Abstract
Schistosoma haematobium eggs and Schistosoma DNA levels were measured in urine samples from 708 girls recruited from 18 randomly sampled primary schools in South Africa. Microscopic analysis of two 10-mL urine subsamples collected on three consecutive days confirmed high day-to-day variation; 103 (14.5%) girls had positive results at all six examinations, and at least one positive sample was seen in 225 (31.8%) girls. Schistosoma-specific DNA, which was measured in a 200-μL urine subsample by using real-time polymerase chain reaction, was detected in 180 (25.4%) cases, and levels of DNA corresponded significantly with average urine egg excretion. In concordance with microscopic results, polymerase chain reaction results were significantly associated with history of gynecologic symptoms and confirmed highly focal distribution of urogenital schistosomiasis. Parasite-specific DNA detection has a sensitivity comparable to single urine microscopy and could be used as a standardized high-throughput procedure to assess distribution of urogenital schistosomiasis in relatively large study populations by using small sample volumes.
Introduction
Schistosomiasis is endemic to 76 countries and 85% of those infected live in rural areas of sub-Saharan Africa, where at least 100 million women and girls are at risk of having this helminth infection.1 Those who are most vulnerable to infection are pre-school and primary school children, adolescent girls, and women of childbearing age. To reduce morbidity the World Health Organization recommends annual treatment of school-age children in areas of high endemicity.2
In sub-Saharan Africa, Schistosoma haematobium is the predominant species.3 Adult worms lodge in the venules surrounding the pelvic organs, where they deposit their eggs. Some of the eggs are released into the urine whereas others get trapped in the bladder mucosa where they give rise to granulomatous inflammation causing hematuria and urinary symptoms. Up to 75% of the women who excrete S. haematobium eggs in the urine may also have Schistosoma eggs in the uterus, cervix, vagina, or vulva.4 The genital manifestations may mimic cancer-like lesions and the different sexually transmitted diseases, such as ulcers, genital warts, polyps, and also cause mucosal immune activation and blood vessel friability.5–7 Several studies have indicated that genital manifestation of schistosomiasis may make women susceptible to human immunodeficiency virus (HIV) infection and they may possibly also develop infertility.8–10 Because S. haematobium affects the urinary and genital tracts, urinary schistosomiasis has been renamed urogenital schistosomiasis.11
The gold standard for diagnosing gynecologic schistosomiasis has been the demonstration of eggs in a crushed biopsy specimen.6 However, this procedure is controversial because it is invasive and could make the cervical mucosa more susceptible to infections with other sexually transmitted infections, such as those with HIV or human papillomavirus.4 The colposcope for investigating gynecologic morbidity has limitations because it is highly observer-dependent, requires extensive training and expensive equipment, and cannot be used among children because intra-vaginal inspections are normally not performed before the onset of sexual activity.
For urinary tract infection with S. haematobium, microscopic examination for eggs is considered the diagnostic gold standard.12 However, the extent to which the presence and intensity of excreted eggs in urine is associated with the actual degree of genital morbidity is still under debate because studies have shown a substantial proportion of adult women with genital lesions to be urine microscopy negative.4 In adults, gynecologic symptoms are often seen many years after reported water exposure.4 Conversely, in school-age girls, one would expect infections to be more recently acquired, with adult worms actively producing eggs that are deposited at different tissues in the pelvic region and excreted into the urine in relatively large quantities.
The association between urine microscopy–detected S. haematobium infection and genital symptoms has been recently studied on a group of 10–12-year-old school girls in South Africa.13 One third of the interviewed girls reported to have a history of genital symptoms, and multivariate regression analysis showed a significant association with the urine microscopy results. To avoid suboptimal diagnosis and not to miss light infections, intense microscopy was performed as generally recommended, which required repeated urine sample collection and examination.12,13 Microscopy has the limitations of being an observer-dependent procedure, as well as laborious, when applied to large-scale population based surveys. For this purpose there is a clear need for more standardized and highly sensitive high-throughput diagnostic procedures.14,15
In recent years, several nucleic acid–based diagnostic tests have been established for specific and sensitive detection and quantification of a broad range of parasite DNA in clinical samples, including an internal transcribed spacer (ITS)–based Schistosoma-specific multiplex real-time polymerase chain reaction (PCR).16,17 In this study, we further explored real-time PCR as a diagnostic tool for mapping school-based distribution of urogenital schistosomiasis by comparing urine Schistosoma DNA levels in the same cohort of school girls from South Africa with the findings of multi-sampling microscopy, as well as reported gynecologic symptoms.
Materials and Methods
Study population.
The study design is a school-based, cluster, randomized, cross-sectional study of girls 10–12 years of age in rural primary schools in KwaZulu-Natal, South Africa. Participants were recruited from 18 randomly selected schools, all situated in a coastal area of 5,866 km2 within Ugu district, south of Durban.13 The region is known to be endemic for S. haematobium and HIV.18,19 Other common helminths are Ascaris lumbricoides and Trichuris trichiura but only occasional cases of infection with S. mansoni have been reported.20 Recruitment of the study participants and their clinical symptoms have been described in detail elsewhere.13 In brief, data and sample collection were performed during September 2009–November 2010. Before the study, information meetings were organized at each school and girls were invited to participate if their parents provided consent.
Interview.
