Abstract
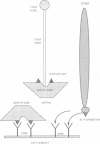
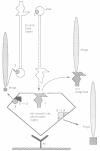
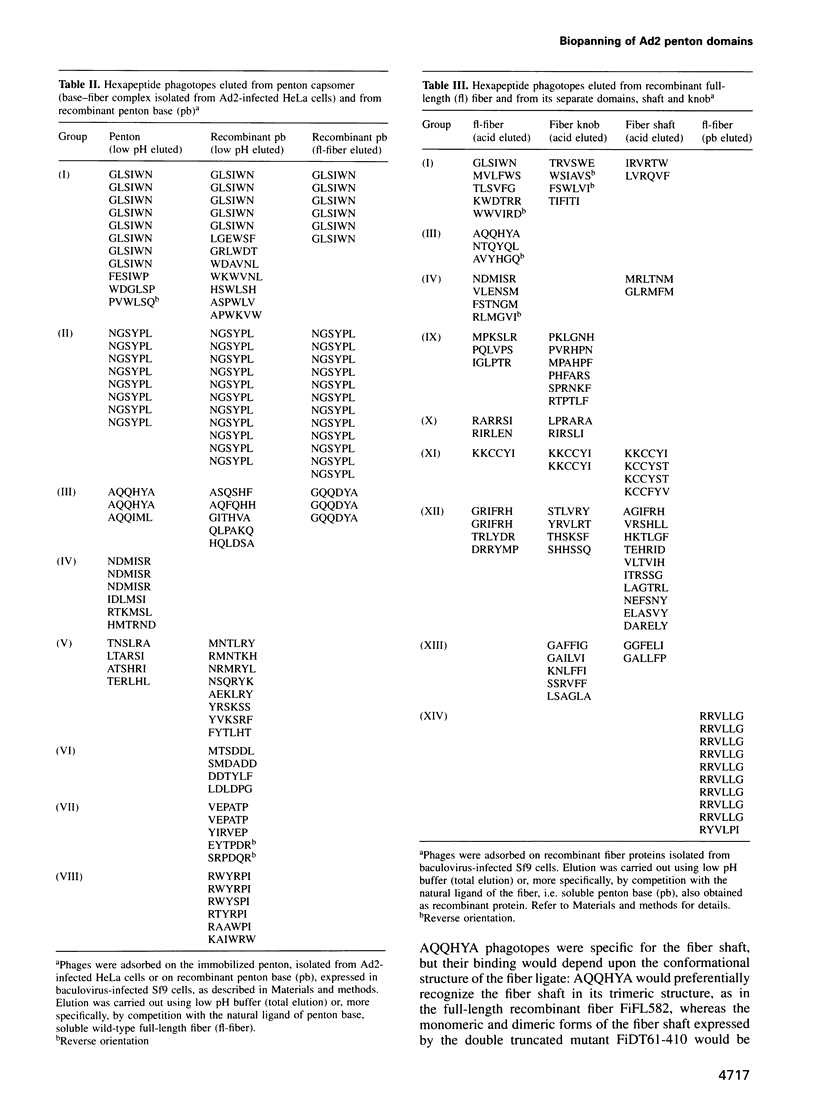
A filamentous phage-displayed random hexapeptide library was screened on the adenovirus type 2 (Ad2) penton capsomer and its separate domains, penton base, full-length fiber, fiber shaft and fiber knob. Affinity supports were designed to immobilize the penton ligate with a preferred orientation, via immuno-adsorption to pre-coated antibody. Three classes of phagotopes were distinguished in the eluates from the penton and fiber domains. (i) The first class represented peptide sequences identified in certain Ad2 capsid proteins, protein IIIa, protein pVIII, penton base and penton fiber. Data from specific ligand elution of phages bound to fiber and penton base wild-types and mutants suggested that the region overlapping the RLSNLLG motif at residues 254-260 in the penton base and the FNPVYP motif at residues 11-16 in the fiber tail formed mutual interacting sites in the penton capsomer. (ii) The second class consisted of phagotopes homologous to peptide sequences found in host cell membrane proteins involved in receptor or adhesion functions. One of the most abundant species corresponded to a conserved motif present in the beta-strand B of type III modules of human fibronectin. In addition, phages which were screened for their failure to bind to penton base RGD mutants were found to carry consensus motifs to peptide sequences present in the RGD recognition site of human integrin beta subunits. (iii) The third class comprised peptide motifs common to both viral and cellular proteins, suggesting that a mechanism of ligand exchange could occur during virus entry and uncoating, and virus assembly and release.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W. The proteinase polypeptide of adenovirus serotype 2 virions. Virology. 1990 Jul;177(1):259–272. doi: 10.1016/0042-6822(90)90479-b. [DOI] [PubMed] [Google Scholar]
- Bai M., Campisi L., Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994 Sep;68(9):5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M., Harfe B., Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993 Sep;67(9):5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin M. T., Boulanger P. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J Gen Virol. 1993 Aug;74(Pt 8):1485–1497. doi: 10.1099/0022-1317-74-8-1485. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S., Cwirla S. E., Dower W. J., Lipshutz R. J., Sprang S. R., Sambrook J. F., Gething M. J. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993 Nov 19;75(4):717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Bork P., Doolittle R. F. Proposed acquisition of an animal protein domain by bacteria. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8990–8994. doi: 10.1073/pnas.89.19.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudin M. L., Boulanger P. Antibody-triggered dissociation of adenovirus penton capsomer. Virology. 1981 Sep;113(2):781–786. doi: 10.1016/0042-6822(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Boulanger P. Assembly of adenovirus penton base and fiber. Virology. 1982 Jan 30;116(2):589–604. doi: 10.1016/0042-6822(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., D'Halluin J. C., Cousin C., Boulanger P. Human adenovirus type 2 protein IIIa. II. Maturation and encapsidation. Virology. 1980 Feb;101(1):144–156. doi: 10.1016/0042-6822(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., Moncany M., D'Halluin J. C., Boulanger P. A. Isolation and characterization of adenovirus type 2 vertex capsomer (penton base). Virology. 1979 Jan 15;92(1):125–138. doi: 10.1016/0042-6822(79)90219-8. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Puvion F. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur J Biochem. 1973 Nov 1;39(1):37–42. doi: 10.1111/j.1432-1033.1973.tb03100.x. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Torpier G., Rimsky A. Crystallographic study of intranuclear adenovirus type 5 crystals. Intervirology. 1974;2(1):56–62. doi: 10.1159/000149406. [DOI] [PubMed] [Google Scholar]
- Caillet-Boudin M. L. Complementary peptide sequences in partner proteins of the adenovirus capsid. J Mol Biol. 1989 Jul 5;208(1):195–198. doi: 10.1016/0022-2836(89)90095-8. [DOI] [PubMed] [Google Scholar]
