Abstract
Introduction
Although working memory (WM) impairments are well documented in schizophrenic patients (PSZ), the underlying mechanisms are poorly understood. The aim of this study was to investigate the role of target salience during encoding to determine whether impaired visual attention in PSZ leads to poor WM.
Methods
31 PSZ and 28 demographically matched healthy controls (HC) performed a spatial delayed-response task. Attentional demands were manipulated during WM encoding by presenting high salient (novel) or low salient (familiar) targets. Participants also rated their level of response confidence at the end of each trial, allowing us to analyse different response types.
Results
WM was impaired in PSZ. Increasing target salience by increasing novelty improved WM performance in HC but not in PSZ. Poor WM performance in PSZ was largely due to an increase in the proportion of incorrect but high confident responses most likely reflecting a failure to encode the correct target.
Conclusions
Our findings suggest that dysfunctions of non-mnemonic attentional processes during encoding contribute to WM impairments in schizophrenia and may represent an important target for cognitive remediation strategies.
Keywords: spatial working memory, encoding, attention, schizophrenia, relatives, false memory
INTRODUCTION
Impairments in working memory (WM) are core cognitive deficits in schizophrenia (Goldman-Rakic, 1994; Lee & Park, 2005) with significant consequences on social and occupational functioning (Cervellione, Burdick, Cottone, Rhinewine, & Kumra, 2007). Spatial WM deficits are present in high-risk populations (Smith, Park, & Cornblatt, 2006), in spectrum disorders (Mitropoulou et al., 2005), and in unaffected relatives (Myles-Worsley & Park, 2002; Park, Holzman, & Goldman-Rakic, 1995; Snitz, MacDonald, & Carter, 2006), and therefore have been proposed to be a potential endophenotypic marker of schizophrenia (Glahn, Therman, & Manninen, 2002; Meyer-Lindenberg & Weinberger, 2006; Saperstein et al., 2006). Although WM deficits in PSZ are well documented, it is still unclear what causes these deficits and whether they can be ameliorated. WM is a complex system of various subprocesses (Bledowski, Kaiser, & Rahm, 2010). For a clear understanding of the nature of WM impairments and to develop targeted remediation strategies, it is therefore crucial to determine which specific cognitive processes are responsible for the performance deficits in schizophrenia.
There is considerable evidence for impaired WM maintenance (Park & Holzman, 1992; Park, Püschel, Sauter, Rentsch, & Hell, 1999; Piskulic, Olver, Norman, & Maruff, 2007) and executive functions that are linked to prefrontal dysfunctions (Barch, 2005). However, performance deficits also occur with very short delays and do not necessarily increase with long delays (Gold et al., 2010; Javitt, Liederman, Cienfuegos, & Shelley, 1999; Lee & Park, 2005; Park & Holzman, 1992) pointing to a major locus of impairment at the encoding stage. Indeed, the perceptual encoding of information and its transfer into a more durable WM representation are slower (Fuller, Luck, McMahon, & Gold, 2005; Hartman, Steketee, Silva, Lanning, & McCann, 2003; Tek et al., 2002) and less precise in PSZ (Javitt, Strous, Grochowski, Ritter, & Cowan, 1997, Javitt et al., 1999; Lencz et al., 2003). These findings converge with results from electrophysiological studies (Haenschel & Linden, 2011) that implicate a role of early-stage visual processing and/or higher-level cognitive processes in abnormal encoding.
During WM encoding the selection of task-relevant information is critical for consolidation (Sperling, 1960). The selection process allows limiting of processing to items that are salient and currently relevant in order to deal with the limited capacity of WM. Recent evidence suggests that both bottom-up cues based on perceptual stimulus features and top-down cues driven by expectations, knowledge, and current goals, can increase the salience of the items to be encoded and facilitate visual WM performance in healthy participants (Fine & Minnery, 2009; Mayer, Kim, & Park, 2011; Schmidt, Vogel, Woodman, & Luck, 2002; Vogel, McCollough, & Machizawa, 2005). PSZ are also able to use simple and salient visual cues to select relevant information for WM encoding (Gold et al., 2006; Smith, Eich, Cebenoyan, & Malapani, 2011) and WM encoding improves when the task involves perceptually salient targets (Lee & Park, 2006). However, when the selection process requires a high degree of top-down control, performance is markedly impaired in PSZ both at the level of perception (Fuller et al., 2006; Gold, Fuller, Robinson, Braun, & Luck, 2007; Tanaka et al., 2007) and WM encoding (Hahn et al., 2010). Thus, difficulties in selecting relevant information or deploying attention to the relevant feature efficiently (Nestor et al., 1992; Sereno & Holzman, 1996) may result in imprecise or incorrect encoding. On the other hand, if impaired attentional processing leads to WM deficits in schizophrenia by influencing encoding, facilitating this process should improve WM performance. A recent study (Mayer et al., 2011) tested this hypothesis in healthy participants who performed a spatial delayed-response task (DRT) that manipulated orthogonally the degree of target salience and the demands on WM encoding. Target salience was manipulated by varying the degree of target novelty/familiarity (high salient = novel target, low salient = familiar target) whilst keeping the basic stimulus properties physically identical. Thus, this task was designed to probe top-down attentional processing driven by expectations rather than stimulus-dependent factors (Treisman & Gormican, 1988; Frith, 1974; Reicher, Snyder & Richards, 1976). WM load was manipulated by varying the number of targets. This study showed that increasing the salience of the targets by increasing their novelty improves spatial WM encoding in healthy participants.