Research assistants invited consenting girls to face-to-face private interviews performed in the local language isiZulu. In brief, in this study we used four key variables: 1) living with both parents, 2) reported water contact, 3) history of red urine, and 4) history of genital symptoms because these variables potentially reflect social status, exposure to and clinical outcome of S. haematobium infection.
Each girl was questioned about her living conditions and relationship with her biological parents and about her own observations concerning red urine. High-risk water contact was defined as reported regular exposure to potentially infective water bodies covering at least 10% of the body surface or being in the risk water at least 60 minutes per exposure. History of genital symptoms consisted of reported symptoms of bloody discharge, malodorous discharge, genital itch or burning sensation in the genitals, and some additional symptoms, which were only occasionally mentioned. Further details about the interview are given elsewhere.13
Ethical considerations and permissions.
This study project drew from a larger project that was approved by the Biomedical Research Ethics Administration, University of KwaZulu Natal (Ref BF029/07). The Departments of Health and Education (Ref HRKM010-08) of KwaZulu Natal also approved the study. Ethical permission was also granted from The Norwegian Ethics Committee, Regional Etisk Komité Øst-Norge (REK-Øst), (Ref IRB 0000 1870) and The European Group on Ethics in Science and New Technologies (Ref IRSES-2010:269245).
Parents or guardians provided written informed consent and consent was given by each girl. All girls were informed of the right to withdraw and to abstain from answering questions without negative consequences. To protect children from stigmatization, the disease was discussed in general terms as urinary schistosomiasis, known as isichenene in isiZulu. Treatment of schistosomiasis with praziquantel was offered to all participants, and all were informed about possible side effects. Support of a private psychologist was available to assist with cases that required psychological support. The project also had a referral system with local clinics and hospitals for those participants that required this system. Consent forms were returned and signed by 92% of the parents in the first 13 schools, but because of teacher strikes, the turnout was only 17% in the remaining five schools.13
Sample collection procedure and microscopy.
A team of trained field research assistants and school nurses visited each school for general study information and urine collection between 10:00 am and 2:00 pm on three consecutive days. Samples were transported to the laboratory on the same day in dark cooler boxes to ensure optimal processing. After arrival, two 10-mL urine samples (A and B) were preserved with 1 mL of 2% tincture of merthiolate in 5% formalin solution.21 Within the same week, samples were centrifuged for 10 minutes at 4,000 rpm and microscopically investigated at a magnification of 10× for S. haematobium eggs. The egg counts were recorded for each 10 mL of urine separately. When more than 1, 000 eggs were seen, counting was stopped. Microscopy was performed blinded to previous results and by separate technicians. Quality control was performed by an independent senior microscopy expert on 10% of randomly chosen samples.
Detection of Schistosoma DNA.
Aliquots of 1 mL of each first-day urine sample, before the merthiolate-formalin was added, were transferred into cryotubes. The aliquots were stored at 4°C for maximum of one week in the dark and thereafter stored at −80°C for several months, before being transported to the Netherlands in frozen conditions for DNA isolation and detection. For a subselection of 85 urine samples, 49 of them that were microscopy negative at all examinations, a second aliquot was tested by PCR at a local laboratory in South Africa.
In the Netherlands, DNA isolation and the set-up of the PCR was performed at the Leiden University Medical Center with a custom-made automated liquid handling station (Hamilton, Bonaduz, Switzerland). DNA was isolated from a 200-μL subsample of each urine sample by using a proteinase K heating step and QI Amp spin columns (QIAGEN, Hilden, Germany) as described.16 Phocin herpes virus 1 (PhHV-1) was added to the lysis buffer as an internal control.22 For real-time PCR, Schistosoma-specific primers Ssp48F and Ssp124R were used to amplify a 77-basepair fragment of ITS2. The double-labeled probe Ssp78T was used to detect amplification.16 For the internal control, PhHV-1-specific primers PhHV-267S and PhHV-337AS and the specific double-labeled probe PhHV-1-305TQ were included in each reaction mixture. The amplification and detection of each DNA sample, with use of the Bio-Rad (Herciules, CA) CFX Manager, was performed as described.16
Similar DNA isolation and PCR procedures were used at the laboratory in South Africa, with some minor modifications. All steps were done manually. The PhHV-1 internal DNA control was replaced by Lambda DNA (1 μL Lambda DNA; dilution of 10−6 per 400 μL of lysis buffer). Therefore Lambda-specific primers and the Lambda-specific CAL Fluor Orange 560–labeled probe Lambda-TMp were used in the reaction mixture. The amplification of each DNA sample was performed in a 25-μL reaction mixture containing PCR buffer (containing 10× Gold Buffer, dNTPs, and TaqGold), 5 mM MgCl2, 2.5 μg bovine serum albumin (Roche Diagnostics Nederland BV, Almere, The Netherlands), 1.5 pmol of each Schistosoma-specific primer, 1.5 pmol of each Lambda-specific primer, 1.25 pmol of each of the Schistosoma-specific and Lambda-specific probes, and 5 μL of the DNA sample. The thermocycler was set for 15 minutes at 95°C, followed by 50 cycles, each for 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. Amplification, detection, and data analysis were performed with the Corbett Rotor Gene 3000 (QIAGEN).
