investigations. We previously showed the usefulness of collecting capillary blood samples in the field for dengue diagnosis and epidemiological purposes—the simplicity of sample collection and shipment from remote areas and the possibility of using routine techniques for dengue diagnosis.14,18 However, the kinetics of biological markers of dengue infection, such as NS1 antigen, IgM, and IgA, in the capillary compartment had not been assessed during the time course of the disease. This issue is especially important, because none of these biological markers are sensitive enough in venous samples to diagnose dengue infection during the period of defervescence (days 4–7) frequently associated with clinical complications.20
First, we observed a high overall performance of the NS1 antigen detection in the capillary blood samples from dengue-infected patients over the course of the disease, with 96% sensitivity (95% CI = 88.3–100%) and 100% specificity. During the acute phase of disease, the sensitivity was 98.7%. These results are consistent with studies performed on venous samples during the same phase of dengue disease.6–8,21–24 Second, we show here that this technique also exhibits a good sensitivity (92.3%) with capillary samples collected during the early convalescent phase of the disease. Although some studies have already reported the possibility of detecting the viral protein NS1 in venous samples after the acute phase of the disease, the sensitivities were lower than those sensitivities reported with capillary blood samples.24–27 In particular, we noted a very high proportion of NS1 antigen-positive samples (24/25; 96%; 95% CI = 88.3–100%) among those samples collected on day 5 of the disease, which corresponds to a blind window for diagnosis with venous samples. Indeed, both direct and indirect methods exhibit a low sensitivity during this period.20 This blind window is a major issue, because this period is also frequently associated with clinical complications, including vascular leakage.20 Altogether, our results strongly suggest that tests with capillary samples may outperform tests with venous samples.
Previous studies have suggested that the presence of pre-existing circulating anti-NS1 antibodies during secondary infections may explain the apparent lower sensitivity of NS1 antigen detection assays with venous samples.21,22,28,29 In the present study, we provide serological evidence that all infected patients were secondary dengue cases, which could be expected, because dengue infection is endemic in Vietnam. Nevertheless, contrary to previous studies,27,30,31 our results suggest that the sensitivity of NS1 detection assays is not significantly affected by the immune status of the patient, which is in agreement with a previous work by Castro-Jorge and others.30 This finding may be because of higher viremia in our cohort or alternatively, the accumulation of NS1 at the endothelium surface of the capillary compartment, which was suggested in the work by Avirutnan and others.32 Finally, we did not observe any significant difference in the detection of NS1 antigen according to dengue serotype, which was previously described with venous samples.23,25,26,33 However, this conclusion has to be taken with caution because of the unequal distribution of the DENV serotypes in our study (only one DENV-4 patient and only four DENV-1 and DENV-4 coinfected patients).
Specific IgM and IgA antibodies in capillary samples were also sensitive markers of dengue infection from day 5, when the detection rates were 92.3% for IgM and 73% for IgA. As previously reported in venous samples, dengue IgM antibodies are detected earlier than dengue IgA antibodies (Figure 1).11 Our study confirms that anti-dengue IgA may also be a useful marker of dengue infection in capillary blood samples.11,13,34 However, our results raise important questions regarding this assay. Indeed, IgA could not be detected in four patients infected by DENV-1 serotype, suggesting that the sensitivity of the IgA detection assay in capillary samples may depend on the virus serotype. This finding is in agreement with the finding in the work by de la Cruz Hernández and others,34 which also reported an impact of DENV serotype on the sensitivity of IgA detection assay. Thus, additional studies are required to address this issue.