- Cuillel M., Cortolezzis B., Chroboczek J., Langowski J., Ruigrok R. W., Jacrot B. Purification and characterization of wild-type and ts 112 mutant protein IIIa of human adenovirus 2 expressed in Escherichia coli. Virology. 1990 Mar;175(1):222–231. doi: 10.1016/0042-6822(90)90202-3. [DOI] [PubMed] [Google Scholar]
- Cuzange A., Chroboczek J., Jacrot B. The penton base of human adenovirus type 3 has the RGD motif. Gene. 1994 Sep 2;146(2):257–259. doi: 10.1016/0378-1119(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Cwirla S. E., Peters E. A., Barrett R. W., Dower W. J. Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6378–6382. doi: 10.1073/pnas.87.16.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Lam S. C., Plow E. F. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988 Oct 7;242(4875):91–93. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- D'Souza S. E., Haas T. A., Piotrowicz R. S., Byers-Ward V., McGrath D. E., Soule H. R., Cierniewski C., Plow E. F., Smith J. W. Ligand and cation binding are dual functions of a discrete segment of the integrin beta 3 subunit: cation displacement is involved in ligand binding. Cell. 1994 Nov 18;79(4):659–667. doi: 10.1016/0092-8674(94)90551-7. [DOI] [PubMed] [Google Scholar]
- Defer C., Belin M. T., Caillet-Boudin M. L., Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990 Aug;64(8):3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Devaux C., Caillet-Boudin M. L., Jacrot B., Boulanger P. Crystallization, enzymatic cleavage, and the polarity of the adenovirus type 2 fiber. Virology. 1987 Nov;161(1):121–128. doi: 10.1016/0042-6822(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Dickinson C. D., Veerapandian B., Dai X. P., Hamlin R. C., Xuong N. H., Ruoslahti E., Ely K. R. Crystal structure of the tenth type III cell adhesion module of human fibronectin. J Mol Biol. 1994 Mar 4;236(4):1079–1092. doi: 10.1016/0022-2836(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Folgori A., Tafi R., Meola A., Felici F., Galfré G., Cortese R., Monaci P., Nicosia A. A general strategy to identify mimotopes of pathological antigens using only random peptide libraries and human sera. EMBO J. 1994 May 1;13(9):2236–2243. doi: 10.1002/j.1460-2075.1994.tb06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Willetts M., Webster P., Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993 Nov 5;75(3):477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Green N. M., Wrigley N. G., Russell W. C., Martin S. R., McLachlan A. D. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 1983;2(8):1357–1365. doi: 10.1002/j.1460-2075.1983.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan C., Raptis L. H., Déry C. V., Weber J. Biological and structural studies with an adenovirus type 2 temperature-sensitive mutant defective for uncoating. Intervirology. 1983;19(4):213–223. doi: 10.1159/000149363. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994 Jan;68(1):1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A. Unpacking the incoming influenza virus. Cell. 1992 May 15;69(4):577–578. doi: 10.1016/0092-8674(92)90219-3. [DOI] [PubMed] [Google Scholar]
- Hennache B., Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977 Aug 15;166(2):237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L. J., Xia D., Wilke M. E., Deisenhofer J., Gerard R. D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994 Aug;68(8):5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Engler J. A. The amino terminus of the adenovirus fiber protein encodes the nuclear localization signal. Virology. 1991 Dec;185(2):758–767. doi: 10.1016/0042-6822(91)90547-o. [DOI] [PubMed] [Google Scholar]
- Karayan L., Gay B., Gerfaux J., Boulanger P. A. Oligomerization of recombinant penton base of adenovirus type 2 and its assembly with fiber in baculovirus-infected cells. Virology. 1994 Aug 1;202(2):782–795. doi: 10.1006/viro.1994.1400. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Chroboczek J., Cusack S., Ruigrok R. W. Adenovirus type 40 virions contain two distinct fibers. Virology. 1993 Jan;192(1):73–84. doi: 10.1006/viro.1993.1009. [DOI] [PubMed] [Google Scholar]
