Abstract
Neurodegenerative diseases and spinal cord injury affect approximately 50 million people worldwide, bringing the total healthcare cost to over 600 billion dollars per year. Nervous system growth factors, that is, neurotrophins, are a potential solution to these disorders, since they could promote nerve regeneration. An average of 500 publications per year attests to the significance of neurotrophins in biomedical sciences and underlines their potential for therapeutic applications. Nonetheless, the poor pharmacokinetic profile of neurotrophins severely restricts their clinical use. On the other hand, small molecules that modulate neurotrophic activity offer a promising therapeutic approach against neurological disorders. Nature has provided an impressive array of natural products that have potent neurotrophic activities. This Review highlights the current synthetic strategies toward these compounds and summarizes their ability to induce neuronal growth and rehabilitation. It is anticipated that neurotrophic natural products could be used not only as starting points in drug design but also as tools to study the next frontier in biomedical sciences: the brain activity map project.
Keywords: Alzheimer’s disease, dementia, drug discovery, neurodegenerative disease, total synthesis
1. Introduction
Neurons are the basic building blocks of the nervous system, which includes the brain, the spinal cord, and the peripheral ganglia. Neurodegenerative disease (NDD) is a catchall term for heterogeneous and often sporadic disorders that are characterized by progressive nervous system dysfunction resulting from loss of neural structure and function as well as from neural death.[1] Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis are notable examples of these disorders. On the other hand, spinal cord injury arises from extreme trauma to the spinal cord.[2] Currently, neurodegenerative diseases and spinal cord injuries affect about 50 million people worldwide bringing the total annual health-care cost to over 600 billion dollars.[2a,3] Efforts to understand the progression of these diseases point to a series of molecular and cellular events, such as accumulation of misfolded proteins, protofibril formation, dysfunction of the ubiquitin-proteasome system, mitochondrial damage, oxidative stress, and synaptic failure.[4] Nonetheless, the etiologies of these diseases still remain under investigation.
Typically, a neuron consists of a cell body, dendrites, and an axon. Although the selective pruning of exuberant neural projections is a fundamental mechanism for maintaining neural plasticity, aberrant axon degeneration, neuronal atrophy, and loss of synapses are a common scenario of acute injury or chronic NDDs.[5] Currently, the therapies for NDDs and spinal cord injuries mainly relieve disease symptoms and control the damage. It is well-known that promoting the regeneration of neural axons, dendrites, and synapses within the adult nervous system would be a highly desirable therapeutic approach.[6] Nonetheless, at present such therapeutic strategies have not yet been successful.[2a,7]
Neurotrophins are a family of proteins that regulate neuron survival, development, and function.[8] Despite their overall promise, the advancement of neurotrophins as therapeutics has encountered significant problems, likely because of their suboptimal pharmacological properties.[9] These include poor serum stability, poor oral bioavailability, and limited penetration of the central nervous system (CNS).[10,11] On the other hand, small molecules, such as dopamine (DOPA), γ-aminobutyric acid (GABA), and acetylcholine (ACh), can overcome these limitations and were shown to play an important role in the management of NDDs.[12] Nature has yielded a fascinating collection of small molecules that are able to mimic neurotrophins or modulate neural cellular signaling. Thus, therapeutic strategies based on neurotrophic small molecules could bypass the limitations of protein-based therapeutics and could lead to clinical applications.
In this Review we will introduce the representative neurodegenerative diseases and spinal cord injuries as well as the major neurotrophins. We will then highlight the main families of neurotrophic natural products, focusing in particular on compounds that have been shown to enhance neurite outgrowth, and will summarize approaches toward their chemical syntheses and biological profiles.
1.1. Neurodegenerative Diseases and Spinal Cord Injury
1.1.1. Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia and affects approximately 36 million people world-wide.[13] From a biological standpoint, pathogenic aggregation of amyloid-β (Aβ) and tau protein in the form of extracellular amyloid plaques and intracellular neurofibrillary tangles characterize this disease.[3a] As these aggregations advance, the neurons gradually undergo dysfunction and cell death, thereby resulting in memory loss, cognitive deficit and ultimately death. Neuronal death in AD occurs mainly in the hippocampus and cerebral cortex.[14] Currently, there are several hypotheses of pathological pathways of AD that include the cholinergic, the amyloid-β, and the tau protein hypotheses.[15] The cholinergic hypothesis implicates decrease of acetylcholine levels as a significant factor in AD pathogenesis, based on the fact that the level of this neurotransmitter is significantly low in AD patients.[15] The second hypothesis involves accumulation of Aβ aggregates, formed upon cleavage of amyloid precursor protein by two secretase enzymes, that form toxic oligomeric species and plaques in neuronal tissue, ultimately resulting in loss of cognitive function.[7b] The third hypothesis attributes the pathology of AD to hyperphosphorylation of the microtubule-associated protein tau.[13] These hypotheses have led to the evaluation of small-molecule-based inhibitors of cholinesterase,[16] secretase,[17] and tau aggregation.[18] Although these compounds have shown symptom improvements or slowed disease progress, they are not capable of stopping, reversing, or curing AD.[19]
1.1.2. Parkinson’s Disease
Parkinson’s disease (PD) is considered to be one of the most common neurodegenerative diseases and affects approximately 1% of the population over 65 years of age.[20] Death of dopamine-secreting neural cells (dopaminergic neurons) in the substantia nigra, has been proposed to occur early in PD pathogenesis.[21] The distinctive feature of PD that differentiates it from other NDDs is the development of intraneuronal proteinaceous cytoplasmic inclusions (referred to as Lewy bodies) in the affected brain area.[22] Certain genetic mutations have been suggested to cause PD, such as mutations in genes for α-synuclein and ubiquitin C-terminal hydrolase like 1 (UCH-L1), parkin (PRKN), PINK1, and DJ-1.[23] The encoded proteins from the above genes are thought to contribute significantly to the three major dysfunctions observed in PD, which are oxidative stress, mitochondrial failure, and proteasomal dysfunction.[7e] The most common treatment of PD is targeting the dopamine receptors with various receptor agonists. Nevertheless, this strategy does not slow or stop the progressive neuron death and, more disturbingly, it produces several severe side effects.[3b] Moreover, monoamine oxidase (MAO) inhibitors,[24,25] catechol-O-methyl transferase (COMT) inhibitors,[26] and amantadine[27] are also used to manage the progression of PD.
1.1.3. Huntington’s Disease
Huntington’s disease (HD) is the most common genetic neurodegenerative disease and affects about 4 to 8 persons per 100000 people.[28] HD is characterized by progressive motor, cognitive, and psychiatric symptoms.[29] At its early stage, HD affects the striatum and subsequently other areas of the brain.[30] This disease is characterized by expansion of a cytosine-adenine-guanine (CAG) repeat in the coding area of the huntingtin gene located on chromosome 4. These CAG repeats are translated into a polyglutamine sequence in the N-terminal region of huntingtin (HTT) that interferes with the various regulatory processes of HTT and produces the defects associated with HD.[7f,28] Currently available therapies only relieve HD symptoms.[7f,28] For example, a small synthetic molecule, tetrabenazine, is currently used to combat the chorea symptoms.[31]
1.1.4. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) affects roughly 2 in 100000 individuals annually.[32] Pathologically, ALS is characterized by degeneration of the large pyramidal neurons in the motor cortex, loss of large motoneurons of the anterior horns in the spinal cord, and motor nuclei in the brainstem.[33–35] The progressive degeneration of the motor neurons weakens the nervous and muscular system, resulting in paralysis, respiratory dysfunction, and eventually death.[32] The cause of ALS remains unknown, although several specific gene mutations or immune responses associated with this disease have been suggested. For example, mutations in the gene encoding Cu/ Zn superoxide dismutase (SOD1) have been implicated in certain cases of familial ALS.[36] Recently, mutations of the TDP-43[37] and FUS[38] genes have also been linked to ALS. Current drug therapies of ALS[7g] only help to alleviate the general symptoms, but do not target the disease itself. For example, riluzole is the only FDA approved drug for this condition which acts on ion channels.[39] Moreover, encouraging phase II clinical data have been reported with dexpramipexole, a putative mitochondrial regulator.[40] Nonetheless, these agents do not provide direct treatment of the motor neuron degeneration.
1.1.5. Spinal Cord Injury
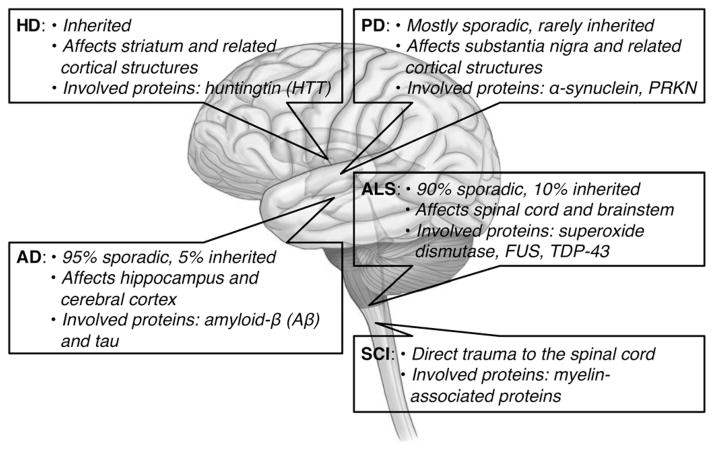
Approximately 2.5 million people live with spinal cord injury (SCI), and more than 130000 new injuries are reported annually.[2a] Unlike NDDs, SCI is produced by direct trauma to the spinal cord, which commonly results in paralysis and death. Damage to the spinal cord results in the production of various growth inhibitors from the myelin and glial scars, which generate an undesirable environment for the regeneration of nerve cells.[41,42] Potential therapeutic strategies include the control of glial scars, the inhibition of myelin-associated proteins, the use of neurotrophic factors and related small molecules, as well as cell replacements and transplantation of peripheral nerves, Schwann cells, and stem cells.[7a,42a,43] Nevertheless, at present there is no effective method for fully regenerating healthy neuronal cells or regaining full mobility.[41,42] Figure 1 shows a summary of neurodegenerative diseases and spinal cord injuries.
Figure 1.
Summary of neurodegenerative diseases (NDDs; AD =Alzheimer’s Disease, ALS =amyotrophic lateral sclerosis, HD =Huntington’s Disease, PD =Parkinson’s Disease) and spinal cord injury (SCI).[44]
1.2. Neurotrophins and Cell Signaling
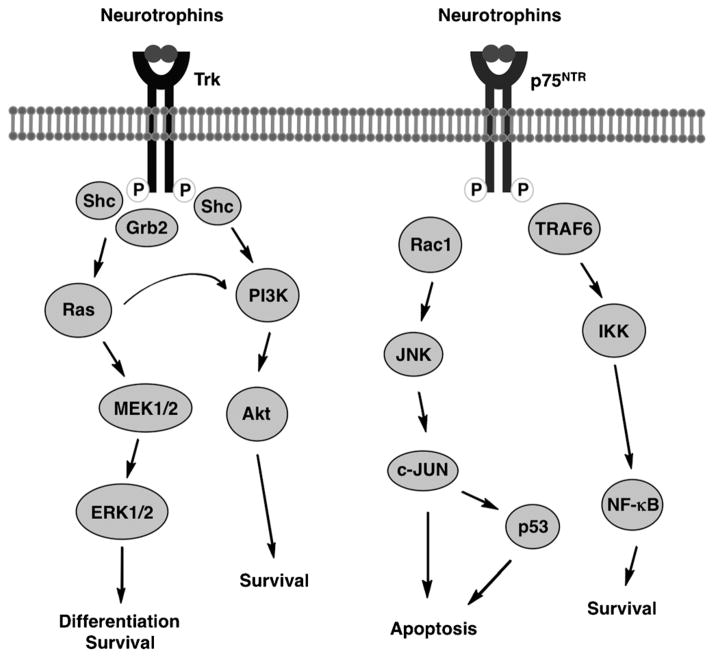
Neurotrophins are a class of small proteins that act on cell membrane receptors that elicit cell growth, differentiation, and survival.[8] Well-known neurotrophins include nerve growth factor (NGF), brain-derived growth factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5).[45] Each neurotrophin binds selectively to its tyrosine kinase receptor (Trk) and nonselectively to a 75 kDa neurotrophin receptor (p75NTR; Figure 2). Trk signaling occurs through two major pathways: the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3) pathway. These pathways regulate the fate of neurotrophin signaling in terms of cell survival and cell differentiation.[46] On the other hand, neurotrophin binding to p75NTR can trigger neuron apoptosis.[47]
Figure 2.
Neurotrophin signaling pathways.
2. Neurotrophic Natural Products
2.1. Lactacystin
From a historical standpoint, lactacystin (1, Scheme 1) is the first nonprotein neurotrophic natural product. Isolated by the Omura research group from a culture broth of Streptomyces sp., 1 was shown to exhibit significant neuritogenic activity at a concentration of 1.3 μM in mouse neuroblastoma cell line Neuro 2A.[48] Specifically, cells treated with lactacystin for one day displayed a predominantly bipolar (two-neurite-bearing) morphology, in which two neurites project at opposite sites of the cell body. Upon longer exposure (3–4 days) the cells displayed a multipolar (multiple-neurite-bearing) morphology, while the neurites became increasingly branched. Lactacystin was found to increase intracellular cAMP levels at a time point that coincides with the development of maximal bipolar morphology, but did not affect protein kinase C (PKC) and did not inhibit proteinases such as thrombin and the plasminogen activator. The lactacystin-induced neurite outgrowth appears to be dependent upon microtubule assembly, actin polymerization, and de novo protein synthesis.[49,50]
Scheme 1.
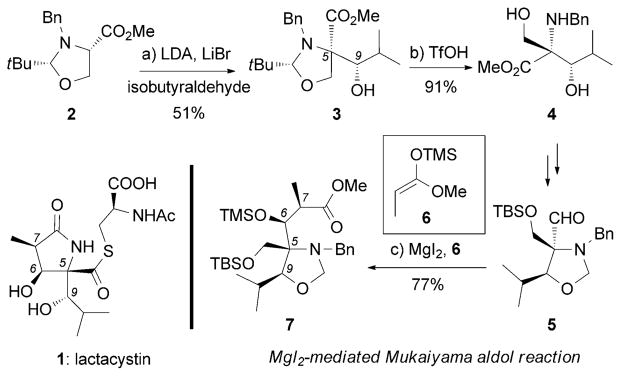
Part of the total synthesis of (+)-lactacystin by Corey et al.[52] Bn =benzyl, LDA =lithium diisopropylamide, TBS =tert-butyl-dimethylsilyl, TfOH =trifluoromethanesulfonic acid, TMS =trimethyl-silyl.
The promising biological profile of lactacystin (1) and its interesting γ-lactam thioester motif triggered numerous biological and synthetic efforts.[51] The first total synthesis of (+)-lactacystin was accomplished by Corey et al. one year after its isolation.[52] The C5-C9 trans-aminol moiety of key intermediate 3 was achieved through an aldol reaction between oxazolidine 2 and isobutyraldehyde (Scheme 1). Another key synthetic step was a MgI2-mediated Mukaiyama aldol reaction[53] between oxazolidine aldehyde 5 and the TMS enolate 6 that produced the desired C6 and C7 stereocenters in 7. Subsequent to this pioneering synthesis by Corey et al., several additional syntheses and formal syntheses of 1 were reported.[54]
Studies on the structure–activity relationships on lactacystin have been reported by Corey, Schreiber, and coworkers[49a] as well as by the Omura and Smith research groups.[54d,55] Biological investigation of lactone 8, referred to as clasto-lactacystin β-lactone (formally the product of elimination of N-acetylcysteine), unveiled that this bicyclic motif represents the active form of lactacystin (Figure 3).[49a,50c] Certain synthetic analogues, such as 9 and 10, were found to be about ten times more active than lactacystin.[49a,54d,55] On the other hand, the dihydroxy acid 11 was inactive, even at a concentration of 100 μM.[49a] Furthermore, it has been shown that the C6 hydroxy group, the C7 methyl group, and the hydroxyisobutyl side chain are essential for the neurotrophic activity.
Figure 3.
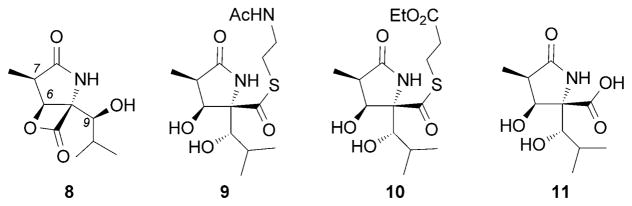
Selected lactacystin analogues.
Important information regarding the mechanism of action of lactacystin was disclosed by Schreiber and co-workers.[49a] Working with Neuro 2A and MG-63 osteosarcoma cells, these authors found that 1 inhibits cell-cycle progression at both the G0/G1 and G2/M phases of the cell cycle. Based on structure–activity relationship screening studies, the authors suggested that the mode of action of 1 could involve acylation of a cellular target through attack of the β-lactone by a nucleophilic group on the target molecule and subsequent opening of the β-lactone ring.[49a] To identify the target of lactacystin, the authors synthesized radiolabeled versions of 1 and used them as covalent affinity labels.[50a] This strategy allowed identification of the 20S proteasome as the main observed target of 1. In particular, the N-terminal threonine residue of the mammalian proteasome subunit X (also known as MB1) was found to be esterified on its side-chain hydroxy group by 1.[50a] This acylation results in inhibition of several proteasome peptidase activities that are critical to protein degradation, cellular differentiation, and apoptosis. In turn, these findings point to a connection between proteasome inhibition and neuronal differentiation. Fellutamide B (12; Figure 4), a marine fungal metabolite,[56,57] provides further evidence in support of this connection; this compound binds to the 20S proteasome at a distinct site, which results in inhibition of its hydrolytic activity[58] and at a concentration of 10 μM induces synthesis of NGF in fibroblasts and cultured brain cells.[59] Perhaps more importantly, these findings suggest that controlled inhibition of proteasome activity by small molecules represents a promising therapeutic approach against various diseases,[59,60] including NDDs.[61]
Figure 4.
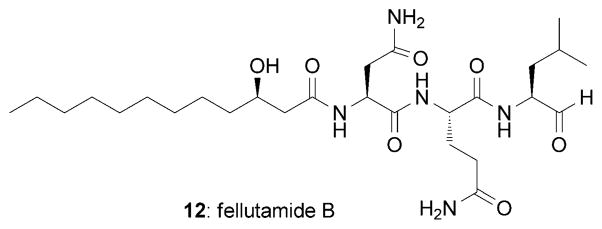
Structure of fellutamide B (12).
2.2. Illicium Sesquiterpenes
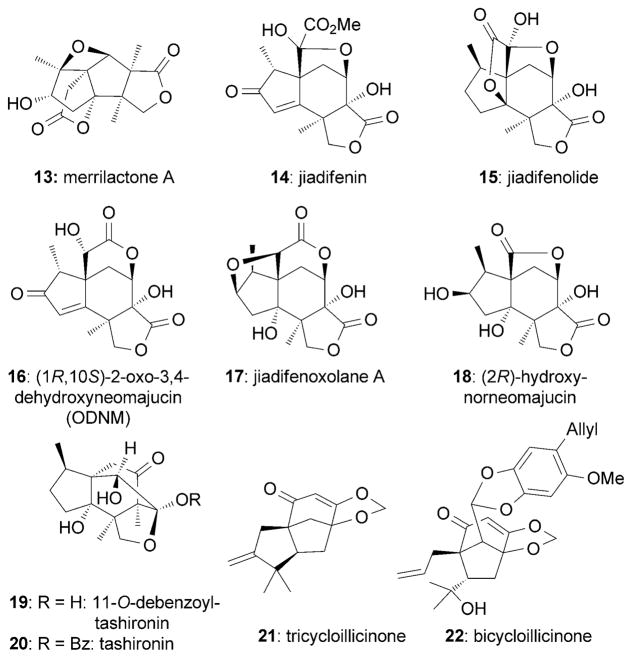
The Illicium family of natural products has attracted considerable attention[62] since several of its members possess potent neurotrophic activities. These include merrilactone A (13),[63] jiadifenin (14),[64] jiadifenolide (15),[65] (1R,10S)-2-oxo-3,4-dehydroxyneomajucin (16, ODNM),[66] jiadifenoxolane A (17),[65] (2R)-hydroxynorneomajucin (18),[67] 11-O-debenzoyltashironin (19),[68] tricycloillicinone (21),[69] and bicycloillicinone (22)[70] (Figure 5). In addition to their complex caged architectures, several natural products of this family were shown to promote neurite outgrowth at low nanomolar to low micromolar concentration in primary cultures of cortical neurons of fetal rats.
Figure 5.
Representative neurotrophic Illicium sesquiterpenes.
Isolated from Illicium merrillianum, a plant indigenous to China and Myanmar, merrilactone A (13) was found to promote neurite outgrowth in primary cultures of cortical neurons of fetal rats at concentrations ranging from 0.1 to 10 μM.[63a] A pioneering synthesis of 13 was reported by the Danishefsky research group that allowed synthetic access to several Illicium natural products (Scheme 2).[71] This approach builds upon an intermolecular Diels–Alder reaction between 23 and 24[72] to afford 25. A subsequent ozonolysis of 26 produced the corresponding dialdehyde, which underwent an intramolecular aldol condensation to yield 27, which contains the BC ring system of the natural product.[73] After formation of the D ring, an impressive AIBN/nBu3SnH-induced free-radical cyclization[74] allowed formation of the A ring of 29 from vinyl bromide 28.
Scheme 2.
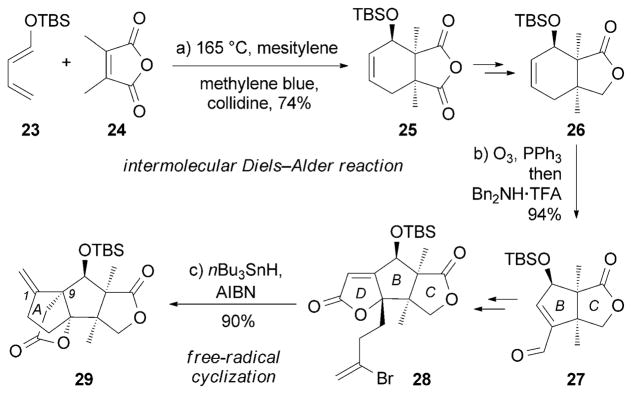
Part of the total synthesis of merrilactone A (13) by Danishefsky and co-workers.[71] AIBN=azobis(isobutyronitrile), TFA = trifluoroacetic acid.
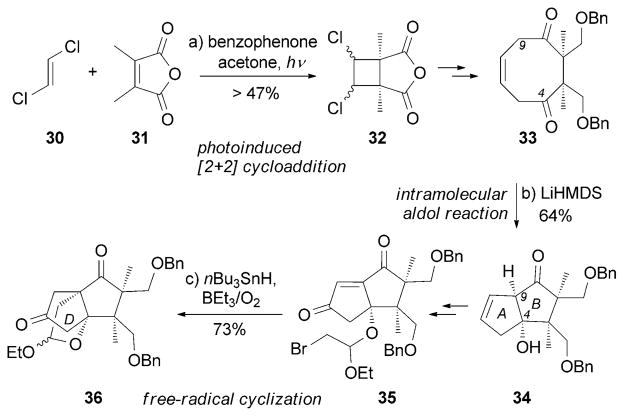
The synthesis of merrilactone by Inoue, Hirama et al. is highlighted by an intramolecular aldol reaction that forms the AB ring system in 34 from diketone 33 (Scheme 3).[75] The latter compound was obtained from cyclic anhydride 32 which, in turn, was assembled from a [2+2] photocycloaddition between trans-1,2-dichloro-ethene (30) and 31. The D ring of 36 was formed by an intramolecular radical cyclization of alkyl bromide 35. Interestingly, in subsequent studies, the authors reported that both enantiomers of 13 are equally active in primary cultures of cortical neurons of fetal rats.[75c]
Scheme 3.
Part of the total synthesis of merrilactone A (13) by Inoue, Hirama and co-workers.[75] LiHMDS =lithium hexamethyldisilazide.
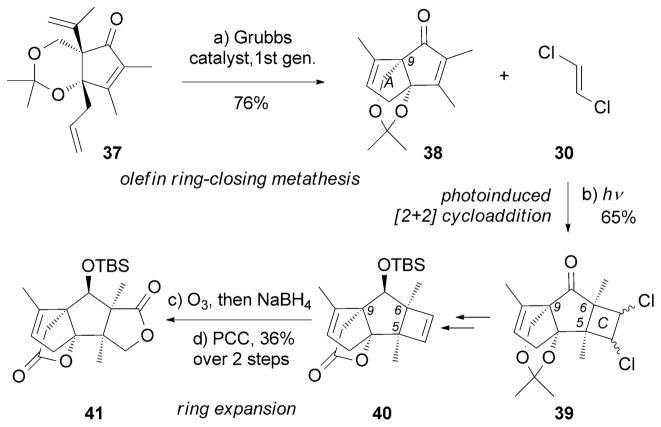
The synthesis of (±)-13[76] by Mehta and Singh relied on a ring-closing metathesis (RCM)[77] to build the A ring and a [2+2] photocyclization to furnish the C-ring framework (Scheme 4). The cyclobutene motif of 40 was then subjected to a ring-expansion strategy that proceeded through ozonolysis and lactol oxidation to afford advanced intermediate 41.
Scheme 4.
Part of the total synthesis of (±)-merrilactone A (13) by Mehta and Singh.[76] PCC=pyridinium chlorochromate.
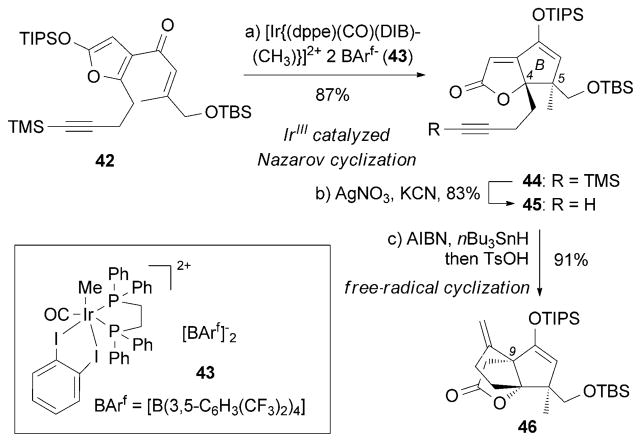
Formation of the B ring of 44 by a Nazarov cyclization[78] constitutes the key step of the total synthesis of (±)-13 reported by Frontier and co-workers.[79] IrIII complex 43[80] was pivotal for the success of this cyclization.[81] After removal of the TMS group, alkyne 45 was subjected to an AIBN/nBu3SnH-induced free-radical cyclization to afford the critical C9 quaternary center in 46 (Scheme 5).
Scheme 5.
Part of the total synthesis of (±)-merrilactone A (13) by Frontier and co-workers.[79] dppe =1,2-bis(diphenylphosphino)ethane, DIB =o-diiodobenzene, TIPS =triisopropylsilyl, TsOH =p-toluenesul-fonic acid.
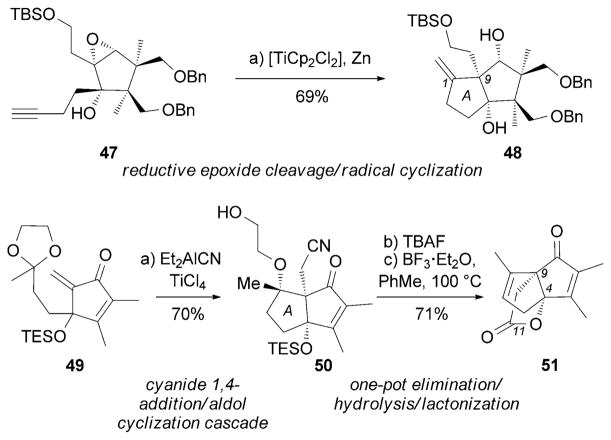
Recently, the Greaney research group reported two interesting synthetic strategies toward (±)-merrilactone A (13).[82] Key to the first-generation synthesis was a Cp2TiCl2/Zn-triggered reductive epoxide cleavage followed by radical cyclization, which furnished the A ring of 48 containing the C9 quaternary center. The second-generation synthesis was based on a cyanide 1,4-addition/aldol reaction cascade[83] that constructed the A ring and the C9 quaternary center. Intermediate 50 then underwent a one-pot elimination/ cyanide hydrolysis/lactonization upon treatment with a Lewis acid and heating (Scheme 6).
Scheme 6.
Total synthesis of (±)-merrilactone A (13) by Greaney and co-workers.[82] Cp =cyclopentadienyl, TBAF =tetrabutylammonium fluoride, TES =triethylsilyl.
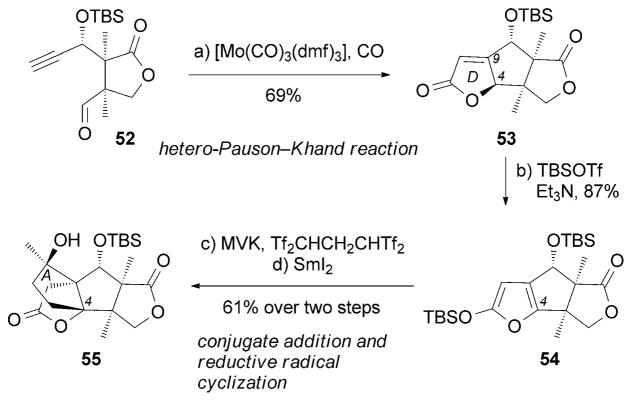
More recently, an interesting synthesis of (±)-13 was described by the Zhai research group,[84] in which a hetero-Pauson–Khand reaction[85] was employed to convert alkyne 52 into the D-ring lactone motif in the presence of [Mo(CO)3-(DMF)3]/CO (Scheme 7). Subsequently, the A-ring frame-work of 55 was constructed through a sequence of reactions that included formation of the extended enol silyl ether 54, conjugate addition of 54 to methyl vinyl ketone (MVK), and SmI2-mediated reductive radical cyclization.[86]
Scheme 7.
Part of the total synthesis of (±)-merrilactone A (13) by Zhai and co-workers.[84] MVK =methyl vinyl ketone.
