Abstract
To evaluate immunity to vaccine-preventable diseases according to nutritional status, a longitudinal study was conducted in Senegalese children ages 1–9 years old. A linear regression analysis predicted that weight for age was positively associated with immunoglobulin G (IgG) response to tetanus toxoid in children born during the rainy season or at the beginning of the dry season. A relationship between village, time of visits, and levels of antibodies to tetanus showed that environmental factors played a role in modulating humoral immunity to tetanus vaccine over time. Moreover, a whole-blood stimulation assay highlighted that the production of interferon-γ (IFN-γ) in response to tetanus toxoid was compromised in stunted children. However, the absence of cytokine modulation in response to Mycobacterium tuberculosis-purified protein derivatives and phytohemagglutinin suggests that the overall ability to produce IFN-γ was preserved in stunted children. Therefore, these results show that nutritional status can specifically alter the efficacy of long-lasting immunity to tetanus.
Introduction
Although the World Health Organization (WHO) has developed an expanded immunization program to improve protection against pathogens, infectious diseases remain a major health and socioeconomic issue in many low-income countries. The effectiveness of vaccines is unquestionable, but evidence suggests that a number of environmental and seasonal factors modulate immunity and limit the strength of vaccine-elicited responses in individuals.1–6
Malnutrition is widely recognized as a major public health concern in many developing countries. This condition mostly affects children, and its hypothesized role as a determinant of immune dysfunctions as well as morbidity and mortality from infectious diseases has been outlined in several recent articles.7–9 Indeed, although early studies reported an impaired cellular response to Bacillus Calmette–Guerin (BCG) in severely malnourished children, recent works on groups of children with moderate nutritional deficiencies show little impact of nutritional status on vaccine response.10
The lack of clear relationship between nutritional status and children's response to immunization might be the result of complex interactions between genetic and seasonal determinants, infections, age of the population, and nutrition itself.2,4,5,11,12 Perinatal undernutrition, in particular, is a possible predictor of immunological alteration.3,13,14 Moreover, most studies have been carried out at the time of vaccination, with no detectable effect of nutrient supplementation or malnutrition on the development of the immune response in early life.10 Indeed, post-vaccination studies that dissect putative associations between malnutrition and established immunity to vaccine antigens during childhood remain scarce. We, therefore, designed a longitudinal study in northern Senegal to investigate the impact of malnutrition, defined by stunting, underweight, or wasting, on the acquired immune response against vaccine-preventable bacterial diseases, including tetanus, diphtheria, and tuberculosis.
Materials and Methods
Study design and population.
This study was conducted in northern Senegal in five villages (Agniam, Fanaye-Diery, Niandane, Ndyane-Pendao, and Guede) in Podor district (Saint-Louis Region). Detailed descriptions of the study are available elsewhere (Clinicaltrials.gov ID: NCT01545115).15 In this longitudinal survey, 410 children ages 1–9 years were enrolled. In this sub-Saharan region, there is a monomodal rainy season from July to October, whereas the rest of the year is the dry season. The cohort was followed during two rainy seasons and the course of dry seasons from October of 2008 to January of 2010. Each village was visited five times (Ts): T1 (October of 2008), T2 (January of 2009), T3 (May and June of 2009), T4 (October of 2009), and T5 (January of 2010). Additional visits in June and July of 2009 were carried out in four villages (Agniam, Fanaye-Diery, Niandane, and Ndyane-Pendao) for whole-blood collection from 74 children. Children recruited for whole-blood cell assays were initially selected on the basis of a complete vaccination schedule and an age less than 5 years. Blood collection was authorized after an individual medical examination.
The WHO Expanded Program on Immunization (EPI) recommends BCG at birth and a schedule of diphtheria–tetanus–whole pertussis vaccine (DTwP) vaccination at 6, 10, and 14 weeks of age. Children from the study zone have been vaccinated since 2005 with Quinvaxem (Crucell), a pentavalent DTwP-hepatitis B-haemophilus influenza type B (HepB-Hib) vaccine, and before that time, trivalent whole-cell pertussis vaccine from Pasteur-Mérieux (Lyon, France) was used. No additional vaccine was administered to the children of the cohort during the study. Vaccination status was ascertained based on vaccination cards available for review (N = 228) or oral communication (N = 7) of the child's mother. Among vaccinated children, 2.2% and 4.9% have not received the second and third doses of DTwP vaccine, respectively. Children without vaccination cards and children whose mothers did not know whether they had received DTwP vaccine for routine immunization were referred to as children with unknown vaccination status. The project was approved by the National Ethics Committee of Senegal (Approval Number: SEN26/08). Written individual informed consent was obtained from each participant's parent or legal guardian at the beginning of the survey and for blood collection for whole-blood cell assays. At each visit, parent's approval was sought orally as well as the consent of the child when it was appropriate.
Serological analysis.
Blood was collected by finger prick on BD Microtainer Tubes (Beckton Dickinson). Specific serum antibody concentrations were analyzed by enzyme-linked immunosorbent assay (ELISA). Immunoglobulin G (IgG) antibodies to Tetanus toxoid (TT) were measured by an in-house ELISA. Microtiter plates (Nunc Maxisorp, Denmark) were coated with TT at a concentration of 5 μg/mL in phosphate-buffered saline (PBS; pH 7.4) and incubated for 1.5 hours at 37°C. Plates were then saturated with food grade Gelatin (Merck, Germany) for 1 hour at 25°C. Sera were diluted to 1:200 in PBS containing 0.1% Tween20. On every plate, a standard positive serum sample from Virion/Serion GmbH (Germany) was run along with the samples. The plates were sealed and incubated overnight at 4°C. After washing the plates, goat peroxidase-conjugated anti-human IgG (Sigma-Aldrich) was added at a 1/4,000 dilution, and the mixture was incubated for 1.5 hours at 37°C. The system was developed with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS; Sigma-Aldrich) and read at 405 nm after 60 minutes. IgG antibodies directed against the diphtheria toxoid (DT) were measured using a commercial kit (Virion/Serion GmbH) on 1:200 diluted sera incubated in duplicates and run along a reference serum provided by the manufacturer to get antibody concentrations. Indeed, serum antibody concentration values for TT and DT were assigned in international units per milliliter according to the Serion Activity Quantification Software v8.1, which allowed for converting of optical density (OD) units into international units per milliliter according to the OD of a standard sample run on the same plate using a four-parameter logistic model. Children were rated as negative or poorly immunized (< 0.1 IU/mL), adequately immunized (between 0.1 and 1 IU/mL), or long-term protected (> 1 IU/mL) as detailed by the manufacturer. All ELISA plates were run on a semiautomated washer (Skatron Skanwasher 400; Molecular Devices, United Kingdom) and read on a Multiskan MS Plate Reader (Thermo Labsystems).
