Abstract
We investigated the clinical characteristics and serologic types of tsutsugamushi disease on the largest island of South Korea. There were 141 patients with tsutsugamushi disease at Jeju National University Hospital and Seogwipo Medical Center between November of 2003 and December of 2012. Median age of patients was 61 years, and 59% were women. The major clinical manifestations were fever (80.5%) and skin rash (55.7%), with eschars evident in 75.8% of the patients. Genotype analysis of Orientia tsutsugamushi was conducted in 33 specimens. The genotype was identified as Boryong type in 17 of 33 patients and Taguchi type in 15 of 33 patients. In our study, although the Taguchi genotype is rarely reported in the endemic area, it was common on Jeju Island. This genotype may be associated with mild and moderate forms of tsutsugamushi disease.
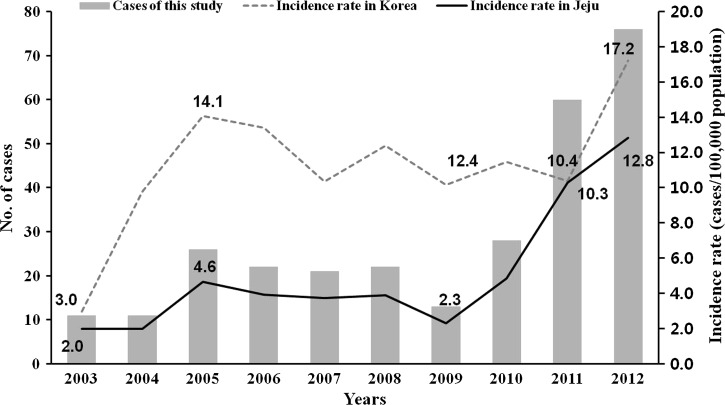
Tsutsugamushi disease, also known as scrub typhus, is an acute febrile illness caused by Orientia tsutsugamushi, which is transmitted to humans through chigger bites.1 It is distributed throughout the Asia Pacific rim, and it is endemic in Korea.1,2 This disease occurs in all regions of South Korea, including Jeju Island.3,4 Since the first identification of tsutsugamushi disease in native Koreans in 1985, the official annual incidence of disease has increased. Along with this change, annual incidence rate has increased on Jeju Island, and the number of patients with tsutsugamushi disease has increased in our hospital (Figure 1).3 This disease is complicated by interstitial pneumonitis, meningoencephalitis, circulatory collapse, multiple organ failure, and death in untreated cases.2 Although there are many reports about serotyping of O. tsutsugamushi and clinical characteristics of tsutsugamushi disease in South Korea, no report of tsutsugamushi disease in the Jeju area has been published. This study is aimed at investigating the epidemiologic, serologic, and clinical characteristics of tsutsugamushi disease on Jeju Island.
Figure 1.
The annual case numbers in this study and annual incidence rate per 100,000 persons in South Korea and Jeju Island for 10 years. The gray bars, gray dotted line, and black line indicate the number of patients in this study, incidence rate of tsutsugamushi disease in Korea, and incidence rate on Jeju Island, respectively.
Jeju Island is the largest island in South Korea, with a population of approximately 600,000. The yearly mean temperature is high compared with other domestic areas.5 We reviewed medical records of 79 patients with tsutsugamushi disease who visited Jeju National University Hospital (JNUH) and Seogwipo Medical Center (SMC) from November of 2003 to October of 2011. Another 62 patients from November of 2011 to December of 2012 were introduced with informed consent. The two hospitals are located in an urban area with a temperate climate (JNUH: mean air temperature, 15.7°C [13.1–18.6°C]; mean relative humidity, 71%; SMC: mean air temperature, 16.7°C [13.8–20.0°C]; mean relative humidity, 70%).5 For four consecutive seasons from November of 2011 to December of 2012, when tsutsugamushi disease was suspected, we routinely tested for the presence of O. tsutsugamushi in eschar, cerebrospinal fluid (CSF), and blood (3 mL) using ethylenediamineteteraacetic acid tubes, when possible, in patients with informed consent. In addition, clinical, microbiological, and epidemiological data were collected for the comparative analysis. Diagnosis of tsutsugamushi disease was made by clinical manifestation (fever, skin rash, or eschar), positive serology, or blood nested polymerase chain reaction (PCR). A positive serology test was defined as a fourfold or greater change in the titer or a titer ≥ 1:80 using the indirect fluorescence antibody test (IFA), which measured whole globulin in a single serum sample.6 Positive nested PCR results were subsequently confirmed by sequencing of the 56 kDa type-specific antigen.6 The PCR products were purified using a QIAquick gel extraction kit (QIAGEN, Hilden, Germany) and sequenced. Multiple alignments of the sequences were performed using the sequences obtained in this study and those sequences obtained from the GenBank database. The genotypes of the O. tsutsugamushi were identified according to the identity of the DNA sequences of 56-kDa type-specific antigen. This study was approved by the institutional review board at each participating hospital.