We also examined different types of correct and erroneous responses. For this purpose, we asked participants to rate their level of response confidence at the end of each trial. Our classification of behavioural responses was as follows: if a subject gave the correct response with high confidence this was classified as true memory, in contrast to a correct response that was given without confidence (correct/ not confident response). If an incorrect response was giving with confidence, this was classified as false memory, in contrast to an incorrect response that was given without confidence (incorrect/ not confident response). We were particularly interested in the rate of false memory responses because false memory errors most likely reflect a problem at the encoding stage such as less precise encoding of the stimuli as demonstrated previously in PSZ (Mayer & Park, 2012). If one successfully transfers imprecisely encoded information into WM, it is possible to maintain and retrieve this information from WM, resulting in an error response that is coupled with a high degree of confidence concerning the veracity of the response. Consistent with this idea, in our previous study with healthy participants we found that the improvement in WM performance under conditions of high target salience was accompanied by a decrease in the percentage of false memory errors rather than a decrease in incorrect/ not confident responses (Mayer et al., 2011). Moreover, Lee, Folley, Gore, & Park (2008) reported similar cortical activation patterns for correct memory responses and false memory responses in a spatial DRT in PSZ, suggesting that the maintenance of the internal representation was intact whether that representation was correctly or incorrectly encoded. Therefore, if reduced WM performance in PSZ was specifically due to difficulties during encoding, we expected a higher percentage of false memory errors rather than incorrect/ not confident responses in PSZ compared to controls.
MATERIAL AND METHODS
Participants
Thirty-one outpatients with schizophrenia (n = 25) or schizoaffective disorder (n = 6) (PSZ) and 28 demographically matched healthy controls (HC) participated in this study. Diagnoses were made according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994) using structured clinical interviews. Demographic and clinical information is summarised in Table 1. The groups were matched on age, F(1, 58) = 1.05, p = .31, premorbid IQ, F(1,58) = 0.79, p = .38, and handedness, F(1, 58) = 1.54, p = .22. There was a significant difference in years of education between groups, F(1, 58) = 5.0, p < .05, which presumably reflects the effects of schizophrenia on educational attainment rather than a premorbid demographic difference.
TABLE 1.
Group demographics and clinical information
|
PSZ Mean (SD) |
HC Mean (SD) |
|
|---|---|---|
| Age | 40.23 (9.10) | 37.89 (8.35) |
| Range | 24–57 | 25–55 |
| Female/male | 11/20 | 12/16 |
| AA: A: C: O | 15: 1: 15: 0 | 8: 1: 17: 2 |
| Handednessa | 51.74 (61.05) | 70.36 (53.33) |
| Years of education | 13.90 (2.71) | 15.29 (1.92) |
| IQb | 104.61 (9.43) | 106.54 (6.84) |
| SAPS | 14.45 (11.12) | n/a |
| SANS | 25.06 (14.95) | n/a |
| BPRS | 14.32 (7.28) | n/a |
| SPQ | n/a | 10.79 (8.01) |
The same HC participated in the DRT and the detection task. 27 PSZ who had participated in the WM task, also performed the detection task.
measured with the Edinburgh Handedness Inventory (Oldfield, 1971).
measured with the National Adult Reading Test (Nelson, 1982). AA = African American; A = Asian; C = Caucasian; O = Other; SAPS = Scale for the Assessment of Positive Symptoms (Andreasen, 1984); SANS = Scale for the Assessment of Negative Symptoms (Andreasen, 1983); BPRS = Brief Psychiatric Rating Scale (Overall & Gorman, 1962); SPQ = Schizotypal Personality Questionnaire (Raine, 1991).
PSZ were clinically stable (mean duration of illness: 17.57, SD = 11.33). Twenty-nine patients were medicated, 3 with a first-generation and 25 with a second-generation antipsychotic. 12 patients also received antidepressants, 2 patients benzodiazepines, and 1patient lithium. 1 patient was treated with antidepressant only. The mean Chlorpromazine equivalent daily dose (CPE) was 329.23 (SD = 299.36).
HC were recruited from the community, had no history of DSM-IV Axis 1 disorder and were medication-free.