Whole-blood cell assay and cytokine detection.
Three- to five-milliliter blood samples were collected in either heparinized tubes (Becton Dickinson) for whole-blood cell culture on the day or ethylenediaminetetraacetic acid (EDTA) tubes for whole-blood cell count. For detection of cytokine released by whole-blood cells, 1 mL blood diluted 10-fold in RPMI 1640 supplemented with 100 U/mL penicillin G and 100 μg/mL streptomycin (Life Technologies, France) was incubated in polypropylene tubes (Becton Dickinson) for either 48 hours with phytohemagglutinin (PHA) or medium alone (2 μg/mL; Sigma-Aldrich) or 6 days with TT (10 μg/mL), DT (10 μg/mL), Mycobacterium tuberculosis-purified protein derivatives (PPDs; 10 μg/mL; Statens Serum Institut, Copenhagen, Denmark) or medium alone at 37°C in 5% CO2 atmosphere. The whole-blood cell supernatants were then harvested and kept at −20°C. Levels of interferon-γ (IFN-γ) and interleukin-10 (IL-10) in supernatants of stimulated whole-blood cells were determined by ELISA using a standard curve (Gen-Probe Diaclone, France). The lower limit of detection was 5 pg/mL for both IFN-γ and IL-10. Duplicates for each antigen were averaged, and background production of cytokines measured in control wells was subtracted from the measurement in Ag-stimulated samples.
Anthropometric measurements.
The nutritional status of children was assessed by anthropometric measurements in January (T2), June (T3), and October (T4) of 2009 and January of 2010 (T5). Anthropometric data were collected by two trained measurers according to international recommendations.16 Weight measurements were recorded using an electronic scale to the nearest 100 g (Téfal, Paris, France) in children > 2 years old, whereas infants were weighed in the caregiver's arms when < 2 years old. Recumbent length measurements were taken for children under 2 years of age, whereas standing height was measured beyond that age using locally made wooden boards precise to the nearest millimeter. Height and length measurements were taken two times, and the mean value was used for the analysis. Child nutritional indicators of height for age (HAZ), weight for age Z score (WAZ) and weight for height (WHZ) were calculated according to the WHO 2006 growth standard using Anthro (version 3.2.2.; WHO) for children < 60 months and AnthroPlus (WHO 2007 growth standard) for children ≥ 60 months. We used the most common thresholds: HAZ < −2 Z scores to define stunting or chronic malnutrition, WAZ < −2 Z scores to define underweight or global malnutrition, and WHZ < −2 Z scores for wasting. Children were excluded from the analysis when their records were flagged by the anthropometry software, because their Z scores exceeded the following values: WAZ < −5 SD or > 4 SD and HAZ < −4 SD or > 4 SD.
Statistical analysis.
Categorical variables are described as absolute numbers and proportions, and continuous variables are described as means ± SDs. After checking the normal distribution of data, differences between groups were tested by the Mann–Whitney U test; χ2 tests were used for categorical variables to compare proportions between groups. The repeated measured analysis of variance (ANOVA) test followed by Bonferroni's multiple comparison test or Kruskal–Wallis test followed by Dunn's analysis was used to compare differences among more than two groups as indicated in the text. Spearman's rank correlation coefficient was used to check correlation. All P values were two-tailed, and differences were considered significant when P values were < 0.05. Analyses were performed using GraphPad Prism 5.02 for Microsoft Windows (GraphPad Software, San Diego, CA).
Multivariate analysis.
Anti-TT IgG responses from T2 to T5 were analyzed using repeated measures analysis of covariance (PROC MIXED; SAS Institute, Cary, NC) with a REPEATED statement for within-child correlation over visits and a RANDOM statement for children within families. Data were log10-transformed because of skewed distributions. The repeated measures covariance structure was specified as a spatial power function to handle unequally spaced measurements over time. Parameters of the model were tested with polynomial contrasts. Multivariate models were built by including all predictors and using a backward selection to reduce the model. In case of a significant interaction between two predictors, the two main effect terms remained in the model, even if not significant. Regression-underlying assumptions were visually inspected with residual plots. Statistical significance was set at α = 0.05. Analyses were performed using SAS version 9.1.3.
Results
Children's characteristics.
A total of 410 children were enrolled at T1, but the nutritional status was assessed from T2 to T5. Table 1 shows information collected at T2 on the cohort characteristics. There was no difference between the number of boys and girls enrolled in the survey. Ages of enrolled children, vaccination status and time span since first DTwP administration were different between villages. Participants were stratified into three groups according to birth seasons: season 1, January to June (the end of the dry season); season 2, July to September (the wet season); season 3, October to December (the beginning of the dry season, when the crops are harvested). Table 1 indicates that the number of children born in each season was similar in all villages. Nutritional status was evaluated by measuring WAZ and HAZ, which define general and chronic malnutrition, respectively, when the Z score is below −2. WHZ for children under 5 years old (wasting when WHZ < −2) was also assessed. As a whole, our results show that young Senegalese children in the Senegal River Valley were mildly malnourished: 19.2% of study participants were underweight, 11.8% of study participants were stunted, and 7.7% of study participants were wasted.
Table 1.