The statistical analyses were performed using SPSS version 20.0 (SPSS, Chicago, IL). Continuous variables are reported as mean values ± SDs or median values (interquartile range [IQR]) and compared using the Student t test. Categorical variables were expressed as percentages of total numbers of patients analyzed and compared using the χ2 test. P < 0.05 was considered statistically significant.
There were 141 patients with known current or previous tsutsugamushi disease at JNUH and SMC between November of 2003 and December of 2012. The 136 and 5 patients were admitted to JNUH and SMC, respectively (the city of Jeju is about 40 km away from the city of Seogwipo). Baseline characteristics are shown in Table 1. The median age was 61 years (IQR = 17–83 years), and 59% were female. In addition, 30.0% of the patients had at least one underlying medical condition. In our study, the months of peak incidence were November (55.4%) and October (24.8%). Among the patients with informed consent, 21 patients (60.4%) were exposed to a fruit farm, and 8 patients (22.9%) had history of climbing mountains. Most patients were treated with doxycycline, and only seven patients were treated with azithromycin. The median time to disappearance of fever in patients treated with antibiotics was 2.0 days (IQR = 1–11 days). The mean duration of antibiotics was 7.3 ± 2.9 days. Ten patients were complicated with pneumonitis, shock, renal failure, and meningitis, but they were completely resolved with appropriate antibiotic treatment.
Table 1.
Baseline characteristics of tsutsugamushi disease (N = 141)
| Variables | Results |
|---|---|
| Age (years), median (IQR) | 61.0 (17–83) |
| Female, no. (%) | 83 (59.0) |
| BMI (kg/m2), mean ± SD | 23.9 ± 4.3 |
| Diabetes, no. (%) | 17 (14.3) |
| Hypertension, no. (%) | 34 (22.2) |
| Clinical manifestation, no. (%) | |
| Febrile sense | 107/133 (80.5) |
| Eschar | 69/91 (75.8) |
| Rash | 140/78 (55.7) |
| Myalgia | 62/133 (46.6) |
| Lymphadenopathy | 5/102 (4.9) |
| Place of residence, no. (%) | |
| City | 103/141 (67.3) |
| Rural | 35/141 (22.9) |
| Body temperature (°C), mean ± SD | 38.8 ± 0.7 |
| Laboratory finding, mean ± SD | |
| WBC (×103/μL) | 8.6 ± 4.3 |
| Platelet (×103/μL) | 161.3 ± 79.5 |
| Albumin (g/dL) | 3.5 ± 0.6 |
| Na (mmol/L) | 135.7 ± 4.0 |
| AST (IU/L) | 143.9 ± 687.9 |
| ALT (IU/L) | 119.8 ± 374.9 |
| hs-CRP (mg/dL) | 7.8 ± 5.9 |
| ESR (mm/hour) | 35.6 ± 24.1 |
| Genotype, no. (%) | |
| Boryong | 17/33 (51.5) |
| Taguchi | 15/33 (45.5) |
| Gilliam | 1/33 (3.0) |
| Antibiotics | |
| Duration (day), mean ± SD | 7.3 ± 2.9 |
| Doxycycline | 132/141 (94.3) |
| Azithromycin | 7/141 (5.0) |
| Complication | |
| Pneumonitis | 6/141 (4.2) |
| Acute renal failure | 1/141 (0.7) |
| Shock | 1/141 (0.7) |
| Meningitis | 2/141 (1.4) |
| Outcome | |
| Cure | 138/141 (97.8) |
| Transfer | 3/141 (2.2) |
| Death | 0 |
ALT = alanine transaminase; AST = aspartate transaminase; BMI = body mass index; ESR = erythrocyte sedimentation rate; hs-CRP = high sensitivity C- reactive protein; IQR = interquartile range; IU = international unit; SD = standard deviation; WBC = white blood cell.