In both laboratory settings, the PCR output consisted of a cycle threshold (Ct) value, which represented the amplification cycle in which the level of fluorescent signal exceeded the background fluorescence and thereby indicating the presence of parasite-specific DNA in the sample that was tested. The Ct values of the internal controls (PhHV in the Netherlands, Lambda DNA in South Africa) were within the expected range for all samples and will not be further discussed within the results.
Data analysis and statistical testing.
The PCR analysis was performed blinded from microscopy or other field data. The results of the real-time PCR analysis were stored and grouped in a Microsoft (Redmond, WA) Access database and imported into IBM (Chicago, IL) SPSS 20.0 for statistical analysis together with provided field data, including results of the interviews and urine microscopy examinations.
Based on microscopy, the infection was classified as high intensity if the mean number of eggs of the three specimens was > 50 per 10 mL of urine.23 Based on PCR, the infection was classified as high intensity (Ct < 30), medium intensity (30 ≤ Ct < 35) or low intensity (35 ≤ Ct < 50).15 These categories were chosen arbitrarily, based on previous experiences with protozoal and helminth infections where DNA loads with a Ct value < 30 could generally be microscopy confirmed and DNA loads with a Ct value > than 35 were always microscopy negative. For better visualization in the scatter plots, Ct values were also recalculated into arbitrary units (AU) of copy numbers of Schistosoma DNA. An arbitrary value of 1 AU was assigned to each positive sample with a Ct value ≥ 35. Assuming 100% efficacy of the DNA multiplication process up to 35 cycles, a duplication of AU was calculated for each PCR cycle, meaning for each single Ct value reduction starting with a Ct value of 35.
Because the data were not normally distributed, results were described as the total number, range, interquartile ranges (IQRs), and median value of positive subjects. For the evaluation of S. haematobium infection intensity, concordance between sample egg counts and PCR output (AU) was statistically analyzed by using the non-parametric Spearman's rank order correlation coefficient (ρ) because both variables were skewed even after log transformation. The McNemar statistical test was used to analyze concordance between the different diagnostic procedures. Statistical significance was considered at P < 0.05.
Results
General characteristics of the study population.
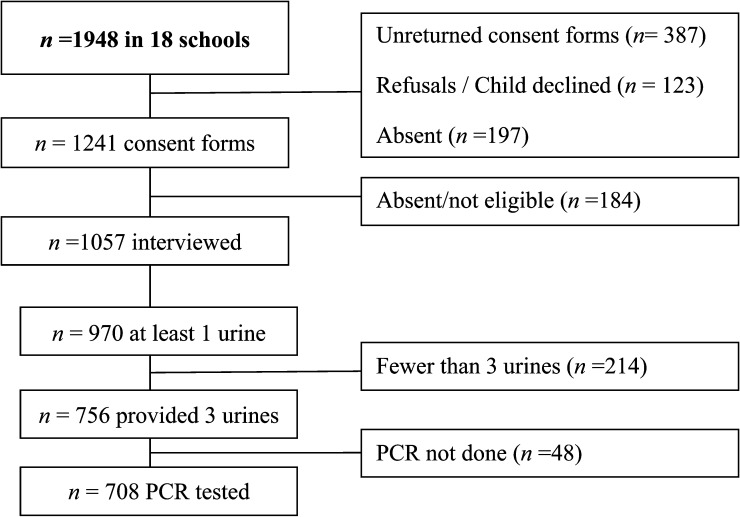
A summary of the sample selection procedure is shown in Figure 1 , and a general description of the study population is shown in Table 1 . A total of 1,948 school girls were included for participation. However, some participants were excluded because unreturned consent forms, refusal to participate, absenteeism, and not being within the specified age range. Of the interviewed girls, 708 pupils provided urine samples for complete microscopy data and PCR, and 13 schools had more than 20 participants. Discrepancy analysis showed no relevant differences between the 708 girls tested and those excluded because of incomplete sampling. At the interview, 27.5% of the 708 girls reported not living with their parents, 60.4% reported possible exposure to infested water, 15.6% reported a history of red urine, and 33.1% reported any history of genital symptoms (Table 1). These characteristics were within the same range as the answers given by all 1,057 interviewed girls, being 27.3%, 62.6%, 17.8% and 35.0%, respectively.
Figure 1.
Flow chart of study participation and adherence by 708 school girls in KwaZulu Natal, South Africa. PCR = polymerase chain reaction.
Table 1.
Epidemiological and parasitological characteristics of urine Schistosoma PCR tested school girls (n=708)
| Characteristics | n | (%) | Range |
|---|---|---|---|
| Number | 708 | ||
| Age (years) | |||
| Median (range) | 11 | 10–12 | |
| Number of schools | 18 | ||
| Participants per school | |||
| Median (range) | 33 | 4–158 | |
| Reported during interview 1) | |||
| Situation at home 2) : | |||
| Living with both biological parents | 163 | (23.1) | |
| Living with one biological parent | 348 | (49.4) | |
| Possible exposure to infected water: | |||
| Low risk water contact | 180 | (25.4) | |
| High risk water contact | 248 | (35.0) | |
| Red urine: | |||
| Sometime before | 50 | (7.1) | |
| Last week | 60 | (8.5) | |
| Genital symptoms3): | |||
| History of bloody discharge | 52 | (7.2) | |
| History of burning sensation in the genitals | 101 | (14.3) | |
| History of any symptoms | 231 | (33.1) | |
| Urine microscopy (S. haematobium eggs) | |||
| 60 mL examination | 225 | (31.8) | |
| Positive in all 6x 10 mL examinations | 103 | (14.5) | |
| Showing mean egg count > 50 eggs/10mL | 60 | (8.5) | |
| 10 mL examination (day 1) | |||
| S. haematobium egg positive cases detected | 171 | (24.2) | |
Full details are presented in Hegertun et al.(2013) definitions describes at material and methods section.