Children's characteristics at T2
| Total (N = 404) | Agniam (N = 50) | Fanaye (N = 105) | Guédé (N = 47) | Niandane (N = 102) | Pendao (N = 100) | P | |
|---|---|---|---|---|---|---|---|
| Age (mean years) | 5.3 ± 2.6 | 4.8 ± 2.5 | 5.6 ± 2.5 | 6.1 ± 3.1 | 5.5 ± 2.4 | 4.8 ± 2.6 | 0.0158* |
| Sex | 0.6105† | ||||||
| Boys | 202 (50.0) | 25 (50.0) | 50 (47.6) | 20 (42.6) | 57 (55.9) | 50 (50.0) | |
| Girls | 202 (50.0) | 25 (50.0) | 55 (52.4) | 27 (57.4) | 45 (44.1) | 50 (50.0) | |
| Vaccinated | < 0.0001† | ||||||
| Yes | 225 (55.7) | 48 (96.0) | 74 (70.5) | 5 (10.6) | 38 (37.2) | 60 (60.0) | |
| No/Unknown | 179 (44.3) | 2 (4.0) | 31 (29.5) | 42 (89.4) | 64 (62.8) | 40 (40.0) | |
| Delay from DTwP vaccine (first administration, mean years) | 3.8 ± 2.3 | 4.1 ± 2.3 | 5.0 ± 2.6 | 2.7 ± 1.1 | 3.0 ± 1.7 | 3.0 ± 1.8 | 0.0001* |
| Mean HAZ | −0.6 ± 1.2 | −0.8 ± 1.0 | −0.5 ± 1.1 | −0.9 ± 1.3 | −0.8 ± 1.3 | −0.5 ± 1.2 | 0.0868* |
| Mean WAZ | −1.1 ± 1.0 | −1.4 ± 1.0 | −1.0 ± 1.0 | −1.3 ± 0.9 | −1.2 ± 1.1 | −0.8 ± 0.9 | 0.0031* |
| Birth season | 0.2086† | ||||||
| 1 | 207 (51.2) | 27 (54.0) | 54 (51.4) | 24 (51.1) | 50 (49.0) | 52 (52.0) | |
| 2 | 96 (23.8) | 12 (24.0) | 16 (15.2) | 15 (31.9) | 30 (29.4) | 23 (23.0) | |
| 3 | 101 (25.0) | 11 (22.0) | 35 (33.3) | 8 (17.0) | 22 (21.6) | 25 (25.0) | |
| IgG TT (mean units) | 1.03 ± 0.6 | 1.3 ± 0.6 | 1.1 ± 0.6 | 0.7 ± 0.5 | 1.1 ± 0.5 | 1.0 ± 0.6 | < 0.0001* |
| n | 369 | 45 | 93 | 45 | 93 | 93 | |
| Long-term protected (> 1 IU/mL, %) | 44.4 | 60.0 | 45.2 | 17.8 | 48.4 | 44.1 | 0.012† |
Difference between villages using the Kruskal–Wallis test. Bold indicates significant differences (P < 0.05).
Differences in the proportion between villages using χ2 test.
Antibody response to tetanus and diphtheria.
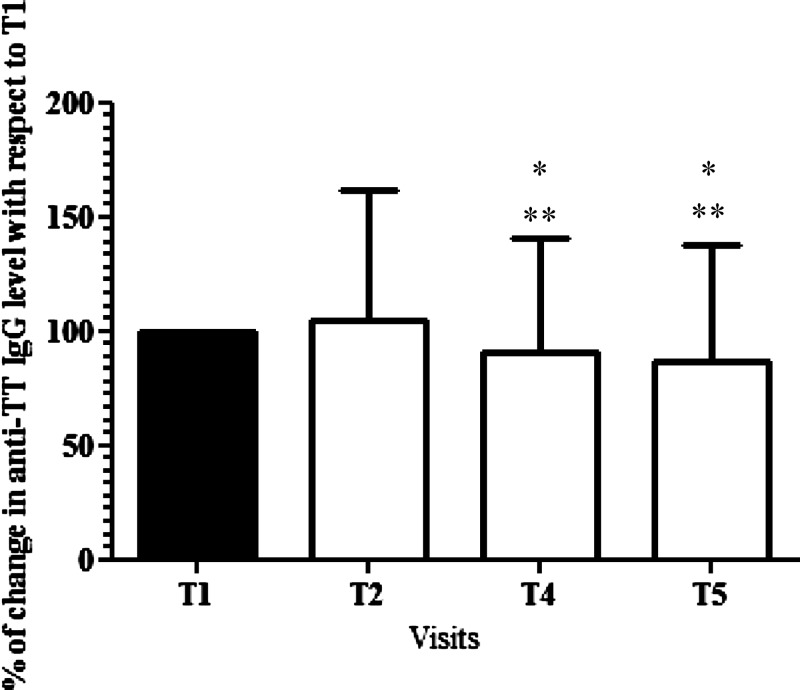
Anti-DT IgGs were predominantly dosed among vaccinated children at T1 and T5 (N = 177 present at both visits). Children immunized to DT significantly declined between T1 and T5: 76.3% of children were adequately immunized (≥ 0.1 IU/mL) at T1, and only 55.9% of children were adequately immunized at T5 (P < 0.0001). We also performed a longitudinal study to evaluate the tetanus-specific IgG response over time. We measured the level of specific antibodies on serological samples collected from 393, 369, 301, 326, and 331 children at T1–T5, respectively. The concentration of IgG to TT at T1 (data not shown) and T2 (Table 1) seemed variable between villages but achieved either long-term or adequate (data not shown) protective levels of antibodies to tetanus at each visit in all children. Evolution of IgG concentration from T1 to T5 was assessed. Children were selected on the basis of their presence at each visit. The proportion of serological change from specific IgG levels at T1 was considered as 100% and used to compare with other visits' anti-TT IgG levels. Figure 1 shows that antibody response to TT was similar at T1 and T2 but became significantly weaker at both T4 and T5. Although children still presented protective IgG levels to tetanus over time, our data indicate a progressive waning of immunity to tetanus from T2 to T5.
Figure 1.