All subjects had normal or corrected-to-normal vision. Exclusion criteria were a history of head injury, neurological disorder or substance abuse within six months. All subjects gave written informed consent approved by the Vanderbilt University Institutional Review Board and were paid.
WM task
Stimuli, task, and procedure
For details on stimuli, task, and procedure see Mayer et al. (2011). Target stimuli were black “A”s of approximately 0.48° visual angle, displayed upright or upside-down on a white background (Figure 1). Stimuli were spaced evenly apart (1.9°) and appeared along an imaginary circle (4.8° radius) including 16 positions around a centrally presented fixation cross (0.36°). The positions of 0°, 90°, 180°, and 270° were excluded.
Figure 1.
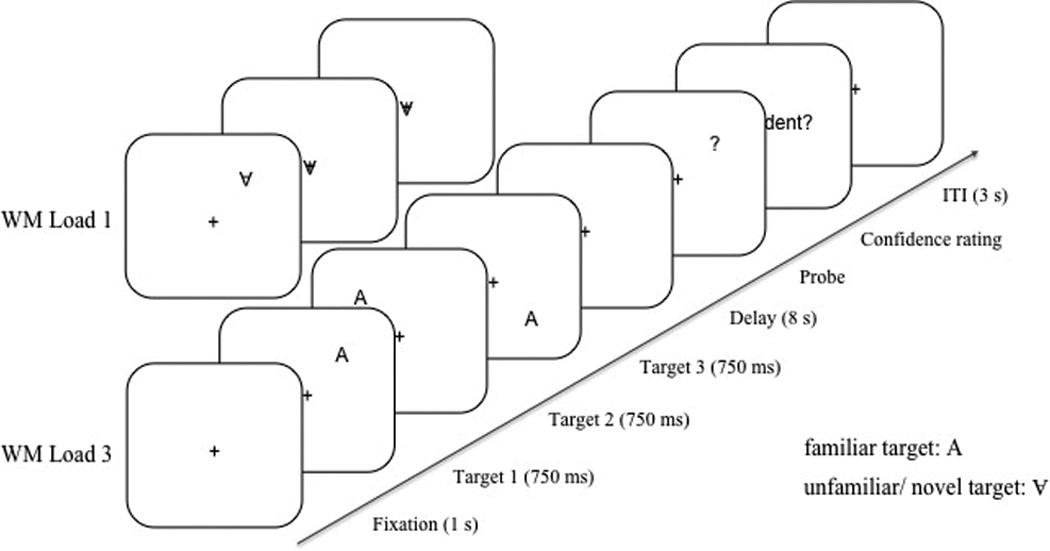
Schematic diagram of the procedure and stimuli used in the WM task.
The design included two within-subjects factors, target familiarity (familiar vs. unfamiliar/ novel targets) and WM load (1 target vs. 3 targets). In the WM load 3 condition, each trial began with presenting a fixation cross at the center position for 1s, then three targets were presented sequentially at three different positions, each for a duration of 750 ms and separated by an inter-stimulus interval of 250 ms. Within each trial, the target positions were determined pseudo-randomly with the constraint that the targets appeared in three different quadrants of the screen and that they appeared at least two positions (3.8°) farther apart from each other on the imaginary circle. In the WM load 1 condition, only the first target appeared at a position on the imaginary circle, while the second and the third target appeared at the center of the screen. In the unfamiliar/novel condition, all “A”s were presented upside-down, while in the familiar condition the “A”s appeared upright (Frith, 1974; Reicher et al. 1976; Shen & Reingold, 2001). After an 8 s delay interval, a question mark (0.48° visual angle) was presented as a probe until a response was given. Participants indicated whether the position of the question mark matched one of the target positions by a left or right key press for match and non-match, respectively. Half of the trials were matches. In the non-match trials, the question mark always appeared one position further apart (1.9°) from one of the target positions along the imaginary circle to hold response difficulty constant. Participants were instructed to respond as fast and accurately as possible. Immediately after the decision, participants indicated the confidence level for their WM response by making a non-speeded response for confident and not confident. An inter-trial interval (ITI) of 3 s followed. There were 32 trials for each experimental condition presented in a randomised order and 10 practice trials.
Detection task
Participants performed a second task using the same stimuli as in the DRT, which required the detection of a target stimulus but did not place any demands on WM. The detection task allowed us to assess whether basic level perceptual processes were intact in PSZ and HC. In addition, we were interested in the effect of target novelty on visual processing and its role for efficient WM encoding. Details on the rationale for the detection task, the stimuli, task procedure, and results are provided as Supplemental Material.
