Abstract
Envenomation by poisonous animals is a neglected condition according to the World Health Organization (WHO). Antivenoms are included in the WHO Essential Medicines List. It has been assumed that immunoglobulin G (IgG) antivenoms could activate the complement system through Fc and induce early adverse reactions (EARs). However, data in the literature indicate that F(ab')2 fragments can also activate the complement system. Herein, we show that several batches of IgG and F(ab')2 antivenoms from the Butantan, Vital Brazil, and Clodomiro Picado Institutes activated the complement classical pathway and induced the production of C3a; however, only those antivenoms from Clodomiro Picado generated C5a. Different protein profiles (IgG heavy chain, protein contaminants, and aggregates) were observed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses. Our results show that various antivenoms from different producers are able to activate the classical pathway of the complement system and generate anaphylatoxins, and these findings suggest that factors, such as composition, contaminant proteins, and aggregates, may influence the anticomplementary activity of antivenoms in vitro. Therefore, there is a need to further improve antivenom production methods to reduce their anticomplementary activity and potential to cause EARs.
Introduction
Envenomation is a relevant public health issue1 that has been included, since 2009, in the list of neglected tropical diseases and conditions of the World Health Organization (WHO).2 The only available treatment of animal envenomation is the parenteral administration of specific antivenom; therefore, animal-derived antivenoms are included on the WHO Essential Medicines List.3 Antivenoms are obtained from the plasma of immunized animals, usually horses or sheep, and may consist of whole immunoglobulin G (IgG) molecules (150 kDa) or F(ab')2 (100 kDa) or F(ab) (50 kDa) fragments; the latter two are devoid of Fc portion and obtained by pepsin and papain digestion, respectively.4,5 Several fractionation methods have been used to purify IgG or its fragments, including salting out with ammonium sulphate, which precipitates Igs, and fractionation with caprylic acid, which precipitates non-IgG proteins from plasma.4,6
The option to produce F(ab')2 or Fab antivenoms is largely based on the hypothesis that early adverse reactions (EARs), observed in a number of patients submitted to the antivenom therapy, could be caused by the generation of anaphylatoxins because of complement activation through the Fc portion of IgG. These reactions range from mild and moderate (characterized by tachycardia, shivering, chills, fever, vomit, and urticaria) to severe anaphylaxis (with bronchospasm, dyspnea, hypotension, and hypoxia) and can result in death.7,8
However, various studies have shown that both types of antivenoms can activate the complement system in vitro9,10 and induce EARs in patients.11,12 Some data highlight the impact of the fractionation protocol on the induction of EARs. It was shown that IgG antivenom precipitated by ammonium sulphate had more aggregates and induced a higher incidence of EARs than IgG antivenom precipitated by caprylic acid.13 Furthermore, depending on the concentration of caprylic acid used in the fractionation step, high-molecular mass protein aggregates also can be formed in IgG preparations.6
It was shown that horse and sheep IgG antivenoms induced higher complement activation and were more immunogenic than camel IgG antivenom.14 There is also a general consensus that adverse effects are directly related to the amount of infused heterologous proteins,15 although it was shown that homologous proteins (human IgG) also activate the complement system.16 The addition of thimerosal and/or phenol to IgG antivenoms increased the formation of dimeric and polymeric IgGs and induced higher complement activation compared with antivenoms formulated without preservatives.17 All of these data indicate the lack of consensus in the literature regarding which types of antivenom induce fewer EARs and in particular, which mechanisms are involved in the genesis of these reactions, including the role of the complement system.
The complement system can initially be activated by three pathways (alternative, classical, and lectin),18 and during its activation, cleavage of the C3, C4, and C5 components results in the generation of anaphylatoxins (C3a, C4a, and C5a). Anaphylatoxins play an important role in the inflammatory response, inducing (1) mast cell degranulation, which releases vasoactive mediators such as histamine; (2) neutrophil and monocyte chemoattraction to inflammatory sites; (3) oxidative burst stimulation; and (4) proinflammatory cytokine release.18 The massive generation of anaphylatoxins can induce non-IgE–dependent anaphylaxis.
Herein, we aimed to evaluate the anticomplementary activity of horse IgG and F(ab')2 antivenoms produced by Butantan, Vital Brazil, and Clodomiro Picado Institutes and correlate the complement activation with some important characteristics of the antivenoms, such as protein content, presence of Fc portion, and amount of protein aggregates.
Materials and Methods
Reagents and buffers.
Mannan, bovine serum albumin (BSA), orthophenylenediamine (OPD), ethylene diamine tetracetic acid (EDTA), ethylene glycol bis-(β-aminoethyl ether)-N,N,N,N'-tetracetic acid (EGTA), and rabbit anti-horse IgG labeled with alkaline phosphatase were purchased from Sigma (St. Louis, MO). Tween 20 was from Labsynth (Diadema, SP, Brazil). Goat IgG anti-human C4 was from Quidel Corporation (San Diego, CA). Rabbit anti-goat IgGs labeled with horseradish peroxidase, nitroblue tetrazolium (NBT), and 5-bromo-4-chloro- 3-indolyl-phosphate (BCIP) were from Promega Corporation (Madison, WI). The following buffers were used: alsever old solution (114 mM sodium citrate, 27 mM d-glucose, 72 mM sodium chloride, pH 6.1); phosphate buffered saline (PBS; 8.1 mM disodium phosphate, 1.5 mM potassium phosphate, 137 mM sodium chloride, 2.7 mM potassium chloride, pH 7.4); veronal buffered saline (VBS++; 2.8 mM barbituric acid, 145.5 mM sodium chloride, 0.8 mM magnesium chloride, 0.3 mM calcium chloride, 0.9 mM Na-barbital, pH 7.2); VBS containing 0.1% BSA (BVB++); and alternative pathway buffer (APB; 150 mM sodium chloride, 7 mM magnesium chloride, 5 mM Na-barbital, 10 mM EGTA, pH 7.4).
Horse IgG and F(ab')2 antivenoms.
Commercial horse antivenoms, produced against the venoms from different animal genera were obtained from the Butantan (São Paulo, Brazil), Vital Brazil (Niterói, Brazil), and Clodomiro Picado (San José, Costa Rica) Institutes, totaling 32 batches (64 vials) produced between 2007 and 2010 (Table 1). Antivenoms from the Butantan and Vital Brazil Institutes consisted of F(ab')2 fragments obtained by pepsin digestion and ammonium sulphate precipitation,19 whereas antivenoms from the Clodomiro Picado Institute contained whole IgG purified by caprylic acid precipitation.20 Antivenoms passed routine quality control tests in manufacturing laboratories and were used within their shelf life.