Analysis of the proportion change in the anti-TT IgG level from T1 to T5. We analyzed the percentage of change compared with T1 of anti-TT IgG at T2, T4, and T5 visits. We considered only children present at each visit (N = 284). Some children were not monitored in one village at T3 (school children from the village of Pendao who did not come to the visit), and therefore, we have excluded this period from this analysis to avoid bias. Histograms represent mean percentage and SD at each visit. *Significant difference with T1 (P < 0.0001) using the repeated measures ANOVA test followed by Bonferroni's multiple comparison test. **Significant difference with T2 (P < 0.0001) using the repeated measures ANOVA test followed by Bonferroni's multiple comparison test.
Influence of the nutritional status on humoral immunity to tetanus.
A longitudinal multivariate linear regression analysis investigated whether childhood malnutrition can impact humoral immunity in response to DTwP vaccination (Table 2). To study variations in the level of anti-TT IgG, we considered only vaccinated children having received at least one injection of DTwP as asserted on vaccination document presentation or mother's oral communication. We used WAZ and HAZ as nutritional variables, and the association between antibody levels and nutritional status was adjusted for potential confounders, including age at T2, time span since the first tetanus dose, time of visits, village (Guede was not included, because information about vaccination status was provided only for a few children), sex, and birth season. Variables that remained significant are highlighted in Table 2. Although we noted the lack of an age-dependent waning in our model, a markedly linear negative association with time of visits confirmed the progressive decrease in concentration of anti-TT IgG over time. The significant interaction between village and time of visits indicates that waning of antibody levels to TT over time was different according to villages. This multivariate analysis did not support a correlation between either HAZ or WAZ and WHZ (the latter was included in a linear regression model taking into consideration only children < 5 years; data not shown) and the rate of the specific antibody waning over time. Conversely, when children displayed a WAZ ≤ −1, a linear association was found between the amount of IgG to TT and WAZ, but it varied negatively or positively according to villages. A significant positive interaction was also determined between season of birth and children with a WAZ ≥ −1. This observation emphasizes that, when children were born in the same villages and at the same visit, the antibody response variation to TT was related to birth seasons 2 and 3 and positively associated with WAZ. Moreover, we did not find any relation between the nutritional status and the level of anti-DT antibodies in children (data not shown). Overall, our results illustrate that time of visit, village, and WAZ among the population born during or just after the rainy season were able to influence immune response to tetanus in vaccinated children.
Table 2.
Linear regression multivariate analysis of prediction for log(anti-TT IgG) for vaccinated children
| Predictor variables | β-coefficient | P |
|---|---|---|
| Visit | ||
| 2 | Reference | |
| 3 | −0.132 | |
| 4 | −0.304 | |
| 5 | −0.410 | |
| Village | < 0.0001 | |
| A | Reference | |
| F | −0.467 | |
| N | 0.228 | |
| P | −0.281 | |
| Birth season | 0.002 | |
| 1 | Reference | |
| 2 | −0.077 | |
| 3 | 0.092 | |
| WAZ* | 0.35 | |
| ≤ −1 | −0.075 | |
| ≥ −1 | −0.081 | |
| Interaction village × passage | 0.008 | |
| Fanaye, visit 3 | 0.019 | 0.10 |
| Fanaye, visit 4 | 0.116 | |
| Fanaye, visit 5 | 0.196 | |
| Niandane, visit 3 | −0.034 | |
| Niandane, visit 4 | 0.042 | |
| Niandane, visit 5 | 0.162 | |
| Pendao, visit 3 | −0.085 | |
| Pendao, visit 4 | 0.154 | |
| Pendao, visit 5 | 0.188 | |
| Interaction village* WAZ ≤ −1 | 0.001 | |
| Fanaye | −0.200 | |
| Niandane | 0.164 | |
| Pendao | −0.000 | |
| Interaction birth season × WAZ ≥ −1 | 0.0002 | |
| 2 | 0.326 | |
| 3 | 0.141 | |
| 0.001 | ||
We used WAZ as a continuous variable representative of the nutritional status. The cutoff value of −1 was not set a priori but came as a result of the analysis: the model shows that there are different tendencies observed for WAZ ≤ −1 and ≥ −1. Bold indicates significant differences (P < 0.05).
Cytokine response to bacterial antigens.
We investigated the ability of whole-blood cells from a subcohort of vaccinated children to produce cytokines on stimulation for 6 days with vaccine antigens or 2 days with PHA as a positive control (Tables 3 and 4). The average age of recruited children was 3.5 ± 1.2 years and ranged from 1.7 to 6.3 years. Most of children produced IFN-γ and to a lesser extent, IL-10 in response to PHA, TT, and PPD, but cytokine amounts were hardly detectable after stimulation with DT (data not shown). Interestingly, there was a marked relationship between the level of antibody and IFN-γ–specific response to TT (Table 3). We also observed a strong correlation in the magnitude of IFN-γ production between each bacterial antigen and PHA that likely reveals an individual host ability to produce IFN-γ (Table 3).
Table 3.
Analysis of correlation between the ability to produce IFN-γ in response to different antigens and the level of TT-specific antibodies
| Coefficient of correlation (R) | PHA | PPD | TT | DT |
|---|---|---|---|---|
| IgG TT | 0.0542 | 0.2124 | 0.4964* | 0.1370 |
| PHA | 0.2678† | 0.3313† | 0.1408 | |
| PPD | 0.3834† | 0.2445† | ||
| TT | 0.3249† |
P < 0.001 using the non-parametric Spearman's rank correlation test.
P < 0.05 using the non-parametric Spearman's rank correlation test.
Table 4.