Among 33 patients who consented to the participation in the study, 55 samples were collected (Table 2). The positive nested PCR detected in eschar, blood, and CSF indicated 96.5%, 47.8%, and 33.3%, respectively. The genotypes of strains identified with the Boryong, Taguchi, and Gilliam were 51.5%, 45.5%, and 3.0%, respectively.
Table 2.
Results for specimen of patients with tsutsugamushi disease
| Result, n (%) | Specimen | ||
|---|---|---|---|
| Eschar | Blood | CSF | |
| Positive | 28 (96.5) | 11 (47.8) | 1* (33.3) |
| Negative | 1 | 12 | 2† |
| Total | 29 | 23 | 3 |
CSF = cerebrospinal fluid; n = number. CSF analysis conducted in patients with severe headache or neck stiffness. Two patients had meningitis.
Boryong genotype.
Taguchi genotype identified in an eschar sample.
In our subgroup analysis, clinical characteristics of Boryong and Taguchi types were analyzed (Table 3), and there were no significant differences except residential district and antibiotics duration between the two genotypes. The increasing trend of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the patients with the Taguchi type was lower than in patients with the Boryong type at the time of presentation. The median duration of antibiotic use was shorter in the Taguchi than the Boryong type. The number of inhabitants in Jeju and Seogwipo was 21 patients and 11 patients, respectively. The Taguchi type was dominant in the inhabitants of Jeju City, and the Boryong type was dominant in Seogwipo City inhabitants. There were no significant differences between Boryong and Taguchi in parameters reflecting severity, including frequency of pneumonitis, meningitis, shock, acute kidney injury, and mean length of hospital stay. For two meningitis patients, one patient was identified with the Taguchi type in an eschar sample, and the other patient was identified with the Boryong type in a CSF sample.
Table 3.
Characteristics of tsutsugamushi disease patients according to genotype
| Boryong (N = 17) | Taguchi (N = 15) | P value | |
|---|---|---|---|
| Age (years), median (IQR) | 64.0 (36–79) | 61.0 (27–72) | 0.41 |
| Female, no. (%) | 6 (35.3) | 6 (40.0) | 0.78 |
| Body temperature (°C), mean ± SD | 38.9 ± 0.8 | 38.9 ± 0.6 | 0.83 |
| Fever, no. (%) | 16 (94.1) | 12 (80.0) | 0.31 |
| Skin rash, no. (%) | 12 (70.6) | 12 (80.0) | 0.53 |
| Lymphadenopathy, no. (%) | 3 (17.6) | 0 | 0.23 |
| Eschar, no. (%) | 13 (76.4) | 12 (70.6) | 0.31 |
| Resident area, no. (%) | |||
| Jeju | 8 (47.1) | 13 (86.7) | 0.02 |
| Seogwipo | 9 (52.9) | 2 (13.3) | 0.03 |
| WBC (×103/μL) | 7.7 ± 3.0 | 7.8 ± 2.9 | 0.90 |
| Hemoglobin (g/dL) | 12.9 ± 1.1 | 13.6 ± 2.1 | 0.28 |
| Platelet (×103/μL) | 161.3 ± 92.6 | 176.5 ± 39.3 | 0.56 |
| hs-CRP (mg/dL) | 8.2 ± 4.4 | 8.2 ± 4.9 | 0.99 |
| Procalcitonin (pg/mL) | 0.6 ± 0.6 | 0.4 ± 0.1 | 0.31 |
| Albumin (g/dL) | 3.5 ± 0.4 | 3.6 ± 0.3 | 0.44 |
| ALP (U/L) | 529 ± 414 | 403 ± 274 | 0.33 |
| AST (IU/L) | 606.6 ± 1,947.2 | 68.6 ± 45.5 | 0.29 |
| ALT (IU/L) | 384.4 ± 1,029.0 | 66.7 ± 49.8 | 0.24 |
| Na (mmol/L) | 132.8 ± 4.6 | 135.4 ± 2.7 | 0.06 |
| Antibiotics duration (day), mean ± SD | 6.6 ± 1.2 | 5.3 ± 1.6 | 0.02 |
| Time to disappearance of fever (day), median (IQR) | 1.0 (1–4) | 1.0 (1–3) | 0.22 |
| Complication, no. (%) | 2 (11.8) | 1 (6.7) | 0.40 |
ALP = alkaline phosphatase; ALT = alanine transaminase; AST = aspartate transaminase; ESR = erythrocyte sedimentation rate; hs-CRP = high sensitivity C- reactive protein; IQR = interquartile range; IU = international unit; SD = standard deviation; WBC = white blood cell count.