In 3 cases no answer was given.
In 5 cases no answer was given.
Schistosoma microscopy.
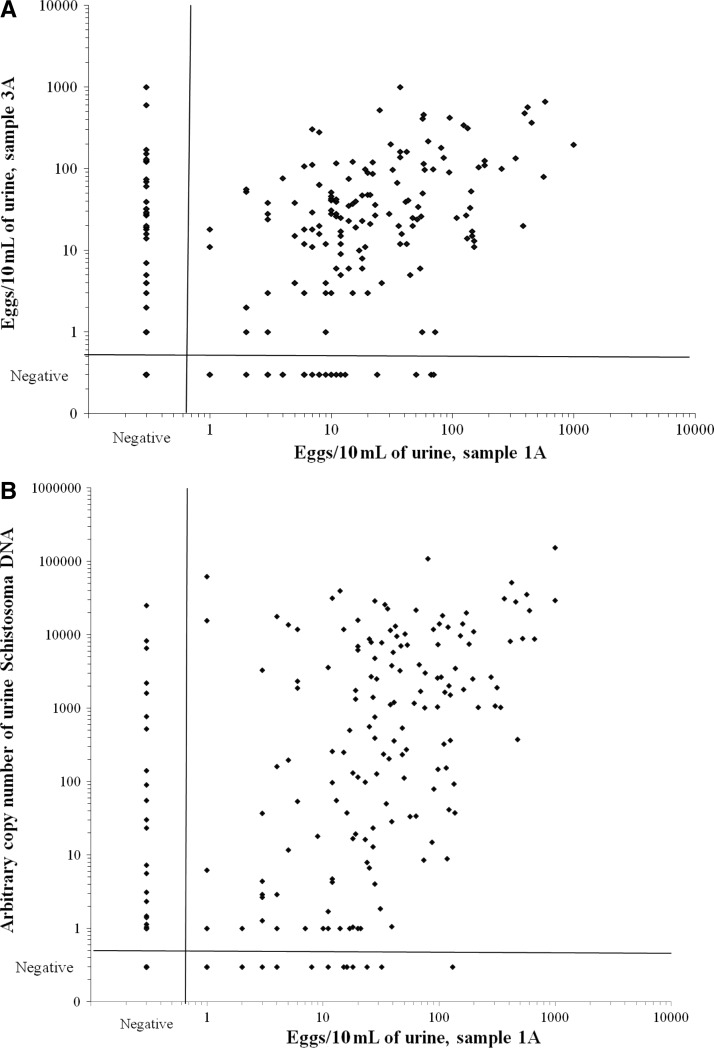
The day-to-day variation in counted S. haematobium eggs is shown in Figure 2A, which depicts egg counts of sample day 1A and sample day 3A. Intensity of infection between the two sample readings correlated significantly in those positive (n = 195; Spearman's ρ = 0.32, P < 0.001). At the same time, discrepancies were seen. Although 139 girls were microscopy positive for both samples, 32 girls were positive in urine sample 1A and negative in sample 3A. In 24 girls, the opposite result was seen. Less than 50 eggs/10 mL of urine were counted in 43 of the 56 girls; there was only one positive urine sample. Identical patterns were seen when microscopy data for other urine readings were compared.
Figure 2.
Scatter plots of 708 school girls 10–12 years of age in KwaZulu-Natal, South Africa. (A) Comparison of urine microscopy of the first 10-mL sample of day 1 (sample 1A) and the first 10-mL sample of day 3 (sample 3A). (B) Comparison of urine microscopy of the first 10-mL sample of day 1 (sample 1A) and urine Schistosoma DNA loads determined in the same day sample (200 μL) by using real-time polymerase chain reaction (PCR).
The overall prevalence of S. haematobium based on the detection of eggs in urine samples collected on three consecutive days was 31.8%, with an average egg count of 0.17–624 eggs/10 mL (median = 20, IQR = 5–55). One hundred and three (45.8%) of the 225 microscopy-positive girls showed eggs in all six urine examinations, and average high intensity infections were seen in 60 (26.7%) infected girls. Based on readings from the three consecutive days, the prevalence of each day was 25.8–26.4%, and based on the individual 10-mL urine examinations, the prevalence was 22.5–24.2%.
Schistosoma PCR.
The PCR analysis of first day urine samples showed Schistosoma DNA in 25.4% of the samples, and 125 of these 180 samples had high DNA loads. The AU of DNA copies ranged from 1 to 154 × 103 (median = 448, IQR = 12–7 × 103). The association between urine Schistosoma DNA load quantified in 200-μL samples and the number of eggs counted in a 10-mL sample of the same portion is shown in Figure 2B. A significant correlation was seen between the two procedures (n = 199; Spearman's ρ = 0.51, P < 0.001). Discrepancies were also observed. Nineteen samples were PCR negative and microscopy positive, all samples except one had egg counts < 50 eggs/10 mL. In addition, Schistosoma DNA was detected in 28 urine samples that were microscopy negative. In 14 of these samples, eggs were seen in at least one of the five other 10-mL urine samples by microscopy, and the remaining 14 had low DNA levels, which indicated that half of them had 1 AU and the maximum seen was 90 AU. The number of detected cases by urine PCR did not statistically differ from the number of detected cases by each of the 10-mL urine microscopy examinations, or each of the three consecutive day readings, but was significantly less compared with the 225 cases detected when all microscopy readings were combined. Also, the average egg counts correlated significantly with the measured urine AU of DNA copies (n = 239; Spearman's ρ = 0.70, P < 0.001).