Table 1.
Samples of antivenoms from Butantan, Vital Brazil, and Clodomiro Picado Institutes
| Antivenom/institute/batch | Fabrication | Additional information |
|---|---|---|
| Anti-Bot | ||
| Butantan | ||
| 0707138/D | 06/2007 | 1 mL neutralizes 5 mg Bothrops jararaca venom |
| 0805063/D | 04/2008 | |
| 0901004/B | 01/2009 | |
| Vital Brazil | ||
| 095103 G | 11/2009 | |
| Anti-Bot–Crot | ||
| Butantan | ||
| 0706120 | 05/2007 | 1 mL neutralizes 5 mg B. jararaca venom and 1.5 mg Crotalus durissus terrificus venom |
| 0905084/A | 04/2009 | |
| Vital Brazil | ||
| 095402 A | 12/2009 | |
| Anti-Bot–Lac | ||
| Butantan | ||
| 0705098/A | 04/2007 | 1 mL neutralizes 5 mg B. jararaca venom and 3 mg Lachesis muta venom |
| 0806068/B | 05/2008 | |
| 0906133 | 06/2009 | |
| Vital Brazil | ||
| 095903 A | 01/2010 | |
| Anti-Crot | ||
| Butantan | ||
| 0710211/B | 09/2007 | 1 mL neutralizes 1.5 mg C. durissus terrificus venom |
| 0810144/B | 09/2008 | |
| 0908162/B | 07/2009 | |
| Vital Brazil | ||
| 095208 A | 11/2009 | |
| Anti-Elap BR | ||
| Butantan | ||
| 0706111/B | 05/2007 | 1 mL neutralizes 1.5 mg Micrurus frontalis venom |
| 0810141 | 09/2008 | |
| 1002031/A | 12/2009 | |
| Anti-Arac | ||
| Butantan | ||
| 0706121 | 05/2007 | 1 mL neutralizes 1.5 MLD Tityus serrulatus venom, 1.5 MLD Phoneutria nigriventer venom, and 15 MND Loxosceles gaucho venom |
| 0806074/A | 05/2008 | |
| 0905100/A | 04/2009 | |
| Anti-Scorp | ||
| Butantan | ||
| 0707135/C | 06/2007 | 1 mL neutralizes 1.5 MLD T. serrulatus venom |
| 0804038/A | 02/2008 | |
| 0905104/A | 04/2009 | |
| Anti-Lon | ||
| Butantan | ||
| 0801006 | 12/2007 | 1 mL neutralizes 0.35 mg Lonomia obliqua venom |
| 0806071 | 05/2008 | |
| 0906113 | 04/2009 | |
| Anti-Elap CR | ||
| Clodomiro Picado | ||
| 4300708ACLQ | 07/2008 | 1 mL neutralizes 0.3 mg Micrurus nigrocinctus venom, 0.3 mg M. d. carinicaudus venom, and 0.125 mg M. fulvius venom |
| 4480909ACLQ | 09/2009 | |
| Anti-Bot–Crot–Lac | ||
| Clodomiro Picado | ||
| 4450609POLQ | 06/2009 | 1 mL neutralizes 3 mg B. asper venom, 2 mg C. durissus venom, and 3 mg L. muta venom |
| 4590410POLQ | 04/2010 | |
| Echi-Tab-Plus | ||
| Clodomiro Picado | ||
| 4600510PALQ | 05/2010 | 1 mL neutralizes 3 mg Echis ocellatus venom, 2 mg Bitis arietans venom, and 0.2 mg Naja nigricollis venom |
Arac = Arachnidic; Bot = Bothropic; Crot = Crotalic; Elap BR = Elapidic Brazil; Elap CR = Elapidic Costa Rica; Lac = Lachetic; Lon = Lonomic; MLD = minimal lethal dose; MND = minimal necrotizing dose; Scorp = Scorpionic.
Ethics statement.
Human blood was obtained from adult healthy donors who knew the objectives of the study and signed the corresponding informed consent form approved by the National Commission on Research Ethics (CAAE02001612.6.0000.0071). All experimental procedures involving animals were in accordance with the ethical principles in animal research adopted by the Brazilian Society of Animal Science and the National Brazilian Legislation (number 11.794/08). Protocols were approved by the Institutional Animal Care and Use Committee (protocol approval number 904/12).
Normal human serum.
Blood samples from adult healthy donors were collected without anticoagulant and allowed to clot for 4 hours at 4°C. After centrifugation, normal human serum (NHS) was collected and stored at −80°C.
Sheep and rabbit erythrocytes.
Sheep and rabbit erythrocytes were used as target cells in classical and alternative pathway hemolytic assays, respectively. Blood was collected in Alsever anticoagulant and stored at 4°C. Sheep blood samples were provided by the São Joaquim Farm (Butantan Institute).
Incubation of antivenoms with NHS.
Antivenoms were incubated with NHS as a source of complement for 1 hour at 37°C. The volume of NHS was the same for all incubations (200 μL), but the sample volume varied between the different antivenoms (Table 2) based on an estimation of the maximum volume of each antivenom administered to patients in severe accidents in proportion to the average volume of circulating plasma in a normal human adult. For practical purposes, a normal human adult was considered to have 2.75 L circulating plasma (55% of 5 L blood). For each control group, NHS was incubated with a corresponding volume of sterile non-pyrogenic saline (0.9% sodium chloride).
Table 2.
Volume of each antivenom incubated with normal human serum in vitro based on the maximum volume administered to patients proportional to the volume of circulating plasma
| Antivenom | Maximum volume administered to patients (volume [mL]/2.75 L plasma) | Incubation volume (volume [μL]/200 μL NHS) |
|---|---|---|
| Anti-Bot | 120 | 8.73 |
| Anti-Bot-Crot | 200 | 14.55 |
| Anti-Bot-Lac | 200 | 14.55 |
| Anti-Crot | 200 | 14.55 |
| Anti-Elap BR | 100 | 7.27 |
| Anti-Arac | 50 | 3.64 |
| Anti-Scorp | 30 | 2.18 |
| Anti-Lon | 100 | 7.27 |
| Anti-Elap CR | 100 | 7.27 |
| Anti-Bot-Crot-Lac | 150 | 10.91 |
| Echi-Tab-Plus | 40 | 2.91 |
Complement activation by the classical, alternative, and lectin pathways.