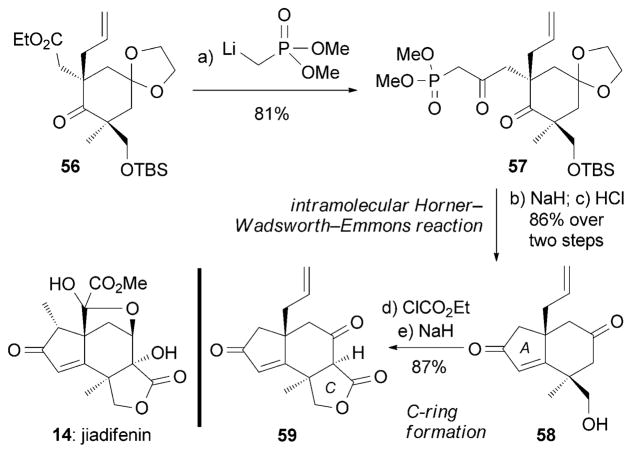
Jiadifenin (14) and ODNM (16) were isolated from the East-Asian plants Illicium jiadifengpi[64] and Illicium majus,[66] respectively. 14 was shown to promote neurite outgrowth in primary cultures of cortical neurons of fetal rats at a concentration of 0.1 μM. The first synthesis of (±)-14 was elegantly accomplished by Danishefsky and co-workers (Scheme 8).[87] Central to the strategy was an intramolecular Horner–Wads-worth–Emmons reaction that formed the A ring of 58. Subsequent formation of the C ring led to construction of key intermediate 59.
Scheme 8.
Part of the total synthesis of (±)-jiadifenin (14) by Dani-shefsky and co-workers.[87]
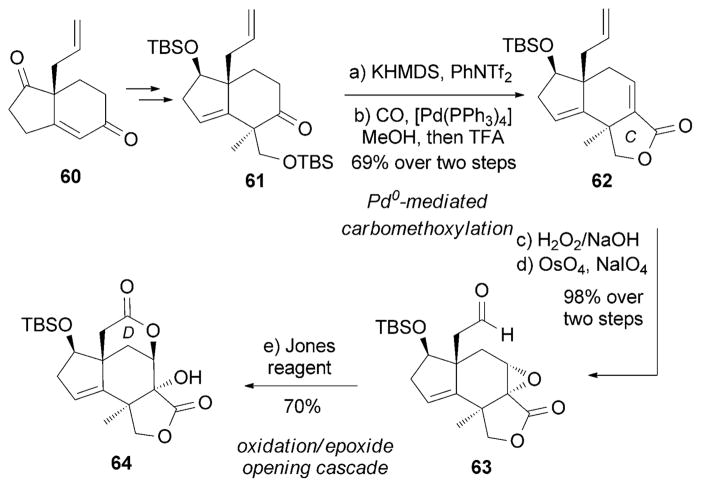
Recently, the Theodorakis research group reported an enantioselective synthesis of (−)-jiadifenin (14) from 60, which was obtained by an organocatalyzed[88] asymmetric Robinson annulation.[89] Palladium-mediated carbomethoxylation[90] followed by an oxidation/epoxide opening cascade was used to build the C/D ring system of 64, an advanced intermediate toward 14 (Scheme 9).
Scheme 9.
Part of the total synthesis of (−)-jiadifenin (14) by Theodorakis and co-workers.[89]
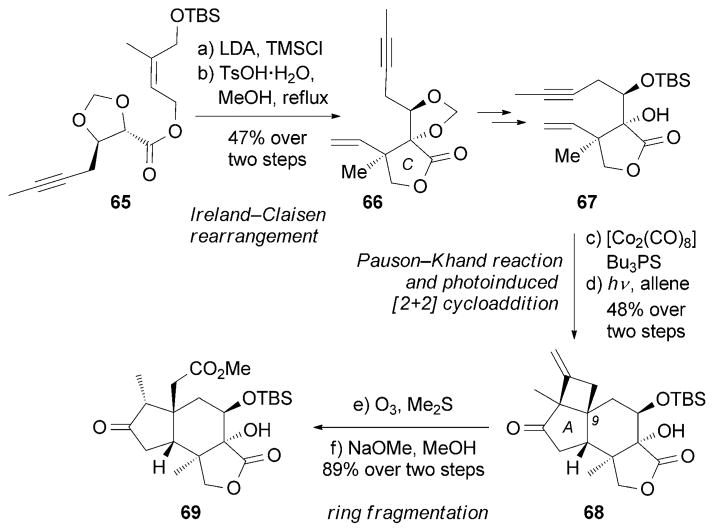
Another enantioselective route toward (−)-14 was recently reported by the Zhai research group (Scheme 10).[91] Critical to this strategy was an Ireland–Claisen rearrangement[92] to form the C ring of 66 followed by a Pauson–Khand reaction[85] to construct the A ring. Subsequently, an interesting photoinduced [2+2] cycloaddition between a pendant enone and an allene motif was used to install the C9 quaternary center in a diastereoselective manner. The cyclobutane moiety was then cleaved through a base-assisted ring fragmentation to afford advanced intermediate 69.
Scheme 10.
Part of the total synthesis of (−)-jiadifenin (14) by Zhai and co-workers.[91]
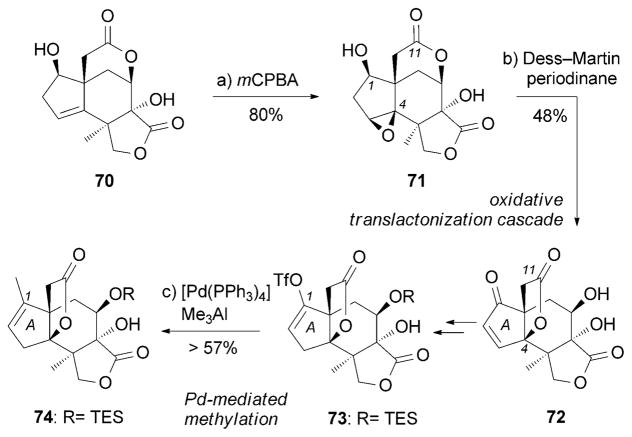
The pentacyclic sesquiterpenoid jiadifenolide (15) was isolated from pericarps of Illicium jiadifengpi.[65] Remarkably, 15 exhibited neurite outgrowth at concentrations as low as 10 nM, thus ranking as one of the most potent neurotrophic small molecules. Theodorakis and co-workers reported an enantioselective synthesis of (−)-15 that is highlighted by an unusual oxidative translactonization cascade (Scheme 11).[93] Treatment of allylic alcohol 70 with mCPBA afforded epoxide 71 in a diastereoselective manner.[94] 71 was then treated with Dess–Martin periodinane to produce the corresponding C1 ketone, which underwent epoxide opening followed by a C4 translactonization to furnish the desired five-membered lactone 72. Further functionalization of the A ring furnished triflate 73, which underwent a Pd0-mediated methylation to install the C1 methyl group in 74. This compound represents a fully functionalized framework of jiadifenolide.
Scheme 11.
Part of the total synthesis of (−)-jiadifenolide (15) by Theodorakis and co-workers.[93] mCPBA =m-chloroperbenzoic acid.
The above strategies paved the way to evaluate structure–activity relationship issues of jiadifenin (Figure 6). Notably, the Danishefsky[87] and Theodorakis research groups[95] have independently performed such studies on the jiadifenin structural motif and have identified several compounds, such as 75, ODNM (16), 76, and 77, which are more potent than the parent natural products in promoting neurite outgrowth. The neurotrophic effects were evaluated by measuring the stimulation of NGF-mediated neurite outgrowth in PC-12 cells.[96,97] These studies suggest that: a) removal of the C1 methyl group increases the neurotrophic activity of the jiadifenin motif, as shown by comparing 14 with 75, and 76 with 77; and b) the α substitution at the C10-position is critical for the activity (Figure 6). These compounds are likely to upregulate NGF signaling rather than mimicking NGF.[87,89,184]
Figure 6.
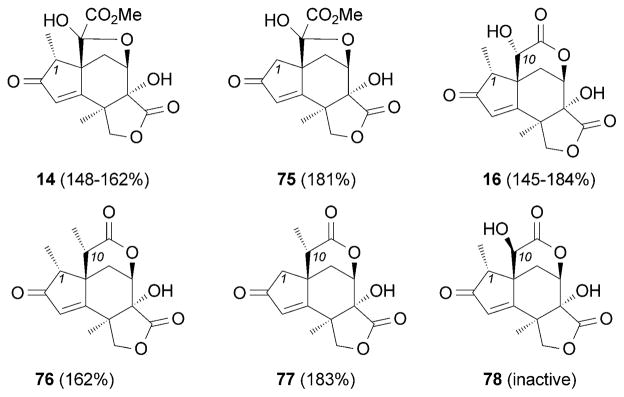
Representative structure–activity relationship studies on jiadifenin and analogues. The numbers in parenthesis indicate the extent of neurite outgrowth compared to a control; inactive indicates less than 110% neurite outgrowth as compared to control.
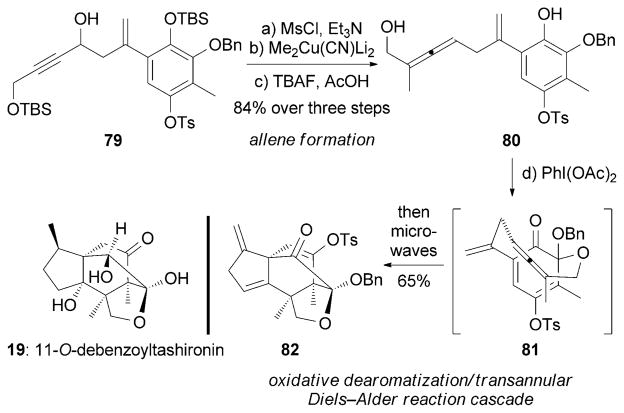
11-O-Debenzoyltashironin (19, Scheme 12) and its benzoate ester (tashironin, 20) were isolated from pericarps of Illicium merrillianum.[68,98] 19 was found to induce neurite outgrowth in cortical neurons of fetal rats at concentrations of as low as 0.1 μM, while 20 was inactive in this assay. Danishefsky and co-workers reported an impressive total synthesis of (±)-19.[99] The key step of their approach is a remarkable oxidative dearomatization/transannular Diels–Alder reaction cascade. This transformation was achieved using PhI(OAc)2 followed by a microwave-assisted intra-molecular Diels–Alder reaction.[100] The enantioselective construction of propargyl alcohol 79 led to an asymmetric synthesis of 19.[101] A synthesis of 11-O-methyldebenzoylta-shironin has also been reported by the Mehta research group.[102]
Scheme 12.
Part of the total synthesis of 11-O-debenzoyltashironin (19) by Danishefsky and co-workers.[99]
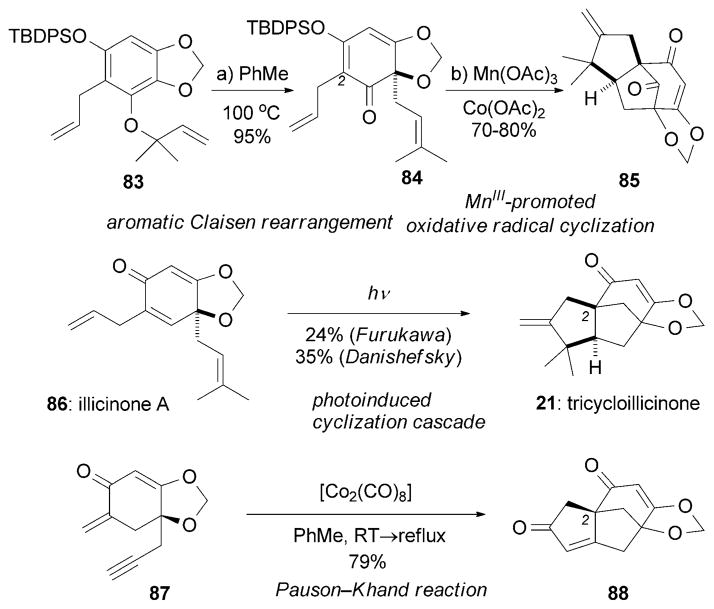
Tricycloillicinone (21) and bicycloillicinone (22) were isolated from the extracts of Illicium tashiroi by Fukuyama et al.[69,70] Interestingly, these compounds have been shown to enhance choline acetyltransferase (ChAT) activity at concentrations as low as 30 μM.[69,70] There is significant evidence that ChAT upregulation induces neurite outgrowth.[103] Specifically, it has been shown that the level of acetylcholine (ACh) is low in AD patients, thus suggesting that upregulation of ChAT represents a possible strategy for the treatment of AD.[104] In 1998, Danishefsky and co-workers reported the first total synthesis of 21.[105] Highlights of their strategy include an aromatic Claisen rearrangement followed by a MnIII-promoted oxidative radical cyclization (Scheme 13).[106] Heating protected phenol 83 in toluene produced the rearranged cyclohexadienone 84 in nearly quantitative yield. In turn, 84 was oxidized by MnIII in the presence of CoII as a co-oxidant to afford a resonance-stabilized radical[107] at C2, which underwent a cyclization cascade to yield the cyclic core of 85. A biomimetic trans formation of 21 from its likely biosynthetic precursor illicinone A (86) was initially described by Furukawa et al.[108] and later optimized by Danishefsky and co-workers.[109] A synthesis of ent-21 has also been reported by the Terashima research group[110] by using a Pauson–Khand reaction[85] to generate the challenging C2 quaternary center (conversion of 87 into 88). In addition, Danishefsky and coworkers have reported the synthesis of bicycloillicinone aldehyde.[105b]
Scheme 13.
Part of the total syntheses of tricycloillicinone (21) by Danishefsky et al. and Terashima and Furuya.[105] TBDPS =tert-butyldi-phenylsilyl.
2.3. Lycopodium Alkaloids
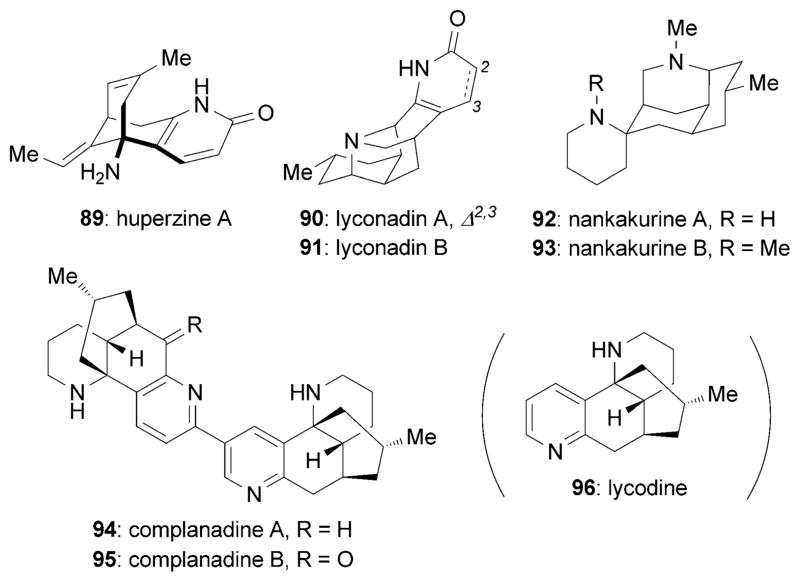
Several members of the Lycopodium alkaloids,[111] such as huperzine A (89),[112] lyconadins (90 and 91),[113] complanadine A (94)[114] and B (95),[115] and nankakurine A (92) and B (93), display highly promising neurotrophic profiles (Figure 7).[116]
Figure 7.
Neurotrophic Lycopodium alkaloids.
Huperzine A (89) was originally isolated from the Chinese plant Huperzia serrate by Liu et al. in 1986.[112] Huperzine A has been found to potently inhibit acetylcholinesterase (AChE)[117] and effectively cross the blood–brain barrier with no signs of cytotoxicity and minimal side effects.[118] Both NGF and P75NTR levels increased in the presence of 89 in studies performed in PC-12 cells.[119,120] Huperzine A was shown to prevent oxidative damage in SHSY5Y cells, presumably as a result of its ability to increase NGF production.[121] Studies in mice demonstrated that huperzine A, administered at 0.2 mgkg−1, increases the concentration of various proteins, including NGF, BDNF, and phosphorylated MAPK.[122] Interestingly, 89 was also reported to promote hippocampal neurogenesis in vitro and in vivo.[120] Moreover, 89 is an approved drug in China to treat cognitive deficiencies associated with AD, and is being offered as a herbal supplement in the USA.[123] The pharmacology and therapeutic potential of huperzine A has recently been reviewed.[124]
The total synthesis of (±)-89 was independently accomplished by Kozikowski and Xia[125] as well as Qian and Ji[126] by using an almost identical strategy to construct the huperzine A core. Specifically, a one-pot Michael addition/aldol reaction of 97 with methacrolein formed the desired carbon bridge of 98 (Scheme 14).
Scheme 14.
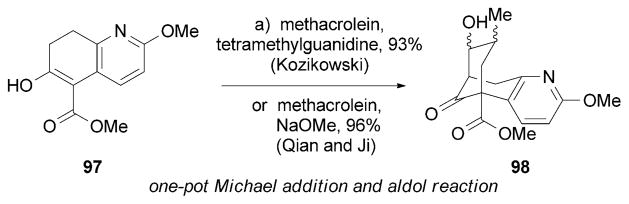
Part of the total syntheses of (±)-huperzine A (89) by Xia and Kozikowski as well as Qian and Ji.[125,126]
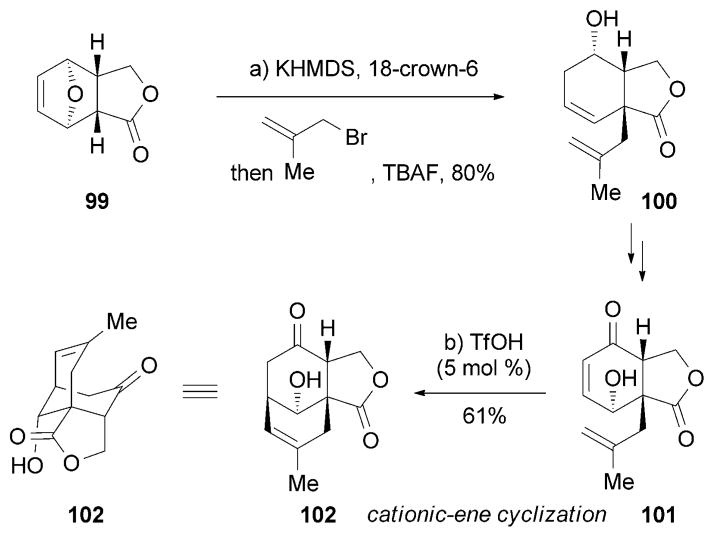
The Fukuyama research group has also reported a synthesis of (−)-89[127] (Scheme 15). Key to this strategy was a TfOH-catalyzed cationic ene cyclization that produced tricyclic lactone 102. Precursor 100 could be constructed from readily available 99 in one step.
Scheme 15.
Part of the total synthesis of (−)-huperzine A (89) by Fukuyama and co-workers.[127]
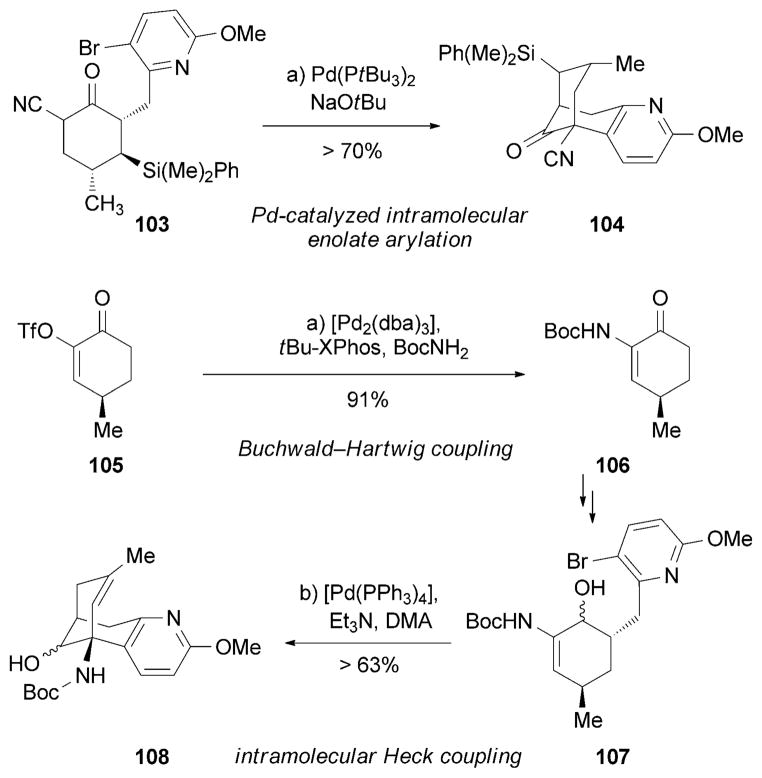
The growing pharmacological interest in (−)-huperzine A (89) prompted two efficient syntheses of this molecule by using palladium-catalyzed coupling reactions as a key step (Scheme 16).[128] Specifically, Herzon and co-workers reported a scalable synthesis of huperzine A[129] that relies on a modified intramolecular Heck coupling of α-cyanoketone 103 to assemble the carbon bridge of 104.[130] Lin and coworkers also disclosed a synthesis of 89[131] by using a Buchwald–Hartwig coupling reaction[132] between enol triflate 105 and Boc-NH2 to form enamine 106. A subsequent intra-molecular Heck reaction[133] of 107 led to the bridged motif of 108. Several other syntheses and formal syntheses of huperzine A (89) have also been reported.[134]
Scheme 16.
Part of the total syntheses of (−)-huperzine A (89) by Herzon and co-workers and Lin and co-workers.[129,131] Boc =tert-butoxycarbonyl.
Lyconadins (90 and 91)[113] and complanadines (94 and 95)[114,115,135] are particularly attractive small molecules since they enhance the mRNA expression of NGF in human glial cells.[113b,115] In addition, 90 displayed modest cytotoxicity against murine lymphoma L1210 and human epidermoid carcinoma KB cells,[113a] while 94 exhibited modest cytotoxicity against murine lymphoma L1210 cells.[114]
From a structural point of view, lyconadins possess a pyridinone-fused tetracyclic caged framework, which constitutes the central synthetic challenge for 90. The Smith research group reported the first total synthesis of (+)-90 and (−)-91 (Scheme 17).[136] The tricyclic intermediate 110 was crafted by an aldol/1,4-addition reaction cascade from aldehyde 109. After a few synthetic steps, amine 111 underwent a NIS-induced cyclization to furnish the tetracyclic motif 112.
Scheme 17.
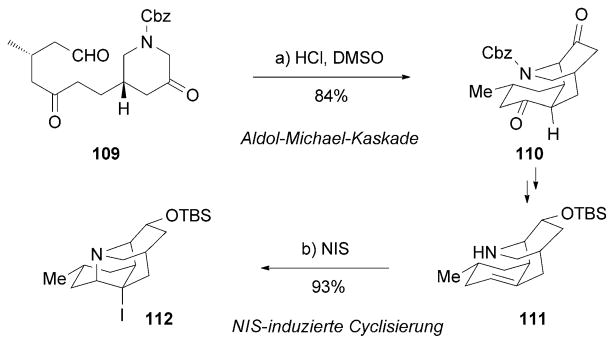
Part of the total synthesis of (+)-lyconadin A (90) and (−)-B (91) by Beshore and Smith.[136] Cbz =carboxybenzyl, NIS =N-iodosuccinimide.
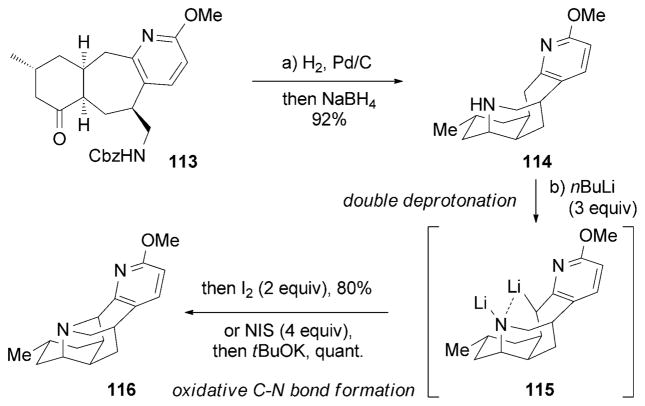
One year later, the Sarpong research group reported the synthesis of (±)- and (+)-90.[137] Double deprotonation of 114 formed dianion 115, which was oxidized with iodine to form the critical C–N bond of 116. This bond formation can also be accomplished by an NIS-mediated SN2 reaction (Scheme 18).
Scheme 18.
Part of the total synthesis of lyconadin A (90) by Sarpong and co-workers.[137]
Another interesting synthesis of (+)-90 and (−)-lyconadins B and C was reported by the Fukuyama research group (Scheme 19)[138] The authors utilized dibromocyclopropane 119 as the key motif, which was prepared from amine 117 by an aza-Prins reaction followed by a dibromocyclopropanation. Subsequently, 119 underwent an acid-promoted ring expansion to produce the tetracyclic core of 120.
Scheme 19.
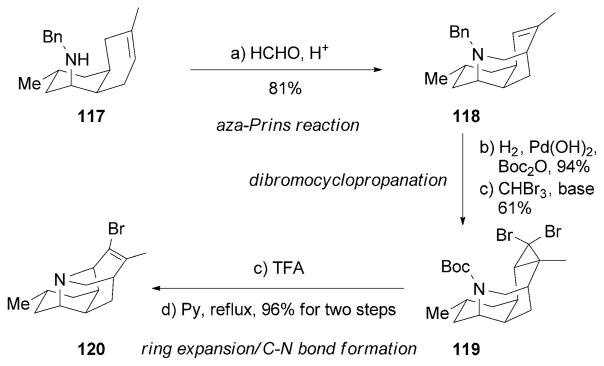
Part of the total syntheses of lyconadins by Fukuyama and co-workers.[138] Py=pyridine.
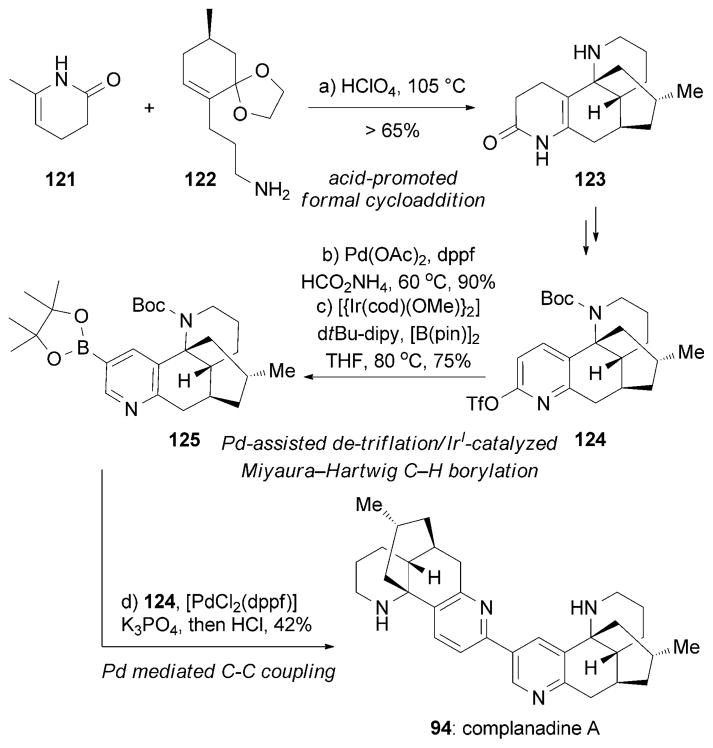
Complanadine A (94) is an unsymmetric dimer of lycodine (96),[139] while complanadine B (95) can be considered as a pseudodimer of 96. Independent studies by the Sarpong and Siegel research groups have yielded two intriguing strategies for the synthesis of (+)-complanadine A. In the former study, Fischer and Sarpong started the synthesis[140] from tetracyclic compound 123 following the procedure developed by Schumann and Naumann (Scheme 20).[141] The authors converted triflated lycodine derivative 124 into boronic ester 125 under reductive detriflation conditions, followed by an IrI-catalyzed Miyaura–Hartwig C–H borylation.[142] Pd-based cross-coupling between 125 and triflate 124, followed by cleavage of the Boc group, produced (+)-94. A synthesis of complanadine B (95) has also been reported by similar strategies.[143]
Scheme 20.
Part of the total synthesis of (+)-complanadine A (94) by Fischer and Sarpong.[140] [B(pin)]2 = diboron pinacolato ester, dppf =1,1′-bis(diphenyl phosphino) ferrocene, dtBu-dipy=di-tert-butyl-bipyridine.
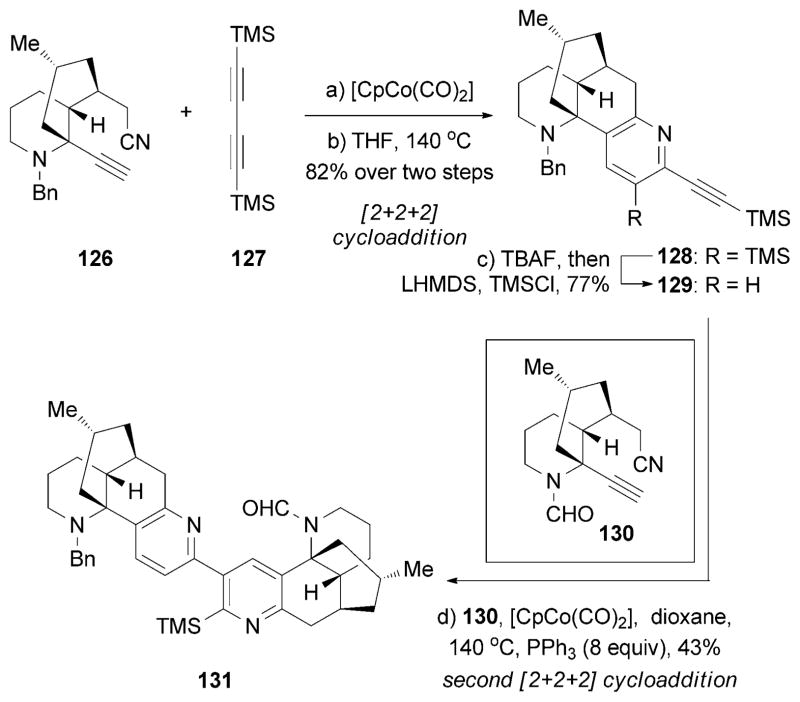
In parallel, the Siegel research group reported a synthesis of (+)-94 (Scheme 21).[144] Key to this strategy was a [2+2+2] cycloaddition of 126 with 127 to produce lycodine analogue 129. This compound was then coupled with 130 to yield complanadine precursor 131. Notably, it was found that both the presence of PPh3 and the formyl motif of 130 were essential for the regioselectivity of the second cycloaddition. More recently, Tsukano and co-workers reported a synthesis of (−)-complanadine A and B[145] by a Pd-catalyzed coupling[146] of N-oxolycodine with α-bromolycodine.[147]
Scheme 21.