RESULTS
WM accuracy and RT
A repeated-measures ANOVA was performed on accuracy and RT as a function of WM load (1 vs. 3 targets), target familiarity (familiar vs. novel target) and group (PSZ vs. HC). Response accuracy and RTs are shown in Figure 2. As expected, accuracy was significantly lower in PSZ compared to HC, F(1, 57) = 22.88, p < .001, η2 = .29. In addition, the analysis revealed a significant main effect of WM load, F(1, 57) = 48.47, p < .001, η2 = .46, with higher accuracy for WM load 1 vs. 3. This effect was similar in both groups as reflected in the lack of a significant interaction between the factors WM load and group, F(1, 57) = 1.13, p = .29. The main effect of target familiarity was not significant, F(1, 57) = 0.89, p = .35. However, the interaction between target familiarity and group was significant, F(1, 57) = 5.45, p < .05, η2 = .09, indicating differential effects of target familiarity in the two groups. In HC, there was an increase in WM accuracy for novel vs. familiar targets, F(1, 27) = 5.31, p < .05, η2 = .16. In contrast, WM accuracy did not differ between the familiarity conditions in PSZ, F(1, 30) = 0.97, p = .33. Planned comparisons using paired t-tests indicated that the beneficial effect of target novelty observed in HC was significant for WM load 3, t(27) = −2.04, p < .05, but not for WM load 1, t(27) = −0.54, p = .59. Interactions between WM load and target familiarity, and between all three factors were not significant (all F-values < 1.59, all p-values > .21).
Figure 2.
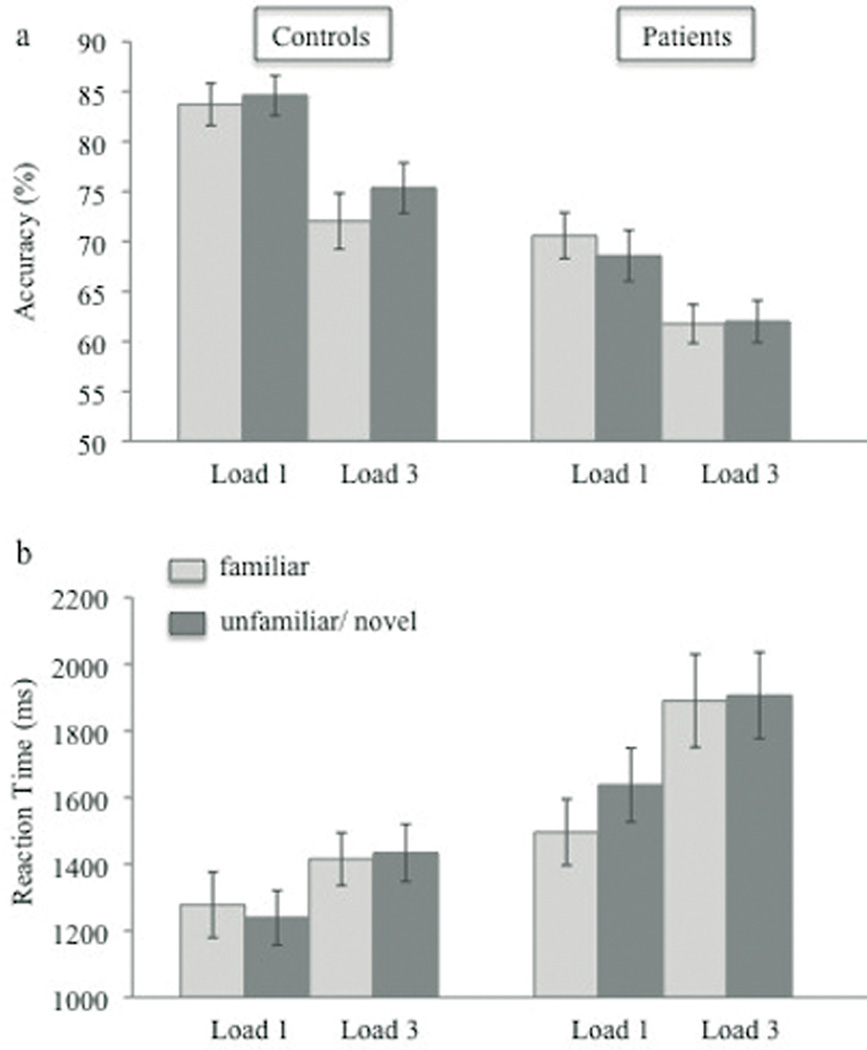
Mean accuracy (a) and RT (b) as a function of WM load (1 vs. 3) and target familiarity (familiar vs. novel) for healthy controls and schizophrenic patients. Vertical bars represent the standard error of the mean.