Activation of the classical and alternative complement pathways by the antivenoms was measured in hemolytic assays by determining the residual hemolytic activity of NHS on sheep and rabbit erythrocytes, respectively. Experiments were performed as previously described.21 The results were expressed as the percentage of CH50/mL (classical pathway) or AP50/mL (alternative pathway) activation compared with NHS incubated with saline (100%). Activation of the lectin complement pathway by the antivenoms was determined by enzyme-linked immunosorbent assay (ELISA), which detected the deposition of C4b in mannan-sensitized plates as previously described.21
Detection of anaphylatoxins.
After incubating NHS with antivenoms or saline (control) as described above, the reactions were stopped by the addition of 10 mM EDTA, and the concentrations of C3a/C3a desArg and C5a/C5a desArg were determined by ELISA (OptEIA ELISA Kit; BD Biosciences, San Jose, CA) following the manufacturer's instructions.
Protein concentration.
To verify whether the complement activation by the antivenoms could be related to the amount of antivenom protein incubated with NHS, the protein concentrations of the antivenoms were determined using the BCA method (Pierce BCA Protein Assay Kit; Pierce, Rockford, IL) according to the manufacturer's instructions with BSA as standard.
Polyacrylamide gel electrophoresis and Western blots.
To determine protein composition profiles, the most recent samples of each antivenom were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis under non-reducing and reducing conditions. Briefly, samples were diluted in saline solution (0.9% sodium chloride) to achieve the protein concentration of 2 mg/mL; 10 μL each diluted sample (20 μg protein) were then mixed with the same volume of reducing or non-reducing buffer and subjected to 12% SDS-PAGE.22 Molecular mass standards (Invitrogen/Life Technologies, Carlsbad, CA) were included in all runs, which were performed at 100 V. Gels were stained with silver.23 For Western blot assays,24 proteins on unstained gels were transferred to nitrocellulose membranes at 150 mA. After transfer, reactions were performed to detect horse IgG as previously described.25
Statistical analysis.
Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey's post-test, and differences with P values that were less than 0.05 were considered to be statistically significant. For correlation analysis, Pearson's correlation coefficient, for which values close to 1.0 or −1.0 indicate positive or negative correlations, respectively, was calculated.
Results
Complement consumption by the antivenoms.
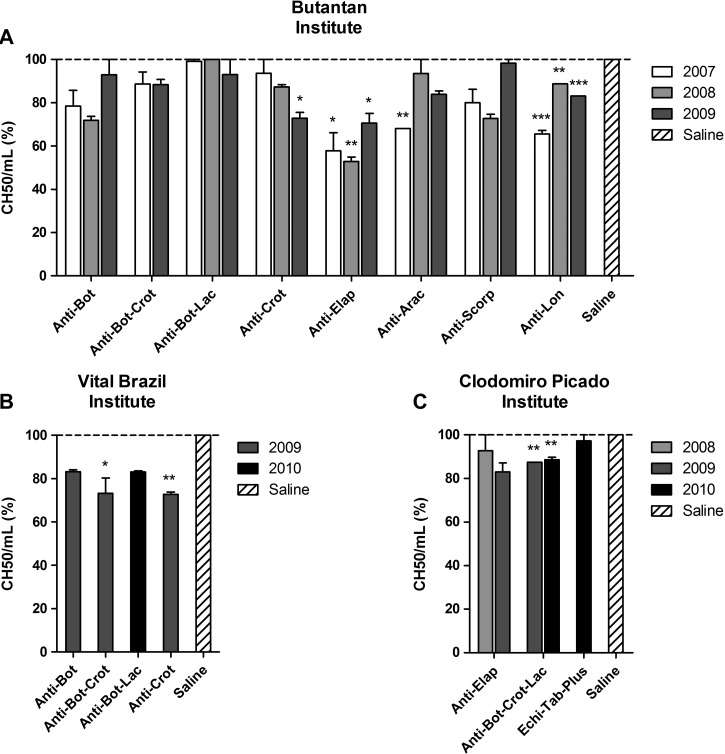
The classical pathway was activated by several antivenoms from the three institutes, which was shown by the reduction of the CH50/mL compared with the control (Figure 1). This reduction was statistically significant for all batches of anti-Elapidic and anti-Lonomic antivenoms from the Butantan Institute as well as for one batch of anti-Crotalic antivenom (2009) and one batch of anti-Arachnidic antivenom (2007) (Figure 1A). Anti-Bothropic–Crotalic and anti-Crotalic antivenoms from the Vital Brazil Institute also significantly interfered with the classical pathway (Figure 1B) as well as the two batches of anti-Bothropic–Crotalic–Lachetic antivenom from the Clodomiro Picado Institute (Figure 1C). None of the antivenoms tested in this study activated the lectin or alternative pathways (data not shown).
Figure 1.
Classical complement consumption by the antivenoms. NHS was incubated with antivenoms from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes or saline as a negative control. The activation of the complement classical pathway was measured by the residual lytic activity of NHS on sheep erythrocytes, and the results were expressed as the percentage of CH50/mL in relation to NHS incubated with saline (100%). Data represent mean ± SD of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.0001 compared with saline. Arac = Arachnidic; Bot = Bothropic; Crot = Crotalic; Elap = Elapidic; Lac = Lachetic; Lon = Lonomic; Scorp = Scorpionic.
Generation of anaphylatoxins by the antivenoms.