Part of the total synthesis of (+)-complanadine A (94) by Siegel and co-workers.[144]
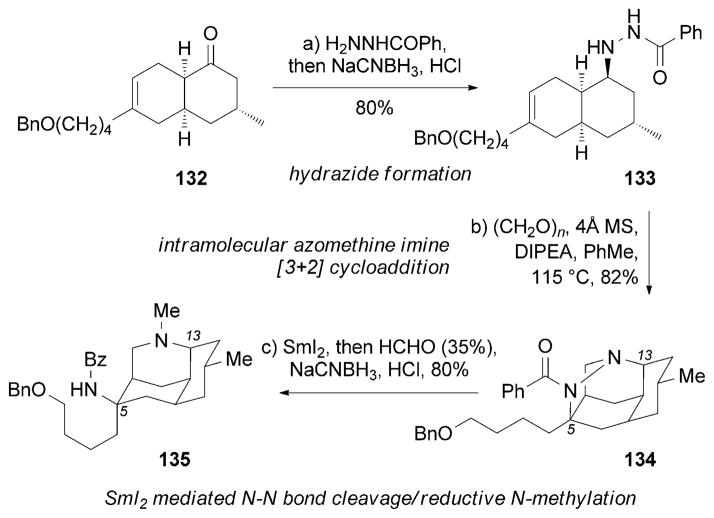
Nankakurine A (92), another Lycopodium natural product, was reported to possess neurotrophic activity in human astrocytoma cells.[116b] 92 was isolated from Lycopodium hamiltonii in 2004,[116a] and its originally proposed structure was revised two years later.[116b] The Overman research group reported the first synthesis of (+)-nankakurine A (92) and B (93), thereby establishing their absolute configurations (Scheme 22).[148] Critical to the synthetic strategy was an intramolecular azomethine imine [3+2] cycloaddition of 133 that installed the two nitrogen atoms at C5 and C13 in a stereoselective manner. Reductive cleavage of the N–N bond in 134 released the free amine at C13, which was reductively methylated to form 135.
Scheme 22.
Part of the total syntheses of (+)-nankakurines by Overman and co-workers.[148]
Cheng and Waters reported a short synthesis of (±)-92 and (±)-93 (Scheme 23).[149] Amine 139 was derived from an intermolecular Diels–Alder reaction between 136 and 137 followed by reductive amination of the resulting ketone 138. Upon treatment with formaldehyde, a Mannich-type cyclization produced luciduline (140), a known precursor of nankakurines.[150]
Scheme 23.
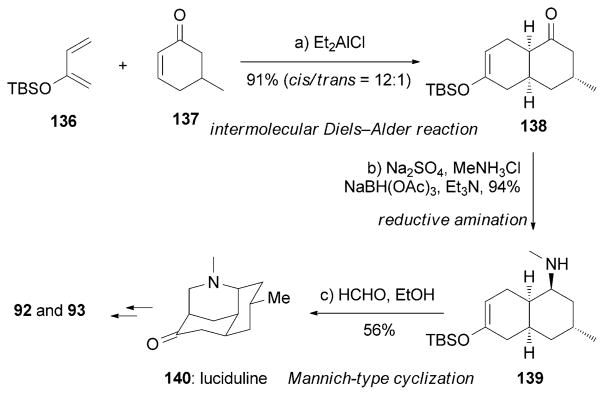
Part of the total syntheses of (±)-nankakurines by Cheng and Waters.[149]
In a recent study, Overman and co-workers reported that 92 and 93 showed no effect on neurite outgrowth in rat hippocampus H-19 cells,[148b] which stands in contrast to the previously reported results.[116b] Certainly, further biological investigation of these intricate Lycodium alkaloids would be necessary to unveil their biological potential.
2.4. Cyathane Diterpenoids
Isolated from fungi, sponges, and fruiting plants, cyathane diterpenes possess a variety of bioactivities including antimicrobial activity and cytotoxicity against certain tumor cells.[151] In addition, erinacines,[152] scabronines,[153] and cyrneines[154] were found to induce NGF synthesis in 1321N1 human astrocytoma cells and mouse astroglial cells as well as promote neurite outgrowth in PC-12 cells at low micromolar concentrations. From a chemical point of view, the cyathane natural product family possess a skeleton with 20 carbon atoms (141) and a 5-6-7 fused tricyclic ring system (Figure 8). Since the first report on this class in 1971,[155] the family of cyathane diterpenoids has prompted intensive synthetic and biological studies.[151]
Figure 8.
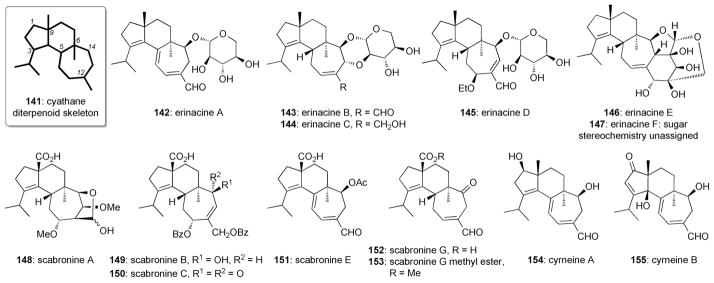
Neurotrophic cyathane diterpenoids.
Erinacines A–F (142–147; Figure 8) were isolated from the cultured mycelia of Hericium erinacium by Kawagishi et al.[152] These cyathane xylosides are able to stimulate NGF secretion in mouse astroglial cells, albeit at rather high concentrations (1–5 mM). The in vivo effect of erinacine A (142) was also studied. Specifically, rats treated with 142 showed an increase in the levels of both noradrenaline and homovanillic acid, and displayed enhanced NGF secretions in both the locus coeruleus and hippocampus.[156] Moreover, erinacine E is a selective agonist of κ-opioid receptors,[157] which are present on the peripheral terminals of primary afferent neurons. It has been reported that activation of these receptors reduces hyperalgesia in a rat model of inflammation.[158]
An efficient synthesis of (+)-erinacine A (142), was reported by the Snider research group (Scheme 24).[159] Key to this strategy was an intramolecular carbonylene reaction[160] of 157 that furnished erinacine A aglycon (158). The synthesis of 158, also known as allocyathin B2, has also been reported by various research groups.[161]
Scheme 24.
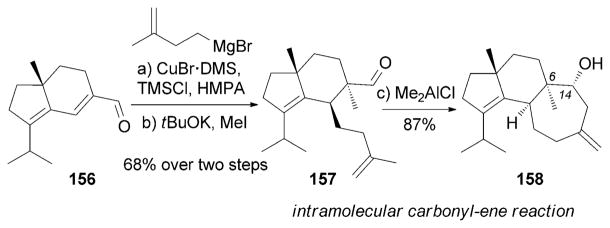
Part of the total synthesis of (+)-erinacine A (142) by Snider et al.[159] DMS=dimethylsulfide, HMPA=hexamethylphosphorus amide.
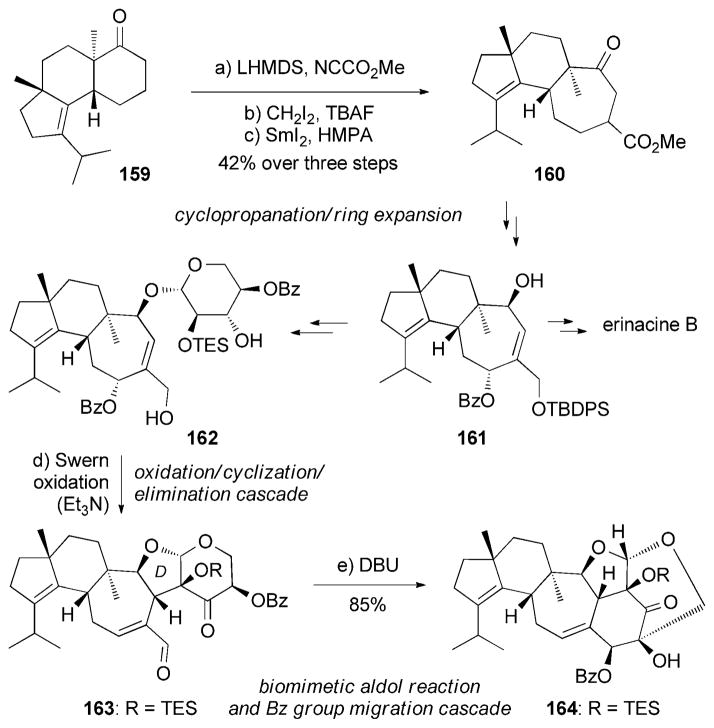
Nakada and co-workers recently disclosed a divergent synthesis of (−)-erinacines B (143)[162] and E (146),[161e,163] by using 161 as the common intermediate (Scheme 25). The tricyclic motif of 161 was constructed through a sequence of three steps that included: a) carbomethoxylation of ketone 159 with Mander’s reagent,[164] b) cyclopropanation, and c) SmI2-assisted ring expansion. Interestingly, the D ring of 163 could be constructed during a Swern oxidation of diol 162, presumably through the presence of triethylamine. In turn, a well-designed biomimetic intramolecular aldol reaction[163] of 163 followed by a 1,2-migration of a benzoyl group (Scheme 25) produced the motif 164, thus paving the way for the synthesis of erinacine E (146).
Scheme 25.
Part of the total syntheses of (−)-erinacine B (143) and (−)-E (146) by Watanabe and Nakada.[163] Bz =benzoyl, DBU =1,8-diazabicyclo[5.4.0]undec-7-ene.
Scabronines A–C (148–150), E (151), and G (152) have been isolated from the mushroom Sarcodon scabrosus.[153] Compounds 148 and 152 potently induce NGF synthesis in 1321N1 human astrocytoma cells in a concentration-dependent manner.[153b,c] It was also found that 148 and 152 enhanced NGF mRNA by 3.2-fold and 3.6-fold, respectively, in the same cell line.[153c] However, it was observed that the concentration of NGF synthesized in response to these compounds was not sufficient to induce differentiation of PC-12 cells. Based on this finding, the authors suggested that other neurotrophic growth factors might be involved in the PC-12 cell differentiation process.[153c]
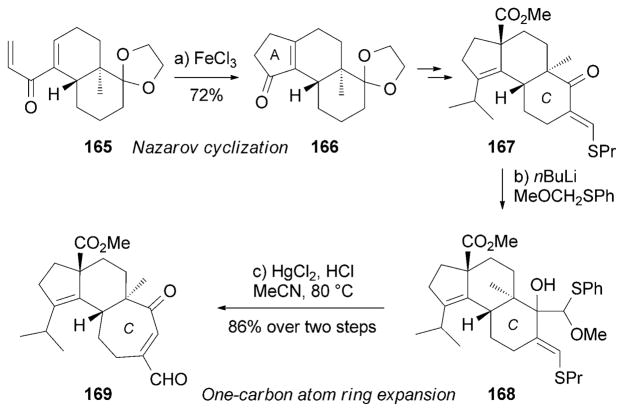
The Danishefsky research group initiated a synthesis of (−)-scabronine G (152)[165] from enone 165, which is available from the Wieland–Miescher ketone (Scheme 26).[166] Nazarov cyclization[78] of 165 produced the A-ring motif of 166 which was transformed into 167 over several steps. 167 was then subjected to lithiated (methoxymethyl)phenyl sulfide to afford alcohol 168. Treatment of 168 with HgCl2 under acidic conditions facilitated the desired one-carbon-atom expansion of the C ring to furnish conjugated cycloheptenone 169, which was then converted into 152.[167]
Scheme 26.
Part of the total synthesis of (−)-scabronine G (152) by Danishefsky and co-workers.[165]
In 2011 another synthesis of (−)-scabronine G (152) was reported by the Kanoh, Iwabuchi et al. (Scheme 27).[168] Key to this synthesis was an intramolecular double 1,4-addition of 170 under TMSI/HMDS conditions, which afforded the bridged tricyclic scaffold of 171. Another critical step was a Lewis acid induced Prins cyclization of 172 to construct the seven-membered ring of 173.[159] More recently, the Nakada research group reported a divergent synthesis of (−)-scabronines A (148) and G (152).[169]
Scheme 27.
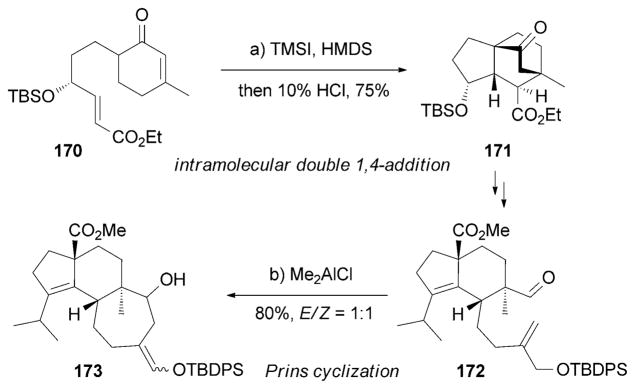
Part of the total synthesis of (−)-scabronine G (152) by Kanoh, Iwabuchi et al.[168] HMDS =hexamethyldisilazane.
In addition, the scabronine G methyl ester (153) was synthesized from 152 and biologically evaluated by the Nakahata research group.[170] This compound displayed stronger neurite outgrowth activity and NGF-synthesis-inducing activity than scabronine G in 1321N cells and PC-12 cells (in the 153-conditioned 1321N cell medium). Similar activities have been reported by Danishefsky and co-workers.[165] Furthermore, the synthetic analogue 169 was found to possess a 30% greater neurite-outgrowth activity than 153.[165] Additional studies on the effect of 153 on NGF synthesis in 1321N cells were reported by Nakahata and co-workers.[170] These authors suggested that the 153-induced NGF synthesis is mediated by the activation of PKC-ζ, an isoform of protein kinase C.[170]
Interestingly, scabronine M (174) significantly inhibited NGF-induced neurite outgrowth in PC-12 cells without showing any cytotoxicity at the concentrations tested (Figure 9).[171] Gao and co-workers suggested that this activity might be due to suppressing the phosphorylation of TrkA and ERK. Moreover, the epoxide motif of 174 was proposed to be the reason for the potent inhibition of neurite outgrowth.
Figure 9.
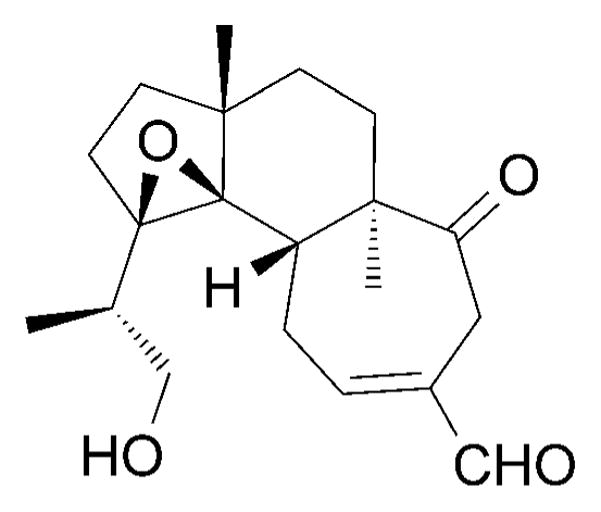
Structure of scabronine M (174).
Cyrneines A (154) and B (155) were isolated from the mushroom Sarcodon cyrneus in 2006.[154] Both compounds induced moderate neurite outgrowth in PC-12 cells at high micromolar concentrations without significant toxicity.[154] It was reported that 154 upregulates the transcription factors activator protein-1 (AP-1) and NF-κB. Moreover, the activity of Rac-1, a small GTPase protein that regulates actin dynamics, was increased, while expression of a dominant-negative Rac-1 mutant significantly inhibited the 154-induced neurite outgrowth in PC-12 cells. Based on these findings, it was suggested that 154 promotes neurite outgrowth through a Rac-1-dependent mechanism.[172]
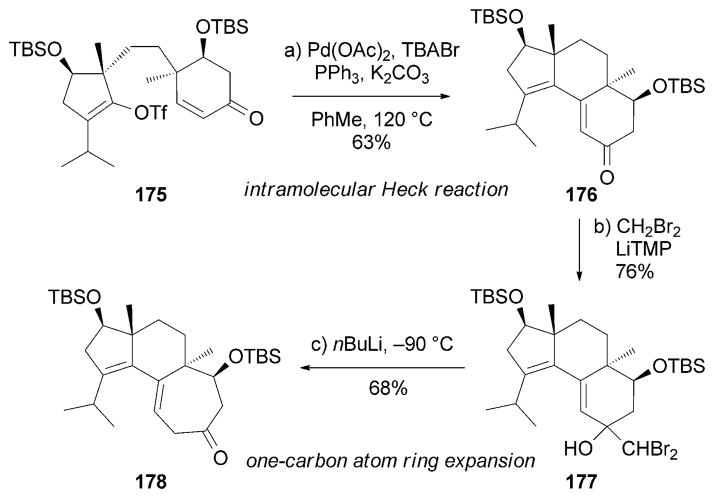
The Gademann research group recently reported a total synthesis of (+)-cyrneine A (154) starting from commercially available (R)-carvone.[173] Critical to this strategy is an intramolecular Heck coupling reaction that constructed the tricyclic core of 176. Treatment of 176 with lithiated dibromo-methane followed by lithium/halogen exchange resulted in a one-carbon-atom ring expansion to form the desired tricyclic motif of 178 (Scheme 28).
Scheme 28.
Part of the total synthesis of (+)-cyrneine A (154) by Gademann and co-workers.[173] TBABr=tetrabutylammonium bromide, TMP =2,2,6,6-tetramethylpiperidine.
2.5. Trichothecanes
Isolated from the fruiting bodies of Paecilomyces tenuipes, the trichothecanes paecilomycine A (179)[174] and spirotenuipesines A (180) and B (181)[175] display significant neurotrophic profiles (Figure 10). Paecilomycine A was reported to stimulate the synthesis of neurotrophic factors, since 1321N medium cultured with 179 (10 nM) was found to promote the differentiation of PC-12 cells.[174] The reported potency of 179 was about 1000 times higher than that of scabronine G (152) in the same NGF-stimulation assay. The related spirotenuipesine A (180) and B (181) were also shown to induce the biosynthesis of neurotrophic factors in 1321N cells, albeit at a higher concentration (1 μM).[175]
Figure 10.
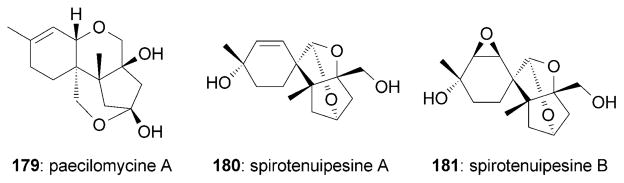
Neurotrophic trichothecanes.
Min and Danishefsky reported an efficient synthesis of (±)-179 starting with a stereoselective Diels–Alder reaction (endo/exo >20:1) between diene 182 and dienophile 183 (Scheme 29).[176] Product 184 was then converted into alkyne 185. An intramolecular Pauson–Khand reaction[85] followed by Luche reduction of the resulting enone produced alcohol 186. Diastereoselective cyclopropanation of 186 followed by ring opening by an oxidation/reduction sequence afforded the desired C5 quaternary center. Inspired by this approach, the Mehta research group also reported a formal synthesis of 179.[177]
Scheme 29.
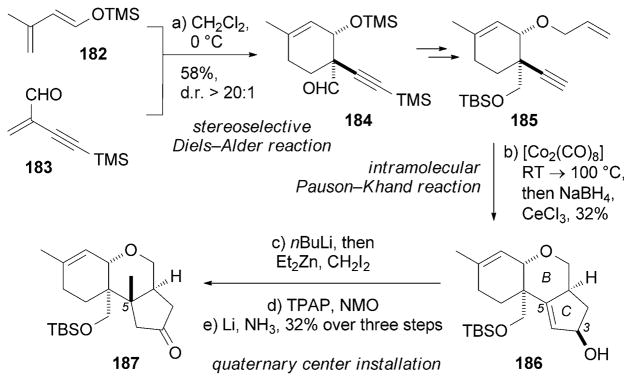
Part of the total synthesis of (±)-paecilomycine A (179) by Min and Danishefsky.[176] NMO =N-methylmorpholine N-oxide, TPAP =tetrapropylammonium perruthenate.
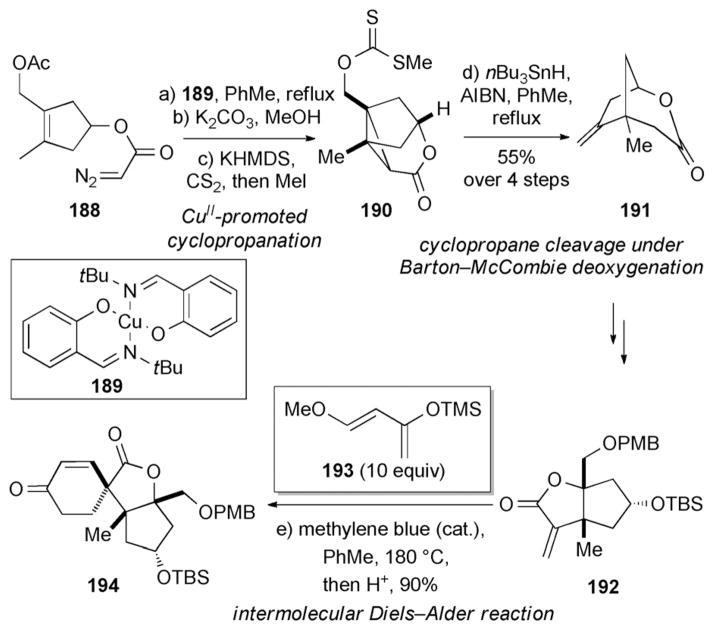
The Danishefsky research group has also achieved the synthesis of (±)-spirotenuipesine A (180) and B (181) (Scheme 30).[178] In this approach the tricyclic motif of 190 was formed through a CuII-promoted intramolecular cyclo-propanation.[179] This was followed by a radical deoxygenation under Barton–McCombie conditions, which also cleaved the strained cyclopropane ring of 190 to afford 191.[180] Another key step involved the construction of enone 194 by a Diels–Alder reaction between 192 and Danishefsky diene 193.[181]
Scheme 30.
Part of the total syntheses of (±)-spirotenuipesine A (180) and B (181) by Danishefsky and co-workers.[178] PMB =p-methoxyben-zyl.
2.6. Manzamine Alkaloids
Isolated from marine sponges of the genera Haliclona and Pellina, the manzamine family is comprised of various structurally complex indole alkaloids.[182] Among them, manzamine A (195)[183] displays a broad biological profile that includes anticancer, antibacterial, insecticidal, antimalarial, anti-inflammatory, and anti-HIV activities.[184] In addition, manzamines A (195), E (197), F (198), and 8-hydroxymanzamine A (196) were shown to exhibit potent neuritogenic activities in Neuro 2A cells at concentrations as low as 1 μM (Figure 11).[185] Furthermore, it was proposed that 195 could act through the same mechanism reported for lactacystin (1).[185]
Figure 11.
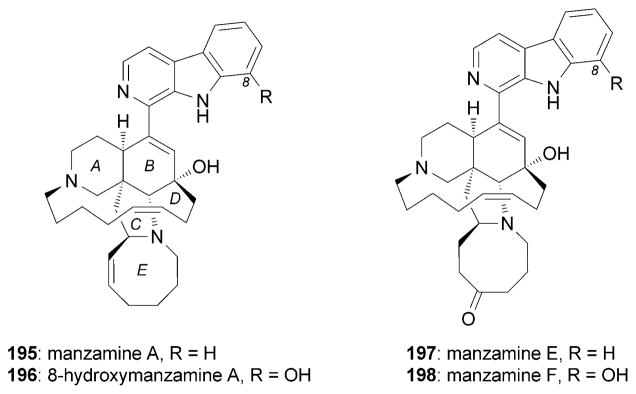
Neurotrophic manzamine natural products.
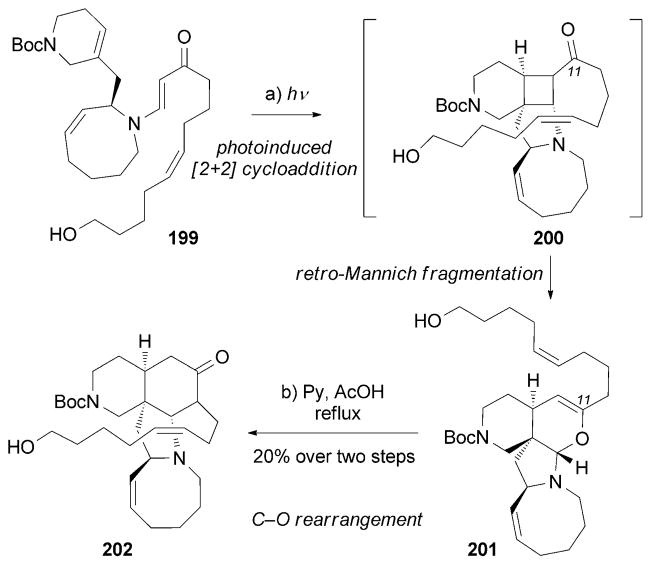
The complex chemical architecture and exciting biological profile of manzamine alkaloids attracted considerable attention from the synthetic community.[182a–c] Winkler and Axten accomplished the first total synthesis of (+)-195 in 1998 (Scheme 31).[186] The construction of the tetracyclic core of 201 was elegantly initiated through a photoinduced [2+2] intramolecular cycloaddition between the enone moiety and the tetrahydropyridine motif of 199. The transiently formed cyclobutane ring of 200 underwent a retro-Mannich fragmentation, and the resulting iminium ion was trapped by the C11 enol to form aminal 201. The corresponding C→O rearrangement furnished the crucial tetracyclic motif 202 in pyridine/ AcOH at elevated temperature.
Scheme 31.
Part of the total synthesis of (+)-manzamine A (195) by Winkler and Axten.[186]
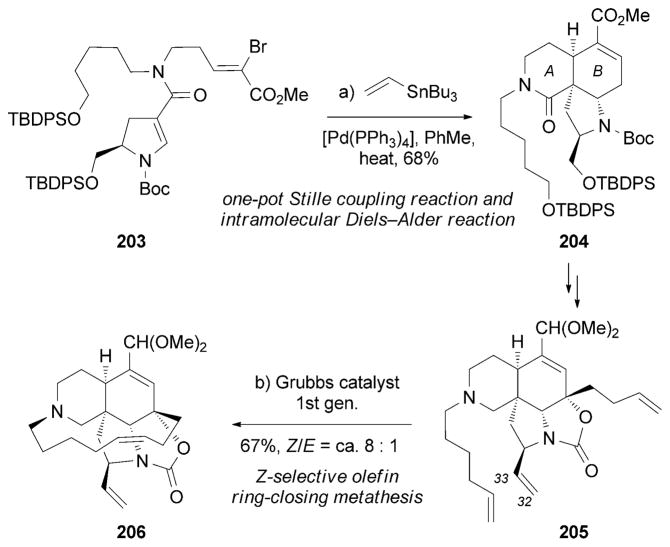
Martin and co-workers also reported an efficient synthesis of (+)-195.[187] The AB ring system of 195 was constructed through a one-pot Stille coupling[188] between vinyl bromide 203 and vinyltributylstannane. This reaction produced in situ the corresponding diene, which underwent an intramolecular Diels–Alder cyclization to form 204. In turn, the macrocyclic ring of 206 was built through the assistance of a Z-selective (ca. 8:1) ring-closing metathesis (RCM) reaction[189] of the two terminal alkenes of 205. Interestingly, the C32/C33 terminal alkene of 205 did not interfere with the formation of this 13-membered ring (Scheme 32).
Scheme 32.
Part of the total synthesis of (+)-manzamine A (195) by Martin et al.[187]
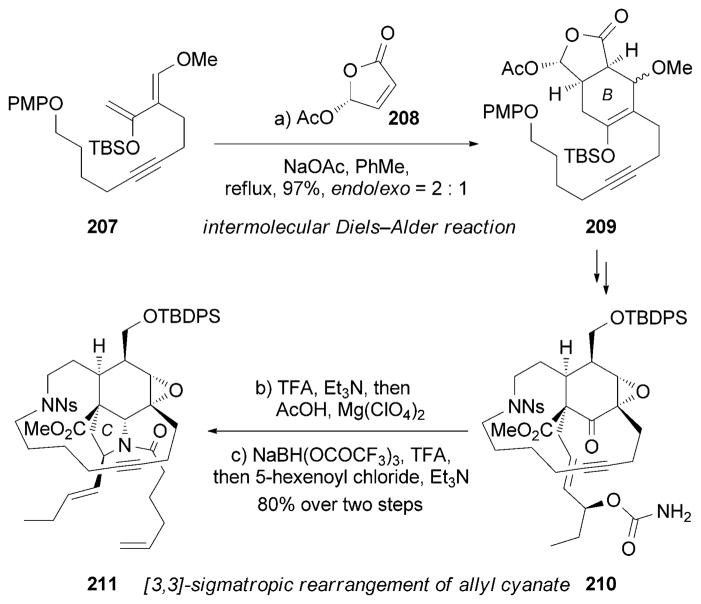
Another interesting synthesis of (+)-195 was reported by the Fukuyama research group.[184] An intermolecular Diels–Alder reaction between the Danishefskydiene-like motif 207 and chiral butenolide 208 provided the B ring of 209. Subsequently, a well-designed [3,3] sigmatropic rearrangement of allyl cyanate 210 furnished the desired pyrrolidine motif of 211. Notably, the stereochemistry of the preformed macrocycle dictated the stereoselectivity during the subsequent chemical steps (Scheme 33).