RTs were significantly higher in PSZ compared to HC, F(1, 57) = 7.47, p < .01, η2 = .12. Consistent with the accuracy data, RTs increased from WM load 1 to WM load 3, F(1, 57) = 53.05, p < .001, η2 = .48, and this effect appeared to be stronger in PSZ than HC [WM load × group interaction, F(1, 57) = 5.91, p < .05, η2 = .09]. The main effect of target familiarity, F(1, 57) = 1.90, p = .17, and the interaction between the factors WM load and familiarity were not significant, F(1, 57) = 0.40, p = .53. Furthermore, the interaction between target familiarity and group, F(1,57) = 3.20, p = .08, as well as the interaction between all three factors did not reach significance, F(1, 57) = 2.84, p = .10. Thus, RTs did not differ between familiarity conditions in either group indicating that the findings for accuracy were not a result of speed-accuracy trade-offs.
Confidence rating
A multifactorial repeated-measures ANOVA was conducted to test whether the percentage of type of response (true memories, correct but not-confident responses, false memories, incorrect and not-confident responses) differed between PSZ and CO. Specifically, we were interested whether PSZ showed an increase in the rate of false memory rather than incorrect/not-confident responses compared to HC reflecting problems specifically at the stage of encoding.
As indicated by a significant interaction between the factors group and response type, F(3, 171) = 5.53, p < .01, η2 = .09, the percentage of type of response differed between PSZ and HC. Post-hoc analyses using separate one-way ANOVAS revealed a significant group difference only in the percentage of false memories, F(1, 58) = 18.13, p < .001, corrected for multiple comparisons using Bonferroni correction. Across conditions, the percentage of false memory errors was about doubled in PSZ compared to HC (Figure 3). In contrast, the percentage of incorrect/not confident responses did not differ between groups, F(1, 58) = 0.28 p = .60. Overall, PSZ gave fewer true memory responses as well as fewer correct/ not confident responses than HC, however the differences did not reach significance, F(1, 58) = 2.67 p = .11, F(1, 58) = 1.96 p = .18, respectively.
Figure 3.
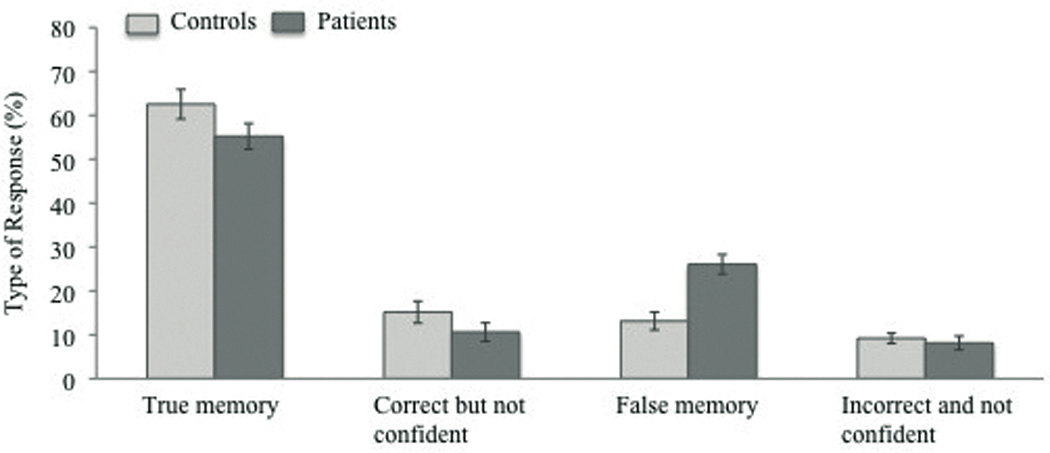
Percentage of response types across conditions for healthy controls and schizophrenic patients. Vertical bars represent the standard error of the mean.
A subsequent analysis tested the effect of target familiarity and WM load on the percentage of false memories in PSZ and HC. The analysis was restricted to the percentage of false memories as a significant group difference emerged only for this type of response. A 2 × 2 × 2 repeated-measures ANOVA revealed a significant main effect of WM load, F(1, 57) = 11.23, p < .01, η2 = .17, with a higher percentage of false memories for load 3 than load 1 in both groups [non-significant interaction between WM load and group, F(1, 57) = 0.01, p = .94] (Figure 4). The effect of target familiarity was not significant, F(1, 57) = 1.33, p = .25. However, the interaction between all three factors was marginally significant, F(1, 57) = 3.09 p = .08, η2 = .05. Planned comparisons using paired t-tests indicated that this interaction effect was driven by the decrease of false memory errors for novel vs. familiar targets in the WM load 1 condition, t(27) = 1.98, p = .058, but not the WM load 3 condition, t(27) = −0.29, p = .78, in HC. In contrast, in PSZ the percentage of false memories did not differ between novel and familiar targets in either the load 1, t(27) = −0.72, p = .48, nor the load 3 condition, t(27) = 1.14, p = .26.
Figure 4.