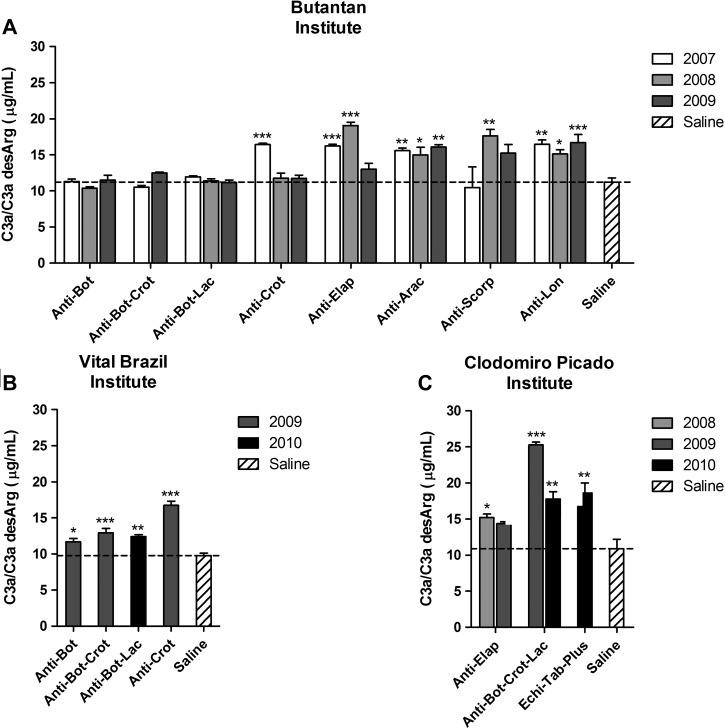
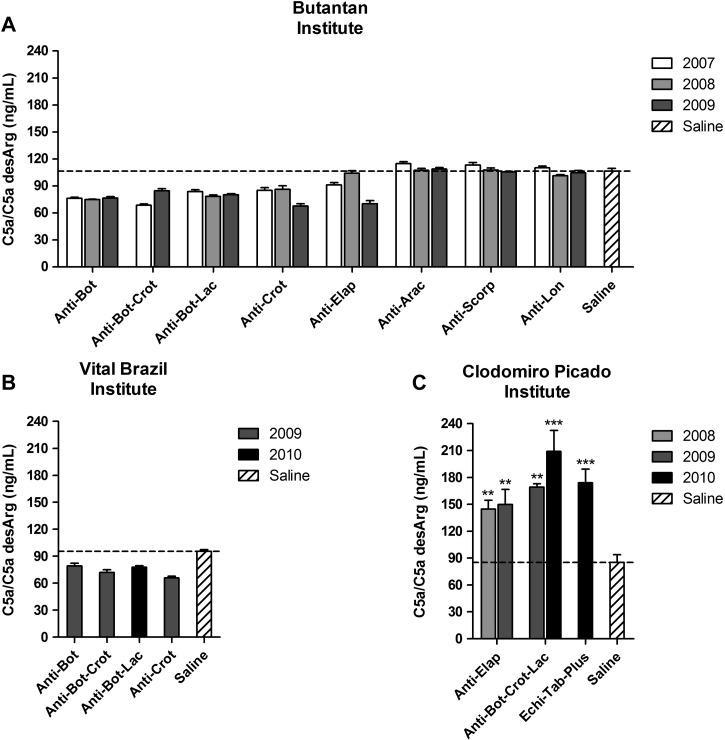
To better investigate the complement activation by the antivenoms, the generation of anaphylatoxins was determined. Most antivenoms from the three institutes induced the generation of C3a/C3a desArg when incubated with NHS (Figure 2 ), which was also observed in samples where the consumption of classical pathway components was not detected (Figure 1). Interestingly, antivenoms from the Butantan and Vital Brazil Institutes did not induce the generation of C5a/C5a desArg (Figure 3A and B), whereas all the antivenoms from the Clodomiro Picado Institute induced this potent anaphylatoxin (Figure 3C).
Figure 2.
Generation of C3a/C3a desArg by antivenoms. NHS was incubated with antivenoms from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes or saline as a negative control. The generation of C3a/C3a desArg was detected by ELISA. Data represent mean ± SD of two independent experiments. *P < 0.05; **P < 0.01; ***P < 0.0001 compared with saline.
Figure 3.
Generation of C5a/C5a desArg by antivenoms. NHS was incubated with antivenoms from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes or saline as a negative control. The generation of C5a/C5a desArg was detected by ELISA. Data represent mean ± SD of two independent experiments. **P < 0.01; ***P < 0.0001 compared with saline.
Protein concentration of the antivenoms.
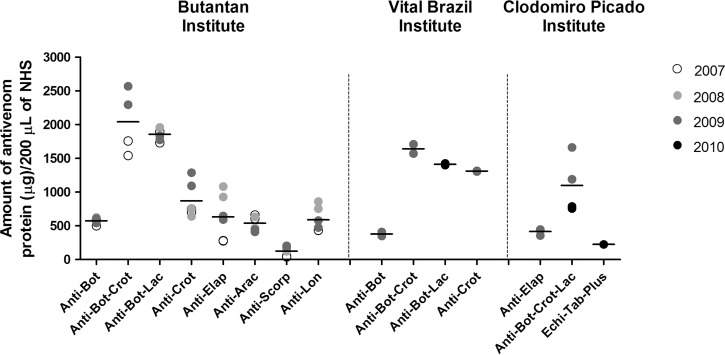
To verify whether the complement activation by the antivenoms could be related to the amount of antivenom protein incubated with NHS, the protein concentration of all the samples was determined (data not shown). Based on the protein concentration of the samples and the volume of each antivenom incubated with NHS (Table 2), the amount of antivenom protein in each incubation was calculated (Figure 4). There was no statistical correlation between the amount of antivenom protein and the consumption of classical pathway components (Pearson r = 0.16, P > 0.05) or the generation of C3a/C3a desArg (Pearson r = −0.15, P > 0.05) or C5a/C5a desArg (Pearson r = −0.30, P > 0.05).
Figure 4.
Amount of antivenom protein in the experimental condition. The protein concentrations of all antivenom samples were determined by the BCA method. Based on the protein concentration of each antivenom and the specific volume used in the incubation with NHS, the amount of antivenom protein in each incubation was calculated. The results are expressed as the amount of antivenom protein (micrograms) per 200 μL NHS and represent the mean of two independent experiments.
SDS-PAGE and Western blot analysis of the antivenoms.