Scheme 33.
Part of the total synthesis of (+)-manzamine A (195) by Fukuyama and co-workers.[184] PMP=p-methoxyphenyl.
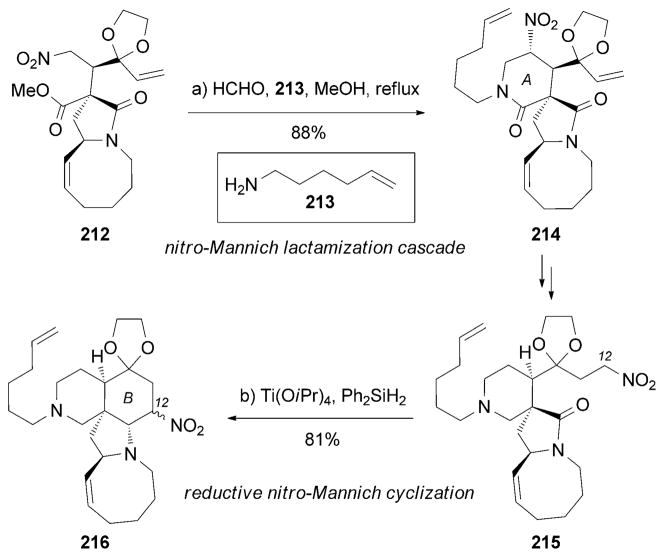
An alternative synthesis of (+)-195 was recently accomplished by Dixon and co-workers.[190] Highlights of this synthesis include: a) a three-component nitro-Mannich lactamization cascade between 212, formaldehyde, and amine 213 to form the A-ring motif in 214; and b) a novel reductive nitro-Mannich cyclization between the C12 alkyl nitrate and the five-membered lactam moiety of 215 in the presence of Ti(OiPr)4/PhSiH2 to generate the B ring of 216 (Scheme 34).
Scheme 34.
Part of the total synthesis of (+)-manzamine A (195) by Dixon and co-workers.[190]
In addition to its neurotrophic activities, 195 was found to be a potent non-ATP competitive inhibitor of GSK-3β, a serine-threonine kinase known to phosphorylate the microtubule-associated protein tau in mammalian cells.[191] It has been found that overexpression of GSK-3 leads to abnormally hyperphosphorylated tau protein, which can no longer bind to microtubules but instead are free and available to undergo filament assembly.[192] Such hyperphosphorylation is believed to be an early event in various neuropathological conditions including brain impairment, learning/memory deficits, depression, AD, and ALS. Thus, inhibiting the abnormally upregulated GSK-3 activity has become an important strategy for NDD treatment.[192] A potential binding site of 195 to GSK-3 was reported.[193]
2.7. Steroids
Various steroids, such as NGA0187 (217) and with anolide A (218), display interesting neurotrophic activities (Figure 12). NGA0187 was isolated from Acremonium sp. TF-0356[194] and is considered to be the first neurotrophic steroid.[10b,195] It was found that 217 induces neurite outgrowth in PC-12 cells at a concentration of 30–60 μM.[194,195] The Danishefsky research group reported a synthesis of (+)-217 starting from adrenosterone (Scheme 35).[195] The strategy is highlighted by a conjugate addition of the lithium-copper complex of 220 to enone 219 to produce advanced intermediate 221. Interestingly, 220 was obtained in an enantiomerically pure form from oxidative cleavage of the side chain of ergosterol (222). Moreover, several C17 side chain truncated analogues of 217 have been synthesized and evaluated. These analogues have shown no appreciable activities, which suggests that the C17 side chain may play a critical role in the neurotrophic activity of NGA0187.[195]
Figure 12.
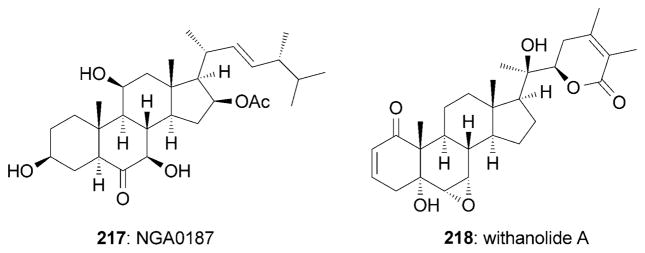
Representative neurotrophic steroids.
Scheme 35.
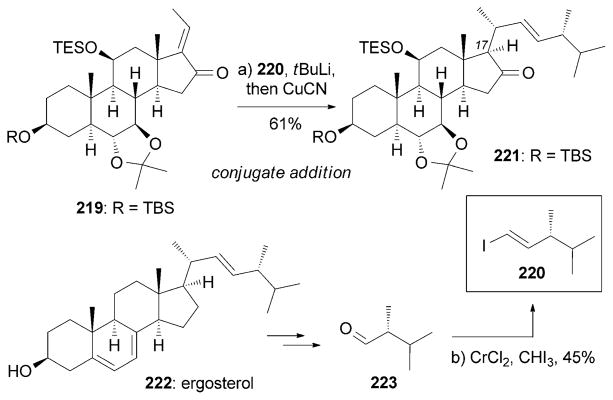
Part of the total synthesis of (+)-NGA0187 (217) by Danishefsky and co-workers.[195]
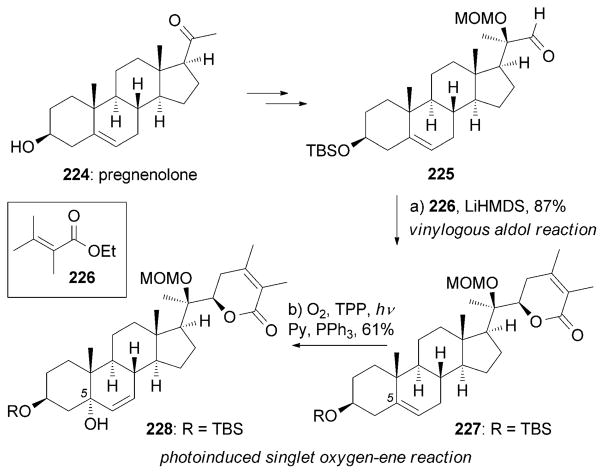
Withanolide A (218) and related steroids were isolated from the roots of Withania somnifera and have shown potent neurite outgrowth activities in human neuroblastoma SH-SY5Y cells at 1 μM.[196] The Gademann research group designed a synthesis of (+)-218 starting from pregnenolone (224; Scheme 36).[197] Crucial steps of this approach include: a) a vinylogous aldol reaction between aldehyde 225 and the lithium enolate of 226 to afford lactone 227; and b) an elegant singlet oxygenene reaction[198] to produce the C5 tertiary alcohol of 228. The singlet oxygen was generated in situ from oxygen under irradiation with a Na lamp in the presence of mesotetraphenylporphyrin (TPP) as the sensitizer.
Scheme 36.
Part of the total synthesis of (+)-withanolide A (218) by Gademann and co-workers.[197] MOM=methoxymethyl, TPP =mesotetraphenylporphyrin.
It has been demonstrated that 218 significantly induces axonal/dendritic regeneration and synaptic reconstruction in the impaired mouse brain and damaged rat cortical neurons.[196b] However, conflicting results precluded a clearer view of the mechanism of action of 218.[197a,199]
Certain steroids, such as β-estradiol, were shown to induce neurite outgrowth through the MAPK pathway (Figure 2)[200] and enhance synaptophysin expression via membrane estrogen receptor and p44 MAP kinase.[201] In addition to 217 and 218, several other steroids,[202] such as deoxygedunin,[203] allopregnanolone,[204] and S19159,[205] have been reported to possess neurotrophic activities.[206] The signaling mechanisms of these steroids are likely similar to that of β-estradiol.[196b,206]
2.8. Polyprenylated Acylphloroglucinols
Polyprenylated acylphloroglucinols (PPAPs) possess an intricate polycyclic structure and a broad range of important bioactivities.[207] Hyperforin (229) and garsubellin A (230) are two representative samples (Figure 13). The neurite outgrowth effects of hyperforin (229) have been well documented.[208] On the other hand, garsubellin A (230), isolated from the wood of Garcinia subelliptica, was proposed to display potent neurotrophic activities by enhancing choline acetyltransferase (ChAT) activity in P10 septal neurons of cultures rats (154% relative to control).[209]
Figure 13.
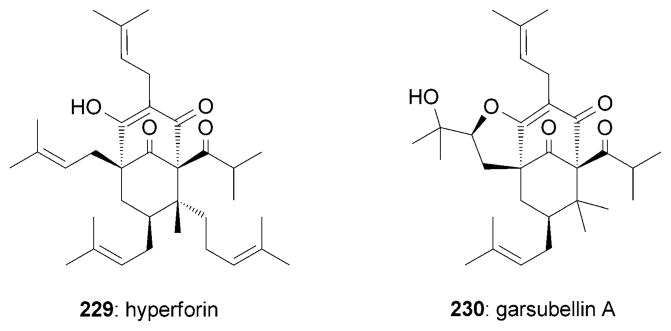
Representative neurotrophic PPAPs.
Hyperforin (229) was isolated from the herb St. John’s wort (Hypericum perforatum) in 1971.[210] In addition to other important bioactivies,[211] 229 was shown to induce promising neurological effects. For example, the antidepressant activity of 229 was attributed to its ability to inhibit neuronal uptake of various neurotransmitters.[208a–f] Furthermore, 229 is known to induce neurite outgrowth (10 μM)[208g] by directly and selectively activating a member of the canonical transient receptor potential (TRPC) family of ion channels.[208g,212] Moreover, 229 was reported to affect the processing of the amyloid precursor protein (APP)[213] and to prevent formation of the amyloid-β (Aβ) deposit, Aβ neurotoxicity, and spatial memory impairments.[214] In addition, 229 stimulates the expression of TrkB, the selective BDNF receptor.[215]
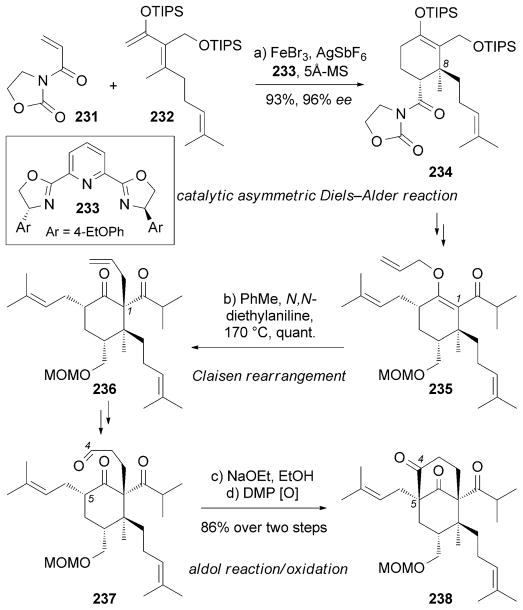
A total synthesis of (−)-229 was accomplished by Kanai, Shibasaki, and co-workers.[216] This approach stems from a catalytic asymmetric Diels–Alder reaction[217] between dienophile 231 and diene 232 promoted by an FeIII complex with PyBOX ligand 233 (Scheme 37). Claisen rearrangement[92] of allyl enol ether 235 installed the C1 quaternary center of 236. The C5 quaternary center of 238 was set through an intramolecular aldol reaction of 237.
Scheme 37.
Part of the total synthesis of (−)-hyperforin (229) by Kanai, Shibasaki and co-workers.[216] DMP=Dess–Martin periodinane.
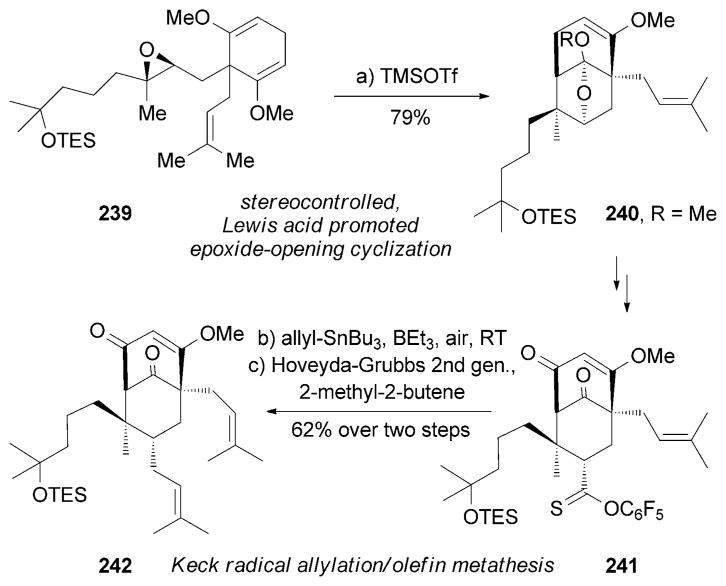
Shair and co-workers reported an efficient approach of (+)-229 (Scheme 38).[218] Key to the hyperforin core 240 was the use of a TMSOTf-mediated epoxide-opening cyclization cascade. During this cyclization, the chair transition state is favored over the boat conformation, which offers perfect stereocontrol. Another key step involves a Keck radical allylation under allyl-SnBu3/BEt3/air conditions. This step was followed by olefin metathesis using the Hoveyda-Grubbs second-generation catalyst[189c] in the presence of 2-methyl-2-butene to yield advanced intermediate 242. More recently, the Nakada research group reported a total synthesis of (±)-229.[219]
Scheme 38.
Part of the total synthesis of (+)-hyperforin (229) by Shair and co-workers.[218]
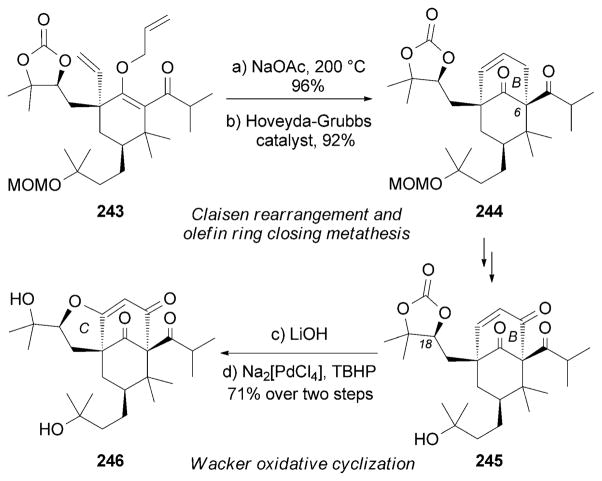
Kanai, Shibasaki, and co-workers also reported a total synthesis of (±)-garsubellin A (230).[220] The B ring of this compound was set through a Claisen rearrangement[92] of 243 followed by a ring-closing metathesis. Cleavage of the carbonate group in 245 and a subsequent Wacker-type oxidative cyclization[221] gave rise to the tricyclic motif of 246 (Scheme 39).
Scheme 39.
Part of the total synthesis of (±)-garsubellin A (221) by Kanai, Shibasaki and co-workers. [220] TBHP =tert-butylhydrogen peroxide.
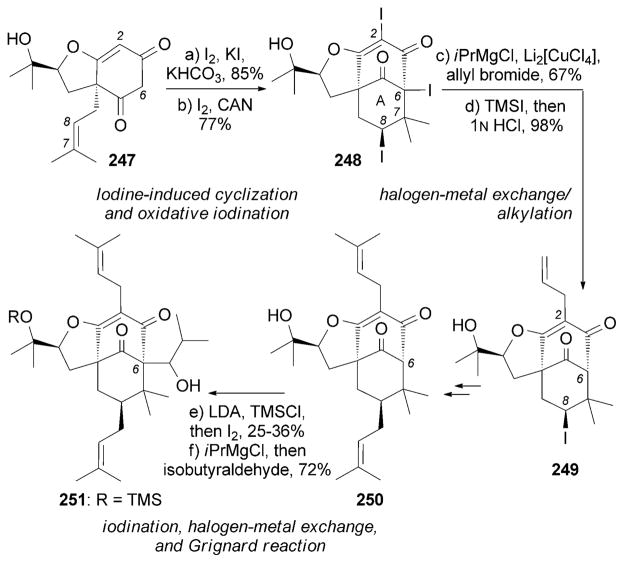
In the total synthesis of (±)-230 by Danishefsky and coworkers the A ring was formed by an iodine-induced cyclization between the C6 center and the C7–C8 olefin of 247 (Scheme 40).[222] A subsequent oxidative iodination[222] installed the vinyl iodide motif in 248 that, upon metalation and allylation, allowed installation of the C2 side chain. A similar sequence was implemented for the conversion of 250 into 251.
Scheme 40.
Part of the total synthesis of (±)-garsubellin A (230) by Siegel and Danishefsky.[222] CAN =cerium(IV) ammonium nitrate.
A formal synthesis of (±)-garsubellin A (230) was accomplished by the Simpkins research group.[224]
2.9. Tricholomalides
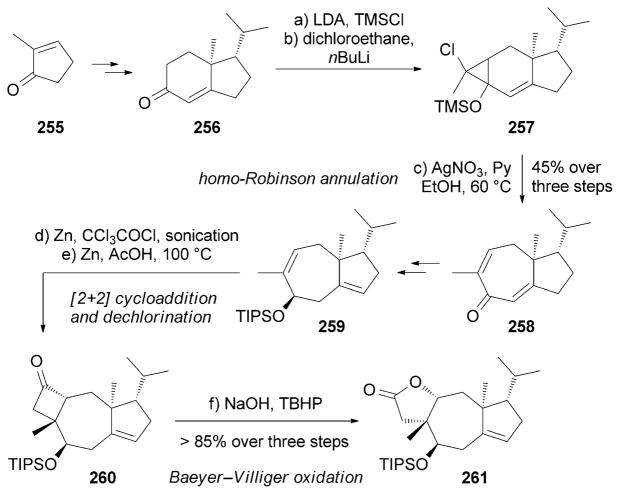
Tricholomalides A–C (252–254) were isolated from the mushroom Tricholoma sp. and were found to promote neurite outgrowth in PC-12 cell lines at concentrations of 100 μM (Figure 14).[225] A total synthesis of (±)-252 and (±)-253 was reported by Danishefsky and co-workers, thus allowing revision of the C2 configuration (Scheme 41).[226] The bicyclic motif of 258 was produced through a homo-Robinson annulation.[226b] Compound 259 was then subjected to a [2+2] cycloaddition followed by dechlorination to afford the tricyclic motif of 260. Subsequently, the cyclobutanone motif of 260 underwent a Baeyer–Villiger oxidation[227] under basic TBHP conditions to yield lactone 261.
Figure 14.
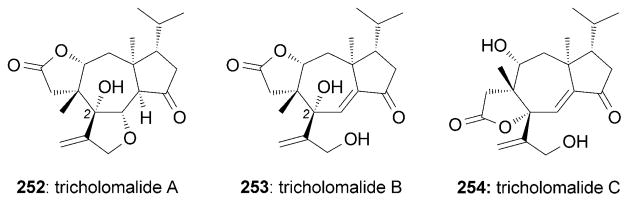
Neurotrophic tricholomalides.
Scheme 41.
Part of the total syntheses of (±)-tricholomalides by Danishefsky and co-workers.[226] TBHP =tert-butylhydrogen peroxide.
2.10. Neovibsanines
Neovibsanin A (262) and B (263) were isolated from the leaves of the poisonous plants Viburnum awabuki (Figure 15).[228] Both compounds were reported to induce NGF-mediated neurite outgrowth in PC-12 cells at 40 μM.[229]
Figure 15.
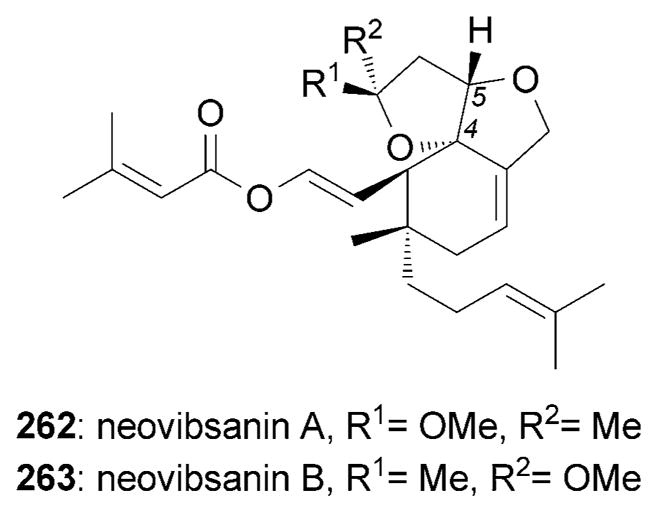
Neurotrophic neovibsanes.
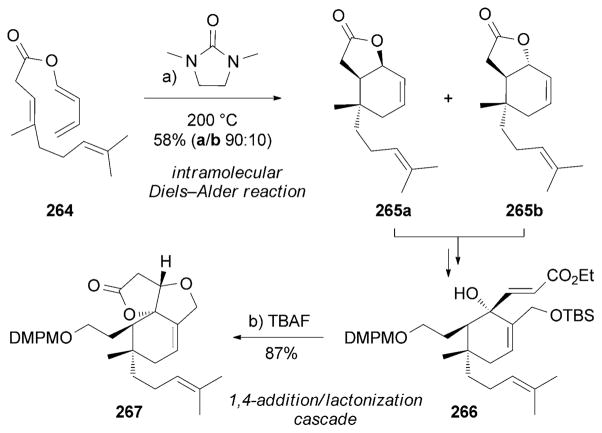
The first total synthesis of (±)-neovibsanin B (263) was accomplished by Imagawa, Nishizawa et al.[229b] This synthesis is based on the formation of lactone 265 through an intramolecular Diels–Alder reaction[72] to yield a mixture of two isomers 265a and 265b, which were both carried forward (Scheme 42). A conjugate addition/lactonization cascade reaction was used to convert 266 into 267. The neurotrophic activity of the racemic 263 was evaluated in PC-12 cells and its activity was found to be comparable to that of enantiomerically pure 263.[229b]
Scheme 42.
Part of the total synthesis of (±)-neovibsanin B (263) by Imagawa, Nishizawa et al.[229b] DMPM=3,4-dimethoxybenzyl.
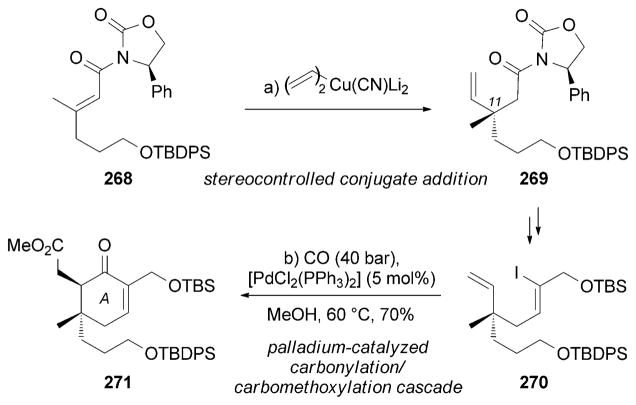
An enantioselective synthesis of (+)-neovibsanin B (263) was accomplished by Esumi, Fukuyama et al.[229c] The strategy features a stereocontrolled conjugate vinylation of chiral oxazolidinone 268 to form the C11 quaternary center (Scheme 43). This reaction was followed by a Pd0-mediated carbonylation/carbomethoxylation cascade reaction to furnish the A ring of 271.
Scheme 43.
Part of the total synthesis of (+)-neovibsanin B (254) by Esumi, Fukuyama et al.[229c]
Moreover, the Williams research group reported the synthesis of (±)-4,5-bis-epi-neovibsanin A and B.[230] These compounds were also shown to promote NGF-mediated neurite outgrowth in PC-12 cells at 40 μM. More recently, a fluorescently labeled and structurally simplified neovibsanin analogue was observed to accumulate around the outer edge prominences of the PC-12 cells.[231]
2.11. Miscellaneous Natural Products
In addition to the above-mentioned natural products, nature has produced other fascinating small molecules that possess various levels of architectural complexity, yet they share a similar level of exciting neurotrophic activity. Representative examples of such natural products are shown in Figure 16.
Figure 16.
Other selected neurotrophic natural products.
Panaxytriol (272) is an active component of red Ginseng[232] and its chemical synthesis has been reported by several research groups.[233] Among them, Danishefsky and co-workers have shown that 272 promotes NGF-induced neurite outgrowth in PC-12 cells at a concentration of 60 μM.[234] Talaumidin (273) is a tetrahydrofuran neolignan that was isolated from the roots of Aristolochia arcuata.[235] It promotes neurite outgrowth of cultured rat cortical neurons at a concentration of 1–30 μM and exhibits neuroprotective effects.[235] As a consequence of its promising biological profiles, various synthetic studies of 273 have been reported.[236] 14-Norpseurotin (274)[237] was isolated from the marine-derived fungus Aspergillus sydowi PFW 1–13 and was reported to induce neurite outgrowth of PC-12 lines at concentrations of 10 μM.[238] A member of the flavonoid family, 7,8-dihydroxyflavone (275), was recently found to be a selective TrKB agonist through Erk1/2 and Akt activation.[239] Moreover, its neuroprotective effects[239,240] and optimized analogues[241] have been recorded. In addition, several other natural flavonoids have been reported to possess activities to promote neurite outgrowth or neurogenesis.[242] Aspermytin A (276) was isolated from a marine-derived fungus Aspergillus sp. and was found to display neurotrophic effects in PC-12 cells at a concentration of 50 μM.[243] A synthesis of 267 has been reported by the Shishido research group.[244] Gelsemiol (277)[245] was isolated from the Paraguayan plant Verbena littoralis H. B. K. and was shown to promote NGF-mediated neurite outgrowth in PC-12 cells at concentrations between 100 and 300 μM. Gademann and co-workers have reported an efficient synthesis of 277.[246]
Various protein kinase C (PKC) activators, such as phorbol ester (PMA, 178)[247] and bryostatin 1 (279),[248] were found to significantly promote neurite outgrowth in PC-12 cell lines[249] and primary culture of chicken dorsal root ganglion (DRG) neurons.[250] Other notable neurotrophic PMA natural analogues include kirkinine (280),[250] kirkinine B, mezerein, and synaptolepis factor K7.[250c] Pioneering syntheses of phorbol and related daphnane natural products have been reported by the research groups of Wender[251] and Cha.[252] In addition, multiple impressive syntheses of bryostatin 1 (270)[253] and its natural analogues have been reported by the research groups of Keck,[253] Masamune,[254] Evans,[255] Nishiyama/Yamamura,[256] Manaviazar,[257] Trost,[258] Krische,[259] and Wender.[260]
Caryolanemagnolol (281) and clovanemagnolol (282) were isolated from Magnolina obovata and have been found to enhance neurite outgrowth as well as increase ChAT activity at a concentration of 0.1 μM.[261] Studies conducted by Siegel and co-workers have led to a bioinspired synthesis of 281 and 282.[262] The authors also confirmed the potent neurotrophic activity of 272 in embryonic hippocampal and cortical neurons at concentrations of 10 nM.[262b] A member of the caged Garcinia xanthones,[263] gambogic amide (283), has shown significant neurotrophic activity at a low nanomolar concentration by binding to and activating TrkA, thereby promoting its dimerization and autophosphorylation.[264] In addition, 283 also exhibited neuroprotective effects.[264]
The clerodane diterpenoid[265] ptychonal (284) was isolated from Ptychopetalum olacoides as a mixture of the hemiacetal and aldehyde form.[266] This equilibrium mixture was shown to exhibit neurite outgrowth promoting activities on NGF-mediated PC-12 cells at concentrations ranging from 0.1 to 10.0 μM. The neurotrophic metabolite epolactaene (285) was isolated from the fungal strain Penicillium sp. BM 1689-P.[267] 285 was shown to promote neurite outgrowth in a human neuroblastoma cell line (SH-SY5Y) at concentrations ranging from 10 μM to 25 μM.[267,268] As a result of its promising biological activity, various syntheses have been reported.[269] Deoxygedunin (286), a natural product isolated from Indian neem trees (Azadirachta indica), was found to act as a BDNF mimic and exhibited TrkB activation in rat hippocampal neurons at nanomolar concentrations.[203] Kansuinin E (287),[270] a macrocyclic jatrophane diterpene,[270,271] was found to promote neurotrophic activity, presumably through TrkA activation.[271c] A related study has also suggested that activation of cyclooxygenase plays an essential role in NGF synthesis.[271b] Tripchlorolide (288), an immunosuppressive extract of Chinese herb Tripterygium wilfordii Hook F, was reported to induce neurotrophic activity in embryonic mesencephalic neurons of rats and displayed neuroprotective effects in dopaminergic neurons from 1 pM to 10 nM. 288 was reported to stimulate BDNF mRNA expression.[272] Moreover, its neuroprotective properties have been evaluated in several studies.[272,273] The neurological activity of 288 might be similar to another immunosuppressant FK506 (tacrolimus).[274]
A series of pyridone alkaloids (289),[275] such as tenellins,[276] bassianins,[276a–c] farinosones,[277] and militarinones,[278] share a common chemical skeleton and promising neurotrophic activities. In 1982, the Williams research group reported an efficient synthesis of tenellin.[279] More recently, a divergent synthesis of various neurotrophic pyridone alkaloids was reported by Gademann and co-workers.[275d,280] The related farinosone C (290)[277] also promotes neurite outgrowth in NGF-mediated PC-12 cell lines at a concentration of 50 μM.[281] The authors reported a synthesis of 290 and have disclosed that L-tyrosinolamide represents its pharmacophore.[281] Studies in PC12 cells suggest that certain militarinones induce persistent neuritic differentiation by elevating the intracellular cAMP levels that, in turn, affect TrkA receptor signaling.[278c] Such persistent activation of the PI3/Akt and MEK/ERK pathways was shown to upregulate p53 and induce the release of AIF from mitochondria, ultimately resulting in cell apoptosis in N2a mouse neuroblastoma cells. Based on these findings, the authors concluded that the effect of militarinones depends on the basal expression of p53; specifically these compounds induce apoptosis in cells with high p53 expression but prompt neuritic differentiation in cells where the p53 levels are low.[278e]
Various macrolides, such as amphotericin B (291) and FK506 (tacrolimus, 292), exhibit remarkable neurotrophic profiles. 291 was originally isolated from the filamentous bacterium Streptomyces nodosus[282–284] and is often used as an antifungal agent.[285] The exciting neurological activity of 291 was recently revealed.[286] This compound was shown to promote neurite outgrowth in rat postnatal cerebellar neurons and rat embryonic cortical neurons. It was also found to prevent the activities of the major myelin- and glial-associated inhibitors, which are the crucial factors that suppress neuron regeneration upon spinal cord injury.[286] A stereoselective synthesis of 291 has been reported by Nicolaou et al.[287] A recent mechanistic study suggested that 291 antagonizes CNS inhibitors to promote axon growth through the activation of the Akt pathway, which suppresses the activity of GSK-3β.[286]
FK506 (tacrolimus, 292) was isolated from Streptomyces tsykubaensis in 1987.[288] In addition to its remarkable function in the immune system, 292 has also shown intriguing neuroprotective and neurotrophic activities, including promotion of neurite outgrowth in PC-12 cells and peripheral neurons.[289] 292 was shown to induce functional improvements of peripheral nerve damage and spinal cord injury.[274] Furthermore, it was reported that 292 promotes the proliferation of cultured Schwann cells and NGF secretion at a concentration of 10 nM.[290] In addition, 292 was reported to promote NGF-induced neurite outgrowth through the MAP kinase pathway.[291] The neuroprotective potential of 292 following CNS injury has been evaluated.[274] Although the detailed mechanism remains unclear, it is noted that binding to two members of the immunophilin family, FKBP-12 and FKBP-52, are critical for its neuroprotective effects.[292] Since CNS injury is associated with the activation of the immune response, it is suggested that the immunosuppressive activity of 292 could induce the observed neuroprotective effects.[274e] Indeed, it has been shown that immunophilin ligands possess neurotrophic activities.[292,293] Therefore, it is reasonable to find that several natural or synthetic analogues of 292, such as rapamycin[289,294] and FK1706,[295] promote neurite outgrowth in PC-12 cells (rapamycin, 1 nM) and human neuroblastoma SH-SY5Y cells (FK1706, 1 nM). A total synthesis of 292 has been accomplished by Merck,[296] Schreiber and co-workers,[297] and Ireland et al.[298] In addition, the research groups of Danishefsky,[299] Sih,[300] and Smith[301] have completed formal total syntheses. The synthetic efforts toward 292 have been reviewed by Nakata[302] and Ley and co-workers.[303]
Stolonidiol (293) was isolated from the Okinawan marine soft coral Clavularia sp.[103a,304] Aside from its potent cytotoxicity to P388 leukemia cells, 293 was reported to possess significant ChAT activity in primary cultured basal forebrain cells and clonal septal SN49 cells at a concentration of 0.3 μM.[103a] A synthesis of stolonidiol was reported by Yamada and co-workers.[305] Xanthofulvin (SM-216289, 294) and vinaxanthone (295) were isolated from the cultured broth of a fungus Penicillium sp. SPF-3059.[306] Significantly, these two compounds were found to strongly inhibit the activity of semaphorin3A (Sema3A), an inhibitor of axonal regeneration, with no observable cytotoxicity. Studies in animals validated the clinical promise of 294.[307] These findings may pave the way for the development of agents to treat injured spinal cords. Recently, Siegel and co-workers have reported an impressive synthesis of 294 and 295.[308]
3. Conclusions and Perspectives
It is widely accepted that natural products have evolved to perform, often in synergy, a wide array of functions that are beneficial to the producing organism. In fact, they are considered to be “privileged structures”[309] which, upon subtle changes aimed to improve their pharmacokinetic and pharmacodynamic profile, can lead to the development of new medicines.[310] For example, natural products and their structural relatives still comprise more than 50% of the drugs used clinically.[311] Equally important is the role of natural products as tools in chemical biology, where the aims are to develop new bioassays, to characterize cell signaling pathways, and to chemically evaluate biological events.