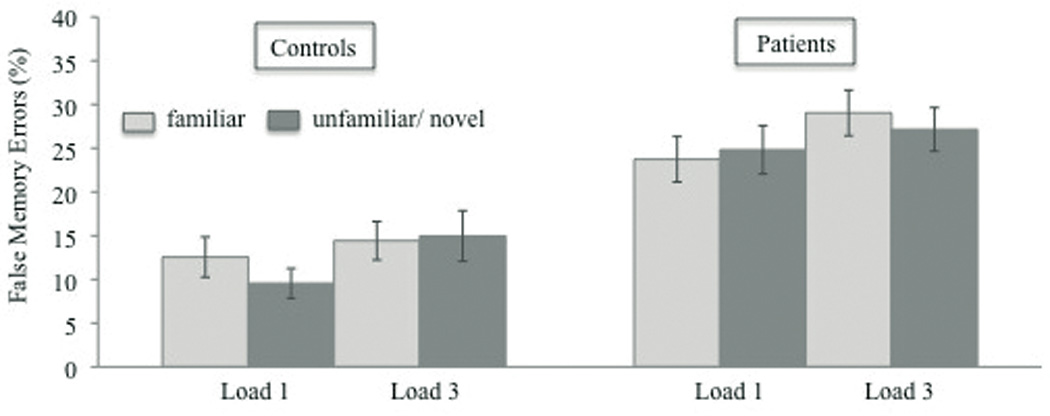
Percentage of false memory responses as a function of WM load (1 vs. 3) and target familiarity (familiar vs. novel) for healthy controls and schizophrenic patients. Vertical bars represent the standard error of the mean.
Correlations
In PSZ and HC, the percentage of false memory responses correlated negatively with overall WM performance in the low (PSZ, r = −.83, p < .001; HC, r = −.63, p < .001) and the high WM load condition (PSZ, r = −.55, p < .01; HC r = −.66, p < .001). In contrast, there was no relationship between the percentage of incorrect/ not confident responses and WM performance in PSZ (all p-values > .12). In HC, the percentage of incorrect/ not confident responses did not correlate with performance in the WM load 1 condition (p = .20), however there was a weak relationship with WM performance in the WM load 3 condition, r = −.38, p < .05.
In PSZ, there was no relationship between response accuracy and symptom severity (all p-values > .14). However, the increase in false memories from WM load 1 to load 3 significantly correlated with the severity of negative symptoms, r = .48, p < .01.
There was a trend for a negative correlation between response accuracy and the daily CPE dose only in the condition WM load 1/ novel targets, r = −.38, p = .06. In all other experimental conditions we did not find significant correlations between task performance and medication doses (all p-values > .11). There was also no relationship between WM accuracy and duration of illness (all p-values > .11).
DISCUSSION
In this study we investigated the effect of target salience on spatial WM in schizophrenia by manipulating the novelty of the targets to be encoded in a DRT. WM performance was markedly impaired in PSZ. Increasing target salience by increasing the novelty improved WM performance in HC but not in PSZ. Thus, in contrast to our hypothesis, PSZ failed to benefit from increased novelty of the target during WM encoding. In addition, the beneficial effect of target novelty on visual processing as assessed in the detection task was positively correlated with performance in the DRT in HC rather than PSZ. Thus, healthy participants who benefit more from novel targets in the detection task showed better WM performance (see Supplemental Material, Figure S1a).
The findings from the confidence rating revealed that impaired WM performance in PSZ was mainly due to an increase in the number of false memory responses. The percentage of false memories increased with WM load and was doubled in PSZ compared to HC, whereas the amount of incorrect responses that were given without being confident did not differ between groups. Also, the percentage of false memory responses rather than the percentage of incorrect/ not confident responses correlated negatively with WM performance in both WM load conditions. This finding indicates that the degree to which participants made false memory errors was related to their degree of WM reduction. In HC the percentage of false memory errors was reduced for novel vs. familiar targets, however this effect was small and appeared only in the load 1 condition. More importantly, the percentage of false memory responses also correlated negatively with the difference in RT between novel and familiar targets in the detection task when calculated across participants (Figure S1b). Thus, those participants who benefit little from novel targets in the detection task made more false memory errors in the WM task. This provides some evidence that the increased rate of false memory errors likely reflects difficulties in deploying attention, which then may lead to inefficient WM encoding. This is consistent with our previous findings indicating that the impairments in spatial WM observed in PSZ as well as healthy first-degree relatives of PSZ can be attributed at least to some degree, to deficits in processes associated with WM encoding such as less precise encoding (Lee & Park, 2005; Mayer & Park, 2012). This finding is also consistent with the results from neuroimaging studies that compared true memory and false memory responses in PSZ and observed similar activation patterns in the prefrontal cortex for both types suggesting that the mechanisms that support the maintenance of the internal representation were intact whether that representation was correctly or incorrectly encoded (Lee et al., 2008).