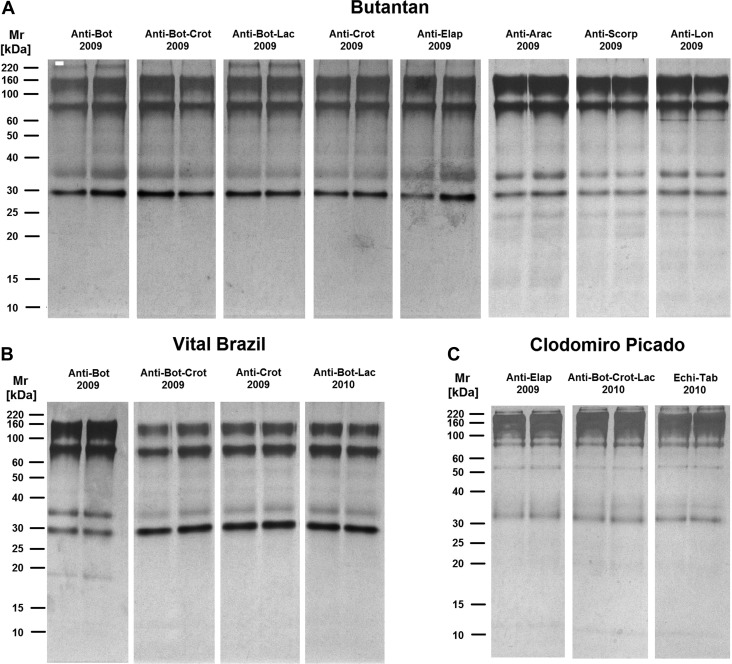
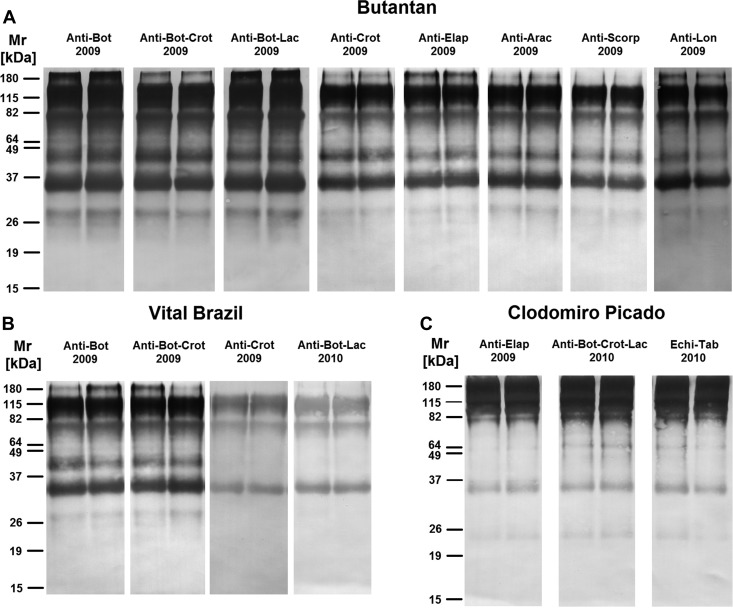
To determine the protein composition profiles and their possible correlation with complement activation, the most recent samples of each antivenom were submitted to SDS-PAGE and Western blot analyses under non-reducing and reducing conditions. Under non-reducing condition, various bands, with molecular masses between 30 and 160 kDa, were observed in all the antivenoms analyzed, and some antivenoms also showed a high-molecular mass band with approximately 220 kDa (Figure 5 ), indicating the presence of high-molecular mass aggregates.13 Most of the non-reduced bands were recognized by the anti-horse IgG antibody in the Western blot reactions (Figure 6 ), suggesting the presence of IgG or its fragments on aggregates of different molecular masses. These aggregates included high-molecular mass aggregates, with more than 180 kDa (Figure 6).
Figure 5.
SDS-PAGE analyses of antivenoms in non-reduction conditions. The most recent antivenom samples from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes were subjected to 12% PAGE under non-reducing condition. Proteins on gels were stained with silver. Molecular mass standards are shown on the left.
Figure 6.
Western blot analyses of antivenoms in non-reduction conditions. The most recent antivenom samples from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes were subjected to 12% PAGE under non-reducing condition. Proteins on gels were transferred to nitrocellulose membranes for the detection of horse IgG. Molecular mass standards are shown on the left.
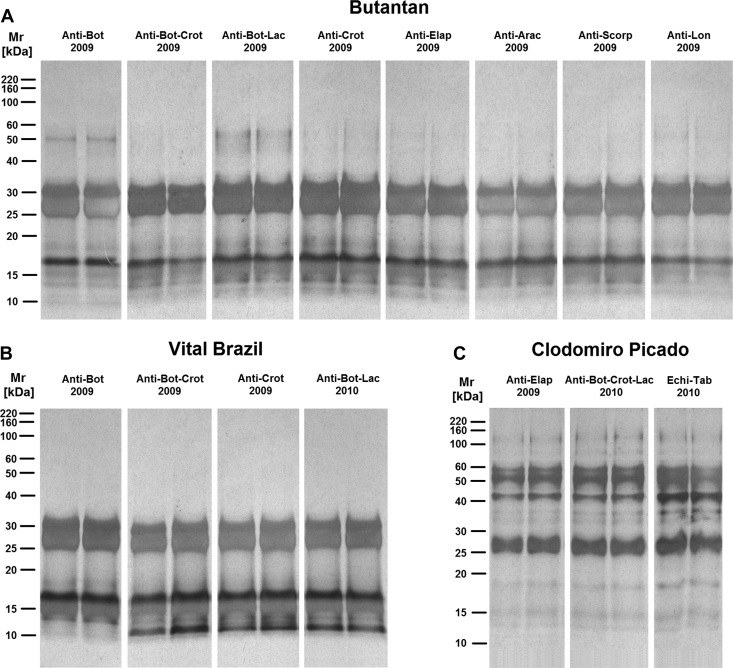
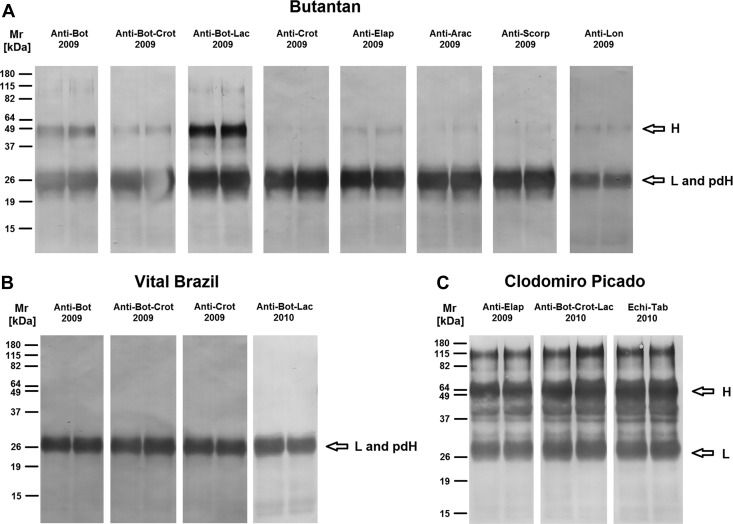
The electrophoretic analysis, under reducing condition, revealed the presence of two major bands with molecular masses between 25 and 30 kDa in all samples from the Butantan and Vital Brazil Institutes as well as several other proteins with molecular masses lower than 20 kDa (Figure 7A and B). One minor band of approximately 50 kDa was also observed in some antivenoms from the Butantan Institute (Figure 7A). Antivenoms from the Clodomiro Picado Institute exhibited a major band of approximately 25–30 kDa, three bands from 40 to 60 kDa, and also, several other minor components with different molecular masses (Figure 7C ). Western blot analysis under reducing condition revealed the existence of IgG heavy (50 kDa) and light (25 kDa) chains26 in several antivenoms from the Butantan Institute (Figure 8A ), indicating that the cleavage of the antibodies for the production of F(ab')2 fragments was not complete. In the antivenoms from the Vital Brazil Institute, only one band of approximately 25 kDa was detected (Figure 8B), which most likely includes IgG light chain and pepsin-digested IgG heavy chain fragments. As expected, IgG heavy and light chains were detected in all the samples from the Clodomiro Picado Institute (Figure 8C), because they contain whole IgGs. However, several other bands were also detected in these samples, including one of approximately 115 kDa (Figure 8C), suggesting that the aggregates present in these antivenoms were not completely disrupted in our experiments.