- Koivunen E., Gay D. A., Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem. 1993 Sep 25;268(27):20205–20210. [PubMed] [Google Scholar]
- Koivunen E., Wang B., Ruoslahti E. Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. J Cell Biol. 1994 Feb;124(3):373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Crowell R. L., Philipson L. Unrelated animal viruses share receptors. Nature. 1976 Feb 26;259(5545):679–681. doi: 10.1038/259679a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis N., Fender P., Barge A., Kitts P., Chroboczek J. Cell-binding domain of adenovirus serotype 2 fiber. J Virol. 1994 Jun;68(6):4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Main A. L., Harvey T. S., Baron M., Boyd J., Campbell I. D. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992 Nov 13;71(4):671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- Mathias P., Wickham T., Moore M., Nemerow G. Multiple adenovirus serotypes use alpha v integrins for infection. J Virol. 1994 Oct;68(10):6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Cheresh D. A., Wickham T. J. Adenovirus entry into host cells: a role for alpha(v) integrins. Trends Cell Biol. 1994 Feb;4(2):52–55. doi: 10.1016/0962-8924(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Neumann R., Chroboczek J., Jacrot B. Determination of the nucleotide sequence for the penton-base gene of human adenovirus type 5. Gene. 1988 Sep 15;69(1):153–157. doi: 10.1016/0378-1119(88)90389-7. [DOI] [PubMed] [Google Scholar]
- Norrby E. The structural and functional diversity of Adenovirus capsid components. J Gen Virol. 1969 Sep;5(2):221–236. doi: 10.1099/0022-1317-5-2-221. [DOI] [PubMed] [Google Scholar]
- Novelli A., Boulanger P. A. Assembly of adenovirus type 2 fiber synthesized in cell-free translation system. J Biol Chem. 1991 May 15;266(14):9299–9303. [PubMed] [Google Scholar]
- Novelli A., Boulanger P. A. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology. 1991 Nov;185(1):365–376. doi: 10.1016/0042-6822(91)90784-9. [DOI] [PubMed] [Google Scholar]
- O'Bryan J. P., Frye R. A., Cogswell P. C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M. M., Earp H. S., Liu E. T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg K. R., Loganathan D., Goldstein I. J., Schultz P. G., Gallop M. A. Peptide ligands for a sugar-binding protein isolated from a random peptide library. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5393–5397. doi: 10.1073/pnas.89.12.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley S. F., Smith G. P. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene. 1988 Dec 20;73(2):305–318. doi: 10.1016/0378-1119(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Partanen J., Armstrong E., Mäkelä T. P., Korhonen J., Sandberg M., Renkonen R., Knuutila S., Huebner K., Alitalo K. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992 Apr;12(4):1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson R., Wohlfart C., Svensson U., Everitt E. Virus-receptor interaction in the adenovirus system: characterization of the positive cooperative binding of virions on HeLa cells. J Virol. 1985 Apr;54(1):92–97. doi: 10.1128/jvi.54.1.92-97.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN L. A hemagglutination-inhibition technique for typing adenoviruses. Am J Hyg. 1960 Jan;71:120–128. doi: 10.1093/oxfordjournals.aje.a120085. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Barge A., Albiges-Rizo C., Dayan S. Structure of adenovirus fibre. II. Morphology of single fibres. J Mol Biol. 1990 Oct 20;215(4):589–596. doi: 10.1016/S0022-2836(05)80170-6. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. K., Loganathan D., Easley R. B., Gong X., Goldstein I. J. A family of concanavalin A-binding peptides from a hexapeptide epitope library. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5398–5402. doi: 10.1073/pnas.89.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. K., Smith G. P. Searching for peptide ligands with an epitope library. Science. 1990 Jul 27;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- Seth P. Adenovirus-dependent release of choline from plasma membrane vesicles at an acidic pH is mediated by the penton base protein. J Virol. 1994 Feb;68(2):1204–1206. doi: 10.1128/jvi.68.2.1204-1206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D., Ginsberg H., Willingham M., Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984 Aug;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Pastan I., Willingham M. C. Adenovirus-dependent increase in cell membrane permeability. J Biol Chem. 1985 Aug 15;260(17):9598–9602. [PubMed] [Google Scholar]