Neurodegenerative diseases is a generic term applied to a variety of conditions that arise from a chronic breakdown and deterioration of the nerve cells, in particular those of the central nervous system.[312] Most commonly, these diseases manifest in elderly people and in industrialized societies, where the life expectancy is long, and they impose severe strains on the social welfare systems. Preventive approaches against neurodegeneration require consideration of various genetic and epidemiological studies that are not easy to correlate. In addition, such approaches are difficult to implement in the case of a traumatic event, such as spinal cord injury. Research into the underlying cause of neurodegeneration is far from complete and no cures for these diseases have been found. The current approach is to produce symptomatic relief or correct neurotransmitter deficiencies and enzymatic breakdown.[2a,7]
Neurotrophins have been identified as promoters of neuronal survival, development, and function, including synapse formation and remodeling of the synaptic network.[8] The importance of these molecules is underscored by the severe neurological effects observed in animals in which neurotrophins or their receptors have been deleted.[313] Despite their unambiguous importance in biology and physiology, drug development approaches based on neurotrophins have encountered several problems associated with oral availability, insufficient delivery to the brain, and high manufacturing cost.[314] Natural products and related molecules with neurotrophic activity can, in principle, overcome these obstacles.[10,11,315] In fact, several compounds are currently in clinical trials for neurodegenerative diseases.[316] A recent example is bryostatin 1 (279),[317,318] which, in turn, highlights the importance of developing robust synthetic strategies that can be used to evaluate and optimize the biological properties of the parent structure.[319]
The synthetic strategies reviewed above offer confidence that the privileged motifs of various neurotrophic natural products can be constructed in the laboratory. Constant advances in the area of synthetic methods promise to further improve these strategies, particularly as they relate to efficiency, flexibility, and overall cost. Apart from certain notable exceptions that have been discussed in this Review, the chemical biology of neurotrophic natural products has not been explored methodically. This could be due to the complexity of pathways that account for neural degeneration and the difficulties related to conducting biological experiments in primary neural cells. The readily available PC-12 cell line offers an attractive and reliable alternative to the primary cell cultures.[96] On the other hand, several key proteins that regulate neuronal growth have been identified as potential targets for therapeutic intervention against neurodegeneration. In addition, advances in high-throughput screening,[320] cellular phenotype imaging,[321] stem cell technology, and regenerative medicine promise to facilitate and accelerate such studies.[322] It is critical to take advantage of this progress on all fronts and push the frontiers of chemical neurobiology further. This task is particularly important and timely due to the recently announced Brain Activity Map (BAM) project.[323] Reminiscent of the Human Genome Project, this initiative will attempt to reconstruct the entire neural activity in the brain. With such an ambitious goal in mind, chemical neurotrophins are expected to play a critical role in the chemical evaluation of neural circuit remodeling, cognitive functions, and behavior.
Acknowledgments
Financial support from the National Institutes of Health (CA 133002) is gratefully acknowledged. We also thank the U.S. Department of Education for a Fellowship to M.H.L. through GAANN grant P200A120223. We thank the National Science Foundation for instrumentation grants CHE9709183 and CHE0741968. We also thank Dr. Lynnie Trzoss, Weng K. Chang, and Prof. William C. Mobley for contributions to the research described in this Review, and Professors Jerry Yang and Carlos A. Guerrero (UCSD) for useful comments.
Biographies
 Jing Xu received his BSc from Nanchang University (2000) and MSc from Tongji University (2004) working under the direction of Professor Ronghua Zhang. He received his PhD from Leipzig University with Professor Athanassios Giannis (2009). He then joined the group of Prof. Emmanuel A. Theodorakis at UCSD as a postdoctoral scientist. His research interests include the synthesis of bioactive natural products and their biological applications.
Jing Xu received his BSc from Nanchang University (2000) and MSc from Tongji University (2004) working under the direction of Professor Ronghua Zhang. He received his PhD from Leipzig University with Professor Athanassios Giannis (2009). He then joined the group of Prof. Emmanuel A. Theodorakis at UCSD as a postdoctoral scientist. His research interests include the synthesis of bioactive natural products and their biological applications.
 Michelle H. Lacoske received her BSc in chemistry from Stony Brook University, where she carried out undergraduate research with Prof. Nancy S. Goroff. She is currently a third-year graduate student at the University of California at San Diego under the direction of Prof. Emmanuel A. Theodorakis and is focusing on natural product synthesis.
Michelle H. Lacoske received her BSc in chemistry from Stony Brook University, where she carried out undergraduate research with Prof. Nancy S. Goroff. She is currently a third-year graduate student at the University of California at San Diego under the direction of Prof. Emmanuel A. Theodorakis and is focusing on natural product synthesis.
 Emmanuel A. Theodorakis received his BSc in Chemistry from the University of Athens (Greece) and his MSc (D.E.A.) and PhD in Organic Chemistry from Paris XI University (France). His MSc studies were performed at the Institute of Chemistry of Natural Products (ICSN-CNRS) under the direction of Prof. H.-P. Husson, and his PhD studies at Texas A&M University under the direction of Sir Derek H. R. Barton. After postdoctoral research at TSRI with Prof. K. C. Nicolaou, in 1995 he joined the faculty at UCSD. His research focuses on the synthesis and biological studies of natural products and designed small molecules.
Emmanuel A. Theodorakis received his BSc in Chemistry from the University of Athens (Greece) and his MSc (D.E.A.) and PhD in Organic Chemistry from Paris XI University (France). His MSc studies were performed at the Institute of Chemistry of Natural Products (ICSN-CNRS) under the direction of Prof. H.-P. Husson, and his PhD studies at Texas A&M University under the direction of Sir Derek H. R. Barton. After postdoctoral research at TSRI with Prof. K. C. Nicolaou, in 1995 he joined the faculty at UCSD. His research focuses on the synthesis and biological studies of natural products and designed small molecules.
Footnotes
Dedicated to Professor Samuel J. Danishefsky
References
- 1.a) Thompson LM. Nature. 2008;452:707–708. doi: 10.1038/452707a. [DOI] [PubMed] [Google Scholar]; b) Palop JJ, Chin J, Mucke L. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]; c) Martin JB. N Engl J Med. 1999;340:1970–1980. doi: 10.1056/NEJM199906243402507. [DOI] [PubMed] [Google Scholar]
- 2.a) Thuret S, Moon LD, Gage FH. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]; b) Cadotte DW, Fehlings MG. Clin Orthop. 2011;469:732–741. doi: 10.1007/s11999-010-1674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Querfurth HW, LaFerla FM. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]; b) Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, Weiner DM, Tison F, Bezard E. Nat Rev Drug Discovery. 2011;10:377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]; c) Executive Summary. Published by Alzheimer’s Disease International; London: 2013. World Alzheimer Report 2013: An analysis of long-term care for dementia. [Google Scholar]
- 4.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Nat Med. 2004;10:S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 5.Luo LQ, O’Leary DDM. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Warrington AE, Bieber AJ, Rodriguez M. CNS Drugs. 2011;25:555–573. doi: 10.2165/11587830-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Bradbury EJ, McMahon SB. Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]; b) Citron M. Nat Rev Drug Discovery. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]; c) Lang AE. Nat Med. 2010;16:1223–1226. doi: 10.1038/nm.2220. [DOI] [PubMed] [Google Scholar]; d) Lindvall O, Kokaia Z, Martinez-Serrano A. Nat Med. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]; e) Esposito E, Cuzzocrea S. Curr Med Chem. 2010;17:2764–2774. doi: 10.2174/092986710791859324. [DOI] [PubMed] [Google Scholar]; f) Ramaswamy S, Shannon KM, Kordower JH. Cell Transplantation. 2007;16:301–312. doi: 10.3727/000000007783464687. [DOI] [PubMed] [Google Scholar]; g) Jackson M, Llado J, Rothstein JD. Expert Opin Invest Drugs. 2002;11:1343–1364. doi: 10.1517/13543784.11.10.1343. [DOI] [PubMed] [Google Scholar]
- 8.a) Huang EJ, Reichardt LF. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chao MV. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 9.Weissmiller AM, Wu C. Transl Neurodegeneration. 2012;1:14. doi: 10.1186/2047-9158-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longo FM, Yang T, Knowles JK, Xie YM, Moore LA, Massa SM. Curr Alzheimer Res. 2007;4:503–506. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RM, Danishefsky SJ. Acc Chem Res. 2006;39:539–549. doi: 10.1021/ar068018n. [DOI] [PubMed] [Google Scholar]
- 12.Young AB. J Neurosci. 2009;29:12722–12728. doi: 10.1523/JNEUROSCI.3767-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Alzheimer Report. Alzheimer’s Disease International; 2012. [Google Scholar]
- 14.Hyman BT, Vanhoesen GW. Neurobiol Aging. 1987;8:555–556. doi: 10.1016/0197-4580(87)90133-3. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed T, Rao PPN. Curr Med Chem. 2011;18:4299–4320. doi: 10.2174/092986711797200435. [DOI] [PubMed] [Google Scholar]
- 16.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Br Med J. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita T. Expert Rev Neurother. 2009;9:661–679. doi: 10.1586/ern.09.24. [DOI] [PubMed] [Google Scholar]
- 18.Bulic B, Pickhardt M, Schmidt B, Mandelkow EM, Waldmann H, Mandelkow E. Angew Chem. 2009;121:1772–1785. doi: 10.1002/anie.200802621. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:1740–1752. doi: 10.1002/anie.200802621. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 20.Shulman JM, De Jager PL, Feany MB. Annu Rev Pathol Mech Dis. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 21.Davie CA. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 22.Dauer W, Przedborski S. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 23.See Ref. [21].
- 24.Youdim MBH, Riederer PF. Neurology. 2004;63:S32–S35. doi: 10.1212/wnl.63.7_suppl_2.s32. [DOI] [PubMed] [Google Scholar]
- 25.Youdim MB, Edmondson D, Tipton KF. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 26.Waters C. J Am Geriatr Soc. 2000;48:692–698. doi: 10.1111/j.1532-5415.2000.tb04732.x. [DOI] [PubMed] [Google Scholar]
- 27.Crosby N, Deane KH, Clarke CE. Cochrane Database Syst Rev. 2003:CD003468. doi: 10.1002/14651858.CD003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landles C, Bates GP. EMBO Rep. 2004;5:958–963. doi: 10.1038/sj.embor.7400250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak MJU, Tabrizi SJ. Br Med J. 2010;340:c3109. doi: 10.1136/bmj.c3109. [DOI] [PubMed] [Google Scholar]
- 30.Myers RH, Vonsattel JP, Paskevich PA, Kiely DK, Stevens TJ, Cupples LA, Richardson EP, Bird ED. J Neuropathol Exp Neurol. 1991;50:729–742. doi: 10.1097/00005072-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan A, Stockwell BR. Prog Neurobiol. 2012;99:262–280. doi: 10.1016/j.pneurobio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden MR, Leavitt BR, Yasothan U, Kirkpatrick P. Nat Rev Drug Discovery. 2009;8:17–18. doi: 10.1038/nrd2784. [DOI] [PubMed] [Google Scholar]
- 33.Rothstein JD. Ann Neurol. 2009;65:S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 34.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 35.Orrell RW. Br Med Bull. 2010;93:145–159. doi: 10.1093/bmb/ldp049. [DOI] [PubMed] [Google Scholar]
- 36.Gurney ME, Pu HF, Chiu AY, Dalcanto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen WJ, Zhai P, Sufit RL, Siddique T. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 37.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, Blumbergs PC, Vucic S, Kiernan MC, Nicholson GA. J Neurol Neurosurg Psychiatry. 2010;81:639–645. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- 39.Bryson HM, Fulton B, Benfield P. Drugs. 1996;52:549–563. doi: 10.2165/00003495-199652040-00010. [DOI] [PubMed] [Google Scholar]
- 40.Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, Moore DH, Schoenfeld D, Mather JL, Archibald D, Sullivan M, Amburgey C, Moritz J, Gribkoff VK. Nat Med. 2011;17:1652–U1169. doi: 10.1038/nm.2579. [DOI] [PubMed] [Google Scholar]
- 41.a) Schwab ME, Bartholdi D. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]; b) Fawcett JW, Asher RA. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 42.a) McKerracher L. Neurobiol Dis. 2001;8:11–18. doi: 10.1006/nbdi.2000.0359. [DOI] [PubMed] [Google Scholar]; b) Silver J, Miller JH. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 43.Cadotte DW, Fehlings MG. Clin Orthop. 2011;469:732–741. doi: 10.1007/s11999-010-1674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.a) Soto C. Nat Rev Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]; b) Mattson MP, Magnus T. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sofroniew MV, Howe CL, Mobley WC. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 46.Huang EJ, Reichardt LF. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 47.Dechant G, Barde YA. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- 48.a) Omura S, Fujimoto T, Otoguro K, Matsuzaki K, Moriguchi R, Tanaka H, Sasaki Y. J Antibiot. 1991;44:113–116. doi: 10.7164/antibiotics.44.113. [DOI] [PubMed] [Google Scholar]; b) Omura S, Matsuzaki K, Fujimoto T, Kosuge K, Furuya T, Fujita S, Nakagawa A. J Antibiot. 1991;44:117–118. doi: 10.7164/antibiotics.44.117. [DOI] [PubMed] [Google Scholar]
- 49.a) Fenteany G, Standaert RF, Reichard GA, Corey EJ, Schreiber SL. Proc Natl Acad Sci USA. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hashimoto K, Guroff G, Katagiri Y. J Neurochem. 2000;74:92–98. doi: 10.1046/j.1471-4159.2000.0740092.x. [DOI] [PubMed] [Google Scholar]
- 50.a) Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]; b) Katagiri M, Hayashi M, Matsuzaki K, Tanaka H, Omura S. J Antibiot. 1995;48:344–346. doi: 10.7164/antibiotics.48.344. [DOI] [PubMed] [Google Scholar]; c) Dick LR, Cruikshank AA, Grenier L, Melandri FD, Nunes SL, Stein RL. J Biol Chem. 1996;271:7273–7276. doi: 10.1074/jbc.271.13.7273. [DOI] [PubMed] [Google Scholar]
- 51.a) Fenteany G, Schreiber SL. J Biol Chem. 1998;273:8545–8548. doi: 10.1074/jbc.273.15.8545. [DOI] [PubMed] [Google Scholar]; b) Corey EJ, Li WDZ. Chem Pharm Bull. 1999;47:1–10. doi: 10.1248/cpb.47.1. [DOI] [PubMed] [Google Scholar]; c) Masse CE, Morgan AJ, Adams J, Panek JS. Eur J Org Chem. 2000:2513–2528. [Google Scholar]; d) Shibasaki M, Kanai M, Fukuda N. Chem Asian J. 2007;2:20–38. doi: 10.1002/asia.200600310. [DOI] [PubMed] [Google Scholar]; e) Kang SH, Kang SY, Lee HS, Buglass AJ. Chem Rev. 2005;105:4537–4558. doi: 10.1021/cr040608g. [DOI] [PubMed] [Google Scholar]; f) Tomoda H, Omura S. Yakugaku Zasshi. 2000;120:935–949. doi: 10.1248/yakushi1947.120.10_935. [DOI] [PubMed] [Google Scholar]
- 52.a) Corey EJ, Reichard GA. J Am Chem Soc. 1992;114:10677–10678. [Google Scholar]; b) Corey EJ, Li WD, Nagamitsu T. Angew Chem. 1998;110:1784–1787. doi: 10.1002/(SICI)1521-3773(19980703)37:12<1676::AID-ANIE1676>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:1676–1679. doi: 10.1002/(SICI)1521-3773(19980703)37:12<1676::AID-ANIE1676>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; c) Corey EJ, Li WD, Reichard GA. J Am Chem Soc. 1998;120:2330–2336. [Google Scholar]; d) Corey EJ, Li WDZ. Tetrahedron Lett. 1998;39:7475–7478. [Google Scholar]
- 53.a) Mukaiyama T. Org React. 1982;28:203–331. [Google Scholar]; b) Kato J, Mukaiyama T. Chem Lett. 1983:1727–1728. [Google Scholar]; c) Iwasawa N, Mukaiyama T. Chem Lett. 1982:1441–1444. [Google Scholar]; d) Beutner GL, Denmark SE. Angew Chem. 2013;125:9256–9266. [Google Scholar]; Angew Chem Int Ed. 2013;52:9086–9096. doi: 10.1002/anie.201302084. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Matsuo JI, Murakami M. Angew Chem. 2013;125:9280–9289. [Google Scholar]; Angew Chem Int Ed. 2013;52:9109–9118. doi: 10.1002/anie.201303192. [DOI] [PubMed] [Google Scholar]
- 54.a) Sunazuka T, Nagamitsu T, Matsuzaki K, Tanaka H, Omura S, Smith AB. J Am Chem Soc. 1993;115:5302–5302. [Google Scholar]; b) Uno H, Baldwin JE, Russell AT. J Am Chem Soc. 1994;116:2139–2140. [Google Scholar]; c) Chida N, Takeoka J, Tsutsumi N, Ogawa S. J Chem Soc Chem Commun. 1995:793–794. [Google Scholar]; d) Nagamitsu T, Sunazuka T, Tanaka H, Omura S, Sprengeler PA, Smith AB. J Am Chem Soc. 1996;118:3584–3590. [Google Scholar]; e) Chida N, Takeoka J, Ando K, Tsutsumi N, Ogawa S. Tetrahedron. 1997;53:16287–16298. [Google Scholar]; f) Kang SH, Jun HS. Chem Commun. 1998:1929–1930. [Google Scholar]; g) Panek JS, Masse CE. Angew Chem. 1999;111:1161–1163. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1093::AID-ANIE1093>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1999;38:1093–1095. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1093::AID-ANIE1093>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; h) Soucy F, Grenier L, Behnke ML, Destree AT, McCormack TA, Adams J, Plamondon L. J Am Chem Soc. 1999;121:9967–9976. [Google Scholar]; i) Green MP, Prodger JC, Hayes CJ. Tetrahedron Lett. 2002;43:6609–6611. [Google Scholar]; j) Brennan CJ, Pattenden G, Rescourio G. Tetrahedron Lett. 2003;44:8757–8760. [Google Scholar]; k) Page PCB, Hamzah AS, Leach DC, Allin SM, Andrews DM, Rassias GA. Org Lett. 2003;5:353–355. doi: 10.1021/ol027387q. [DOI] [PubMed] [Google Scholar]; l) Bulman Page PCB, Leach DC, Hayman CM, Hamzah AS, Allin SM, McKee V. Synlett. 2003:1025–1027. [Google Scholar]; m) Donohoe TJ, Sintim H, Sisangia L, Harling JD. Angew Chem. 2004;116:2343–2346. doi: 10.1002/anie.200453843. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:2293–2296. doi: 10.1002/anie.200453843. [DOI] [PubMed] [Google Scholar]; n) Donohoe TJ, Sintim HO, Sisangia L, Ace KW, Guyo PM, Cowley A, Harling JD. Chem Eur J. 2005;11:4227–4238. doi: 10.1002/chem.200401119. [DOI] [PubMed] [Google Scholar]; o) Wardrop DJ, Bowen EG. Chem Commun. 2005:5106–5108. doi: 10.1039/b508300a. [DOI] [PubMed] [Google Scholar]; p) Balskus EP, Jacobsen EN. J Am Chem Soc. 2006;128:6810–6812. doi: 10.1021/ja061970a. [DOI] [PubMed] [Google Scholar]; q) Fukuda N, Sasaki K, Sastry TVRS, Kanai M, Shibasaki M. J Org Chem. 2006;71:1220–1225. doi: 10.1021/jo0524223. [DOI] [PubMed] [Google Scholar]; r) Miyaoka H, Yamanishi M, Hoshino A, Mitome H, Kawashima E. Chem Pharm Bull. 2008;56:738–741. doi: 10.1248/cpb.56.738. [DOI] [PubMed] [Google Scholar]; s) Hameed A, Blake AJ, Hayes CJ. Synlett. 2010:535–538. [Google Scholar]; t) Hayes CJ, Sherlock AE, Selby MD. Org Biomol Chem. 2006;4:193–195. doi: 10.1039/b516311k. [DOI] [PubMed] [Google Scholar]; u) Hayes CJ, Sherlock AE, Green MP, Wilson C, Blake AJ, Selby MD, Prodger JC. J Org Chem. 2008;73:2041–2051. doi: 10.1021/jo7027695. [DOI] [PubMed] [Google Scholar]
- 55.Nagamitsu T, Sunazuka T, Stump H, Obata R, Arima S, Matsuzaki K, Tanaka H, Omura S. J Antibiot. 1995;48:747–748. doi: 10.7164/antibiotics.48.747. [DOI] [PubMed] [Google Scholar]
- 56.Shigemori H, Wakuri S, Yazawa K, Nakamura T, Sasaki T, Kobayashi J. Tetrahedron. 1991;47:8529–8534. [Google Scholar]
- 57.Schneekloth JS, Sanders JL, Hines J, Crews CM. Bioorg Med Chem Lett. 2006;16:3855–3858. doi: 10.1016/j.bmcl.2006.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hines J, Groll M, Fahnestock M, Crews CM. Chem Biol. 2008;15:501–512. doi: 10.1016/j.chembiol.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi K, Tsuji T, Wakuri S, Yazawa K, Kondo K, Shigemori H, Kobayashi J. Biosci Biotechnol Biochem. 1993;57:195–199. doi: 10.1271/bbb.57.195. [DOI] [PubMed] [Google Scholar]
- 60.a) Delcros JG, Floc’h MB, Prigent C, Arlot-Bonnemains Y. Curr Med Chem. 2003;10:479–503. doi: 10.2174/0929867033368231. [DOI] [PubMed] [Google Scholar]; b) Kim KB, Crews CM. J Med Chem. 2008;51:2600–2605. doi: 10.1021/jm070421s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee DH, Goldberg AL. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]; d) Myung J, Kim KB, Crews CM. Med Res Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Schneekloth JS, Crews CM. Curr Drug Targets. 2011;12:1581–1594. doi: 10.2174/138945011798109491. [DOI] [PubMed] [Google Scholar]
- 61.a) Ciechanover A, Brundin P. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]; b) Meiners S, Ludwig A, Stangl V, Stangl K. Med Res Rev. 2008;28:309–327. doi: 10.1002/med.20111. [DOI] [PubMed] [Google Scholar]; c) Crews CM. Chem Biol. 2010;17:551–555. doi: 10.1016/j.chembiol.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kisselev AF, van der Linden WA, Overkleeft HS. Chem Biol. 2012;19:99–115. doi: 10.1016/j.chembiol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.a) Urabe D, Inoue M. Tetrahedron. 2009;65:6271–6289. [Google Scholar]; b) Fukuyama Y, Huang J-M. In: Studies in Natural Products Chemistry. Attaur-Rahman, editor. Vol. 32. Elsevier; Amsterdam: 2005. pp. 395–429. [Google Scholar]
- 63.a) Huang JM, Yokoyama R, Yang CS, Fukuyama Y. Tetrahedron Lett. 2000;41:6111–6114. [Google Scholar]; b) Huang JM, Yang CS, Tanaka M, Fukuyama Y. Tetrahedron. 2001;57:4691–4698. [Google Scholar]
- 64.Yokoyama R, Huang JM, Yang CS, Fukuyama Y. J Nat Prod. 2002;65:527–531. doi: 10.1021/np010571k. [DOI] [PubMed] [Google Scholar]
- 65.Kubo M, Okada C, Huang JM, Harada K, Hioki H, Fukuyama Y. Org Lett. 2009;11:5190–5193. doi: 10.1021/ol9021029. [DOI] [PubMed] [Google Scholar]
- 66.Kouno I, Baba N, Hashimoto M, Kawano N, Takahashi M, Kaneto H, Yang CS. Chem Pharm Bull. 1990;38:422–425. doi: 10.1248/cpb.38.291. [DOI] [PubMed] [Google Scholar]
- 67.Kubo M, Kobayashi K, Huang JM, Harada K, Fukuyama Y. Tetrahedron Lett. 2012;53:1231–1235. [Google Scholar]
- 68.Huang JM, Yokoyama R, Yang CS, Fukuyama Y. J Nat Prod. 2001;64:428–431. doi: 10.1021/np0005715. [DOI] [PubMed] [Google Scholar]
- 69.Fukuyama Y, Shida N, Kodama M, Chaki H, Yugami T. Chem Pharm Bull. 1995;43:2270–2272. doi: 10.1248/cpb.43.2270. [DOI] [PubMed] [Google Scholar]
- 70.Fukuyama Y, Hata Y, Kodama M. Planta Med. 1997;63:275–277. doi: 10.1055/s-2006-957675. [DOI] [PubMed] [Google Scholar]
- 71.a) Birman VB, Danishefsky SJ. J Am Chem Soc. 2002;124:2080–2081. doi: 10.1021/ja012495d. [DOI] [PubMed] [Google Scholar]; b) Meng ZY, Danishefsky SJ. Angew Chem. 2005;117:1535–1537. [Google Scholar]; Angew Chem Int Ed. 2005;44:1511–1513. doi: 10.1002/anie.200462509. [DOI] [PubMed] [Google Scholar]