Taken together, the present findings support and extend previous reports on the relevance of processes associated with the early phase of encoding for WM deficits in schizophrenia (Fuller et al., 2005; Haenschel & Linden, 2011; Hahn et al., 2010; Hartman et al., 2003; Javitt et al., 1999; Lee & Park, 2005; Lencz et al., 2003; Mayer, Fukuda, Vogel, & Park, 2012, Mayer & Park, 2012; Tek et al., 2002). Analysing overall performance and types of responses we demonstrate that dysfunctions of visual attention in the service of WM encoding, rather than a failure of WM storage per se, contribute to WM impairments in schizophrenia.
It might be argued that the deficit in WM performance in PSZ was due to slowed perceptual encoding rather than attentional processing. However, given that the target detection time in PSZ was well below the target exposure time of 750 ms (see Supplemental Material), we can rule out that slowed perceptual encoding was a limiting factor for WM performance in our task.
The increased rate of false memories might reflect an overall response bias for confident responses in PSZ rather than a specific deficit of attentional processing. In this case we would have expected a similar distribution of confident and not confident responses among correct and incorrect responses in PSZ. The results were not consistent with this hypothesis. Across conditions, 84% of the correct responses were true memories and 16% of the correct responses were given without being confident. In contrast, among the errors about 75% were false memory errors whereas 25% were incorrect and not confident responses.
Our findings pinpoint a circumscribed deficit in the processing of novelty in PSZ. It is important to note that in the present task the influence of low-level physical features on target salience was minimised as novel and familiar targets differed only with regard to a local change in orientation. The attentional salience of the novel target was likely driven by expectations regarding a previously stored concept of the letter “A” (Frith, 1974; Mayer et al., 2011; Reicher et al., 1976; Shen & Reingold, 2001). Thus, the patients’ deficit in assigning attentional salience to novel targets in order to facilitate WM encoding adds to the growing body of evidence that top-down processes function abnormally in schizophrenia when visual inputs are selected for further perceptual (Fuller et al., 2006; Gold et al., 2007; Tanaka et al., 2007) and memory processing (Hahn et al., 2010). In contrast, when the selection process is driven by perceptually salient stimulus features and/ or the distractors do not strongly compete for attentional resources, attentional selection is intact in PSZ and facilitates WM encoding (Gold et al., 2006; Lee & Park, 2006; Smith et al., 2011).
All PSZ except for three were taking antipsychotic medication. However, the profile of WM deficit observed in PSZ cannot be explained solely in terms of medication. First, we did not find a consistent relationship between WM performance and daily medication dosage. Second, preliminary data in a group of healthy relatives (REL) of PSZ who were medication-free showed that WM accuracy was also lower in this group compared to HC and similar to PSZ this deficit was also accompanied by an increase in false memories (see Supplemental Material). Consistent with previous reports (Mayer & Park, 2012) these findings suggest that PSZ and REL might be comparable in terms of spatial WM deficits as well as the underlying mechanisms.
Understanding the processes that contribute to impaired WM in schizophrenia is crucial in the search for cognitive remediation strategies. The present findings suggest that non-mnemonic attentional processes in the service of WM encoding represent an important target for improving WM.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01 MH073028 to S.P. and P30 HD15052 to the Vanderbilt Kennedy Center for Research on Human Development). We thank Lindsey Gilling McIntosh for help with data collection, Heath Nichols, Katy Thakkar, and Joel Peterman for their assistance with clinical interviews and subject recruitment, and Professor Stephan Heckers with his help in recruitment.
Footnotes
Supplementary material
Supplementary (Figure SI and S2 and further content) is available via the ‘Supplementary’ tab on the article’s online page (http://dx.doi.org/10.1080/13546805.2013.854199R.).