Figure 7.
SDS-PAGE analyses of antivenoms in reduction conditions. The most recent antivenom samples from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes were subjected to 12% PAGE under reducing condition. Proteins on gels were stained with silver. Molecular mass standards are shown on the left.
Figure 8.
Western blot analyses of antivenoms in reduction conditions. The most recent antivenom samples from the (A) Butantan, (B) Vital Brazil, and (C) Clodomiro Picado Institutes were subjected to 12% PAGE under reducing condition. Proteins on gels were transferred to nitrocellulose membranes for the detection of horse IgG. Molecular mass standards are shown on the left. H = IgG heavy chain; L = IgG light chain; pdH = pepsin-digested IgG heavy chain.
Discussion
In the present work, the anticomplementary activity of commercial horse antivenoms produced by the Butantan, Vital Brazil, and Clodomiro Picado Institutes between 2007 and 2010 was evaluated in in vitro assays. Antivenoms from the Butantan and Vital Brazil Institutes consist of F(ab')2 fragments obtained by pepsin digestion and ammonium sulphate precipitation,19 whereas antivenoms from the Clodomiro Picado Institute contain whole IgGs purified by caprylic acid fractionation.20
Analysis of complement activation was performed after incubating the antivenoms with NHS. The proportion of antivenom to NHS was based on the estimation of the maximum volume of each antivenom administered to patients in severe accidents in proportion to the average volume of circulating plasma in normal adult humans. This protocol results in different amounts of antivenom protein added to each incubation mixture. Although this limitation concerns the comparison between different samples on the basis of the amount of protein, our experimental rationale was adopted as an effort to partially reproduce, in vitro the conditions to which patients are subjected during antivenom therapy.
Under these experimental conditions, none of the antivenoms tested activated the lectin or alternative pathways, but some of them interfered with the classical pathway. Interestingly, classical pathway consumption was also induced by antivenoms devoid of Fc portion (i.e., antivenoms in which the IgG heavy chain was not detected in the Western blot reactions). It has been shown that the hinge portion of IgG, which is preserved in F(ab')2 fragments, has an important role in C1q binding.27 Additionally, F(ab')2-containing immune complexes, together with naturally occurring antihinge antibodies, can induce complement activation.28 These findings corroborate that removal of Fc from equine IgGs by pepsin digestion does not eliminate complement activation in vitro.
The potential clinical relevance of complement activation by antivenoms is related to the possible generation of anaphylatoxins. C5a is the most potent anaphylatoxin, and it is followed by C3a and C4a.29 These anaphylatoxins contain a C-terminal arginine residue that is rapidly cleaved by serum carboxypeptidases, resulting in desArg derivatives. This mechanism is involved in the regulation of the complement system,18,29 and the detection of desArg derivatives also reflects the generation of anaphylatoxins. In this study, the generation of C3a and C5a by the antivenoms was analyzed by detecting C3a/C3a desArg and C5a/C5a desArg.
Several antivenoms from the three institutes induced the production of C3a, which was also observed in samples where the consumption of classical pathway components was not detected. This production probably occurred because of different analytical sensitivity of the two methodologies used (i.e., hemolytic assay and ELISA). The release of C3a was not related to the presence of Fc, once antivenoms from Vital Brazil Institute, none of which contained the Fc fraction, also induced the production of this anaphylatoxin. The generation of C5a was only induced by the antivenoms from the Clodomiro Picado Institute. This finding can be related to the presence of higher amounts of IgG heavy chain and protein aggregates in Clodomiro Picado Institute antivenoms, an issue that deserves additional investigation.
The generation of anaphylatoxins by antivenoms might be related to the induction of EARs in patients, because it could be involved with the non-IgE–dependent anaphylatic reactions. Thus, it could be reasonable to suggest that the severity of these reactions might be related to the amount and type of anaphylatoxins (C3a or C5a) generated in vivo. In this case, the concomitant release of C5a and C3a might induce more severe reactions than the release of C3a alone. Based on this hypothesis and our observations, it could be suggested that Clodomiro Picado antivenoms might cause a higher incidence of severe EARs than Butantan and Vital Brazil antivenoms. However, clinical trials using IgG antivenoms produced at the Clodomiro Picado Institute found that severe EARs were infrequent.13,30 A study comparing the concentrations of anaphylatoxins and other inflammatory mediators in plasma from Sri Lanka snakebite victims before and after antivenom therapy was recently published.31 Sri Lankan snake envenoming induced complement activation and release of inflammatory mediators, and antivenom therapy further increased the levels of these mediators but not anaphylatoxins. The differences between our results and the results from the in vivo studies can be because of differences in the sensitivities of the methods used for the determination of complement activation and generation of anaphylatoxins and/or types of antivenoms. Moreover, it cannot be ruled out that the in vitro results do not translate into in vivo effects.