To our knowledge, this study is the first descriptive study that evaluates the kinetics of three dengue biological markers in the capillary compartment of dengue-confirmed patients over the course of the disease. We confirmed that analysis of capillary blood on filter paper is a very convenient and effective approach to diagnose dengue infection; it would be particularly valuable in remote areas, where diagnostic infrastructures are limited. Strikingly, the results obtained between day 4 and day 7 and particularly, on day 5 of the disease, which is considered to be a critical clinic phase, showed the value of tests using capillary blood; during this period, most patients scored negative if venous samples are tested. Nevertheless, our study presents some limitations: the number of samples was small, our study did not include primary dengue patients, which would have allowed analysis of the influence of dengue immune status on the kinetics of the biological markers, and venous samples were not tested in parallel. Additional work is, therefore, needed to confirm these promising results.
ACKNOWLEDGMENTS
The authors thank all patients for their participation in this study. The authors also thank Prof. Nguyen Tran Chinh (Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam) and Dr. Nguyen Quoc Khanh and Dr. Phan Ngoc Nam (People's Hospital 115, Ho Chi Minh City, Vietnam) for their help and support during this study.
Footnotes
Financial support: This study was supported by Conseil Régional de la Guyane Agreement 60/2007/CR.
Authors' addresses: Séverine Matheus and Bhetty Labeau, Laboratoire de Virologie, Centre National de Référence des Arbovirus, Laboratoire Associé, Institut Pasteur de la Guyane, Cayenne, French Guiana, E-mails: smatheus@pasteur-cayenne.fr and blabeau@pasteur-cayenne.fr. Thai Binh Pham, Far East Medical Vietnam Limited, Ho Chi Minh City, Vietnam, E-mail: binh.pham@fvhospital.com. Vu Thi Que Huong, Laboratoire de Virologie, Institut Pasteur Vietnam, Ho Chi Minh City, Vietnam, E-mail: huongpasteur@hotmail.com. Vincent Lacoste, Laboratoire des Interactions Virus-Hôtes, Institut Pasteur de la Guyane, Cayenne, French Guiana, E-mail: vlacoste@pasteur-cayenne.fr. Xavier Deparis, Centre d'Epidémiologie et de Santé Publique des Armées, Marseille, France, E-mail: xavier.deparis@wanadoo.fr. Vincent Marechal, UMRS 872, Pôle 4, Equipe 16, Institut National de la Santé et de la Recherche Médicale, Centre de Recherches Biomédicales des Cordeliers, Paris, France, E-mail: vincent.marechal@upmc.fr.
References
- 1.Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Kyle J, Harris E. Global spread and persistence. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 4.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henchal EA, McCown JM, Seguin MC, Gentry MK, Brandt WE. Rapid identification of dengue virus isolates by using monoclonal antibodies in an indirect immunofluorescence assay. Am J Trop Med Hyg. 1983;32:164–169. doi: 10.4269/ajtmh.1983.32.164. [DOI] [PubMed] [Google Scholar]
- 6.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–381. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dussart P, Labeau B, Lagathu G, Louis P, Nunes MR, Rodrigues SG, Storck-Herrmann C, Cesaire R, Morvan J, Flamand M, Baril L. Evaluation of an enzyme immunoassay for detection of dengue virus NS1 antigen in human serum. Clin Vaccine Immunol. 2006;13:1185–1189. doi: 10.1128/CVI.00229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumarasamy V, Chua SK, Hassan Z, Wahab AH, Chem YK, Mohamad M, Chua KB. Evaluation of a commercial dengue NS1 antigen-capture ELISA for laboratory diagnosis of acute dengue virus infection. J Virol Methods. 2007;140:75–79. doi: 10.1016/j.jviromet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez S, Ruiz D, Barrero R, Ramirez R, Calzada N, del Rosario Peña B, Reyes S, Guzman MG. Kinetics of dengue virus NS1 protein in dengue 4-confirmed adult patients. Diagn Microbiol Infect Dis. 2010;68:46–49. doi: 10.1016/j.diagmicrobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Kuno G, Gomez I, Gubler DJ. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods. 1991;33:101–113. doi: 10.1016/0166-0934(91)90011-n. [DOI] [PubMed] [Google Scholar]