In the Asia Pacific rim, Karp and Gilliam serotypes are prevalent in Taiwan, the Gilliam serotype is prevalent in China, the Karp serotype is prevalent in Japan,1 and the Boryong serotype dominates in South Korea.1,6,7 Serotypes in the domestic areas also vary. Although the Gilliam and Karp serotypes are reportedly more common in the central part,1,8 the Boryong type predominates in the southern part of South Korea.4,9 In addition, Kato, Neimeng-65, and Kawasaki genotypes have been identified in South Korea.10 Although a prior study reported that the Boryong genotype rather than the Karp genotype predominated on Jeju Island,7 we found that the Taguchi type was also common on Jeju Island. Previously, reports indicated that the Taguchi genotype is uncommon in endemic areas and does not show clinical manifestation.6,11 Park and others6 reported that the Taguchi and Kanda/Kawasaki serotypes caused less severe complications compared with the Boryong serotype. Our study revealed no significant differences in demographic data, clinical manifestations, and laboratory results between the Boryong and Taguchi serotypes. Analysis of the genotype differences according to the domestic distribution has not yet been reported. We presume that the difference between the vector species and climate change was associated with the genotype variety.12,13 Whereas Leptotrombidium pallidum and L. scutellare are dominant in South Korea,13 the predominant chigger mite species through consecutive seasons were L. zetum (43.3%) followed by L. orientale (27.4%) and L. scutellare (26.6%) on Jeju Island.14 L. zetum dominated on Jeju Island compared with other domestic area. This mite peaked in winter (46.2%), and the levels were maintained in spring (42.9%).14 Actually, 15.7% of tsutsugamushi disease developed in winter and spring in our study. However, most cases of tsutsugamushi disease are reported in autumn. The Boryong serotype has been found where both L. pallidum and L. scutellare are present, whereas the Karp serotype was found with L. pallidum.7 Additional studies are required to determine whether the Taguchi serotype is mediated by L. zetum or other vectors. Presently, there is no significant difference in season between the Boryong and Taguchi serotypes (data not shown).
Our study has some limitations. It was a non-randomized, retrospective study involving convenience samples of a relatively small number of inpatients seen in two centers, and analysis of genotype included only those samples collected in the last 2 years.
Despite the limitations, the strength of the report is that it is the first attempted genotypic analysis of O. tsutsugamushi on Jeju Island, showing that the Taguchi genotype was also a dominant strain. This report explains the possibility for reinfection and clinical course, which could be used in patient management in clinical settings. In the past, all of the isolated serologic types of O. tsutsugamushi from vectors on Jeju Island were only the Boryong serotype, but those types isolated from humans were the Taguchi and Gilliam serotypes in addition to the Boryong serotype. Whereas the most common serologic type on the mainland was the Boryong serotype (with only 20% being the Taguchi serotype), the dominant type on Jeju Island was the Taguchi serotype, which comprised more than 50% of the isolates. This report provides a foundation and directions for future studies on the effects of vector and climate change; it also shows the difference between vector and human infection to explain the difference in isolated serologic types of O. tsutsugamushi between the mainland and Jeju Island.
In conclusion, this study is the first that suggests a clinical manifestation of Taguchi type in an endemic area. We determined the genotypes of O. tsutsugamushi; the Taguchi genotype predominated, unlike other regions in Korea. Clinical manifestation of this genotype compared with the Boryong serotype was not a significant difference, and it improved with treatment with the usual antibiotics. These preliminary findings must be confirmed with more studies performed in different regions of Korea.