Subcohort characteristics and cytokine production in June and July of 2009
| Total number of children* | Stunted (n) | Not stunted (n) | P† | |
|---|---|---|---|---|
| Age | 74 | 3.1 ± 1.1‡ (9) | 3.6 ± 1.3 (53) | 0.2674 |
| Sex | ||||
| Male | 39 | nd | −0.6 ± 0.9 (28) | |
| Female | 35 | nd | −0.9 ± 0.6 (25) | |
| BCG and first dose of DTwP vaccine | ||||
| Yes | 72 | nd | nd | |
| Unknown | 2 | nd | nd | |
| Nutritional status at T3 (May of 2009) | < 0.0001 | |||
| WAZ | 64 | −2.4 ± 0.6 (9) | −1.1 ± 0.8 (53) | |
| HAZ | 62 | −2.6 ± 0.5 (9) | −0.7 ± 0.8 (53) | |
| Anti-TT IgG at T3 | 61 | 0.9 ± 0.5 (9) | 1.2 ± 0.6 (49) | 0.2209 |
| Leukocyte number (Giga/L) | 70 | 9.9 ± 1.1 (7) | 9.1 ± 2.9 (51) | 0.1891 |
| IFN-γ (pg/mL) | ||||
| PHA | 72 | 66.7 ± 96.0 (8) | 144.3 ± 157.0 (52) | 0.2716 |
| PPD | 74 | 584.8 ± 540.0 (9) | 979.7 ± 736.1 (53) | 0.1678 |
| TT | 73 | 20.2 ± 32.5 (9) | 156.0 ± 149.5 (53) | 0.0017 |
| DT | 72 | 6.8 ± 17.8 (8) | 29.7 ± 71.0 (53) | 0.1276 |
| IL-10 (pg/mL) | ||||
| PHA | 67 | 194.8 ± 199.0 (8) | 198.0 ± 245.9 (46) | 0.5838 |
| PPD | 68 | 55.7 ± 56.7 (9) | 52.1 ± 78.7 (46) | 0.6651 |
| TT | 68 | 25.9 ± 57.9 (9) | 26.2 ± 97.8 (46) | 0.9524 |
| DT | 68 | 27.6 ± 75.3 (9) | 7.2 ± 14.9 (46) | 0.8759 |
nd = not determined.
Because we lack complete information about the nutritional status measured at T3 for some children, we observed a discrepancy between the number of samples stimulated with PHA or vaccine antigens and the total number of children with data for HAZ.
P < 0.05 value of the Mann–Whitney U test between stunted and not stunted children. Bold indicates the significant difference.
Data are represented as mean ± SD.
Stunting is correlated with the ability to produce IFN-γ in response to TT.
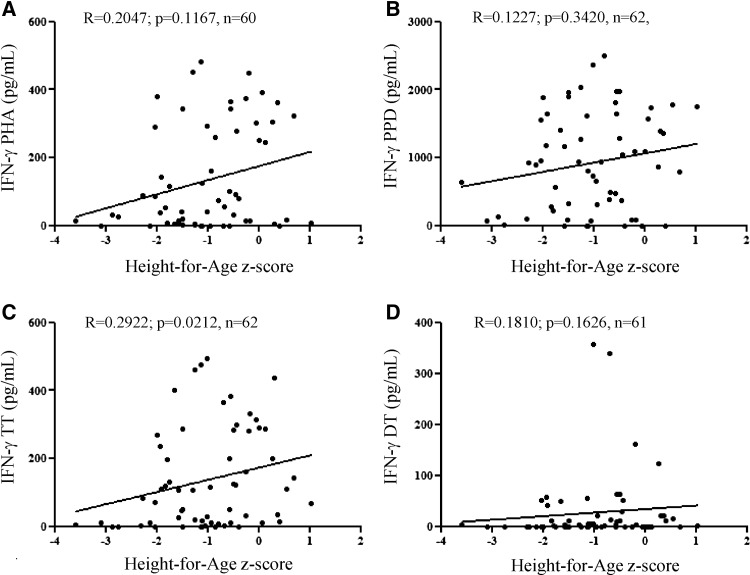
We next explored the magnitude of IFN-γ and IL-10 response according to the nutritional status measured at T3 (N = 62; information about stunting was not available for all children who had blood taken) near the period of whole-blood collection. General information on this subcohort in relation with stunting is indicated in Table 4. A weaker level of IFN-γ production after stimulation with TT was detected among stunted children compared with well-nourished children (P = 0.0017) (Table 4). IFN-γ response to other antigens also tended to be reduced in stunted children, but the difference with not stunted children did not reach statistical significance. These modulations could not be explained by a different leukocyte number, because the count was similar between not stunted and stunted children (Table 4). Moreover, there were no differences in IL-10 response to any antigen between both groups of children. To confirm a negative association between stunting and cytokine production to TT, we further examined the correlation between the degrees of IFN-γ or IL-10 production and children's HAZ scores (Figure 2). Unlike IL-10 (data not shown), the ability of whole-blood cells to release IFN-γ after stimulation with TT was positively correlated with HAZ (P = 0.0212). Our data also suggest a trend to a linear correlation between the ability to produce IFN-γ to PHA and HAZ. The lack of significant correlation could be because of a great heterogeneity among responders (Figure 2). We can, for example, distinguish high (IFN-γ > 200 pg/mL) from low (IFN-γ < 200 pg/mL) responders to PHA (Figure 2A). Children that displayed a higher response to PHA also had a greater ability to produce IFN-γ on stimulation with PPD and TT (data not shown). This group of children was significantly older (4.1 ± 1.3 years, P = 0.0309) and had an increased mean HAZ (−0.5 ± 0.8, P = 0.0120) than the group with a low response to antigens (3.3 ± 1.1 years and −1.2 ± 1.0, respectively). However, no linear association between IFN-γ production and HAZ was found inside either group. Moreover, there was no correlation between WAZ and the intensity of IFN-γ and IL-10 response to antigens. Therefore, these results show that stunting, but not underweight, is specifically associated with the ability to produce IFN-γ in response to TT.
Figure 2.
Correlations between IFN-γ production and HAZ in response to PHA or bacterial antigens. IFN-γ production was measured after whole-blood cell stimulation for (A) 48 hours with PHA or 6 days with (B) PPD, (C) TT, or (D) DT. The coefficient of correlation and the P values are indicated. The Spearman's rank correlation coefficient was used to assess correlation. P < 0.05 means a significant correlation.
Discussion
The main objective of this study was to evaluate whether immunity to vaccine-preventable diseases was modulated according to malnutrition. As a whole, our current analysis identifies WAZ as a modulating factor of antibody responses to TT according to children's birth season. However, cellular immunity to TT was compromised in chronically malnourished children, whereas the overall ability to produce the Th1-type cytokine IFN-γ was not affected by malnutrition.