A comparison between PCR outputs in the two PCR laboratories showed only minor discrepancies. Four of the 36 microscopy-positive urine samples were missed in South Africa; these samples showed positive results in the Netherlands. Conversely, 1 of the 49 microscopy-negative samples had a high DNA level in South Africa, which could not be confirmed when tested in the Netherlands.
School level analysis.
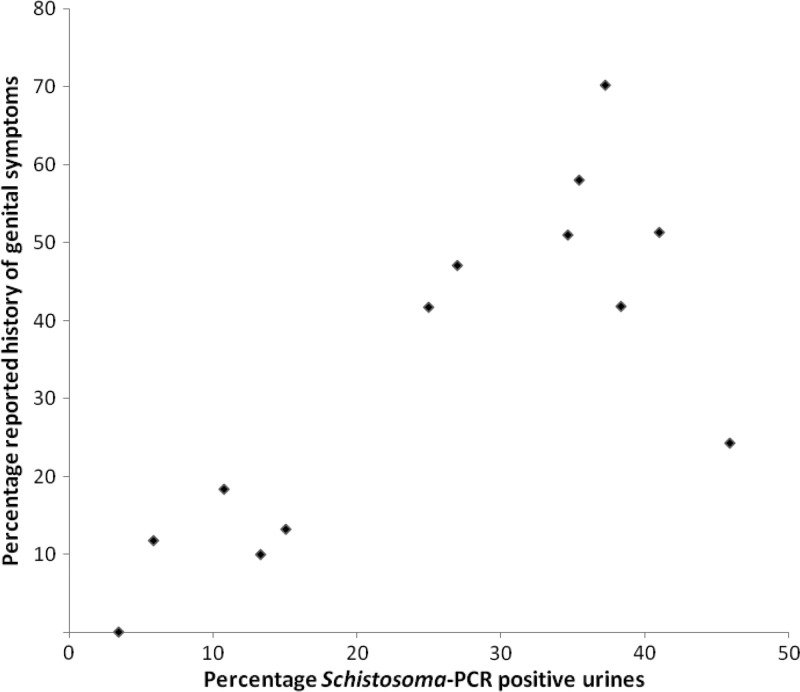
To fully explore the diagnostic value of urine PCR to identify high-risk communities, findings were further analyzed at school level. Data for 13 schools with at least 20 participants per school, representing 666 school girls, were aggregated. The prevalence of S. haematobium per school based on urine PCR ranged from 3.4% to 45.9% (median = 27.0%, IQR = 12.0–37.8%) and showed a significant correlation with the prevalence range based on complete microscopy (n = 13; Spearman's ρ = 0.95, P < 0.001). The prevalence of detectable Schistosoma DNA showed a significant correlation with reported history of genital symptoms at school level (Figure 3) (n = 13; Spearman's ρ = 0.74, P < 0.01). Also, reported history of high-risk water contact and history of red urine showed a significant correlation with school-based Schistosoma prevalence determined by PCR or microscopy (n = 13; Spearman's ρ = 0.62–0.73, P < 0.03). A total of 58.6–83.8% of the girls reported living with at least one parent, and 13.9–34.5% reported living with both parents. No association was seen between household composition and prevalence of schistosomiasis determined by PCR or microscopy.
Figure 3.
Scatter plot comparing school-based prevalence of Schistosoma DNA in urine samples and reported history of genital symptoms in school girls in KwaZulu Natal, South Africa. Schools (n = 13) with at least 20 participants were included, which included 666 school girls of 10–12 years of age. PCR = polymerase chain reaction.
Discussion
In this study, we used a PCR with 708 urine samples collected from girls in 18 primary schools from KwaZulu-Natal, South Africa. Microscopy was performed six times on a 10-mL urine sample collected over three consecutive days. We found substantial dispersion of counted eggs in urine; < 15% of the girls showed positive results for all six 10-mL urine sample examinations. This high day-to-day variability in Schistosoma eggs shedding is generally acknowledged, but data have been published only sparsely.24
Interestingly, we found the sensitivity of the ITS-based PCR performed with a 200-μL subsample to be comparable to that of each of single 10-mL microscopy examinations. However, the sensitivity was lower when compared with repeated microscopy (complete 60-mL urine sample examination). DNA amplification was detected in 98.3% and 88.3% of the samples with > 50 eggs/10 mL of urine and ≤ 50 eggs/10 mL of urine, respectively, and in 28 (5.2%) of 537 urine samples in which eggs were not detected at the first 10-mL urine sample examination. In one considers that our population of school girls was truly infected if at least one of the six microscopy examination results was positive, or if Schistosoma DNA was detected in urine, this resulted in a negative predictive values for complete 60-mL microscopy, 10-mL microscopy, and urine PCR of 98%, 86%, and 88%, respectively.