Footnotes
Financial support: This work was supported by a research grant from Jeju National University in 2012.
Authors' addresses: Jeong Rae Yoo, Division of Infectious Disease, Samsung Medical Center, Sungkynkwan University School of Medicine, Seoul, Republic of Korea, E-mail: mdyoojr@gmail.com. Sang Taek Heo, Department of Internal Medicine, School of Medicine, Jeju National University, Jeju, Republic of Korea, E-mail: neosangtaek@naver.com. Young-Sang Koh, and Sohyun Kim, Departments of Microbiology and Immunology, School of Medicine, Jeju National University, Jeju, Republic of Korea, E-mails: yskoh7@jejunu.ac.kr and just_so@naver.com. Seok Kim, Department of Internal Medicine, Segwipo Medical Center, Jeju, Republic of Korea, E-mail: gimuseog@daum.net.
References
- 1.Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48((Suppl 3)):S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 2.Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001;3:11–21. doi: 10.1016/s1286-4579(00)01352-6. [DOI] [PubMed] [Google Scholar]
- 3.Korea Centers for Disease Control and Prevention Acute Febrile Illness in Fall Recommendations. 2012. http://www.cdc.go.kr/CDC/cms /content/54/9954_view.html Available at. Accessed March 10, 2012.
- 4.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10:227–238. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korea Meteorological Administration Annual Climatological Report. 2012. http://www.kma.go.kr/weather/observation/data_monthly.jsp Available at. Accessed April 9, 2013.
- 6.Park SW, Lee CK, Kwak YG, Moon C, Kim BN, Kim ES, Kang JM, Lee CS. Antigenic drift of Orientia tsutsugamushi in South Korea as identified by the sequence analysis of a 56-kDa protein-encoding gene. Am J Trop Med Hyg. 2010;83:930–935. doi: 10.4269/ajtmh.2010.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ree HI, Kim TE, Lee IY, Jeon SH, Hwang UW, Chang WH. Determination and geographical distribution of Orientia tsutsugamushi serotypes in Korea by nested polymerase chain reaction. Am J Trop Med Hyg. 2001;65:528–534. doi: 10.4269/ajtmh.2001.65.528. [DOI] [PubMed] [Google Scholar]
- 8.Jeong HW, Choi YK, Baek YH, Seong MH. Phylogenetic analysis of the 56-kDa type-specific protein genes of Orientia tsutsugamushi in Central Korea. J Korean Med Sci. 2012;27:1315–1319. doi: 10.3346/jkms.2012.27.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SW, Lee CS, Lee CK, Kwak YG, Moon C, Kim BN, Kim ES, Kang JM, Oh MD. Severity predictors in eschar-positive scrub typhus and role of serum osteopontin. Am J Trop Med Hyg. 2011;85:924–930. doi: 10.4269/ajtmh.2011.11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YM, Kim DM, Lee SH, Jang MS, Neupane GP. Phylogenetic analysis of the 56 kDa protein genes of Orientia tsutsugamushi in Southwest Area of Korea. Am J Trop Med Hyg. 2011;84:250–254. doi: 10.4269/ajtmh.2011.09-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enatsu T, Urakami H, Tamura A. Phylogenetic analysis of Orientia tsutsugamushi strains based on the sequence homologies of 56-kDa type-specific antigen genes. FEMS Microbiol Lett. 1999;180:163–169. doi: 10.1111/j.1574-6968.1999.tb08791.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Jang JY. Correlations between climate change-related infectious diseases and meteorological factors in Korea. J Prev Med Public Health. 2010;43:436–444. doi: 10.3961/jpmph.2010.43.5.436. [DOI] [PubMed] [Google Scholar]
- 13.Lee IY, Kim HC, Lee YS, Seo JH, Lim JW, Yong TS, Klein TA, Lee WJ. Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J Parasitol. 2009;47:381–386. doi: 10.3347/kjp.2009.47.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ree HI, Lee IY, Cho MK. Study on vector mites of tsutsugamushi disease in Cheju Island, Korea. Korean J Parasitol. 1992;30:341–348. doi: 10.3347/kjp.1992.30.4.341. [DOI] [PubMed] [Google Scholar]