TT, DT, and PPD were chosen as microbial antigens, because they are good indicators of immunity generated by vaccination. Our follow-up clearly shows a decrease over time of the levels of both anti-TT and anti-DT antibodies. This finding suggests an absence of natural challenge to the etiological agents of tetanus and diphtheria, and it also suggests that immunity to TT and DT antigens was merely dependent of the time elapsed since last DTwP vaccine challenge.17 An efficient cellular immunity was also intended in response to TT, DT, and PPD.17,18 In our study, all children produced IFN-γ in response to PPD. Most of the children were vaccinated with BCG at birth, but background exposure to environmental mycobacteria could also be involved in the persistence of cellular immunity and the amount of in vitro IFN-γ production in response to PPD.19 In the present study, a clinical examination before blood collection did not reveal any signs of active tuberculosis in any of the children; however, latent tuberculosis could also influence individual immunity to PPD. The absence of positive response to PHA observed in some African children (data not shown) has been previously described in Malawi and The Gambia, but it seems to be less evident in industrialized countries.4,18 This finding might highlight the role of the environment in children's ability to produce INF-γ.
Environmental factors have, indeed, been suggested as being related to antibody production to TT in early life.5 Herein, the linear regression analysis underlines differences in IgG response to TT according to villages and times of visits. These observations emphasize that geographical and environmental factors were plausible causal factors at the origin of individual variations of humoral immunity generated by tetanus vaccine. In northern Senegal, differences in ethnicity, access to drinking water, access to healthcare, and exposure to infectious risks are many potential causes of immune changes. Interactions between village and WAZ ≤ −1 also show that underweight was an important immunomodulatory factor. Among possible risk factors, stunting and underweight have been previously shown to negatively affect the mean level of antibody titers to TT in Ecuadorian children younger than 5 years of age.20 Herein, as in other studies,21,22 we did not provide evidence that the level of antibodies to TT and DT was impaired by stunting in children. However, our analysis highlights a close interaction among well-nourished children (WAZ ≥ −1) between WAZ and level of IgG against TT for children born during and after the rainy seasons. In Senegal, the wet season between July and the beginning of October coincides with the lean season because of the low availability of food, and it could be characterized by a loss of weight in women and fetal growth retardation.23,24 Being born during the rainy season also leads to variations in the duration of breastfeeding.25 The role of hunger season during fetal life or early infancy on children immunity later in life has been the focus of other research works: numerous findings have hypothesized that critical periods of nutritional starvation in early life influence the maturation of immunity or response to vaccination.3,13,14,26 For instance, the work by Moore and others14 has described that adults born with a low birth weight displayed a reduced antibody response to a polysaccharide vaccine, but this result remains debated for other vaccines.27 However, the season of crops and harvesting after the rainy seasons could favor a greater nutritional and immune development of newborns. Indeed, to understand whether nutritional deficiencies early in life could influence the long-lasting acquired immune response to tetanus related to individual's weight in our current study, it would have been interesting to consider birth weight among children as a possible predictive modulating factor of immunity against tetanus in rural Senegal.
Other than conflicting findings, it is also intriguing that the influence of stunting and underweight on cellular immunity was poorly explored. A report has documented a negative association between stunting and IFN-γ production to Amoeba antigens.8 Previous studies suggest that malnourished children have an impaired cellular immunity to tuberculosis10,28,29 and that vitamin A deficiency depressed immunity to TT.30 Our survey emphasizes, for the first time, that stunting (but not underweight) may determine the long-term persistence of cell-mediated immunity to T-dependent antigens, such as TT, in the absence of a natural boost, which was likely not the case for immunity to PPD. The production of the regulatory cytokine IL-10 was conversely not affected, suggesting that the regulatory network was not directly under nutritional status influence. In addition, no general cellular immune deficiency was measured in the present analysis, because the cytokine response to PHA and PPD was maintained in both stunted and underweight children. These general inconsistencies regarding vaccine antigens are in accordance with a lack of consensus about a clear cut modulating effect of a nutritional deficit or supplementation with nutrients on vaccine immunity.10,22
Discrepancies between our work and the works of others could also be explained by the absence of severe malnutrition in our cohort or the difference in the age range of previous studies' populations. A different use of nutritional definitions might also explain such disagreements between studies. Indeed, we have generated Z scores with the WHO child growth standards, the most up-to-date anthropometric reference tool, instead of the previously recommended National Center for Health Statistics/WHO international growth reference.31 The effect of stunting on children's immunity may also be magnified by other risk factors, such as parasite infections, that could play a predominant role in modulating the development of immunity to vaccine antigens.11 We are confident that malaria did not play a major role in children's immunity to vaccines, because malaria prevalence in this region of northern Senegal at the time of the study was very low.32 Moreover, immune responses to TT vaccine were reported to be robust in malaria-infected African children.21 However, we cannot rule out the possibility that the burden of Schistosoma haematobium, the most prevalent helminthic infection in the studied villages, could play a role as a modulator of IFN-γ production.33
In conclusion, our study provides little evidence that underweight or stunting can influence the rate of anti-TT IgG waning over time. However, weight seems to be closely and positively related to an established IgG response against tetanus. In addition, we have shown that stunting can alter cellular immune memory. However, this effect was not consistent across vaccine antigens and seems to be dependent on a weak frequency of natural boosters, which occurs with tetanus. Whether immune correlates of protection to infections were affected by poor maternal nutritional status or nutritional deficiencies early in infancy remains unknown, but the implication of these factors should be considered when studying vaccine effectiveness.
ACKNOWLEDGMENTS
We thank the children, families, and healthcare staff in the visited villages for their kind collaboration as well as Simon Senghor, Daba Diagne, Charles Bouganali, and Sidi Ndongue (deceased) for their devoted work in the field, Mathieu Cassin for his assistance with the building of the database, and Nicholas Philipson for his help with the experiments along with whole Centre de Recherche Biomédicale Espoir Pour la Santé team. We also thank Emily C. Griffiths for critical review of the manuscript.