- 72.a) Brieger G, Bennett JN. Chem Rev. 1980;80:63–97. [Google Scholar]; b) Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem. 2002;114:1742–1773. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; c) Juhl M, Tanner D. Chem Soc Rev. 2009;38:2983–2992. doi: 10.1039/b816703f. [DOI] [PubMed] [Google Scholar]
- 73.Corey EJ, Danheiser RL, Chandrasekaran S, Siret P, Keck GE, Gras JL. J Am Chem Soc. 1978;100:8031–8034. [Google Scholar]
- 74.a) Marinovic NN, Ramanathan H. Tetrahedron Lett. 1983;24:1871–1874. [Google Scholar]; b) Jasperse CP, Curran DP, Fevig TL. Chem Rev. 1991;91:1237–1286. [Google Scholar]; c) Curran DP, Sisko J, Yeske PE, Liu H. Pure Appl Chem. 1993;65:1153–1159. [Google Scholar]; d) Koert U. Angew Chem. 1996;108:441–443. [Google Scholar]; Angew Chem Int Ed Engl. 1996;35:405–407. [Google Scholar]
- 75.a) Inoue M, Sato T, Hirama M. J Am Chem Soc. 2003;125:10772–10773. doi: 10.1021/ja036587+. [DOI] [PubMed] [Google Scholar]; b) Inoue M, Sato T, Hirama M. Angew Chem. 2006;118:4961–4966. [Google Scholar]; Angew Chem Int Ed. 2006;45:4843–4848. doi: 10.1002/anie.200601358. [DOI] [PubMed] [Google Scholar]; c) Inoue M, Lee N, Kasuya S, Sato T, Hirama M, Moriyama M, Fukuyama Y. J Org Chem. 2007;72:3065–3075. doi: 10.1021/jo0700474. [DOI] [PubMed] [Google Scholar]
- 76.Mehta G, Singh SR. Angew Chem. 2006;118:967–969. doi: 10.1002/anie.200503618. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:953–955. doi: 10.1002/anie.200503618. [DOI] [PubMed] [Google Scholar]
- 77.a) Nicolaou KC, Bulger PG, Sarlah D. Angew Chem. 2005;117:4564–4601. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:4490–4527. doi: 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]; b) Samojlowicz C, Bieniek M, Grela K. Chem Rev. 2009;109:3708–3742. doi: 10.1021/cr800524f. [DOI] [PubMed] [Google Scholar]; c) Vougioukalakis GC, Grubbs RH. Chem Rev. 2010;110:1746–1787. doi: 10.1021/cr9002424. [DOI] [PubMed] [Google Scholar]
- 78.a) Frontier AJ, Collison C. Tetrahedron. 2005;61:7577–7606. [Google Scholar]; b) Pellissier H. Tetrahedron. 2005;61:6479–6517. [Google Scholar]; c) Tius MA. Eur J Org Chem. 2005:2193–2206. [Google Scholar]; d) Vaidya T, Eisenberg R, Frontier AJ. ChemCatChem. 2011;3:1531–1548. [Google Scholar]
- 79.a) He W, Huang J, Sun XF, Frontier AJ. J Am Chem Soc. 2007;129:498–499. doi: 10.1021/ja068150i. [DOI] [PubMed] [Google Scholar]; b) He W, Huang J, Sun XF, Frontier AJ. J Am Chem Soc. 2008;130:300–308. doi: 10.1021/ja0761986. [DOI] [PubMed] [Google Scholar]
- 80.a) Janka M, He W, Frontier AJ, Eisenberg R. J Am Chem Soc. 2004;126:6864–6865. doi: 10.1021/ja049643v. [DOI] [PubMed] [Google Scholar]; b) Janka M, He W, Haedicke IE, Fronczek FR, Frontier AJ, Eisenberg R. J Am Chem Soc. 2006;128:5312–5313. doi: 10.1021/ja058772o. [DOI] [PubMed] [Google Scholar]; c) Vaidya T, Atesin AC, Herrick IR, Frontier AJ, Eisenberg R. Angew Chem. 2010;122:3435–3438. doi: 10.1002/anie.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:3363–3366. doi: 10.1002/anie.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.a) He W, Sun XF, Frontier AJ. J Am Chem Soc. 2003;125:14278–14279. doi: 10.1021/ja037910b. [DOI] [PubMed] [Google Scholar]; b) He W, Sun XF, Frontier AJ. J Am Chem Soc. 2004;126:10493–10493. [Google Scholar]; c) He W, Herrick IR, Atesin TA, Caruana PA, Kellenberger CA, Frontier AJ. J Am Chem Soc. 2008;130:1003–1011. doi: 10.1021/ja077162g. [DOI] [PubMed] [Google Scholar]
- 82.a) Shi L, Meyer K, Greaney MF. Angew Chem. 2010;122:9436–9439. doi: 10.1002/anie.201005156. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:9250–9253. doi: 10.1002/anie.201005156. [DOI] [PubMed] [Google Scholar]; b) Nazef N, Davies RDM, Greaney MF. Org Lett. 2012;14:3720–3723. doi: 10.1021/ol301513h. [DOI] [PubMed] [Google Scholar]
- 83.a) Wasilke JC, Obrey SJ, Baker RT, Bazan GC. Chem Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem. 2006;118:7292–7344. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Chen JS. Chem Soc Rev. 2009;38:2993–3009. doi: 10.1039/b903290h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen JW, Gao P, Yu FM, Yang Y, Zhu SZ, Zhai HB. Angew Chem. 2012;124:5999–6001. [Google Scholar]; Angew Chem Int Ed. 2012;51:5897–5899. doi: 10.1002/anie.201200378. [DOI] [PubMed] [Google Scholar]
- 85.Blanco-Urgoiti J, Anorbe L, Perez-Serrano L, Dominguez G, Perez-Castells J. Chem Soc Rev. 2004;33:32–42. doi: 10.1039/b300976a. [DOI] [PubMed] [Google Scholar]
- 86.a) Molander GA, Harris CR. Chem Rev. 1996;96:307–338. doi: 10.1021/cr950019y. [DOI] [PubMed] [Google Scholar]; b) Edmonds DJ, Johnston D, Procter DJ. Chem Rev. 2004;104:3371–3403. doi: 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Ellery SP, Chen JS. Angew Chem. 2009;121:7276–7301. doi: 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:7140–7165. doi: 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.a) Cho YS, Carcache DA, Tian Y, Li YM, Danishefsky SJ. J Am Chem Soc. 2004;126:14358–14359. doi: 10.1021/ja045939p. [DOI] [PubMed] [Google Scholar]; b) Carcache DA, Cho YS, Hua ZH, Tian Y, Li YM, Danishefsky SJ. J Am Chem Soc. 2006;128:1016–1022. doi: 10.1021/ja056980a. [DOI] [PubMed] [Google Scholar]
- 88.a) Dalko PI, Moisan L. Angew Chem. 2004;116:5248–5286. [Google Scholar]; Angew Chem Int Ed. 2004;43:5138–5175. doi: 10.1002/anie.200400650. [DOI] [PubMed] [Google Scholar]; b) List B. Acc Chem Res. 2004;37:548–557. doi: 10.1021/ar0300571. [DOI] [PubMed] [Google Scholar]; c) Lelais G, MacMillan DWC. Aldrichimica Acta. 2006;39:79–87. [Google Scholar]; d) Taylor MS, Jacobsen EN. Angew Chem. 2006;118:1550–1573. [Google Scholar]; Angew Chem Int Ed. 2006;45:1520–1543. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; e) Marion N, Diez-Gonzalez S, Nolan IP. Angew Chem. 2007;119:3046–3058. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]; f) Gaunt MJ, Johansson CCC, McNally A, Vo NT. Drug Discovery Today. 2007;12:8–27. doi: 10.1016/j.drudis.2006.11.004. [DOI] [PubMed] [Google Scholar]; g) Bertelsen S, Jorgensen KA. Chem Soc Rev. 2009;38:2178–2189. doi: 10.1039/b903816g. [DOI] [PubMed] [Google Scholar]
- 89.Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Org Lett. 2011;13:4554–4557. doi: 10.1021/ol201742j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.a) Cowell A, Stille JK. J Am Chem Soc. 1980;102:4193–4198. [Google Scholar]; b) Cacchi S, Morera E, Ortar G. Tetrahedron Lett. 1985;26:1109–1112. [Google Scholar]
- 91.Yang Y, Fu XN, Chen JW, Zhai HB. Angew Chem. 2012;124:9963–9966. [Google Scholar]; Angew Chem Int Ed. 2012;51:9825–9828. doi: 10.1002/anie.201203176. [DOI] [PubMed] [Google Scholar]
- 92.Matín Castro AM. Chem Rev. 2004;104:2939–3002. doi: 10.1021/cr020703u. [DOI] [PubMed] [Google Scholar]
- 93.Xu J, Trzoss L, Chang WK, Theodorakis EA. Angew Chem. 2011;123:3756–3760. doi: 10.1002/anie.201100313. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:3672–3676. doi: 10.1002/anie.201100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307–1370. [Google Scholar]
- 95.a) Trzoss L, Xu J, Lacoske MH, Mobley WC, Theodorakis EA. Chem Eur J. 2013;19:6398–6408. doi: 10.1002/chem.201300198. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Trzoss L, Xu J, Lacoske MH, Theodorakis EA. Beilstein J Org Chem. 2013;9:1135–1140. doi: 10.3762/bjoc.9.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greene LA, Tischler AS. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westerink RH, Ewing AG. Acta Physiol. 2008;192:273–285. doi: 10.1111/j.1748-1716.2007.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fukuyama Y, Shida N, Kodama M. Tetrahedron Lett. 1995;36:583–586. [Google Scholar]
- 99.Cook SP, Polara A, Danishefsky SJ. J Am Chem Soc. 2006;128:16440–16441. doi: 10.1021/ja0670254. [DOI] [PubMed] [Google Scholar]
- 100.Cook SP, Danishefsky SJ. Org Lett. 2006;8:5693–5695. doi: 10.1021/ol062067i. [DOI] [PubMed] [Google Scholar]
- 101.Polara A, Cook SP, Danishefsky SJ. Tetrahedron Lett. 2008;49:5906–5908. doi: 10.1016/j.tetlet.2008.07.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehta G, Maity P. Tetrahedron Lett. 2011;52:1749–1752. [Google Scholar]
- 103.a) Yabe T, Yamada H, Shimomura M, Miyaoka H, Yamada Y. J Nat Prod. 2000;63:433–435. doi: 10.1021/np990263a. [DOI] [PubMed] [Google Scholar]; b) Iwasaki Y, Ikeda K, Ichikawa Y, Igarashi O, Kinoshita M, Marubuchi S, Ono S. Neurochem Res. 2002;27:225–228. doi: 10.1023/a:1014884504879. [DOI] [PubMed] [Google Scholar]
- 104.a) Baskin DS, Browning JL, Pirozzolo FJ, Korporaal S, Baskin JA, Appel SH. Arch Neurol. 1999;56:1121–1123. doi: 10.1001/archneur.56.9.1121. [DOI] [PubMed] [Google Scholar]; b) Dekosky ST, Ikonamovic MD, Styren SD, Paulin M, Cochran E, Bennett D, Mufson EJ. Ann Neurol. 1999;46:452–452. [Google Scholar]; c) Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Neurobiol Aging. 2000;21:11–17. doi: 10.1016/s0197-4580(00)00090-7. [DOI] [PubMed] [Google Scholar]; d) Fu AL, Li Q, Dong ZH, Huang SJ, Wang YX, Sun MJ. Neurosci Lett. 2004;368:258–262. doi: 10.1016/j.neulet.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 105.a) Pettus TRR, Chen XT, Danishefsky SJ. J Am Chem Soc. 1998;120:12684–12685. [Google Scholar]; b) Pettus TRR, Inoue M, Chen XT, Danishefsky SJ. J Am Chem Soc. 2000;122:6160–6168. [Google Scholar]
- 106.a) Iqbal J, Bhatia B, Nayyar NK. Chem Rev. 1994;94:519–564. [Google Scholar]; b) Snider BB. Chem Rev. 1996;96:339–363. doi: 10.1021/cr950026m. [DOI] [PubMed] [Google Scholar]
- 107.a) Xu J, Caro-Diaz EJE, Lacoske MH, Hung CI, Jamora C, Theodorakis EA. Chem Sci. 2012;3:3378–3386. doi: 10.1039/C2SC21308G. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xu J, Caro-Diaz EJE, Trzoss L, Theodorakis EA. J Am Chem Soc. 2012;134:5072–5075. doi: 10.1021/ja300807e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yin J, Wang C, Kong LL, Cai SJ, Gao SH. Angew Chem. 2012;124:7906–7909. [Google Scholar]; Angew Chem Int Ed. 2012;51:7786–7789. doi: 10.1002/anie.201202455. [DOI] [PubMed] [Google Scholar]
- 108.Yakushijin K, Furukawa H, Mcphail AT. Chem Pharm Bull. 1984;32:23–30. [Google Scholar]
- 109.Lei XG, Dai MJ, Hua ZH, Danishefsky SJ. Tetrahedron Lett. 2008;49:6383–6385. doi: 10.1016/j.tetlet.2008.07.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furuya S, Terashima S. Tetrahedron Lett. 2003;44:6875–6878. [Google Scholar]
- 111.a) Ayer WA. Nat Prod Rep. 1991;8:455–463. doi: 10.1039/np9910800455. [DOI] [PubMed] [Google Scholar]; b) Ma X, Gang DR. Nat Prod Rep. 2004;21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]; c) Kobayashi J, Morita H. Alkaloids Chem Biol. 2005;61:1–57. doi: 10.1016/s1099-4831(05)61001-2. [DOI] [PubMed] [Google Scholar]
- 112.Liu JS, Zhu YL, Yu CM, Zhou YZ, Han YY, Wu FW, Qi BF. Can J Chem. 1986;64:837–839. [Google Scholar]
- 113.a) Kobayashi J, Hirasawa Y, Yoshida N, Morita H. J Org Chem. 2001;66:5901–5904. doi: 10.1021/jo0103874. [DOI] [PubMed] [Google Scholar]; b) Ishiuchi K, Kubota T, Hoshino T, Obara Y, Nakahata N, Kobayashi J. Bioorg Med Chem. 2006;14:5995–6000. doi: 10.1016/j.bmc.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 114.Kobayashi J, Hirasawa Y, Yoshida N, Morita H. Tetrahedron Lett. 2000;41:9069–9073. [Google Scholar]
- 115.Morita H, Ishiuchi K, Haganuma A, Hoshino T, Obara Y, Nakahata N, Kobayashi J. Tetrahedron. 2005;61:1955–1960. [Google Scholar]
- 116.a) Hirasawa Y, Morita H, Kobayashi J. Org Lett. 2004;6:3389–3391. doi: 10.1021/ol048621a. [DOI] [PubMed] [Google Scholar]; b) Hirasawa Y, Kobayashi J, Obara Y, Nakahata N, Kawahara N, Goda Y, Morita H. Heterocycles. 2006;68:2357–2364. [Google Scholar]
- 117.Liang YQ, Tang XC. Neurosci Lett. 2004;361:56–59. doi: 10.1016/j.neulet.2003.12.071. [DOI] [PubMed] [Google Scholar]
- 118.Xu SS, Gao ZX, Zheng W, Du ZM, Xu WA, Yang JS, Zhang ML, Tong ZH, Fang YS, Chai XS, Li SL. Acta Pharm Sin. 1995;16:391–395. [PubMed] [Google Scholar]
- 119.Tang LL, Wang R, Tang XC. Acta Pharm Sin. 2005;26:673–678. doi: 10.1111/j.1745-7254.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 120.Ma T, Gong K, Yan YF, Zhang LH, Tang PF, Zhang XF, Gong YD. Brain Res. 2013;1506:35–43. doi: 10.1016/j.brainres.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 121.Tang LL, Wang R, Tang XC. Eur J Pharmacol. 2005;519:9–15. doi: 10.1016/j.ejphar.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 122.Wang ZF, Tang LL, Yan H, Wang YJ, Tang XC. Pharmacol Biochem Behavior. 2006;83:603–611. doi: 10.1016/j.pbb.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 123.a) Ma XQ, Tan CH, Zhu DY, Gang DR, Xiao PG. J Ethnopharmacol. 2007;113:15–34. doi: 10.1016/j.jep.2007.05.030. [DOI] [PubMed] [Google Scholar]; b) Ha GT, Wong RK, Zhang Y. Chem Biodiversity. 2011;8:1189–1204. doi: 10.1002/cbdv.201000269. [DOI] [PubMed] [Google Scholar]; c) Jiang HL, Luo XM, Bai DL. Curr Med Chem. 2003;10:2231–2252. doi: 10.2174/0929867033456747. [DOI] [PubMed] [Google Scholar]
- 124.Tun MKM, Herzon SB. J Exp Pharmacol. 2012;4:113–123. doi: 10.2147/JEP.S27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia Y, Kozikowski AP. J Am Chem Soc. 1989;111:4116–4117. [Google Scholar]
- 126.Qian LG, Ji RY. Tetrahedron Lett. 1989;30:2089–2090. [Google Scholar]
- 127.Koshiba T, Yokoshima S, Fukuyama T. Org Lett. 2009;11:5354–5356. doi: 10.1021/ol9022408. [DOI] [PubMed] [Google Scholar]
- 128.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem. 2005;117:4516–4563. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:4442–4489. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 129.Maung KM, Wustmann DJ, Herzon SB. Chem Sci. 2011;2:2251–2253. [Google Scholar]
- 130.a) Kawatsura M, Hartwig JF. J Am Chem Soc. 1999;121:1473–1478. [Google Scholar]; b) Fox JM, Huang XH, Chieffi A, Buchwald SL. J Am Chem Soc. 2000;122:1360–1370. [Google Scholar]; c) Bellina F, Rossi R. Chem Rev. 2010;110:3850–3850. doi: 10.1021/cr9000836. [DOI] [PubMed] [Google Scholar]
- 131.Ding R, Sun BF, Lin GQ. Org Lett. 2012;14:4446–4449. doi: 10.1021/ol301951r. [DOI] [PubMed] [Google Scholar]
- 132.a) Hartwig JF. Acc Chem Res. 1998;31:852–860. [Google Scholar]; b) Hartwig JF. Angew Chem. 1998;110:2154–2177. [Google Scholar]; Angew Chem Int Ed. 1998;37:2046–2067. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; c) Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL. Acc Chem Res. 1998;31:805–818. [Google Scholar]; d) Yang BH, Buchwald SL. J Organomet Chem. 1999;576:125–146. [Google Scholar]
- 133.a) De Meijere A, Meyer FE. Angew Chem. 1994;106:2473–2506. [Google Scholar]; Angew Chem Int Ed Engl. 1994;33:2379–2411. [Google Scholar]; b) Cabri W, Candiani I. Acc Chem Res. 1995;28:2–7. [Google Scholar]; c) Beletskaya IP, Cheprakov AV. Chem Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- 134.a) Yamada F, Kozikowski AP, Reddy ER, Pang YP, Miller JH, Mckinney M. J Am Chem Soc. 1991;113:4695–4696. [Google Scholar]; b) Campiani G, Sun LQ, Kozikowski AP, Aagaard P, Mckinney M. J Org Chem. 1993;58:7660–7669. [Google Scholar]; c) Kaneko S, Yoshino T, Katoh T, Terashima S. Heterocycles. 1997;46:27–30. [Google Scholar]; d) Kaneko S, Yoshino T, Katoh T, Terashima S. Tetrahedron: Asymmetry. 1997;8:829–832. [Google Scholar]; e) Kaneko S, Yoshino T, Katoh T, Terashima S. Tetrahedron. 1998;54:5471–5484. [Google Scholar]; f) Chassaing C, Haudrechy A, Langlois Y. Tetrahedron Lett. 1999;40:8805–8809. [Google Scholar]; g) Haudrechy A, Chassaing C, Riche C, Langlois Y. Tetrahedron. 2000;56:3181–3187. [Google Scholar]; h) He XC, Wang B, Yu GL, Bai DL. Tetrahedron: Asymmetry. 2001;12:3213–3216. [Google Scholar]; i) Lee IYC, Jung MH, Lee HW, Yang JY. Tetrahedron Lett. 2002;43:2407–2409. [Google Scholar]; j) Pan QB, Ma DW. Chin J Chem. 2003;21:793–796. [Google Scholar]; k) Lucey C, Kelly SA, Mann J. Org Biomol Chem. 2007;5:301–306. doi: 10.1039/b615059d. [DOI] [PubMed] [Google Scholar]; l) Ding XH, Li X, Liu D, Cui WC, Ju X, Wang SZ, Yao ZJ. Tetrahedron. 2012;68:6240–6248. [Google Scholar]; m) Tudhope SR, Bellamy JA, Ball A, Rajasekar D, Azadi-Ardakani M, Meera HS, Gnanadeepam JM, Saiganesh R, Gibson F, He LL, Behrens CH, Underiner G, Marfurt J, Favre N. Org Process Res Dev. 2012;16:635–642. [Google Scholar]; n) White JD, Li Y, Kim J, Terinek M. Org Lett. 2013;15:882–885. doi: 10.1021/ol400012s. [DOI] [PubMed] [Google Scholar]
- 135.Ishiuchi K, Kubota T, Ishiyama H, Hayashi S, Shibata T, Mori K, Obara Y, Nakahata N, Kobayashi J. Bioorg Med Chem. 2011;19:749–753. doi: 10.1016/j.bmc.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 136.a) Beshore DC, Smith AB., III J Am Chem Soc. 2007;129:4148–4149. doi: 10.1021/ja070336+. [DOI] [PubMed] [Google Scholar]; b) Beshore DC, Smith AB., III J Am Chem Soc. 2008;130:13778–13789. doi: 10.1021/ja804939r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.a) Bisai A, West SP, Sarpong R. J Am Chem Soc. 2008;130:7222–7223. doi: 10.1021/ja8028069. [DOI] [PubMed] [Google Scholar]; b) West SP, Bisai A, Lim AD, Narayan RR, Sarpong R. J Am Chem Soc. 2009;131:11187–11194. doi: 10.1021/ja903868n. [DOI] [PubMed] [Google Scholar]
- 138.a) Nishimura T, Unni AK, Yokoshima S, Fukuyama T. J Am Chem Soc. 2011;133:418–419. doi: 10.1021/ja109516f. [DOI] [PubMed] [Google Scholar]; b) Nishimura T, Unni AK, Yokoshima S, Fukuyama T. J Am Chem Soc. 2013;135:3243–3247. doi: 10.1021/ja312065m. [DOI] [PubMed] [Google Scholar]
- 139.a) Anet FAL, Eves CR. Can J Chem. 1958;36:902–909. [Google Scholar]; b) Anet FAL, Rao MV. Tetrahedron Lett. 1960;1:9–12. [Google Scholar]; c) Ayer WA, Iverach GG. Can J Chem. 1960;38:1823–1826. [Google Scholar]; d) Kleinman E, Heathcock CH. Tetrahedron Lett. 1979;20:4125–4128. [Google Scholar]; e) Heathcock CH, Kleinman EF, Binkley ES. J Am Chem Soc. 1982;104:1054–1068. [Google Scholar]; f) Tsukano C, Zhao L, Takemoto Y, Hirama M. Eur J Org Chem. 2010:4198–4200. [Google Scholar]
- 140.Fischer DF, Sarpong R. J Am Chem Soc. 2010;132:5926–5927. doi: 10.1021/ja101893b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schumann D, Naumann A. Liebigs Ann Chem. 1983:220–225. [Google Scholar]
- 142.a) Takagi J, Sato K, Hartwig JF, Ishiyama T, Miyaura N. Tetrahedron Lett. 2002;43:5649–5651. [Google Scholar]; b) Murphy JM, Liao X, Hartwig JF. J Am Chem Soc. 2007;129:15434. doi: 10.1021/ja076498n. [DOI] [PubMed] [Google Scholar]; c) Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J Am Chem Soc. 2002;124:390–391. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]; d) Ishiyama T, Takagi J, Hartwig JF, Miyaura N. Angew Chem. 2002;114:3182–3184. doi: 10.1002/1521-3773(20020816)41:16<3056::AID-ANIE3056>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:3056–3058. doi: 10.1002/1521-3773(20020816)41:16<3056::AID-ANIE3056>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 143.Newton JN, Fischer DF, Sarpong R. Angew Chem. 2013;125:1770–1774. doi: 10.1002/anie.201208571. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:1726–1730. doi: 10.1002/anie.201208571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan C, Chang CT, Axelrod A, Siegel D. J Am Chem Soc. 2010;132:5924–5925. doi: 10.1021/ja101956x. [DOI] [PubMed] [Google Scholar]
- 145.Zhao L, Tsukano C, Kwon E, Takemoto Y, Hirama M. Angew Chem. 2013;125:1766–1769. doi: 10.1002/anie.201208297. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:1722–1725. doi: 10.1002/anie.201208297. [DOI] [PubMed] [Google Scholar]
- 146.Thompson ALS, Kabalka GW, Akula MR, Huffman JW. Synthesis. 2005:547–550. [Google Scholar]
- 147.Aranyos A, Old DW, Kiyomori A, Wolfe JP, Sadighi JP, Buchwald SL. J Am Chem Soc. 1999;121:4369–4378. [Google Scholar]
- 148.a) Nilsson BL, Overman LE, deAlaniz JR, Rohde JM. J Am Chem Soc. 2008;130:11297. doi: 10.1021/ja804624u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Altman RA, Nilsson BL, Overman LE, deAlaniz JR, Rohde JM, Taupin V. J Org Chem. 2010;75:7519–7534. doi: 10.1021/jo101619d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cheng XY, Waters SP. Org Lett. 2010;12:205–207. doi: 10.1021/ol902455y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.a) Ayer WA, Masaki N, Nkunika DS. Can J Chem. 1968;46:3631–3642. [Google Scholar]; b) Scott WL, Evans DA. J Am Chem Soc. 1972;94:4779–4780. doi: 10.1021/ja00768a083. [DOI] [PubMed] [Google Scholar]; c) Oppolzer W, Petrzilka M. J Am Chem Soc. 1976;98:6722–6723. [Google Scholar]; d) Oppolzer W, Petrzilka M. Helv Chim Acta. 1978;61:2755–2762. [Google Scholar]; e) Szychowski J, Maclean DB. Can J Chem. 1979;57:1631–1637. [Google Scholar]; f) Schumann D, Naumann A. Liebigs Ann Chem. 1984:1519–1528. [Google Scholar]; g) Comins DL, Brooks CA, Alawar RS, Goehring RR. Org Lett. 1999;1:229–231. doi: 10.1021/ol990028j. [DOI] [PubMed] [Google Scholar]; h) Barbe G, Fiset D, Charette AB. J Org Chem. 2011;76:5354–5362. doi: 10.1021/jo200745n. [DOI] [PubMed] [Google Scholar]
- 151.a) Wright DL, Whitehead CR. Org Prep Proced Int. 2000;32:307–330. [Google Scholar]; b) Enquist JA, Jr, Stoltz BM. Nat Prod Rep. 2009;26:661–680. doi: 10.1039/b811227b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.a) Kawagishi H, Shimada A, Shirai R, Okamoto K, Ojima F, Sakamoto H, Ishiguro Y, Furukawa S. Tetrahedron Lett. 1994;35:1569–1572. [Google Scholar]; b) Kawagishi H, Shimada A, Hosokawa S, Mori H, Sakamoto H, Ishiguro Y, Sakemi S, Bordner J, Kojima N, Furukawa S. Tetrahedron Lett. 1996;37:7399–7402. [Google Scholar]; c) Kawagishi H, Simada A, Shizuki K, Mori H, Okamoto K, Sakamoto H, Furukawa S. Heterocycl Commun. 1996;2:51–54. [Google Scholar]
- 153.a) Kita T, Takaya Y, Oshima Y, Ohta T, Aizawa K, Hirano T, Inakuma T. Tetrahedron. 1998;54:11877–11886. [Google Scholar]; b) Ohta T, Kita T, Kobayashi N, Obara Y, Nakahata N, Ohizumi Y, Takaya Y, Oshima Y. Tetrahedron Lett. 1998;39:6229–6232. [Google Scholar]; c) Obara Y, Nakahata N, Kita T, Takaya Y, Kobayashi H, Hosoi S, Kiuchi F, Ohta T, Oshima Y, Ohizumi Y. Eur J Pharmacol. 1999;370:79–84. doi: 10.1016/s0014-2999(99)00077-1. [DOI] [PubMed] [Google Scholar]
- 154.Marcotullio MC, Pagiott R, Maltese F, Obara Y, Hoshino T, Nakahata N, Curini M. Planta Med. 2006;72:819–823. doi: 10.1055/s-2006-946681. [DOI] [PubMed] [Google Scholar]
- 155.Allbutt AD, Ayer WA, Brodie HJ, Johri BN, Taube H. Can J Microbiol. 1971;17:1401–1407. doi: 10.1139/m71-223. [DOI] [PubMed] [Google Scholar]
- 156.Shimbo M, Kawagishi H, Yokogoshi H. Nutr Res. 2005;25:617–623. [Google Scholar]
- 157.Saito T, Aoki F, Hirai H, Inagaki T, Matsunaga Y, Sakakibara T, Sakemi S, Suzuki Y, Watanabe S, Suga O, Sujaku T, Smogowicz AA, Truesdell SJ, Wong JW, Nagahisa A, Kojima Y, Kojima N. J Antibiot. 1998;51:983–990. doi: 10.7164/antibiotics.51.983. [DOI] [PubMed] [Google Scholar]
- 158.Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- 159.a) Snider BB, Vo NH, O’Neil SV, Foxman BM. J Am Chem Soc. 1996;118:7644–7645. [Google Scholar]; b) Snider BB, Vo NH, O’Neil SV. J Org Chem. 1998;63:4732–4740. [Google Scholar]
- 160.a) Snider BB. Acc Chem Res. 1980;13:426–432. [Google Scholar]; b) Johnson JS, Evans DA. Acc Chem Res. 2000;33:325–335. doi: 10.1021/ar960062n. [DOI] [PubMed] [Google Scholar]
- 161.a) Tori M, Toyoda N, Sono M. J Org Chem. 1998;63:306–313. [Google Scholar]; b) Takano M, Umino A, Nakada M. Org Lett. 2004;6:4897–4900. doi: 10.1021/ol048010i. [DOI] [PubMed] [Google Scholar]; c) Trost BM, Dong L, Schroeder GM. J Am Chem Soc. 2005;127:2844–2845. doi: 10.1021/ja0435586. [DOI] [PubMed] [Google Scholar]; d) Trost BM, Dong L, Schroeder GM. J Am Chem Soc. 2005;127:10259–10268. doi: 10.1021/ja051547m. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Watanabe H, Takano M, Nakada M. J Synth Org Chem Jpn. 2008;66:1116–1125. [Google Scholar]