- 11.Talarmin A, Labeau B, Lelarge J, Sarthou JL. Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever. J Clin Microbiol. 1998;36:1189–1192. doi: 10.1128/jcm.36.5.1189-1192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balmaseda A, Guzmán MG, Hammond S, Robleto G, Flores C, Téllez Y, Videa E, Saborio S, Pérez L, Sandoval E, Rodriguez Y, Harris E. Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clin Diagn Lab Immunol. 2003;10:317–322. doi: 10.1128/CDLI.10.2.317-322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawa M, Takasaki T, Ito M, Inoue S, Morita K, Kurane I. Immunoglobulin A antibody responses in dengue patients: a useful marker for serodiagnosis of dengue virus infection. Clin Diagn Lab Immunol. 2005;12:1235–1237. doi: 10.1128/CDLI.12.10.1235-1237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matheus S, Meynard JB, Lacoste V, Morvan J, Deparis X. Use of capillary blood samples as a new approach for diagnosis of dengue virus infection. J Clin Microbiol. 2007;45:887–890. doi: 10.1128/JCM.02063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vázquez S, Cabezas S, Pérez AB, Pupo M, Ruiz D, Calzada N, Bernardo L, Castro O, González D, Serrano T, Sanchez A, Guzmán MG. Kinetics of antibodies in sera, saliva, and urine samples from adult patients with primary or secondary dengue 3 virus infections. Int J Infect Dis. 2007;11:256–262. doi: 10.1016/j.ijid.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Balmaseda A, Saborio S, Tellez Y, Mercado JC, Pérez L, Hammond SN, Rocha C, Kuan G, Harris E. Evaluation of immunological markers in serum, filter-paper blood spots, and saliva for dengue diagnosis and epidemiological studies. J Clin Virol. 2008;43:287–291. doi: 10.1016/j.jcv.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Anders KL, Nguyet NM, Quyen NT, Ngoc TV, Tram TV, Gan TT, Tung NT, Dung NT, Chau NV, Wills B, Simmons CP. An evaluation of dried blood spots and oral swabs as alternative specimens for the diagnosis of dengue and screening for past dengue virus exposure. Am J Trop Med Hyg. 2012;87:165–170. doi: 10.4269/ajtmh.2012.11-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matheus S, Meynard JB, Lavergne A, Girod R, Moua D, Labeau B, Dussart P, Lacoste V, Deparis X. Dengue-3 outbreak in Paraguay: investigations using capillary blood samples on filter paper. Am J Trop Med Hyg. 2008;79:685–687. [PubMed] [Google Scholar]
- 19.Matheus S, Deparis X, Labeau B, Lelarge J, Morvan J, Dussart P. Discrimination between primary and secondary dengue virus infection by an immunoglobulin G avidity test using a single acute-phase serum sample. J Clin Microbiol. 2005;43:2793–2797. doi: 10.1128/JCM.43.6.2793-2797.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Geneva: World Health Organization; 2009. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. New edition. [Google Scholar]
- 21.Kumarasamy V, Chua SK, Hassan Z, Wahab AH, Chem YK, Mohamad M, Chua KB. Evaluating the sensitivity of a commercial dengue NS1 antigen-capture ELISA for early diagnosis of acute dengue virus infection. Singapore Med J. 2007;48:669–673. [PubMed] [Google Scholar]
- 22.Chuansumrit A, Chaiyaratana W, Pongthanapisith V, Tangnararatchakit K, Lertwongrath S, Yoksan S. The use of dengue nonstructural protein 1 antigen for the early diagnosis during the febrile stage in patients with dengue infection. Pediatr Infect Dis J. 2008;27:43–48. doi: 10.1097/INF.0b013e318150666d. [DOI] [PubMed] [Google Scholar]