To verify the correlation between complement activation and the amount of antivenom protein added in the incubations, the protein concentrations of the samples were determined (data not shown). Based on the protein concentrations of the samples and the volume of each antivenom incubated with NHS, the amount of antivenom protein in each incubation was calculated. The amount of antivenom protein did not significantly correlate with the percentage of CH50/mL or the generation of C3a and C5a, suggesting that it is not the main factor involved in the complement activation by the antivenoms in vitro.
SDS-PAGE and Western blot analyses of the antivenoms under non-reducing condition showed the presence of high-molecular mass aggregates containing IgG molecules in several samples, mainly those samples from the Clodomiro Picado Institute; however, previous studies showed that the amount of protein aggregate in antivenoms from this manufacturer is ∼3%.13,32 IgG aggregates can be formed by F(ab)–F(ab) or Fc–Fc interactions and occur after prolonged storage times, increasing the immunogenicity and modifying the physical properties of antivenoms.33 Low-molecular mass proteins were also detected in reduced samples, but they did not correspond to Ig degradation products, which was shown by the Western blot reactions; thus, they were considered protein contaminants.
In conclusion, our results show that different types of antivenoms can activate the classical pathway of the complement system and generate anaphylatoxins, events that may be associated with various antivenom characteristics, such as composition, contaminant proteins, and aggregates. The role of the generation of C5a by the antivenoms in the EARS still needs additional investigation; however, the detection of C5a may be a useful mark for the presence of protein aggregates, which can help the quality control process for antivenom production.
ACKNOWLEDGMENTS
We thank Guillermo León for critical reading of the manuscript.
Footnotes
Financial support: This work was supported by São Paulo Research Foundation (FAPESP) Grant 2011/51869-1.
Authors' addresses: Carla Cristina Squaiella-Baptistão and Denise V. Tambourgi, Laboratório de Imunoquímica, Instituto Butantan, São Paulo, SP, Brazil, E-mails: carla.baptistao@butantan.gov.br and denise.tambourgi@butantan.gov.br. José Roberto Marcelino, Seção de Processamento de Plasmas Hiperimunes, Instituto Butantan, São Paulo, SP, Brazil, E-mail: jose.marcelino@butantan.gov.br. Luiz Eduardo Ribeiro da Cunha, Instituto Vital Brazil, Niterói, RJ, Brazil, E-mail: luis.eduardo@vitalbrazil.rj.gov.br. José María Gutiérrez, Instituto Clodomiro Picado, Facultad de Microbiología, Universidad de Costa Rica, San José, Costa Rica, E-mail: jose.gutierrez@ucr.ac.cr.
References
- 1.World Health Organization Rabies and Envenomings: A Neglected Public Health Issue: Report of a Consultative Meeting. 2007. http://www.who.int/bloodproducts/animal_sera/Rabies.pdf Available at. Accessed February 5, 2013.
- 2.World Health Organization The 17 Neglected Tropical Diseases. 2009. http://www.who.int/neglected_diseases/diseases/en Available at. Accessed February 5, 2013.
- 3.World Health Organization WHO Model List of Essential Medicines. 2011. http://whqlibdoc.who.int/hq/2011/a95053_eng.pdf Available at. Accessed February 5, 2013.
- 4.World Health Organization Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. 2010. http://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguideline.pdf Available at. Accessed February 5, 2013. [DOI] [PubMed]
- 5.Lavonas EJ. Antivenoms for snakebite: design, function and controversies. Curr Pharm Biotechnol. 2012;13:1980–1986. doi: 10.2174/138920112802273227. [DOI] [PubMed] [Google Scholar]
- 6.Eursakun S, Simsiriwong P, Ratanabanangkoon K. Studies on the fractionation of equine antivenom IgG by combinations of ammonium sulfate and caprylic acid. Toxicon. 2012;60:1022–1029. doi: 10.1016/j.toxicon.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso JL, Fan HW, França FO, Jorge MT, Leite RP, Nishioka SA, Avila A, Sano-Martins IS, Tomy SC, Santoro ML, Chudzinski AM, Castro SCB, Kamiguti AS, Kelen EMA, Hirata MH, Mirandola RMS, Theakston RDG, Warrell DA. Randomized comparative trial of three antivenoms in the treatment of envenoming by lance-headed vipers (Bothrops jararaca) in São Paulo, Brazil. Q J Med. 1993;86:315–325. [PubMed] [Google Scholar]
- 8.Isbister GK, Shahmy S, Mohamed F, Abeysinghe C, Karunathilake H, Ariaratnam A. A randomised controlled trial of two infusion rates to decrease reactions to antivenom. PLoS One. 2012;7:e38739. doi: 10.1371/journal.pone.0038739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morais JF, de Freitas MC, Yamaguchi IK, dos Santos MC, da Silva WD. Snake antivenoms from hyperimmunized horses: comparison of the antivenom activity and biological properties of their whole IgG and F(ab')2 fragments. Toxicon. 1994;32:725–734. doi: 10.1016/0041-0101(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 10.León G, Monge M, Rojas E, Lomonte B, Gutiérrez JM. Comparison between IgG and F(ab')2 polyvalent antivenoms: neutralization of systemic effects induced by Bothrops asper venom in mice, extravasation to muscle tissue, and potential for induction of adverse reactions. Toxicon. 2001;39:793–801. doi: 10.1016/s0041-0101(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 11.Otero-Patiño R, Cardoso JLC, Higashi HG, Núñez V, Díaz A, Toro MF, Garcia ME, Sierra A, Garcia LF, Moreno AM, Medina MC, Castañeda N, Silva-Diaz JF, Murcia M, Cardenas SY, Dias da Silva WD. A randomized, blinded, comparative trial of one pepsin-digested and two whole IgG antivenoms for Bothrops snake bites in Uraba, Colombia. Trop Med Hyg. 1998;58:183–189. doi: 10.4269/ajtmh.1998.58.183. [DOI] [PubMed] [Google Scholar]