- Signäs C., Akusjärvi G., Pettersson U. Adenovirus 3 fiber polypeptide gene: implications for the structure of the fiber protein. J Virol. 1985 Feb;53(2):672–678. doi: 10.1128/jvi.53.2.672-678.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P., Scott J. K. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- Sprengel J., Schmitz B., Heuss-Neitzel D., Zock C., Doerfler W. Nucleotide sequence of human adenovirus type 12 DNA: comparative functional analysis. J Virol. 1994 Jan;68(1):379–389. doi: 10.1128/jvi.68.1.379-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen C. W., Lane D. P. Mutant conformation of p53. Precise epitope mapping using a filamentous phage epitope library. J Mol Biol. 1992 Jun 5;225(3):577–583. doi: 10.1016/0022-2836(92)90386-x. [DOI] [PubMed] [Google Scholar]
- Stewart P. L., Burnett R. M., Cyrklaff M., Fuller S. D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991 Oct 4;67(1):145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- Stewart P. L., Fuller S. D., Burnett R. M. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993 Jul;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouten P. F., Sander C., Ruigrok R. W., Cusack S. New triple-helical model for the shaft of the adenovirus fibre. J Mol Biol. 1992 Aug 20;226(4):1073–1084. doi: 10.1016/0022-2836(92)91053-r. [DOI] [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- Varga M. J., Weibull C., Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991 Nov;65(11):6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. STRUCTURE OF TYPE 5 ADENOVIRUS. I. ANTIGENIC RELATIONSHIP OF VIRUS-STRUCTURAL PROTEINS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:295–306. doi: 10.1084/jem.118.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M., Talbot B. G., Delorme L. The orientation of the adenovirus fiber and its anchor domain identified through molecular mimicry. Virology. 1989 Jan;168(1):180–182. doi: 10.1016/0042-6822(89)90419-4. [DOI] [PubMed] [Google Scholar]
- Webster A., Russell S., Talbot P., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: substrate specificity. J Gen Virol. 1989 Dec;70(Pt 12):3225–3234. doi: 10.1099/0022-1317-70-12-3225. [DOI] [PubMed] [Google Scholar]
- White J. M. Integrins as virus receptors. Curr Biol. 1993 Sep 1;3(9):596–599. doi: 10.1016/0960-9822(93)90007-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Filardo E. J., Cheresh D. A., Nemerow G. R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994 Oct;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Xia D., Henry L. J., Gerard R. D., Deisenhofer J. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure. 1994 Dec 15;2(12):1259–1270. doi: 10.1016/s0969-2126(94)00126-x. [DOI] [PubMed] [Google Scholar]
- Yayon A., Aviezer D., Safran M., Gross J. L., Heldman Y., Cabilly S., Givol D., Katchalski-Katzir E. Isolation of peptides that inhibit binding of basic fibroblast growth factor to its receptor from a random phage-epitope library. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10643–10647. doi: 10.1073/pnas.90.22.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew P. R., Kao C. C., Berk A. J. Dissection of functional domains in the adenovirus 2 early 1B 55K polypeptide by suppressor-linker insertional mutagenesis. Virology. 1990 Dec;179(2):795–805. doi: 10.1016/0042-6822(90)90147-j. [DOI] [PubMed] [Google Scholar]
- van Oostrum J., Burnett R. M. Molecular composition of the adenovirus type 2 virion. J Virol. 1985 Nov;56(2):439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oostrum J., Smith P. R., Mohraz M., Burnett R. M. The structure of the adenovirus capsid. III. Hexon packing determined from electron micrographs of capsid fragments. J Mol Biol. 1987 Nov 5;198(1):73–89. doi: 10.1016/0022-2836(87)90459-1. [DOI] [PubMed] [Google Scholar]