- 162.Watanabe H, Takano M, Umino A, Ito T, Ishikawa H, Nakada M. Org Lett. 2007;9:359–362. doi: 10.1021/ol0628816. [DOI] [PubMed] [Google Scholar]
- 163.Watanabe H, Nakada M. J Am Chem Soc. 2008;130:1150–1151. doi: 10.1021/ja7102795. [DOI] [PubMed] [Google Scholar]
- 164.Crabtree SR, Chu WLA, Mander LN. Synlett. 1990:169–170. [Google Scholar]
- 165.Waters SP, Tian Y, Li YM, Danishefsky SJ. J Am Chem Soc. 2005;127:13514–13515. doi: 10.1021/ja055220x. [DOI] [PubMed] [Google Scholar]
- 166.Wieland P, Miescher K. Helv Chim Acta. 1950;33:2215–2228. [Google Scholar]
- 167.Guerrero A, Parrilla A, Camps F. Tetrahedron Lett. 1990;31:1873–1876. [Google Scholar]
- 168.Kanoh N, Sakanishi K, Iimori E, Nishimura K, Iwabuchi Y. Org Lett. 2011;13:2864–2867. doi: 10.1021/ol200873y. [DOI] [PubMed] [Google Scholar]
- 169.Kobayakawa Y, Nakada M. Angew Chem. 2013;125:7717–7721. doi: 10.1002/anie.201303224. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:7569–7573. doi: 10.1002/anie.201303224. [DOI] [PubMed] [Google Scholar]
- 170.Obara Y, Kobayashi H, Ohta T, Ohizumi Y, Nakahata N. Mol Pharmacol. 2001;59:1287–1297. doi: 10.1124/mol.59.5.1287. [DOI] [PubMed] [Google Scholar]
- 171.Liu L, Shi XW, Zong SC, Tang JJ, Gao JM. Bioorg Med Chem Lett. 2012;22:2401–2406. doi: 10.1016/j.bmcl.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 172.Obara Y, Hoshino T, Marcotullio MC, Pagiotti R, Nakahata N. Life Sci. 2007;80:1669–1677. doi: 10.1016/j.lfs.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 173.Elamparuthi E, Fellay C, Neuburger M, Gademann K. Angew Chem. 2012;124:4147–4149. doi: 10.1002/anie.201200515. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:4071–4073. doi: 10.1002/anie.201200515. [DOI] [PubMed] [Google Scholar]
- 174.Kikuchi H, Miyagawa Y, Sahashi Y, Inatomi S, Haganuma A, Nakahata N, Oshima Y. Tetrahedron Lett. 2004;45:6225–6228. [Google Scholar]
- 175.Kikuchi H, Miyagawa Y, Sahashi Y, Inatomi S, Haganuma A, Nakahata N, Oshima Y. J Org Chem. 2004;69:352–356. doi: 10.1021/jo035137x. [DOI] [PubMed] [Google Scholar]
- 176.Min SJ, Danishefsky SJ. Angew Chem. 2007;119:2249–2252. [Google Scholar]; Angew Chem Int Ed. 2007;46:2199–2202. doi: 10.1002/anie.200605058. [DOI] [PubMed] [Google Scholar]
- 177.a) Mehta G, Samineni R, Srihari P. Tetrahedron Lett. 2012;53:829–832. [Google Scholar]; b) Mehta G, Samineni R, Srihari P, Reddy RG, Chakravarty S. Org Biomol Chem. 2012;10:6830–6833. doi: 10.1039/c2ob26107c. [DOI] [PubMed] [Google Scholar]
- 178.a) Dai M, Danishefsky SJ. J Am Chem Soc. 2007;129:3498–3499. doi: 10.1021/ja069164r. [DOI] [PubMed] [Google Scholar]; b) Dai M, Krauss IJ, Danishefsky SJ. J Org Chem. 2008;73:9576–9583. doi: 10.1021/jo8016814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Danishefsky S. Acc Chem Res. 1979;12:66–72. [Google Scholar]
- 180.Barton DHR, Mccombie SW. J Chem Soc Perkin Trans 1. 1975:1574–1585. [Google Scholar]
- 181.Danishefsky S, Kitahara T. J Am Chem Soc. 1974;96:7807–7808. [Google Scholar]
- 182.a) Tsuda M, Kobayashi J. Heterocycles. 1997;46:765–794. [Google Scholar]; b) Magnier E, Langlois Y. Tetrahedron. 1998;54:6201–6258. [Google Scholar]; c) Matzanke N, Gregg RJ, Weinreb SM. Org Prep Proced Int. 1998;30:1–51. [Google Scholar]; d) Baldwin JE, Whitehead RC. Tetrahedron Lett. 1992;33:2059–2062. [Google Scholar]; e) Baldwin JE, Ciaridge TDW, Culshaw AJ, Heupel FA, Lee V, Spring DR, Whitehead RC, Boughtflower RJ, Mutton IM, Upton RJ. Angew Chem. 1998;110:2806–2808. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2661::AID-ANIE2661>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2661–2663. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2661::AID-ANIE2661>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 183.a) Sakai R, Higa T. J Am Chem Soc. 1986;108:6404–6405. [Google Scholar]; b) Nakamura H, Deng SZ, Kobayashi J, Ohizumi Y, Tomotake Y, Matsuzaki T, Hirata Y. Tetrahedron Lett. 1987;28:621–624. [Google Scholar]
- 184.Toma T, Kita Y, Fukuyama T. J Am Chem Soc. 2010;132:10233–10235. doi: 10.1021/ja103721s. [DOI] [PubMed] [Google Scholar]
- 185.Zhang B, Higuchi R, Miyamoto T, Van Soest RWM. Chem Pharm Bull. 2008;56:866–869. doi: 10.1248/cpb.56.866. [DOI] [PubMed] [Google Scholar]
- 186.Winkler JD, Axten JM. J Am Chem Soc. 1998;120:6425–6426. doi: 10.1021/ja981303k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.a) Martin SF, Humphrey JM, Ali A, Hillier MC. J Am Chem Soc. 1999;121:866–867. [Google Scholar]; b) Humphrey JM, Liao YS, Ali A, Rein T, Wong YL, Chen HJ, Courtney AK, Martin SF. J Am Chem Soc. 2002;124:8584–8592. doi: 10.1021/ja0202964. [DOI] [PubMed] [Google Scholar]
- 188.a) Milstein D, Stille JK. J Am Chem Soc. 1978;100:3636–3638. [Google Scholar]; b) Stille JK. Angew Chem. 1986;98:504–519. [Google Scholar]; Angew Chem Int Ed Engl. 1986;25:508–523. [Google Scholar]; c) Espinet P, Echavarren AM. Angew Chem. 2004;116:4808–4839. doi: 10.1002/anie.200300638. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:4704–4734. doi: 10.1002/anie.200300638. [DOI] [PubMed] [Google Scholar]
- 189.a) Schwab P, Grubbs RH, Ziller JW. J Am Chem Soc. 1996;118:100–110. [Google Scholar]; b) Meek SJ, O’Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168–8179. [Google Scholar]
- 190.Jakubec P, Hawkins A, Felzmann W, Dixon DJ. J Am Chem Soc. 2012;134:17482–17485. doi: 10.1021/ja308826x. [DOI] [PubMed] [Google Scholar]
- 191.a) Rao KV, Donia MS, Peng J, Garcia-Palomero E, Alonso D, Martinez A, Medina M, Franzblau SG, Tekwani BL, Khan SI, Wahyuono S, Willett KL, Hamann MT. J Nat Prod. 2006;69:1034–1040. doi: 10.1021/np0601399. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hamann M, Alonso D, Martin-Aparicio E, Fuertes A, Perez-Puerto MJ, Castro A, Morales S, Navarro ML, del Monte-Millan M, Medina M, Pennaka H, Balaiah A, Peng JN, Cook J, Wahyuono S, Martinez A. J Nat Prod. 2007;70:1397–1405. doi: 10.1021/np060092r. [DOI] [PubMed] [Google Scholar]; c) Osolodkin DI, Shulga DA, Palyulin VA, Zefirov NS. Russ Chem Bull. 2010;59:1983–1993. [Google Scholar]
- 192.a) Alonso M, Martinez A. Curr Med Chem. 2004;11:755–763. doi: 10.2174/0929867043455738. [DOI] [PubMed] [Google Scholar]; b) Meijer L, Flajolet M, Greengard P. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]; c) Martinez A, Perez DI. J Alzheimer’s Dis. 2008;15:181–191. doi: 10.3233/jad-2008-15204. [DOI] [PubMed] [Google Scholar]; d) Palomo V, Perez DI, Gil C, Martinez A. Curr Med Chem. 2011;18:3028–3034. doi: 10.2174/092986711796391697. [DOI] [PubMed] [Google Scholar]; e) Cohen P, Frame S. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]; f) Eldar-Finkelman H, Martinez A. Front Mol Neurosci. 2011;4:32. doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Martinez A, Castro A, Medina M, editors. Glycogen Synthase Kinase 3 (GSK-3) and Its Inhibitors: Drug Discovery and Development. Wiley; Hoboken: 2011. [PubMed] [Google Scholar]
- 193.Peng J, Kudrimoti S, Prasanna S, Odde S, Doerksen RJ, Pennaka HK, Choo YM, Rao KV, Tekwani BL, Madgula V, Khan SI, Wang B, Mayer AM, Jacob MR, Tu LC, Gertsch J, Hamann MT. J Med Chem. 2010;53:61–76. doi: 10.1021/jm900672t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nozawa Y, Sakai N, Matsumoto K, Mizoue K. J Antibiot. 2002;55:629–634. doi: 10.7164/antibiotics.55.629. [DOI] [PubMed] [Google Scholar]
- 195.Hua ZH, Carcache DA, Tian Y, Li YM, Danishefsky SJ. J Org Chem. 2005;70:9849–9856. doi: 10.1021/jo051556d. [DOI] [PubMed] [Google Scholar]
- 196.a) Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K. Chem Pharm Bull. 2002;50:760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]; b) Kuboyama T, Tohda C, Komatsu K. Br J Pharmacol. 2005;144:961–971. doi: 10.1038/sj.bjp.0706122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.a) Jana CK, Hoecker J, Woods TM, Jessen HJ, Neuburger M, Gademann K. Angew Chem. 2011;123:8557–8561. doi: 10.1002/anie.201101869. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:8407–8411. doi: 10.1002/anie.201101869. [DOI] [PubMed] [Google Scholar]; b) Liffert R, Hoecker J, Jana CK, Woods TM, Burch P, Jessen HJ, Neuburger M, Gademann K. Chem Sci. 2013;4:2851–2857. [Google Scholar]
- 198.a) Adam W, Staab E. Liebigs Ann Chem. 1988:757–759. [Google Scholar]; b) Prein M, Adam W. Angew Chem. 1996;108:519–538. [Google Scholar]; Angew Chem Int Ed Engl. 1996;35:477–494. [Google Scholar]
- 199.Patil SP, Maki S, Khedkar SA, Rigby AC, Chan C. J Nat Prod. 2010;73:1196–1202. doi: 10.1021/np900633j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dominguez R, Jalali C, de Lacalle S. J Neurosci. 2004;24:982–990. doi: 10.1523/JNEUROSCI.2586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Mol Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- 202.a) Zou K, Zhu S, Meselhy MR, Tohda C, Cai SQ, Komatsu K. J Nat Prod. 2002;65:1288–1292. doi: 10.1021/np0201117. [DOI] [PubMed] [Google Scholar]; b) Li YS, Ishibashi M, Satake M, Chen XG, Oshima Y, Ohizumi Y. J Nat Prod. 2003;66:696–698. doi: 10.1021/np020577p. [DOI] [PubMed] [Google Scholar]
- 203.Jang SW, Liu X, Chan CB, France SA, Sayeed I, Tang WX, Lin X, Xiao G, Andero R, Chang QA, Ressler KJ, Ye KQ. PLoS One. 2010;5:e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun CY, Ou XM, Farley JM, Stockmeier C, Bigler S, Brinton RD, Wang JM. Curr Alzheimer Res. 2012;9:473–480. doi: 10.2174/156720512800492567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.a) Sato T, Hanada T, Arioka M, Ando K, Sugiyama J, Uramoto M, Yamasaki M, Kitamoto K. J Antibiot. 1998;51:897–901. doi: 10.7164/antibiotics.51.897. [DOI] [PubMed] [Google Scholar]; b) Sato T, Hanada T, Arioka M, Morita TI, Koshino H. J Antibiot. 1998;51:1047–1050. doi: 10.7164/antibiotics.51.1047. [DOI] [PubMed] [Google Scholar]
- 206.a) Toran-Allerand CD. Dev Neurosci. 1996;18:36–48. doi: 10.1159/000111393. [DOI] [PubMed] [Google Scholar]; b) Lee SJ, McEwen BS. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]; c) Scharfman HE, MacLusky NJ. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ciochina R, Grossman RB. Chem Rev. 2006;106:3963–3986. doi: 10.1021/cr0500582.Richard JA, Pouwer RH, Chen DYK. Angew Chem. 2012;124:4612–4638.Angew Chem Int Ed. 2012;51:4536–4561. doi: 10.1002/anie.201103873.Polyprenylated Phloroglucinols and Xanthones : Dakanali M, Theodorakis EA. In: Biomimetic Organic Synthesis. Poupon E, Nay B, editors. Wiley-VCH; Weinheim: 2011.
- 208.a) Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Muller WE. Life Sci. 1998;63:499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]; b) Muller WE, Singer A, Wonnemann M, Hafner U, Rolli M, Schafer C. Pharmacopsychiatry. 1998;31:16–21. doi: 10.1055/s-2007-979341. [DOI] [PubMed] [Google Scholar]; c) Muller WE, Singer A, Wonnemann M, Rolli M, Schafer C, Hafner U. Psychopharmakotherapy. 1998;5:40–45. doi: 10.1055/s-2007-979341. [DOI] [PubMed] [Google Scholar]; d) Rodriguez-Landa JF, Contreras CA. Phytomedicine. 2003;10:688–699. doi: 10.1078/0944-7113-00340. [DOI] [PubMed] [Google Scholar]; e) Verotta L. Curr Top Med Chem. 2003;3:187–201. doi: 10.2174/1568026033392589. [DOI] [PubMed] [Google Scholar]; f) Beerhues L. Phytochemistry. 2006;67:2201–2207. doi: 10.1016/j.phytochem.2006.08.017. [DOI] [PubMed] [Google Scholar]; g) Kumar S, Chakraborty S, Barbosa C, Brustovetsky T, Brustovetsky N, Obukhov AG. J Cell Physiol. 2012;227:1408–1419. doi: 10.1002/jcp.22855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.a) Fukuyama Y, Kuwayama A, Minami H. Chem Pharm Bull. 1997;45:947–949. doi: 10.1248/cpb.45.947. [DOI] [PubMed] [Google Scholar]; b) Fukuyama Y, Minami H, Kuwayama A. Phytochemistry. 1998;49:853–857. [Google Scholar]
- 210.a) Gurevich AI, Dobrynin VN, Kolosov MN, Popravko SA, Ryabova ID, Chernov BK, Derbents NA, Aizenman BE, Garaguly AD. Antibiotiki. 1971;16:510–513. [PubMed] [Google Scholar]; b) Bystrov NS, Chernov BK, Dobrynin VN, Kolosov MN. Tetrahedron Lett. 1975;16:2791–2794. [Google Scholar]; c) Brondz I, Greibrokk T, Groth PA, Aasen AJ. Tetrahedron Lett. 1982;23:1299–1300. [Google Scholar]; d) Brondz I, Greibrokk T, Groth P, Aasen AJ. Acta Chem Scand Ser A. 1983;37:263–265. [Google Scholar]; e) Adam P, Arigoni D, Bacher A, Eisenreich W. J Med Chem. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- 211.a) Gartner M, Muller T, Simon JC, Giannis A, Sleeman JP. ChemBioChem. 2005;6:171–177. doi: 10.1002/cbic.200400195. [DOI] [PubMed] [Google Scholar]; b) Gey C, Kyrylenko S, Hennig L, Nguyen LHD, Butner A, Pham HD, Giannis A. Angew Chem. 2007;119:5311–5314. doi: 10.1002/anie.200605207. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:5219–5222. doi: 10.1002/anie.200605207. [DOI] [PubMed] [Google Scholar]; c) Rothley M, Schmid A, Thiele W, Schacht V, Plaumann D, Gartner M, Yektaoglu A, Bruyere F, Noel A, Giannis A, Sleeman JP. Int J Cancer. 2009;125:34–42. doi: 10.1002/ijc.24295. [DOI] [PubMed] [Google Scholar]; d) Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. Proc Natl Acad Sci USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Verotta L, Appendino G, Bombardelli EO, Brun R. Bioorg Med Chem Lett. 2007;17:1544–1548. doi: 10.1016/j.bmcl.2006.12.100. [DOI] [PubMed] [Google Scholar]; f) Medina MA, Martinez-Poveda B, Amores-Sanchez MI, Quesada AR. Life Sci. 2006;79:105–111. doi: 10.1016/j.lfs.2005.12.027. [DOI] [PubMed] [Google Scholar]; g) Zanoli P. CNS Drug Rev. 2004;10:203–218. doi: 10.1111/j.1527-3458.2004.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.a) Leuner K, Foik A, Harteneck C, Muller WE. Neuropsychopharmacology. 2006;31:S237–S237. [Google Scholar]; b) Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, Harteneck C, Muller WE. FASEB J. 2007;21:4101–4111. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]; c) Leuner K, Muller M, Kasanzki V, Harteneck C, Muller WE. Pharmacopsychiatry. 2007;40:213–213. [Google Scholar]; d) Leuner K, Muller M, Henke B, Harteneck C, Muller WE. N-S Arch Pharmacol. 2008;377:35–35. [Google Scholar]; e) Heiser JH, Muller WE, Leuner K. Eur Neuropsychopharmacol. 2010;20:S92–S92. [Google Scholar]; f) Leuner K, Li W, Amaral MD, Rudolph S, Calfa G, Schuwald AM, Harteneck C, Inoue T, Pozzo-Miller L. Hippocampus. 2012;23:40–52. doi: 10.1002/hipo.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.a) Froestl B, Steiner B, Muller WE. Biochem Pharmacol. 2003;66:2177–2184. doi: 10.1016/j.bcp.2003.08.010. [DOI] [PubMed] [Google Scholar]; b) Froestl B, Steiner B, Mueller WE. N-S Arch Pharmacol. 2002;365:R85–R85. [Google Scholar]
- 214.Dinamarca MC, Cerpa W, Garrido J, Hancke JL, Inestrosa NC. Mol Psychiatry. 2006;11:1032–1048. doi: 10.1038/sj.mp.4001866. [DOI] [PubMed] [Google Scholar]
- 215.Gibon J, Deloulme JC, Chevallier T, Ladeveze E, Abrous DN, Bouron A. Int J Neuropsychopharmacol. 2013;16:189–198. doi: 10.1017/S146114571100188X. [DOI] [PubMed] [Google Scholar]
- 216.Shimizu Y, Shi SL, Usuda H, Kanai M, Shibasaki M. Angew Chem. 2010;122:1121–1124. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:1103–1106. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]
- 217.a) Kagan HB, Riant O. Chem Rev. 1992;92:1007–1019. [Google Scholar]; b) Jørgensen KA. Angew Chem. 2000;112:3702–3733. [Google Scholar]; Angew Chem Int Ed. 2000;39:3558–3588. doi: 10.1002/1521-3773(20001016)39:20<3558::aid-anie3558>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 218.Sparling BA, Moebius DC, Shair MD. J Am Chem Soc. 2013;135:644–647. doi: 10.1021/ja312150d. [DOI] [PubMed] [Google Scholar]
- 219.Uwamori M, Nakada M. Tetrahedron Lett. 2013;54:2022–2025. [Google Scholar]
- 220.Kuramochi A, Usuda H, Yamatsugu K, Kanai M, Shibasaki M. J Am Chem Soc. 2005;127:14200–14201. doi: 10.1021/ja055301t. [DOI] [PubMed] [Google Scholar]
- 221.a) Takacs JM, Jiang XT. Curr Org Chem. 2003;7:369–396. [Google Scholar]; b) Keith JA, Nielsen RJ, Oxgaard J, Goddard WA. J Am Chem Soc. 2007;129:12342–12343. doi: 10.1021/ja072400t. [DOI] [PubMed] [Google Scholar]
- 222.Siegel DR, Danishefsky SJ. J Am Chem Soc. 2013;135:644–647. [Google Scholar]
- 223.Asakura J, Robins MJ. Tetrahedron Lett. 1988;29:2855–2858. [Google Scholar]
- 224.Ahmad NM, Rodeschini V, Simpkins NS, Ward SE, Blake AJ. J Org Chem. 2007;72:4803–4815. doi: 10.1021/jo070388h. [DOI] [PubMed] [Google Scholar]
- 225.Tsukamoto S, Macabalang AD, Nakatani K, Obara Y, Nakahata N, Ohta T. J Nat Prod. 2003;66:1578–1581. doi: 10.1021/np030140x. [DOI] [PubMed] [Google Scholar]
- 226.a) Wang Z, Min SJ, Danishefsky SJ. J Am Chem Soc. 2009;131:10848. doi: 10.1021/ja9049433. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Min SJ, Danishefsky SJ. Tetrahedron Lett. 2008;49:3496–3499. doi: 10.1016/j.tetlet.2008.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Renz M, Meunier B. Eur J Org Chem. 1999:737–750. [Google Scholar]
- 228.Fukuyama Y, Minami H, Takeuchi K, Kodama M, Kawazu K. Tetrahedron Lett. 1996;37:6767–6770. [Google Scholar]
- 229.a) Fukuyama Y, Kubo M, Minami H, Yuasa H, Matsuo A, Fujii T, Morisaki M, Harada K. Chem Pharm Bull. 2005;53:72–80. doi: 10.1248/cpb.53.72. [DOI] [PubMed] [Google Scholar]; b) Imagawa H, Saijo H, Kurisaki T, Yamamoto H, Kubo M, Fukuyama Y, Nishizawa M. Org Lett. 2009;11:1253–1255. doi: 10.1021/ol802973f. [DOI] [PubMed] [Google Scholar]; c) Esumi T, Mori T, Zhao M, Toyota M, Fukuyama Y. Org Lett. 2010;12:888–891. doi: 10.1021/ol902960n. [DOI] [PubMed] [Google Scholar]; d) Fukuyama Y, Esumi T. J Synth Org Chem Jpn. 2007;65:585–597. [Google Scholar]; e) Fukuyama Y, Kubo M, Esumi T, Harada K, Hioki H. Heterocycles. 2010;81:1571–1602. [Google Scholar]; f) Mak JYW, Williams CM. Nat Prod Rep. 2012;29:440–448. doi: 10.1039/c2np00067a. [DOI] [PubMed] [Google Scholar]
- 230.a) Chen APJ, Williams CM. Org Lett. 2008;10:3441–3443. doi: 10.1021/ol801117e. [DOI] [PubMed] [Google Scholar]; b) Chen APJ, Muller CC, Cooper HM, Williams CM. Org Lett. 2009;11:3758–3761. doi: 10.1021/ol901406j. [DOI] [PubMed] [Google Scholar]; c) Chen APJ, Muller CC, Cooper HM, Williams CM. Tetrahedron. 2010;66:6842–6850. [Google Scholar]; d) Mak JYW, Williams CM. Eur J Org Chem. 2012:2001–2012. [Google Scholar]; e) Mak JYW, Williams CM. Chem Commun. 2012;48:287–289. doi: 10.1039/c1cc15995j. [DOI] [PubMed] [Google Scholar]
- 231.Imagawa H, Saijo H, Yamaguchi H, Maekawa K, Kurisaki T, Yamamoto H, Nishizawa M, Oda M, Kabura M, Nagahama M, Sakurai J, Kubo M, Nakai M, Makino K, Ogata M, Takahashi H, Fukuyama Y. Bioorg Med Chem Lett. 2012;22:2089–2093. doi: 10.1016/j.bmcl.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 232.a) Matsunaga H, Katano M, Yamamoto H, Mori M, Takata K. Chem Pharm Bull. 1989;37:1279–1281. doi: 10.1248/cpb.37.1279. [DOI] [PubMed] [Google Scholar]; b) Kobayashi M, Mahmud T, Umezome T, Kitagawa I. Chem Pharm Bull. 1995;43:1595–1597. doi: 10.1248/cpb.43.365. [DOI] [PubMed] [Google Scholar]
- 233.a) Satoh M, Takeuchi N, Fujimoto Y. Chem Pharm Bull. 1997;45:1114–1116. [Google Scholar]; b) Lu W, Zheng GR, Cai JC. Synlett. 1998:737–738. [Google Scholar]; c) Gurjar MK, Kumar VS, Rao BV. Tetrahedron. 1999;55:12563–12576. [Google Scholar]; d) Yadav JS, Maiti A. Tetrahedron. 2002;58:4955–4961. [Google Scholar]; e) Prasad KR, Swain B. Tetrahedron: Asymmetry. 2011;22:1261–1265. [Google Scholar]
- 234.a) Yun HD, Danishefsky SJ. J Org Chem. 2003;68:4519–4522. doi: 10.1021/jo0341665. [DOI] [PubMed] [Google Scholar]; b) Yun HD, Chou TC, Dong HJ, Tian Y, Li YM, Danishefsky SJ. J Org Chem. 2005;70:10375–10380. doi: 10.1021/jo0515475. [DOI] [PubMed] [Google Scholar]
- 235.Zhai HF, Nakatsukasa M, Mitsumoto Y, Fukuyama Y. Planta Med. 2004;70:598–602. doi: 10.1055/s-2004-827179. [DOI] [PubMed] [Google Scholar]
- 236.a) Esumi T, Hojyo D, Zhai HF, Fukuyama Y. Tetrahedron Lett. 2006;47:3979–3983. [Google Scholar]; b) Hanessian S, Reddy GJ. Synlett. 2007:0475–0479. [Google Scholar]; c) Kim HS, Wooten CM, Park Y, Hong JY. Org Lett. 2007;9:3965–3968. doi: 10.1021/ol7016388. [DOI] [PubMed] [Google Scholar]; d) Fukuyama Y, Harada K, Esumi T, Hojyo D, Kujime Y, Kubo N, Kubo M, Hioki H. Heterocycles. 2008;76:551–567. [Google Scholar]; e) Matcha K, Ghosh S. Tetrahedron Lett. 2008;49:3433–3436. [Google Scholar]; f) Rye CE, Barker D. Synlett. 2009:3315–3319. [Google Scholar]; g) Xue P, Wang LP, Jiao XZ, Jiang YJ, Xiao Q, Luo ZG, Xie P, Liang XT. J Asian Nat Prod Res. 2009;11:281–287. doi: 10.1080/10286020802675191. [DOI] [PubMed] [Google Scholar]
- 237.Zhang M, Wang WL, Fang YC, Zhu TJ, Gu QQ, Zhu WM. J Nat Prod. 2008;71:985–989. doi: 10.1021/np700737g. [DOI] [PubMed] [Google Scholar]
- 238.Ge HM, Yu ZG, Zhang J, Wu JH, Tan RX. J Nat Prod. 2009;72:753–755. doi: 10.1021/np800700e. [DOI] [PubMed] [Google Scholar]
- 239.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye KQ. Proc Natl Acad Sci USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.a) Chen J, Chua KW, Chua CC, Yu HL, Pei AJ, Chua BHL, Hamdy RC, Xu XS, Liu CF. Neurosci Lett. 2011;499:181–185. doi: 10.1016/j.neulet.2011.05.054. [DOI] [PubMed] [Google Scholar]; b) Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. Hippocampus. 2012;22:399–408. doi: 10.1002/hipo.20906. [DOI] [PubMed] [Google Scholar]; c) Devi L, Ohno M. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) You YY, Gupta V, Li J, Klistorner A, Graham S. Clin Exp Ophthalmol. 2012;40:87–87. [Google Scholar]; e) Zeng Y, Liu YK, Wu MY, Liu J, Hu Q. J Alzheimer’s Dis. 2012;31:765–778. doi: 10.3233/JAD-2012-120886. [DOI] [PubMed] [Google Scholar]; f) Zeng Y, Lv F, Li L, Yu H, Dong M, Fu QH. J Neurochem. 2012;122:800–811. doi: 10.1111/j.1471-4159.2012.07830.x. [DOI] [PubMed] [Google Scholar]; g) Gupta VK, You YY, Li JC, Klistorner A, Graham SL. J Mol Neurosci. 2013;49:96–104. doi: 10.1007/s12031-012-9899-x. [DOI] [PubMed] [Google Scholar]
- 241.a) Liu X, Chan CB, Jang SW, Pradoldej S, Huang JJ, He KY, Phun LH, France S, Xiao G, Jia YH, Luo HBR, Ye KQ. J Med Chem. 2010;53:8274–8286. doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu X, Chan CB, Qi Q, Xiao G, Luo HR, He XL, Ye KQ. J Med Chem. 2012;55:8524–8537. doi: 10.1021/jm301099x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.a) Maher P, Akaishi T, Abe K. Proc Natl Acad Sci USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Maher P. Arch Biochem Biophys. 2008;476:139–144. doi: 10.1016/j.abb.2008.03.023. [DOI] [PubMed] [Google Scholar]; c) Li M, Tsang KS, Choi ST, Li K, Shaw PC, Lau KF. ChemBioChem. 2011;12:449–456. doi: 10.1002/cbic.201000570. [DOI] [PubMed] [Google Scholar]; d) El Omri A, Han J, Kawada K, Ben Abdrabbah M, Isoda H. Brain Res. 2012;1437:16–25. doi: 10.1016/j.brainres.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 243.Tsukamoto S, Miura S, Yamashita Y, Ohta T. Bioorg Med Chem Lett. 2004;14:417–420. doi: 10.1016/j.bmcl.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 244.Inoue A, Kanematsu M, Yoshida M, Shishido K. Tetrahedron Lett. 2010;51:3966–3968. [Google Scholar]
- 245.a) Li YS, Matsunaga K, Kato R, Ohizumi Y. J Pharm Pharmacol. 2001;53:915–919. doi: 10.1211/0022357011776108. [DOI] [PubMed] [Google Scholar]; b) Li Y, Ishibashi M, Satake M, Oshima Y, Ohizumi Y. Chem Pharm Bull. 2003;51:1103–1105. doi: 10.1248/cpb.51.1103. [DOI] [PubMed] [Google Scholar]
- 246.Burch P, Binaghi M, Scherer M, Wentzel C, Bossert D, Eberhardt L, Neuburger M, Scheiffele P, Gademann K. Chem Eur J. 2013;19:2589–2591. doi: 10.1002/chem.201203746. [DOI] [PubMed] [Google Scholar]