Footnotes
Financial support: L.G. received support from the Region Nord-Pas de Calais and the Pasteur Institute of Lille. Centre de Recherche Biomédicale Espoir Pour la Santé supported field investigation, and the Center for Infection and Immunity of Lille provided support for the achievement of the immunological assays.
Authors' addresses: Lobna Gaayeb and Gilles Riveau, Center for Infection and Immunity of Lille (CIIL)—U1019 Inserm, UMR8204 CNRS, Université Lille Nord de France, Institut Pasteur de Lille, Lille, France and Centre de Recherche Biomédicale Espoir Pour la Santé (EPLS), Saint-Louis, Senegal, E-mails: lobna.gaayeb@gmail.com and gilles.riveau@gmail.com. Jean B. Sarr, Modou Seck, and Andre B. Sagna, Centre de Recherche Biomédicale Espoir Pour la Santé (EPLS), Saint-Louis, Senegal, E-mails: sarrjeanbirame@yahoo.fr, modou.seck@espoir-sante.org, and sagna.ab@gmail.com. Cécile Cames, Institut de Recherche pour le Développement UMI233 TransVIHmi, Centre Régional de Recherche et de Formation à la prise en charge Clinique, Centre Hospitalier Universitaire de Fann, Dakar, Senegal, E-mail: Cecile.Cames@ird.fr. Claire Pincon, Department of Biostatistics, Faculté de Pharmacie, Université Lille Nord de France, Lille, France, E-mail: claire.pincon@univ-lille2.fr. Jean-Baptiste Hanon, Fabien Herbert, Anne-Marie Schacht, and Emmanuel Hermann, Center for Infection and Immunity of Lille (CIIL)—U1019 Inserm, UMR8204 CNRS, Université Lille Nord de France, Institut Pasteur de Lille, Lille, France, E-mails: jb.hanon@yahoo.fr, fabien.herbert@pasteur-lille.fr, am.schacht@gmail.com, and emmanuel.hermann@univ-lille2.fr. Mamadou O. Ndiath, UMR198 URMITE, Campus International, Institut de Recherche pour le Développement, UCAD, Institut de Recherche pour le Développement Hann, Dakar, Senegal, E-mail: ousmane.ndiatt@ird.fr. Franck Remoue, UMR MIVEGEC, Institut de Recherche pour le Développement 224, CNRS5290, Université de Montpellier 1 et 2, Institut de Recherche pour le Développement, Centre de Recherche Entomologique de Cotonou (CREC), Cotonou, Benin, E-mail: franck.remoue@ird.fr.
References
- 1.Deming MS, Linkins RW, Jaiteh KO, Hull HF. The clinical efficacy of trivalent oral polio vaccine in The Gambia by season of vaccine administration. J Infect Dis. 1997;175((Suppl 1)):S254–S257. doi: 10.1093/infdis/175.supplement_1.s254. [DOI] [PubMed] [Google Scholar]
- 2.Moore SE, Collinson AC, Fulford AJ, Jalil F, Siegrist CA, Goldblatt D, Hanson LA, Prentice AM. Effect of month of vaccine administration on antibody responses in The Gambia and Pakistan. Trop Med Int Health. 2006;11:1529–1541. doi: 10.1111/j.1365-3156.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 3.Miles DJ, van der Sande M, Crozier S, Ojuola O, Palmero MS, Sanneh M, Touray ES, Rowland-Jones S, Whittle H, Ota M, Marchant A. Effects of antenatal and postnatal environments on CD4 T-cell responses to Mycobacterium bovis BCG in healthy infants in the Gambia. Clin Vaccine Immunol. 2008;15:995–1002. doi: 10.1128/CVI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, Mvula H, Newport MJ, Branson K, McGrath N, Crampin AC, Fine PE, Dockrell HM. Population differences in immune responses to Bacille Calmette-Guerin vaccination in infancy. J Infect Dis. 2009;199:795–800. doi: 10.1086/597069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchant A, Pihlgren M, Goetghebuer T, Weiss HA, Ota MO, Schlegel-Hauter SE, Whittle H, Lambert PH, Newport MJ, Siegrist CA. Predominant influence of environmental determinants on the persistence and avidity maturation of antibody responses to vaccines in infants. J Infect Dis. 2006;193:1598–1605. doi: 10.1086/503775. [DOI] [PubMed] [Google Scholar]
- 6.Collinson AC, Ngom PT, Moore SE, Morgan G, Prentice AM. Birth season and environmental influences on blood leucocyte and lymphocyte subpopulations in rural Gambian infants. BMC Immunol. 2008;9:18. doi: 10.1186/1471-2172-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque R, Mondal D, Shu J, Roy S, Kabir M, Davis AN, Duggal P, Petri WA., Jr Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg. 2007;76:340–344. [PubMed] [Google Scholar]
- 9.Fillol F, Sarr JB, Boulanger D, Cisse B, Sokhna C, Riveau G, Simondon KB, Remoue F. Impact of child malnutrition on the specific anti-Plasmodium falciparum antibody response. Malar J. 2009;8:116. doi: 10.1186/1475-2875-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savy M, Edmond K, Fine PE, Hall A, Hennig BJ, Moore SE, Mulholland K, Schaible U, Prentice AM. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139:2154S–2218S. doi: 10.3945/jn.109.105312. [DOI] [PubMed] [Google Scholar]
- 11.Borkow G, Bentwich Z. Chronic parasite infections cause immune changes that could affect successful vaccination. Trends Parasitol. 2008;24:243–245. doi: 10.1016/j.pt.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Dauby N, Alonso-Vega C, Suarez E, Flores A, Hermann E, Cordova M, Tellez T, Torrico F, Truyens C, Carlier Y. Maternal infection with Trypanosoma cruzi and congenital Chagas disease induce a trend to a type 1 polarization of infant immune responses to vaccines. PLoS Negl Trop Dis. 2009;3:e571. doi: 10.1371/journal.pntd.0000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr. 2001;74:543–548. doi: 10.1093/ajcn/74.4.543. [DOI] [PubMed] [Google Scholar]