In a previous study, the same ITS-based real-time PCR for specific detection of Schistosoma DNA showed excellent sensitivities for 730 urine samples collected from children in five primary schools from different communities in the Greater Accra region of Ghana.17 Compared with our study, infection levels were low in the selected schools in Ghana; the overall prevalence was 7.8% and only 10 of 57 children had > 50 eggs/10 mL of urine. Schistosoma DNA was amplified in 100% and 85.2% of urine samples with > 50 eggs/10 mL of urine and ≤ 50 eggs/10 mL of urine, respectively. In addition, Schistosoma DNA was detected by PCR in 102 (15.2%) of 673 urine samples in which eggs were not detected by single 10-mL urine microscopy. These findings resulted in higher negative predictive values for real-time PCR (> 94.6%) than for microscopy (54.3–95.7%).17
Although Schistosoma DNA was quantified in both studies according to the same PCR procedure, microscopy showed better performance in South Africa. Results could not be explained by the higher overall S. haematobium prevalence in samples in South Africa because concordance between PCR output and microscopy did not shift between the selected schools (e.g., the five schools with a microscopy prevalence < 20% showed a similar range in prevalence based on urine Schistosoma DNA detection, while the five schools tested in Ghana showed substantial higher infection levels based on PCR).17
A possible explanation for the comparatively poor PCR performance in the current study could be the procedure of sample collection (i.e., the time lag between receiving the samples and definitive storage in a frozen condition). Because of logistics, freezing of samples could only be performed a number of days, up to a week, after collection. When mixed with ethanol, fecal samples can be kept at room temperatures for weeks to months, even in tropical conditions, before transported to centralized laboratory facilities for DNA isolation.25 For urine samples, optimal storage conditions without immediate need for a cold chain still need to be further explored.
Alternatively, the ITS-based PCR may not be the most optimal procedure for amplifying Schistosoma DNA in all geographic settings. Other procedures have been described and some of them seem to show promising diagnostic performance.26,27 However, not all of these PCRs have been transformed into a multiplex real-time PCR format. Thus, they are substantially more laborious, still observer-dependent, and lack the option to include a proper internal control.27 Also, some need relatively large serum volumes.28 Thus, their feasibility for large-scale population based surveys still needs to be fully explored.
Human errors, including missed observations during microscopy and mislabeling and swapping of samples, can never be completely excluded. This problem is illustrated by the single sample showing > 50 eggs/10 mL of urine that showed negative results when tested by PCR. This person showed S. haematobium eggs at all six microscopy examinations, almost all with high egg counts. Thus, it is likely that aliquots were swapped during sampling for PCR analysis. The potential influence of human error is also illustrated by the performance of the real-time PCR at the separate laboratory facilities. Overall results were highly reproducible, but in case of discrepancy, the output produced by using an automated liquid handling station has been more in concordance with the microscopy data than the PCR output produced manually.
The use of an automated liquid handling station also enables processing of large sample numbers in a short period (i.e., we completed DNA isolation and detection, including automated data handling, for all 708 samples within 10 working days). The small sample volume required and the potential number of additional DNA targets that can be quantified, in particular when working with fecal samples, makes this approach highly attractive for large-scale population screening for the presence of multiple urinary and intestinal microorganisms.17,29,30 In this study, we also introduced arbitrary units (AU) of copy numbers of Schistosoma DNA as PCR output, which provides a more realistic view on the existing high linear range of intensity of Schistosoma infection than Ct values. In addition, for more in depth comparison between different PCRs, further standardization, including converting DNA loads into egg counts, is needed.
In a previous study, the overall association between urine egg excretion and reported urinary and gynecologic symptoms was described. In particular, bloody discharge and a burning sensation in the genitals were found to be significantly correlated with repeated urine microscopy findings, even after controlling for confounders.13 As expected, we found the same associations between PCR-diagnosed schistosomiasis and reported genital symptoms. Because of the young age of the girls included, we were not able to collect any gynecologic samples, which would be of interest for quantification of Schistosoma DNA levels.31 However, for confirmation, urinary and vaginal samples should be collected from adult women.
Although schools were randomly selected from a relatively small rural area of KwaZulu-Natal that had comparable ecologic and socioeconomic circumstances, we found S. haematobium infection prevalence to range from < 5% up to approximately 60% between the different schools. Schistosoma prevalence and the prevalence of reported urinary and gynecologic symptoms varied greatly between included schools. Conversely, we found no association between household composition, reflecting social status in this particular region, and the prevalence of S. haematobium. Focal distribution of schistosomiasis has been described and besides behavioral components in relation to exposure to infected water bodies, many other factors may influence prevalence and intensity of infection, including micro-level environmental circumstances.32 The consequences are obvious. Although local hot-spots require intensive and repeated mass treatment with appropriate anti-schistosomal drugs in combination with intense control measures and monitoring, other communities will be served sufficiently by targeted treatment or even passive case detection. Based on this knowledge, there is also a clear need for a rapid and field applicable diagnostic test to allocate these hot spots in resource-poor settings without the need of high technology laboratory equipment.33,34
In conclusion, our findings illustrate that the diagnostic potentials of urine Schistosoma real-time PCR is dependent on the quality and intensity of performed microscopy. Repeated microscopy may in certain settings be more sensitive but at the same time it is a highly laborious as well as observer dependent procedure. Although improved sensitivity is still anticipated, parasite-specific DNA detection already seems an attractive and efficient automated high-output system for large screening programs. Only small sample volumes are needed to identify communities at risk for development of genital morbidity caused by S. haematobium infection.