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fourth ed. Washington, DC: American Psychiatric Publishing; 1994. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Kaiser J, Rahm B. Basic operations in working memory: contributions from functional imaging studies. Behavioural Brain Research. 2010;214:172–179. doi: 10.1016/j.bbr.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Cervellione KL, Burdick KE, Cottone JG, Rhinewine JP, Kumra S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:867–878. doi: 10.1097/chi.0b013e318054678d. [DOI] [PubMed] [Google Scholar]
- Fine MS, Minnery BS. Visual salience affects performance in a working memory task. Journal of Neuroscience. 2009;29:8016–8021. doi: 10.1523/JNEUROSCI.5503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. A curious effect with reversed letters explained by a theory of schema. Attention & Psychophysics. 1974;16:113–116. [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. Journal of Abnormal Psychology. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. Journal of Abnormal Psychology. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophrenia Research. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. Journal of Abnormal Psychology. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Gold JM, Hahn B, Zhang WW, Robinson BM, Kappenman ES, Beck VM, Luck SJ. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Archives of General Psychiatry. 2010;67:570–577. doi: 10.1001/archgenpsychiatry.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Linden D. Exploring intermediate phenotypes with EEG: working memory dysfunction in schizophrenia. Behavioural Brain Research. 2011;216:481–495. doi: 10.1016/j.bbr.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, Gold JM. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biological Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M, Steketee M, Silva S, Lanning K, McCann H. Working memory and schizophrenia: evidence for slowed encoding. Schizophrenia Research. 2003;59:99–113. doi: 10.1016/s0920-9964(01)00366-8. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Liederman E, Cienfuegos A, Shelley AM. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophrenia Bulletin. 1999;25:763–775. doi: 10.1093/oxfordjournals.schbul.a033417. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N. Impaired precision, but normal retention, of auditory sensory ("echoic") memory information in schizophrenia. Journal of Abnormal Psychology. 1997;106:315–324. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- Lee J, Folley BS, Gore J, Park S. Origins of spatial working memory deficits in schizophrenia: an event-related FMRI and near-infrared spectroscopy study. PLoS One. 2008;3:e1760. doi: 10.1371/journal.pone.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. The role of stimulus salience in CPT-AX performance of schizophrenia patients. Schizophrenia Research. 2006;81:191–197. doi: 10.1016/j.schres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Archives of General Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Fukuda K, Vogel EK, Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS One. 2012;7:e48586. doi: 10.1371/journal.pone.0048586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Kim J, Park S. Enhancing visual working memory encoding: The role of target novelty. Visual Cognition. 2011;19:863–885. doi: 10.1080/13506285.2011.594459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Park S. Working memory encoding and false memory in schizophrenia and bipolar disorder in a spatial delayed response task. Journal of Abnormal Psychology. 2012;121:784–794. doi: 10.1037/a0028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. American Journal of Psychiatry. 2005;162:1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Park S. Spatial working memory deficits in schizophrenia patients and their first degree relatives from Palau, Micronesia. American Journal of Medical Genetics. 2002;114:609–615. doi: 10.1002/ajmg.10644. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test. Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- Nestor P, Faux S, McCarley R, Penhune V, Shenton M, Pollak S, Sands SF. Attentional cues in chronic schizophrenia: abnormal disengagement of attention. Journal of Abnormal Psychology. 1992;101:682–689. doi: 10.1037//0021-843x.101.4.682. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorman DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Archives of General Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Park S, Püschel J, Sauter B, Rentsch M, Hell D. Spatial working memory deficits and clinical symptoms in schizophrenia: a 4-month follow-up study. Biological Psychiatry. 1999;46:392–400. doi: 10.1016/s0006-3223(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioural studies of spatial working memory dysfunction in schizophrenia: a quantitative literature review. Psychiatry Research. 2007;150:111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: A scale for the assessment of schizotypal personality based on DSMIII-R criteria. Schizophrenia Bulletin. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Reicher GM, Snyder CRR, Richards JT. Familiarity of background characters in visual scanning. Journal of Experimental Psychology: Human Perception and Performance. 1976;2:522–530. doi: 10.1037//0096-1523.2.4.522. [DOI] [PubMed] [Google Scholar]
- Saperstein AM, Fuller RL, Avila MT, Adami H, McMahon RP, Thaker GK, Gold JM. Spatial working memory as a cognitive endophenotype of schizophrenia: assessing risk for pathophysiological dysfunction. Schizophrenia Bulletin. 2006;32:498–506. doi: 10.1093/schbul/sbj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BK, Vogel EK, Woodman GF, Luck SJ. Voluntary and automatic attentional control of visual working memory. Perception & Psychophysics. 2002;64:754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Sereno AB, Holzman PS. Spatial selective attention in schizophrenic, affective disorder, and normal subjects. Schizophrenia Research. 1996;20:33–50. doi: 10.1016/0920-9964(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Shen J, Reingold EM. Visual search asymmetry: The influence of stimulus familiarity and low-level features. Perception & Psychophysics. 2001;63:464–475. doi: 10.3758/bf03194413. [DOI] [PubMed] [Google Scholar]
- Smith EE, Eich TS, Cebenoyan D, Malapani C. Intact and impaired cognitive-control processes in schizophrenia. Schizophrenia Research. 2011;126:132–137. doi: 10.1016/j.schres.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Smith CW, Park S, Cornblatt B. Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophrenia Research. 2006;81:211–215. doi: 10.1016/j.schres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G. The information available in brief visual presentations. Psychological Monographs: General and Applied. 1960;74:1–29. [Google Scholar]
- Tanaka G, Mori S, Inadomi H, Hamada Y, Ohta Y, Ozawa H. Clear distinction between preattentive and attentive process in schizophrenia by visual search performance. Psychiatry Research. 2007;149:25–31. doi: 10.1016/j.psychres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Archives of General Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gormican S. Feature analysis in early vision: evidence from search asymmetries. Psychological Review. 1988;1:15–48. doi: 10.1037/0033-295x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