- 12.Otero-Patiño R, Segura A, Herrera M, Angulo Y, León G, Gutiérrez JM, Barona J, Estrada S, Pereañez A, Quintana JC, Vargas LJ, Gómez JP, Díaz A, Suárez AM, Fernández J, Ramírez P, Fabra P, Perea M, Fernández D, Arroyo Y, Betancur D, Pupo L, Córdoba EA, Ramírez CE, Arrieta AB, Rivero A, Mosquera DC, Conrado NL, Ortiz R. Comparative study on the efficacy and safety of two polyvalent, caprylic acid fractionated [IgG and F(ab')2] antivenoms, in Bothrops asper bites in Colombia. Toxicon. 2012;59:344–355. doi: 10.1016/j.toxicon.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Otero R, Gutiérrez JM, Rojas G, Núñez V, Díaz A, Miranda E, Uribe AF, Silva JF, Ospina JG, Medina Y, Toro MF, Garcia ME, León G, Garcia M, Lizano S, De La Torre J, Márquez J, Mena Y, González N, Arenas LC, Puzón A, Blanco N, Sierra A, Espinal ME, Arboleda M, Jiménez JC, Ramírez P, Díaz M, Guzmán MC, Barros J, Henao S, Ramírez A, Macea U, Lozano R. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon. 1999;37:895–908. doi: 10.1016/s0041-0101(98)00220-7. [DOI] [PubMed] [Google Scholar]
- 14.Herrera M, León G, Segura A, Meneses F, Lomonte B, Chippaux JP, Gutiérrez JM. Factors associated with adverse reactions induced by caprylic acid-fractionated whole IgG preparations: comparison between horse, sheep and camel IgGs. Toxicon. 2005;46:775–781. doi: 10.1016/j.toxicon.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Theakston RDG, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541–557. doi: 10.1016/s0041-0101(02)00393-8. [DOI] [PubMed] [Google Scholar]
- 16.Mollnes TE, Hogasen K, De Carolis C, Vaquero E, Nielsen EW, Fontana L, Perricone R. High-dose intravenous immunoglobulin treatment activates complement in vivo. Scand J Immunol. 1998;48:312–317. doi: 10.1046/j.1365-3083.1998.00386.x. [DOI] [PubMed] [Google Scholar]
- 17.García M, Monge M, León G, Lizano S, Segura E, Solano G, Rojas G, Gutiérrez JM. Effect of preservatives on IgG aggregation, complement-activating effect and hypotensive activity of horse polyvalent antivenom used in snakebite envenomation. Biologicals. 2002;30:143–151. doi: 10.1006/biol.2002.0329. [DOI] [PubMed] [Google Scholar]
- 18.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.dos Santos MC, D'Império Lima MR, Furtado GC, Colletto GM, Kipnis TL, Dias da Silva W. Purification of F(ab')2 anti-snake venom by caprylic acid: a fast method for obtaining IgG fragments with high neutralization activity, purity and yeld. Toxicon. 1989;27:297–303. doi: 10.1016/0041-0101(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 20.Rojas G, Jiménez JM, Gutiérrez JM. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon. 1994;32:351–363. doi: 10.1016/0041-0101(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 21.Pidde-Queiroz G, Furtado MFD, Filgueiras CF, Pessoa LA, Spadafora-Ferreira M, van den Berg CW, Tambourgi DV. Human complement activation and anaphylatoxins generation induced by snake venom toxins from Bothrops genus. Mol Immunol. 2010;47:2537–2544. doi: 10.1016/j.molimm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Morrissey JH. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 24.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from acrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka GD, Pidde-Queiroz G, Furtado MFD, van den Berg C, Tambourgi DV. Micrurus snake venoms activate human complement system and generate anaphylatoxins. BMC Immunol. 2012;13:4. doi: 10.1186/1471-2172-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solano S, Segura A, León G, Gutiérrez JM, Burnouf T. Low pH formulation of whole IgG antivenom: impact on quality, safety, neutralizing potency and viral inactivation. Biologicals. 2012;40:129–133. doi: 10.1016/j.biologicals.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Dall'Acqua WF, Cook KE, Damschroder MM, Woods RM, Wu H. Modulation of the effector functions of a human IgG1 through engineering of its hinge region. J Immunol. 2006;177:1129–1138. doi: 10.4049/jimmunol.177.2.1129. [DOI] [PubMed] [Google Scholar]
- 28.Fumia S, Goede JS, Fischler M, Luginbühl A, Frick S, Fodor P, Lutz HU. Human F(ab')2-containing immune complexes together with anti-hinge natural antibodies stimulate complement amplification in vitro and in vivo. Mol Immunol. 2008;45:2951–2961. doi: 10.1016/j.molimm.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Zhou W. The new face of anaphylatoxins in immune regulation. Immunobiology. 2012;217:225–234. doi: 10.1016/j.imbio.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Otero R, León G, Gutiérrez JM, Rojas G, Toro MF, Barona J, Rodríguez V, Díaz A, Núñez V, Quintana JC, Ayala S, Mosquera D, Conrado LL, Fernández D, Arroyo Y, Paniaqua CA, López M, Ospina CE, Alzate C, Fernández J, Meza JJ, Silva JF, Ramírez P, Fabra PE, Ramírez E, Córdoba E, Arrieta AB, Warrell DA, Theakston RD. Efficay and safety of two whole IgG polyvalent antivenoms, refined by caprylic acid fractionation with or without beta-propiolactone, in the treatment of Bothrops asper bites in Colombia. Trans R Soc Trop Med Hyg. 2006;100:1173–1182. doi: 10.1016/j.trstmh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Stone SF, Isbister GK, Shahmy S, Mohamed F, Abeysinghe C, Karunathilake H, Ariaratnam A, Jacoby-Alner TE, Cotterell CL, Brown SG. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Negl Trop Dis. 2013;7:e2326. doi: 10.1371/journal.pntd.0002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abubakar IS, Abubakar SB, Habib AG, Nasidi A, Durfa N, Yusuf PO, Larnyang S, Garnvwa J, Sokomba E, Salako L, Theakston RD, Juszczak E, Alder N, Warrell DA. Nigeria-UK EchiTab Study Group Randomised controlled double-blind non-inferiority trial of two antivenoms for saw-scaled or carpet viper (Echis ocellatus) envenoming in Nigeria. PLoS Negl Trop Dis. 2010;4:e767. doi: 10.1371/journal.pntd.0000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nezlin R. Interactions between immunoglobulin G molecules. Immunol Lett. 2010;132:1–5. doi: 10.1016/j.imlet.2010.06.006. [DOI] [PubMed] [Google Scholar]