ACKNOWLEDGMENTS
We thank the study community district, including the KwaZulu Natal Department of Health, the Department of Education, and the FGS Project Team in South Africa for data collection; Roy Manyaira for data management; Colleen Archer for re-examining microscopic samples; and Dr. Dennis York for providing laboratory facilities and training for real-time PCR in South Africa.
Footnotes
Financial support: This study was supported by Prof. Dr. P.C. Flu-Foundation, the University of Copenhagen (with the support from the Bill and Melinda Gates Foundation); the South-Eastern Norway Regional Health Authority (Network project no. 2011073); the International Research Staff Exchange Scheme; Marie Curie Actions, Oslo University Hospital Ullevaal (VIRUUS), the Center for Imported and Tropical Diseases, Oslo, Norway; the Norwegian Research Council (ref 213702/H10); and the National Research Foundation, South Africa.
Authors' addresses: Pavitra Pillay, Department of Biomedical and Clinical Technology, Durban University of Technology, KwaZulu Natal, South Africa, E-mail: pillayp@dut.ac.za. Myra Taylor and Siphosenkosi G. Zulu, School of Nursing and Public Health, University of KwaZulu Natal, KwaZulu Natal, South Africa, E-mails: taylormyra@gmail.com and siphogzulu@gmail.com. Svein G. Gundersen, Research Unit, Sorlandet Hospital HF, Kristiansand, Norway, and University of Agder, Kristiansand, Norway, E-mail: svein.g.gundersen@sshf.no. Jaco J. Verweij, St. Elisabeth Hospital, Tilburg, The Netherlands, E-mail: j.verweij@elisabeth.nl. Pytsje Hoekstra, Eric A. T. Brienen, and Lisette van Lieshout, Department of Parasitology, Leiden University Medical Center, Leiden, The Netherlands, E-mails: p.t.hoekstra@amc.uva.nl, e.a.t.brienen@lumc.nl, and e.a.van_lieshout@lumc.nl. Elisabeth Kleppa and Eyrun F. Kjetland, Norwegian Centre for Imported and Tropical Diseases, Department of Infectious Diseases, Ulleval, Oslo University Hospital, Olso, Norway, E-mails: elisabeth.kleppa@medisin.uio.no and e.f.kjetland@medisin.uio.no.
References
- 1.Fenwick A. Waterborne infectious diseases–could they be consigned to history? Science. 2006;313:1077–1081. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 3.Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLos Neg Tropl Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28:58. doi: 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Leutscher PD, Ravaoalimalala VE, Raharisolo C, Ramarokoto CE, Rasendramino M, Raobelison A, Vennervald B, Esterre P, Feldmeier H. Clinical findings in female genital schistosomiasis in Madagascar. Trop Med Int Health. 1998;3:327–332. doi: 10.1046/j.1365-3156.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- 6.Poggensee G, Krantz I, Kiwelu I, Diedrich T, Feldmeier H. Presence of Schistosoma mansoni eggs in the cervix uteri of women in Mwanga District, Tanzania. Trans R Soc Trop Med Hyg. 2001;95:299–300. doi: 10.1016/s0035-9203(01)90239-1. [DOI] [PubMed] [Google Scholar]
- 7.Wright ED, Chipangwi J, Hutt MS. Schistosomiasis of the female genital tract. A histopathological study of 176 cases from Malawi. Trans R Soc Trop Med Hyg. 1982;76:822–829. doi: 10.1016/0035-9203(82)90118-3. [DOI] [PubMed] [Google Scholar]
- 8.Ndhlovu PD, Mduluza T, Kjetland EF, Midzi N, Nyanga L, Gundersen SG, Friis H, Gomo E. Prevalence of urinary schistosomiasis and HIV in females living in a rural community of Zimbabwe: does age matter? Trans R Soc Trop Med Hyg. 2007;101:433–438. doi: 10.1016/j.trstmh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Kjetland EF, Mduluza T, Ndhlovu PD, Gomo E, Gwanzura L, Midzi N, Mason PR, Friis H, Gundersen SG. Genital schistosomiasis in women: a clinical 12-month in vivo study following treatment with praziquantel. Trans R Soc Trop Med Hyg. 2006;100:740–752. doi: 10.1016/j.trstmh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Mbabazi PS, Andan O, Fitzgerald DW, Chitsulo L, Engels D, Downs JA. Examining the relationship between urogenital schistosomiasis and HIV infection. PLos Neg Tropl Dis. 2011;5:e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Statement – WHO Working Group on Urogenital Schistosomiasis and HIV Transmission, October 12, 2009. Geneva: World Health Organization; 2010. [Google Scholar]
- 12.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 13.Hegertun IEA, Gundersen KMS, Kleppa E, Zulu SG, Gundersen SG, Taylor M, Kvalsvig JD, Kjetland EF. S. haematobium as a common cause of genital morbidity in girls: a cross-sectional study of children in South Africa. PLos Neg Tropl Dis. 2013;7:e2401. doi: 10.1371/journal.pntd.0002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Lieshout L, Yazdanbakhsh M. Landscape of neglected tropical diseases: getting it right. Lancet Infect Dis. 2013;13:469–470. doi: 10.1016/S1473-3099(13)70094-X. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcanti MG, Silva LF, Peralta RH, Barreto MG, Peralta JM. Schistosomiasis in areas of low endemicity: a new era in diagnosis. Trends Parasitol. 2013;29:75–82. doi: 10.1016/j.pt.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, Van Dam G, Van Lieshout L. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–633. doi: 10.1179/136485908X337490. [DOI] [PubMed] [Google Scholar]