- 14.Moore SE, Jalil F, Ashraf R, Szu SC, Prentice AM, Hanson LA. Birth weight predicts response to vaccination in adults born in an urban slum in Lahore, Pakistan. Am J Clin Nutr. 2004;80:453–459. doi: 10.1093/ajcn/80.2.453. [DOI] [PubMed] [Google Scholar]
- 15.Gaayeb L, Sarr JB, Ndiath MO, Hanon JB, Debrie AS, Seck M, Schacht AM, Remoue F, Hermann E, Riveau G. Seroprevalence of pertussis in Senegal: a prospective study. PLoS One. 2012;7:e48684. doi: 10.1371/journal.pone.0048684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO . Physical Status: The Use and Interpretation of Anthropometry. WHO Technical Report Series. WHO Technical Report Series 854. Geneva: World Health Organization; 1995. pp. 1–452. [PubMed] [Google Scholar]
- 17.Viana PO, Ono E, Miyamoto M, Salomao R, Costa-Carvalho BT, Weckx LY, de Moraes-Pinto MI. Humoral and cellular immune responses to measles and tetanus: the importance of elapsed time since last exposure and the nature of the antigen. J Clin Immunol. 2010;30:574–582. doi: 10.1007/s10875-010-9420-7. [DOI] [PubMed] [Google Scholar]
- 18.Finan C, Ota MO, Marchant A, Newport MJ. Natural variation in immune responses to neonatal Mycobacterium bovis Bacillus Calmette-Guerin (BCG) vaccination in a cohort of Gambian infants. PLoS One. 2008;3:e3485. doi: 10.1371/journal.pone.0003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black GF, Dockrell HM, Crampin AC, Floyd S, Weir RE, Bliss L, Sichali L, Mwaungulu L, Kanyongoloka H, Ngwira B, Warndorff DK, Fine PE. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J Infect Dis. 2001;184:322–329. doi: 10.1086/322042. [DOI] [PubMed] [Google Scholar]
- 20.Brussow H, Sidoti J, Dirren H, Freire WB. Effect of malnutrition in Ecuadorian children on titers of serum antibodies to various microbial antigens. Clin Diagn Lab Immunol. 1995;2:62–68. doi: 10.1128/cdli.2.1.62-68.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monjour L, Bourdillon F, Korinek AM, Aubonnet-Laignel A, Brousse G, Gentilini M, Bayard P, Ballet JJ. Humoral immunity, 5 years after anti-tetanus vaccination, in a group of malaria-infected and malnourished African children. Pathol Biol (Paris) 1988;36:235–239. [PubMed] [Google Scholar]
- 22.Siekmann JH, Allen LH, Watnik MR, Nestel P, Neumann CG, Shoenfeld Y, Peter JB, Patnik M, Ansari AA, Coppel RL, Gershwin ME. Titers of antibody to common pathogens: relation to food-based interventions in rural Kenyan schoolchildren. Am J Clin Nutr. 2003;77:242–249. doi: 10.1093/ajcn/77.1.242. [DOI] [PubMed] [Google Scholar]
- 23.Simondon KB, Ndiaye T, Dia M, Yam A, Ndiaye M, Marra A, Diallo A, Simondon F. Seasonal variations and trends in weight and arm circumference of non-pregnant rural Senegalese women, 1990–1997. Eur J Clin Nutr. 2008;62:997–1004. doi: 10.1038/sj.ejcn.1602807. [DOI] [PubMed] [Google Scholar]
- 24.Ceesay SM, Prentice AM, Cole TJ, Foord F, Weaver LT, Poskitt EM, Whitehead RG. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. BMJ. 1997;315:786–790. doi: 10.1136/bmj.315.7111.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simondon KB, Simondon F. Mothers prolong breastfeeding of undernourished children in rural Senegal. Int J Epidemiol. 1998;27:490–494. doi: 10.1093/ije/27.3.490. [DOI] [PubMed] [Google Scholar]
- 26.Moore SE, Prentice AM, Wagatsuma Y, Fulford AJ, Collinson AC, Raqib R, Vahter M, Persson LA, Arifeen SE. Early-life nutritional and environmental determinants of thymic size in infants born in rural Bangladesh. Acta Paediatr. 2009;98:1168–1175. doi: 10.1111/j.1651-2227.2009.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore SE, Richards AA, Goldblatt D, Ashton L, Szu SC, Prentice AM. Early-life and contemporaneous nutritional and environmental predictors of antibody response to vaccination in young Gambian adults. Vaccine. 2012;30:4842–4848. doi: 10.1016/j.vaccine.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas TA, Mondal D, Noor Z, Liu L, Alam M, Haque R, Banu S, Sun H, Peterson KM. Malnutrition and helminth infection affect performance of an interferon gamma-release assay. Pediatrics. 2010;126:e1522–e1529. doi: 10.1542/peds.2010-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray DN, Loomis SA, Casazza LJ, Rey H, Miranda R. Development of impaired cell-mediated immunity in mild and moderate malnutrition. Am J Clin Nutr. 1981;34:68–77. doi: 10.1093/ajcn/34.1.68. [DOI] [PubMed] [Google Scholar]
- 30.Semba RD, Muhilal SAL, Natadisastra G, Wirasasmita S, Mele L, Ridwan E, West KP, Jr, Sommer A. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr. 1992;122:101–107. doi: 10.1093/jn/122.1.101. [DOI] [PubMed] [Google Scholar]
- 31.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ndiath MO, Sarr JB, Gaayeb L, Mazenot C, Sougoufara S, Konate L, Remoue F, Hermann E, Trape JF, Riveau G, Sokhna C. Low and seasonal malaria transmission in the middle Senegal River basin: identification and characteristics of Anopheles vectors. Parasit Vectors. 2012;5:21. doi: 10.1186/1756-3305-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diallo TO, Remoue F, Gaayeb L, Schacht AM, Charrier N, De Clerck D, Dompnier JP, Pillet S, Garraud O, N'Diaye AA, Riveau G. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS One. 2010;5:e12764. doi: 10.1371/journal.pone.0012764. [DOI] [PMC free article] [PubMed] [Google Scholar]