- 247.a) Hecker E, Bartsch H, Bresch H, Gschwend M, Harle E, Kreibich G, Kubinyi H, Schairer HU, Vonszcze C, Thielman HW. Tetrahedron Lett. 1967;8:3165–3170. [Google Scholar]; b) Petterse RC, Ferguson G. J Chem Soc Chem Commun. 1967:716–717. [Google Scholar]
- 248.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. J Am Chem Soc. 1982;104:6846–6848. [Google Scholar]
- 249.Burry RW. J Neurosci Res. 1998;53:214–222. doi: 10.1002/(SICI)1097-4547(19980715)53:2<214::AID-JNR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 250.a) He WD, Cik M, Lesage A, Van der Linden I, De Kimpe N, Appendino G, Bracke J, Mathenge SG, Mudida FP, Leysen JE, Van Puyvelde L. J Nat Prod. 2000;63:1185–1187. doi: 10.1021/np000249u. [DOI] [PubMed] [Google Scholar]; b) He W, Cik M, Appendino G, Puyvelde LV, Leysen JE, De Kimpe N. Mini-Rev Med Chem. 2002;2:185–200. doi: 10.2174/1389557024605492. [DOI] [PubMed] [Google Scholar]; c) He WD, Cik M, Van Puyvelde L, Van Dun J, Appendino G, Lesage A, Van der Lindin I, Leysen JE, Wouters W, Mathenge SG, Mudida FP, De Kimpe N. Bioorg Med Chem. 2002;10:3245–3255. doi: 10.1016/s0968-0896(02)00163-3. [DOI] [PubMed] [Google Scholar]
- 251.a) Wender PA, Kogen H, Lee HY, Munger JD, Wilhelm RS, Williams PD. J Am Chem Soc. 1989;111:8957–8958. [Google Scholar]; b) Wender PA, Mcdonald FE. J Am Chem Soc. 1990;112:4956–4958. [Google Scholar]; c) Wender PA, Rice KD, Schnute ME. J Am Chem Soc. 1997;119:7897–7898. [Google Scholar]; d) Wender PA, Jesudason CD, Nakahira H, Tamura N, Tebbe AL, Ueno Y. J Am Chem Soc. 1997;119:12976–12977. [Google Scholar]; e) Wender PA, Buschmann N, Cardin NB, Jones LR, Kan C, Kee JM, Kowalski JA, Longcore KE. Nat Chem. 2011;3:615–619. doi: 10.1038/nchem.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee K, Cha JK. J Am Chem Soc. 2001;123:5590–5591. doi: 10.1021/ja010643u. [DOI] [PubMed] [Google Scholar]
- 253.Keck GE, Poudel YB, Cummins TJ, Rudra A, Covel JA. J Am Chem Soc. 2011;133:744–747. doi: 10.1021/ja110198y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kageyama M, Tamura T, Nantz MH, Roberts JC, Somfai P, Whritenour DC, Masamune S. J Am Chem Soc. 1990;112:7407–7408. [Google Scholar]
- 255.a) Evans DA, Carter PH, Carreira EM, Prunet JA, Charette AB, Lautens M. Angew Chem. 1998;110:2526–2530. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2354::AID-ANIE2354>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2354–2359. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2354::AID-ANIE2354>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; b) Evans DA, Carter PH, Carreira EM, Charette AB, Prunet JA, Lautens M. J Am Chem Soc. 1999;121:7540–7552. [Google Scholar]
- 256.Ohmori K, Ogawa Y, Obitsu T, Ishikawa Y, Nishiyama S, Yamamura S. Angew Chem. 2000;112:2376–2379. [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:2290–2294. [PubMed] [Google Scholar]
- 257.Manaviazar S, Frigerio M, Bhatia GS, Hummersone MG, Aliev AE, Hale KJ. Org Lett. 2006;8:4477–4480. doi: 10.1021/ol061626i. [DOI] [PubMed] [Google Scholar]
- 258.a) Trost BM, Dong GB. Nature. 2008;456:485–488. doi: 10.1038/nature07543. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Trost BM, Dong GB. J Am Chem Soc. 2010;132:16403–16416. doi: 10.1021/ja105129p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lu Y, Woo SK, Krische MJ. J Am Chem Soc. 2011;133:13876–13879. doi: 10.1021/ja205673e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wender PA, Schrier AJ. J Am Chem Soc. 2011;133:9228–9231. doi: 10.1021/ja203034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.a) Fukuyama Y, Otoshi Y, Kodama M, Hasegawa T, Okazaki H. Tetrahedron Lett. 1990;31:4477–4480. [Google Scholar]; b) Fukuyama Y, Otoshi Y, Miyoshi K, Nakamura K, Kodama M, Nagasawa M, Hasegawa T, Okazaki H, Sugawara M. Tetrahedron. 1992;48:377–392. [Google Scholar]
- 262.a) Cheng X, Harzdorf NL, Shaw T, Siegel D. Org Lett. 2010;12:1304–1307. doi: 10.1021/ol100214x. [DOI] [PubMed] [Google Scholar]; b) Khaing Z, Kang D, Camelio AM, Schmidt CE, Siegel D. Bioorg Med Chem Lett. 2011;21:4808–4812. doi: 10.1016/j.bmcl.2011.06.054. [DOI] [PubMed] [Google Scholar]; c) Cheng X, Harzdorf N, Khaing Z, Kang D, Camelio AM, Shaw T, Schmidt CE, Siegel D. Org Biomol Chem. 2012;10:383–393. doi: 10.1039/c1ob06363d. [DOI] [PubMed] [Google Scholar]; d) Zlotkowski K, Pierce-Shimomura J, Siegel D. ChemBioChem. 2013;14:307–310. doi: 10.1002/cbic.201200712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.a) Tisdale EJ, Chowdhury C, Vong BG, Li HM, Theodorakis EA. Org Lett. 2002;4:909–912. doi: 10.1021/ol017278w. [DOI] [PubMed] [Google Scholar]; b) Tisdale EJ, Slobodov I, Theodorakis EA. Org Biomol Chem. 2003;1:4418–4422. doi: 10.1039/b311833a. [DOI] [PubMed] [Google Scholar]; c) Tisdale EJ, Slobodov I, Theodorakis EA. Proc Natl Acad Sci USA. 2004;101:12030–12035. doi: 10.1073/pnas.0401932101. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Batova A, Lam T, Wascholowski V, Yu AL, Giannis A, Theodorakis EA. Org Biomol Chem. 2007;5:494–500. doi: 10.1039/b612903j. [DOI] [PubMed] [Google Scholar]; e) Chantarasriwong O, Cho WC, Batova A, Chavasiri W, Moore C, Rheingold AL, Theodorakis EA. Org Biomol Chem. 2009;7:4886–4894. doi: 10.1039/b913496d. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Batova A, Altomare D, Chantarasriwong O, Ohlsen KL, Creek KE, Lin YC, Messersmith A, Yu AL, Yu J, Theodorakis EA. Mol Cancer Ther. 2010;9:2869–2878. doi: 10.1158/1535-7163.MCT-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Chantarasriwong O, Batova A, Chavasiri W, Theodorakis EA. Chem Eur J. 2010;16:9944–9962. doi: 10.1002/chem.201000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jang SW, Okada M, Sayeed I, Xiao G, Stein D, Jin P, Ye KQ. Proc Natl Acad Sci USA. 2007;104:16329–16334. doi: 10.1073/pnas.0706662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guo Y, Li Y, Xu J, Li N, Yamakuni T, Ohizumi Y. Chem Pharm Bull. 2007;55:1532–1534. doi: 10.1248/cpb.55.1532. [DOI] [PubMed] [Google Scholar]
- 266.a) Tang W, Hioki H, Harada K, Kubo M, Fukuyama Y. J Nat Prod. 2008;71:1760–1763. doi: 10.1021/np8004002. [DOI] [PubMed] [Google Scholar]; b) Tang WX, Kubo M, Harada K, Hioki H, Fukuyama Y. Bioorg Med Chem Lett. 2009;19:882–886. doi: 10.1016/j.bmcl.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 267.Kakeya H, Takahashi I, Okada G, Isono K, Osada H. J Antibiot. 1995;48:733–735. doi: 10.7164/antibiotics.48.733. [DOI] [PubMed] [Google Scholar]
- 268.a) Kakeya H, Onozawa C, Osada H. FASEB J. 1997;11:A1322–A1322. [Google Scholar]; b) Kakeya H, Onozawa C, Sato M, Arai K, Osada H. J Med Chem. 1997;40:391–394. doi: 10.1021/jm960719a. [DOI] [PubMed] [Google Scholar]
- 269.a) Hayashi Y, Narasaka K. Chem Lett. 1998:313–314. [Google Scholar]; b) Marumoto S, Kogen H, Naruto S. J Org Chem. 1998;63:2068–2069. [Google Scholar]; c) Kuramochi K, Nagata S, Itaya H, Takao K, Kobayashi S. Tetrahedron Lett. 1999;40:7371–7374. [Google Scholar]; d) Marumoto S, Kogen H, Naruto S. Tetrahedron. 1999;55:7129–7144. [Google Scholar]; e) Marumoto S, Kogen H, Naruto S. Tetrahedron. 1999;55:7145–7156. [Google Scholar]; f) Hayashi Y, Kanayama J, Yamaguchi J, Shoji M. J Org Chem. 2002;67:9443–9448. doi: 10.1021/jo025641m. [DOI] [PubMed] [Google Scholar]; g) Kuramochi K, Nagata S, Itaya H, Matsubara Y, Sunoki T, Uchiro H, Takao K, Kobayashi S. Tetrahedron. 2003;59:9743–9758. [Google Scholar]; h) Tan Z, Negishi E. Org Lett. 2006;8:2783–2785. doi: 10.1021/ol060856u. [DOI] [PubMed] [Google Scholar]
- 270.Hohmann J, Vasas A, Gunther G, Dombi G, Blazso G, Falkay G, Mathe I, Jerkovich G. Phytochemistry. 1999;51:673–677. doi: 10.1016/s0031-9422(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 271.a) Yamaguchi K, Uemura D, Tsuji T, Kondo K. Biosci Biotechnol Biochem. 1994;58:1749–1751. doi: 10.1271/bbb.60.92. [DOI] [PubMed] [Google Scholar]; b) Yamaguchi K, Tsuji Y, Uemura D, Kondo K. Biosci Biotechnol Biochem. 1996;60:92–94. doi: 10.1271/bbb.60.92. [DOI] [PubMed] [Google Scholar]; c) Pan Q, Ip FCF, Ip NY, Zhu HX, Min ZD. J Nat Prod. 2004;67:1548–1551. doi: 10.1021/np030541c. [DOI] [PubMed] [Google Scholar]
- 272.Li FQ, Cheng XX, Liang XB, Wang XH, Xue B, He QH, Wang XM, Han JS. Exp Neurol. 2003;179:28–37. doi: 10.1006/exnr.2002.8049. [DOI] [PubMed] [Google Scholar]
- 273.a) Chen S, Hong Z. Mov Disord. 2007;22:S71–S71. [Google Scholar]; b) Hong Z, Wang G, Gu J, Pan J, Bai L, Zhang S, Chen SD. Eur J Neurosci. 2007;26:1500–1508. doi: 10.1111/j.1460-9568.2007.05766.x. [DOI] [PubMed] [Google Scholar]; c) Pan XD, Chen XC, Zhu YG, Zhang J, Huang TW, Chen LM, Ye QY, Huang HP. Biochem Pharmacol. 2008;76:362–372. doi: 10.1016/j.bcp.2008.05.018. [DOI] [PubMed] [Google Scholar]; d) Pan XD, Chen XC, Zhu YG, Chen LM, Zhang J, Huang TW, Ye QY, Huang HP. Glia. 2009;57:1227–1238. doi: 10.1002/glia.20844. [DOI] [PubMed] [Google Scholar]
- 274.a) Gold BG. Expert Opin Invest Drugs. 2000;9:2331–2342. doi: 10.1517/13543784.9.10.2331. [DOI] [PubMed] [Google Scholar]; b) Sosa I, Reyes O, Kuffler DP. Exp Neurol. 2005;195:7–15. doi: 10.1016/j.expneurol.2005.04.016. [DOI] [PubMed] [Google Scholar]; c) Hailer NP. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]; d) Toll EC, Seifalian AM, Birchall MA. Regener Med. 2011;6:635–652. doi: 10.2217/rme.11.43. [DOI] [PubMed] [Google Scholar]; e) Saganova K, Galik J, Blasko J, Korimova A, Racekova E, Vanicky I. Life Sci. 2012;91:77–82. doi: 10.1016/j.lfs.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 275.a) Jessen HJ, Gademann K. Nat Prod Rep. 2010;27:1168–1185. doi: 10.1039/b911516c. [DOI] [PubMed] [Google Scholar]; b) Gademann K, Sieber S. Chimia. 2011;65:835–838. doi: 10.2533/chimia.2011.835. [DOI] [PubMed] [Google Scholar]; c) Wach JY, Gademann K. Synlett. 2012:163–170. [Google Scholar]; d) Schmid F, Jessen HJ, Burch P, Gademann K. MedChemComm. 2013;4:135–139. [Google Scholar]; e) Jessen HJ, Schumacher A, Schmid F, Pfaltz A, Gademann K. Org Lett. 2011;13:4368–4370. doi: 10.1021/ol201692h. [DOI] [PubMed] [Google Scholar]
- 276.a) Basyouni SH, Brewer D, Vining LC. Can J Bot. 1968;46:441–448. [Google Scholar]; b) Mcinnes AG, Smith DG, Wat CK, Vining LC, Wright JLC. J Chem Soc Chem Commun. 1974:281–282. [Google Scholar]; c) Jeffs LB, Khachatourians GG. Toxicon. 1997;35:1351–1356. doi: 10.1016/s0041-0101(97)00025-1. [DOI] [PubMed] [Google Scholar]; d) Halo LM, Heneghan MN, Yakasai AA, Song Z, Williams K, Bailey AM, Cox RJ, Lazarus CM, Simpson TJ. J Am Chem Soc. 2008;130:17988–17996. doi: 10.1021/ja807052c. [DOI] [PubMed] [Google Scholar]
- 277.Cheng YX, Schneider B, Riese U, Schubert B, Li ZZ, Hamburger M. J Nat Prod. 2004;67:1854–1858. doi: 10.1021/np049761w. [DOI] [PubMed] [Google Scholar]
- 278.a) Schmidt K, Gunther W, Stoyanova S, Schubert B, Li ZZ, Hamburger M. Org Lett. 2002;4:197–199. doi: 10.1021/ol016920j. [DOI] [PubMed] [Google Scholar]; b) Schmidt K, Riese U, Li ZZ, Hamburger M. J Nat Prod. 2003;66:378–383. doi: 10.1021/np020430y. [DOI] [PubMed] [Google Scholar]; c) Riese U, Ziegler E, Hamburger M. FEBS Lett. 2004;577:455–459. doi: 10.1016/j.febslet.2004.10.045. [DOI] [PubMed] [Google Scholar]; d) Cheng YX, Schneider B, Riese U, Schubert B, Li ZZ, Hamburger M. J Nat Prod. 2006;69:436–438. doi: 10.1021/np050418g. [DOI] [PubMed] [Google Scholar]; e) Kuenzi P, Kiefer S, Koryakina A, Hamburger M. Apoptosis. 2008;13:364–376. doi: 10.1007/s10495-008-0185-x. [DOI] [PubMed] [Google Scholar]
- 279.Williams DR, Sit SY. J Org Chem. 1982;47:2846–2851. [Google Scholar]
- 280.Jessen HJ, Schumacher A, Shaw T, Pfaltz A, Gademann K. Angew Chem. 2011;123:4308–4312. doi: 10.1002/anie.201007671. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:4222–4226. doi: 10.1002/anie.201007671. [DOI] [PubMed] [Google Scholar]
- 281.Jessen HJ, Barbaras D, Hamburger M, Gademann K. Org Lett. 2009;11:3446–3449. doi: 10.1021/ol901277q. [DOI] [PubMed] [Google Scholar]
- 282.Oura M, Sternberg TH, Wright ET. Antibiot Annu. 1955;3:566–573. [PubMed] [Google Scholar]
- 283.Mechlinski W, Schaffner CP, Ganis P, Avitabile G. Tetrahedron Lett. 1970;11:3873–3876. [Google Scholar]
- 284.Ganis P, Avitabile G, Mechlinski W, Schaffner CP. J Am Chem Soc. 1971;93:4560–4564. doi: 10.1021/ja00747a037. [DOI] [PubMed] [Google Scholar]
- 285.a) Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. Antimicrob Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brajtburg J, Bolard J. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Volmer AA, Szpilman AM, Carreira EM. Nat Prod Rep. 2010;27:1329–1349. doi: 10.1039/b820743g. [DOI] [PubMed] [Google Scholar]
- 286.Gao Y, Deng KW, Cao ZX, Graziani EI, Gilbert AM, Koehn FE, Wood A, Doherty P, Walsh FS. J Neurochem. 2010;113:1331–1342. doi: 10.1111/j.1471-4159.2010.06704.x. [DOI] [PubMed] [Google Scholar]
- 287.a) Nicolaou KC, Chakraborty TK, Daines RA, Simpkins NS. J Chem Soc Chem Commun. 1986:413–416. [Google Scholar]; b) Nicolaou KC, Chakraborty TK, Daines RA, Ogawa Y. J Chem Soc Chem Commun. 1987:686–689. [Google Scholar]; c) Nicolaou KC, Daines RA, Chakraborty TK. J Am Chem Soc. 1987;109:2208–2210. [Google Scholar]; d) Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. J Am Chem Soc. 1987;109:2821–2822. [Google Scholar]; e) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J Am Chem Soc. 1987;109:2205–2208. [Google Scholar]; f) Nicolaou KC, Chakraborty TK, Ogawa Y, Daines RA, Simpkins NS, Furst GT. J Am Chem Soc. 1988;110:4660–4672. [Google Scholar]; g) Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. J Am Chem Soc. 1988;110:4685–4696. [Google Scholar]; h) Nicolaou KC, Daines RA, Ogawa Y, Chakraborty TK. J Am Chem Soc. 1988;110:4696–4705. [Google Scholar]; i) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J Am Chem Soc. 1988;110:4672–4685. [Google Scholar]
- 288.Tanaka H, Kuroda A, Marusawa H, Hatanaka H, Kino T, Goto T, Hashimoto M, Taga T. J Am Chem Soc. 1987;109:5031–5033. [Google Scholar]
- 289.Lyons WE, George EB, Dawson TM, Steiner JP, Snyder SH. Proc Natl Acad Sci USA. 1994;91:3191–3195. doi: 10.1073/pnas.91.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jiang HJ, Qu W, Han F, Zhang WG, Lu DC. Neural Regener Res. 2011;6:29–33. [Google Scholar]
- 291.Price RD, Yamaji T, Matsuoka N. Br J Pharmacol. 2003;140:825–829. doi: 10.1038/sj.bjp.0705522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Gold BG, Villafranca JE. Curr Top Med Chem. 2003;3:1368–1375. doi: 10.2174/1568026033451880.Progress in Enhancing the neurotrophic effects of natural FKBP ligands Carter GT. RSC Drug Discovery Series No. 25. In: Genilloud O, Vicente F, editors. Drug Discovery from Natural Products. Royal Society of Chemistry; London: 2012.
- 293.Gold BG, Densmore V, Shou W, Matzuk MM, Gordon HS. J Pharmacol Exp Ther. 1999;289:1202–1210. [PubMed] [Google Scholar]
- 294.Ruan BF, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, Liang S, Chen Y, Mercado ML, Feng XD, Bennettt F, von Schack D, McDonald L, Zaleska MM, Wood A, Reinhart PH, Magolda RL, Skotnicki J, Pangalos MN, Koehn FE, Carter GT, Abou-Gharbia M, Graziani EI. Proc Natl Acad Sci USA. 2008;105:33–38. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Price RD, Yamaji T, Yamamoto H, Higashi Y, Hanaoka K, Yamazaki S, Ishiye M, Aramori I, Matsuoka N, Mutoh S, Yanagihara T, Gold BG. Eur J Pharmacol. 2005;509:11–19. doi: 10.1016/j.ejphar.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 296.a) Jones TK, Mills SG, Reamer RA, Askin D, Desmond R, Volante RP, Shinkai I. J Am Chem Soc. 1989;111:1157–1159. [Google Scholar]; b) Jones TK, Reamer RA, Desmond R, Mills SG. J Am Chem Soc. 1990;112:2998–3017. [Google Scholar]
- 297.a) Nakatsuka M, Ragan JA, Sammakia T, Smith DB, Uehling DE, Schreiber SL. J Am Chem Soc. 1990;112:5583–5601. [Google Scholar]; b) Andrus MB, Schreiber SL. J Am Chem Soc. 1993;115:10420–10421. [Google Scholar]
- 298.a) Ireland RE, Gleason JL, Gegnas LD, Highsmith TK. J Org Chem. 1996;61:6856–6872. doi: 10.1021/jo951646q. [DOI] [PubMed] [Google Scholar]; b) Ireland RE, Liu LB, Roper TD. Tetrahedron. 1997;53:13221–13256. [Google Scholar]; c) Ireland RE, Liu LB, Roper TD, Gleason JL. Tetrahedron. 1997;53:13257–13284. [Google Scholar]
- 299.Jones AB, Villalobos A, Linde RG, Danishefsky SJ. J Org Chem. 1990;55:2786–2797. [Google Scholar]
- 300.Gu RL, Sih CJ. Tetrahedron Lett. 1990;31:3287–3290. [Google Scholar]
- 301.Smith AB, Chen KM, Robinson DJ, Laakso LM, Hale KJ. Tetrahedron Lett. 1994;35:4271–4274. [Google Scholar]
- 302.Nakata T. In: Macrolide Antibiotics. 2. Ômura S, editor. Elsevier; San Diego: 2002. pp. 210–220. [Google Scholar]
- 303.Maddess ML, Tackett MN, Ley SV. Prog Drug Res. 2008;66:15–186. doi: 10.1007/978-3-7643-8595-8_2. [DOI] [PubMed] [Google Scholar]
- 304.Mori K, Iguchi K, Yamada N, Yamada Y, Inouye Y. Tetrahedron Lett. 1987;28:5673–5676. [Google Scholar]
- 305.Miyaoka H, Baba T, Mitome H, Yamada Y. Tetrahedron Lett. 2001;42:9233–9236. [Google Scholar]
- 306.a) Kikuchi K, Kishino A, Konishi O, Kumagai K, Hosotani N, Saji I, Nakayama C, Kimura T. J Biol Chem. 2003;278:42985–42991. doi: 10.1074/jbc.M302395200. [DOI] [PubMed] [Google Scholar]; b) Kumagai K, Hosotani N, Kikuchi K, Kimura T, Saji I. J Antibiot. 2003;56:610–616. doi: 10.7164/antibiotics.56.610. [DOI] [PubMed] [Google Scholar]
- 307.Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He ZG, Toyama Y, Okano H. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 308.Axelrod A, Eliasen AM, Chin MR, Zlotkowski K, Siegel D. Angew Chem. 2013;125:3505–3508. doi: 10.1002/anie.201205837. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:3421–3424. doi: 10.1002/anie.201205837. [DOI] [PubMed] [Google Scholar]
- 309.a) Evans BE, Rittle KE, Bock MG, Dipardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao GQ, Barluenga S, Mitchell HJ. J Am Chem Soc. 2000;122:9939–9953. [Google Scholar]; c) Nicolaou KC, Pfefferkorn JA, Mitchell HJ, Roecker AJ, Barluenga S, Cao GQ, Affleck RL, Lillig JE. J Am Chem Soc. 2000;122:9954–9967. [Google Scholar]; d) Nicolaou KC, Pfefferkorn JA, Barluenga S, Mitchell HJ, Roecker AJ, Cao GQ. J Am Chem Soc. 2000;122:9968–9976. [Google Scholar]; e) Welsch ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Buss AD, Butler MS, editors. Natural Product Chemistry for Drug Discovery. RSC; Cambridge: 2010. [Google Scholar]
- 311.a) Mann J. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]; b) Newman DJ, Cragg GM. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Newman DJ. J Med Chem. 2008;51:2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- 312.Brodski C, Weisenhorn DMV, Dechant G. Expert Rev Neurother. 2002;2:417–426. doi: 10.1586/14737175.2.3.417. [DOI] [PubMed] [Google Scholar]
- 313.Snider WD. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 314.a) Skaper SD. CNS Neurol Disord Drug Targets. 2008;7:46–U10. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]; b) Skaper SD. Curr Pharm Des. 2011;17:2704–2718. doi: 10.2174/138161211797415995. [DOI] [PubMed] [Google Scholar]
- 315.a) Skaper SD, Walsh FS. Mol Cell Neurosci. 1998;12:179–193. doi: 10.1006/mcne.1998.0714. [DOI] [PubMed] [Google Scholar]; b) Xie YM, Longo FM. Prog Brain Res. 2000;128:333–347. doi: 10.1016/s0079-6123(00)28030-8. [DOI] [PubMed] [Google Scholar]; c) Massa SM, Xie YM, Longo FM. J Mol Neurosci. 2002;19:107–111. doi: 10.1007/s12031-002-0019-1. [DOI] [PubMed] [Google Scholar]; d) Massa SM, Xie YM, Longo FM. J Mol Neurosci. 2003;20:323–326. doi: 10.1385/JMN:20:3:323. [DOI] [PubMed] [Google Scholar]; e) Clement JA, Yoder BJ, Kingston DGL. Mini-Rev Org Chem. 2004;1:183–208. [Google Scholar]; f) Longo FM, Massa SM. Curr Alzheimer Res. 2005;2:167–169. doi: 10.2174/1567205053585819. [DOI] [PubMed] [Google Scholar]; g) Tohda C, Kuboyama T, Komatsu K. Neurosignals. 2005;14:34–45. doi: 10.1159/000085384. [DOI] [PubMed] [Google Scholar]; h) Longo FM, Yang T, Xie Y, Massa SM. Curr Alzheimer Res. 2006;3:5–10. doi: 10.2174/156720506775697089. [DOI] [PubMed] [Google Scholar]; i) Longo FM, Massa SM. CNS Neurol Disord Drug Targets. 2008;7:63–70. doi: 10.2174/187152708783885093. [DOI] [PubMed] [Google Scholar]; j) Rishton GM. Recent Pat CNS Drug Discovery. 2008;3:200–208. doi: 10.2174/157488908786242425. [DOI] [PubMed] [Google Scholar]; k) Webster NJG, Pirrung MC. BMC Neurosci. 2008;9:S1. doi: 10.1186/1471-2202-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Allsopp TE, Bunnage ME, Fish PV. MedChemComm. 2010;1:16–29. [Google Scholar]; m) Iriti M, Vitalini S, Fico G, Faoro F. Molecules. 2010;15:3517–3555. doi: 10.3390/molecules15053517. [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Joyner PM, Cichewicz RH. Nat Prod Rep. 2011;28:26–47. doi: 10.1039/c0np00017e. [DOI] [PubMed] [Google Scholar]; o) Qi J, Luo Y, Gao L. Mini-Rev Med Chem. 2011;11:658–677. doi: 10.2174/138955711796268796. [DOI] [PubMed] [Google Scholar]; p) Williams P, Sorribas A, Howes MJR. Nat Prod Rep. 2011;28:48–77. doi: 10.1039/c0np00027b. [DOI] [PMC free article] [PubMed] [Google Scholar]; q) More SV, Koppula S, Kim IS, Kumar H, Kim BW, Choi DK. Molecules. 2012;17:6728–6753. doi: 10.3390/molecules17066728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Butler MS. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f.b)http://www.clinicaltrials.gov/ct2/results?term=neurodegeneration
- 317.Hongpaisan J, Sun MK, Alkon DL. J Neurosci. 2011;31:630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.http://www.brni.org/scientific_research/clinical_trials.aspx and http://www.news-medical.net/news/2009/04/22/48710.aspx.
- 319.DeChristopher BA, Fan AC, Felsher DW, Wender PA. Oncotarget. 2012;3:58–66. doi: 10.18632/oncotarget.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.a) Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. Nat Chem Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]; b) Varma H, Lo DC, Stockwell BR. Comb Chem High Throughput Screening. 2008;11:238–248. doi: 10.2174/138620708783877753. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Varma H. Curr Mol Pharmacol. 2010;3:164–173. doi: 10.2174/1874467211003030164. [DOI] [PubMed] [Google Scholar]
- 321.a) Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]; b) Fumagalli F, Molteni R, Calabrese F, Maj PF, Racagni G, Riva MA. CNS Drugs. 2008;22:1005–1019. doi: 10.2165/0023210-200822120-00004. [DOI] [PubMed] [Google Scholar]; c) Marchetto MC, Winner B, Gage FH. Hum Mol Genet. 2010;19:R71–76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.a) Blelloch R. Nature. 2008;455:604–605. doi: 10.1038/455604a. [DOI] [PubMed] [Google Scholar]; b) Chien KR. Nature. 2008;453:302–305. doi: 10.1038/nature07037. [DOI] [PubMed] [Google Scholar]; c) DeWitt N. Nature. 2008;453:301–301. doi: 10.1038/453301a. [DOI] [PubMed] [Google Scholar]; d) Holden C. Science. 2008;320:437–437. doi: 10.1126/science.320.5875.437a. [DOI] [PubMed] [Google Scholar]; e) Xu Y, Shi Y, Ding S. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]; f) Berthiaume F, Maguire TJ, Yarmush ML. Annu Rev Chem Biomol. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 323.a) Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, Yuste R. Neuron. 2012;74:970–974. doi: 10.1016/j.neuron.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Alivisatos AP, Chun M, Church GM, Deisseroth K, Donoghue JP, Greenspan RJ, McEuen PL, Roukes ML, Sejnowski TJ, Weiss PS, Yuste R. Science. 2013;339:1284–1285. doi: 10.1126/science.1236939. [DOI] [PMC free article] [PubMed] [Google Scholar]