- 23.Dussart P, Petit L, Labeau B, Bremand L, Leduc A, Moua D, Matheus S, Baril L. Evaluation of two new commercial tests for the diagnosis of acute dengue virus infection using NS1 antigen detection in human serum. PLoS Negl Trop Dis. 2008;2:e280. doi: 10.1371/journal.pntd.0000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride WJ. Evaluation of dengue NS1 test kits for the diagnosis of dengue fever. Diagn Microbiol Infect Dis. 2009;64:31–36. doi: 10.1016/j.diagmicrobio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Guzman MG, Jaenisch T, Gaczkowski R, Ty Hang VT, Sekoran SD, Kroeger A, Vazquez S, Ruiz D, Martinez E, Mercado JC, Balmaseda A, Harris E, Dimano E, Leano PS, Yoksan S, Villegas E, Benduzu H, Villalobos I, Farrar J, Simmons CP. Multi-country evaluation of the sensitivity and specificity of two commercially available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis. 2010;4:e811. doi: 10.1371/journal.pntd.0000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osorio L, Ramirez M, Bonelo A, Villar LA, Parra B. Comparison of the diagnostic accuracy of commercial NS1-based diagnostic tests for early dengue infection. Virol J. 2010;7:361. doi: 10.1186/1743-422X-7-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blacksell SD, Mammen MP, Jr, Thongpaseuth S, Gibbons RV, Jarman RG, Jenjaroen K, Nisalak A, Phetsouvanh R, Newton PN, Day NP. Evaluation of the Panbio dengue virus nonstructural 1 antigen detection and immunoglobulin M antibody enzyme-linked immunosorbent assays for the diagnosis of acute dengue infections in Laos. Diagn Microbiol Infect Dis. 2008;60:43–49. doi: 10.1016/j.diagmicrobio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Lapphra K, Sangcharaswichai A, Chokephaibulkit K, Tiengrim S, Piriyakarnsakul W, Chakorn T, Yoksan S, Wattanamongkolsil L, Thamlikitkul V. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn Microbiol Infect Dis. 2008;60:387–391. doi: 10.1016/j.diagmicrobio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, Dung NM, Van Ngoc T, Hien TT, Farrar J, Wills B, Simmons CP. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3:e360. doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro-Jorge LA, Machado PR, Fávero CA, Borges MC, Passos LM. Clinical evaluation of the NS1 antigen-capture ELISA for early diagnosis of dengue virus infection in Brazil. J Med Virol. 2010;82:1400–1405. doi: 10.1002/jmv.21814. [DOI] [PubMed] [Google Scholar]
- 31.Kumarasamy V, Wahab AHA, Chuab SK, Hassan ZA, Chema YK, Mohamada M, Chua KB. Evaluation of a commercial dengue NS1 antigen-capture ELISA for laboratory diagnosis of acutedengue virus infection. J Virol Methods. 2007;140:75–79. doi: 10.1016/j.jviromet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Avirutnan P, Zhang L, Punyadee N, Manuyakorn A, Puttikhunt C, Kasinrerk W, Malasit P, Atkinson JP, Diamond MS. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 2007;3:e183. doi: 10.1371/journal.ppat.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CH, Kuo LL, Yang KD, Lin PS, Lu PL, Lin CC, Chang K, Chen TC, Lin WR, Lin CY, Chen YH, Wu HS. Laboratory diagnostics of dengue fever: an emphasis on the role of commercial dengue virus nonstructural protein 1 antigen rapid test. J Microbiol Immunol Infect. 2013;46:358–365. doi: 10.1016/j.jmii.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 34.de la Cruz Hernández SI, González Mateos S, Flores Aguilar H, López Martinez I, Alpuche Aranda C, Ludert JE, Del Angel RM. Evaluation of a novel commercial rapid test for dengue diagnosis based on specific IgA detection. Diagn Microbiol Infect Dis. 2012;72:150–155. doi: 10.1016/j.diagmicrobio.2011.11.002. [DOI] [PubMed] [Google Scholar]