- 17.Aryeetey YA, Essien-Baidoo S, Larbi IA, Ahmed K, Amoah AS, Obeng BB, Van Lieshout L, Yazdanbakhsh M, Boakye DA, Verweij JJ. Molecular diagnosis of Schistosoma infections in urine samples of school children in Ghana. Am J Trop Med Hyg. 2013;88:1028–1031. doi: 10.4269/ajtmh.12-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appleton CC, Kvalsvig JD. A school-based helminth control programme successfully implemented in KwaZulu-Natal. South Afr J Epidemiol Infect. 2006;21:55–67. [Google Scholar]
- 19.Department of Health South Africa . The 2011 National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa. Johannesburg, South Africa: Department of Health South Africa; 2011. [Google Scholar]
- 20.Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. A health and nutritional profile of rural school children in KwaZulu-Natal, South Africa. Ann Trop Paediatr. 2001;21:50–58. [PubMed] [Google Scholar]
- 21.Thomassen Morgas DE, Kvalsvig JD, Gundersen SG, Taylor M, Kjetland EF. Schistosomiasis and water-related practices in school girls in rural KwaZulu-Natal, South Africa. South Afr J Epidemiol Infect. 2010;25:30–33. [Google Scholar]
- 22.Niesters HG. Clinical virology in real time. J Clin Virol. 2002;25:S3–S12. doi: 10.1016/s1386-6532(02)00197-x. [DOI] [PubMed] [Google Scholar]
- 23.Montresor A, Crompton D, Hall A, Bundy D, Savioli L. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: World Health Organization; 1998. [Google Scholar]
- 24.Doehring E, Reider F, Schmidt-Ehry G, Ehrich JH. Reduction of pathological findings in urine and bladder lesions in infection with Schistosoma haematobium after treatment with praziquantel. J Infect Dis. 1985;152:807–810. doi: 10.1093/infdis/152.4.807. [DOI] [PubMed] [Google Scholar]
- 25.Van Lieshout L, Verweij JJ. Newer diagnostic approaches to intestinal protozoa. Curr Opin Infect Dis. 2010;23:488–493. doi: 10.1097/QCO.0b013e32833de0eb. [DOI] [PubMed] [Google Scholar]
- 26.Cnops L, Tannich E, Polman K, Clerinx J, Van Esbroeck M. Schistosoma real-time PCR as diagnostic tool for international travellers and migrants. Trop Med Int Health. 2012;17:1208–1216. doi: 10.1111/j.1365-3156.2012.03060.x. [DOI] [PubMed] [Google Scholar]
- 27.Ibironke OA, Phillips AE, Garba A, Lamine SM, Shiff C. Diagnosis of Schistosoma haematobium by detection of specific DNA fragments from filtered urine samples. Am J Trop Med Hyg. 2011;84:998–1001. doi: 10.4269/ajtmh.2011.10-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wichmann D, Poppert S, Von Thien H, Clerinx J, Dieckmann S, Jensenius M, Parola P, Richter J, Schunk M, Stich A. Prospective European-wide multicentre study on a blood based real-time PCR for the diagnosis of acute schistosomiasis. BMC Infect Dis. 2013;13:55. doi: 10.1186/1471-2334-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker SL, Vogt J, Knopp S, Panning M, Warhurst DC, Polman K, Marti H, von Müller L, Yansouni CP, Jacobs J. Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect Dis. 2013;13:37. doi: 10.1186/1471-2334-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jex AR, Stanley KK, Lo W, Littman R, Verweij JJ, Campbell BE, Nolan MJ, Pangasa A, Stevens MA, Haydon S. Detection of diarrhoeal pathogens in human faeces using an automated, robotic platform. Mol Cell Probes. 2012;26:11–15. doi: 10.1016/j.mcp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Kjetland EF, Ten Hove RJ, Gomo E, Midzi N, Gwanzura L, Mason P, Friis H, Verweij JJ, Gundersen SG, Ndhlovu PD. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2009;81:1050–1055. doi: 10.4269/ajtmh.2009.09-0081. [DOI] [PubMed] [Google Scholar]
- 32.Standley CJ, Vounatsou P, Gosoniu L, Mckeon C, Adriko M, Kabatereine NB, Stothard JR. Micro-scale investigation of intestinal schistosomiasis transmission on Ngamba and Kimi islands, Lake Victoria, Uganda. Acta Trop. 2012;128:353–364. doi: 10.1016/j.actatropica.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Colley DG, Binder S, Campbell C, King CH, Tchuenté L-AT, N'Goran EK, Erko B, Karanja DM, Kabatereine NB, Van Lieshout L. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg. 2013;88:426–432. doi: 10.4269/ajtmh.12-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dam GJ, de Dood CJ, Lewis M, Deelder AM, Van Lieshout L, Tanke HJ, Van Rooyen LH, Corstjens PL. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol. 2013;135:274–282. doi: 10.1016/j.exppara.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]