Abstract
Cystic fibrosis (CF) airways disease represents an example of polymicrobial infection whereby different bacterial species can interact and influence each other. In CF patients Staphylococcus aureus is often the initial pathogen colonizing the lungs during childhood, while Pseudomonas aeruginosa is the predominant pathogen isolated in adolescents and adults. During chronic infection, P. aeruginosa undergoes adaptation to cope with antimicrobial therapy, host response and co-infecting pathogens. However, S. aureus and P. aeruginosa often co-exist in the same niche influencing the CF pathogenesis. The goal of this study was to investigate the reciprocal interaction of P. aeruginosa and S. aureus and understand the influence of P. aeruginosa adaptation to the CF lung in order to gain important insight on the interplay occurring between the two main pathogens of CF airways, which is still largely unknown. P. aeruginosa reference strains and eight lineages of clinical strains, including early and late clonal isolates from different patients with CF, were tested for growth inhibition of S. aureus. Next, P. aeruginosa/S. aureus competition was investigated in planktonic co-culture, biofilm, and mouse pneumonia model. P. aeruginosa reference and early strains, isolated at the onset of chronic infection, outcompeted S. aureus in vitro and in vivo models of co-infection. On the contrary, our results indicated a reduced capacity to outcompete S. aureus of P. aeruginosa patho-adaptive strains, isolated after several years of chronic infection and carrying several phenotypic changes temporally associated with CF lung adaptation. Our findings provide relevant information with respect to interspecies interaction and disease progression in CF.
Introduction
Chronic airway infections and inflammation cause progressive lung disease and are the leading causes of mortality in patients with cystic fibrosis (CF) [1]. CF disease is characterized by the accumulation of secretion in the lungs and by a decreased mucociliary clearance that lead to an impaired ability to defeat bacterial infections. The viscous CF lung secretions provide an environment that protects bacteria from the assault of antibiotics and immune cells, thus favoring colonization and persistence. CF patients have a unique set of bacterial pathogens that are frequently acquired in an age dependent sequence [2]. The most frequently cultured organisms from the respiratory tract of young children are Staphylococcus aureus and non-typeable Haemophilus influenzae. Later, as the patient ages, infection progresses to involve opportunistic pathogens such as Pseudomonas aeruginosa and Burkholderia cepacia.
It is now becoming clear that the different bacteria coexisting in CF airways have a mutual interaction and contribute to the pathogenesis of the disease [3], [4]. In a context that involves a complex polymicrobial community a single-species microbial analysis could be inadequate, as different microbes within the community can interact each other and the resulting infection pathogenesis differs from that in infections caused by the component species individually [3], [5]. Chronic bacterial infections associated with CF lung disease have been studied by a range of culture-independent profiling methodologies [6]–[12], and each approach has revealed greater microbial diversity than was previously recognized. Overall, the results of these studies suggest that the polymicrobial nature of CF infections could play a key role in driving disease and response to therapy and, in turn, significantly impact upon clinical outcomes [1], [7], [13]. Nevertheless, very little is known about the role of interspecies interactions in the pathogenesis of the CF lung disease [14], [15].
The Gram-positive bacterium S. aureus is the pathogen most commonly isolated in nasopharyngeal samples from young children with CF, and in the preantibiotic era, many CF patients succumbed to S. aureus infection [16]. Recent data demonstrate an increase in S. aureus infections in the CF population, not only in the US but also in Europe, with methicillin-resistant S. aureus (MRSA) strains being on the rise [17], [18], reflecting the overall increase in prevalence and epidemiologic changes in the general population [19], [20].
Of the multiple opportunistic bacteria that may infect CF patients, the Gram-negative bacterium P. aeruginosa is considered to be the most significant as it has clearly been linked to worsening of the pulmonary status [21]. Despite intensive antibiotic treatments, P. aeruginosa infections are difficult to eradicate [22]. The antibiotic treatment may favor the emergence of antimicrobial drug resistance. One of the most striking characteristics of P. aeruginosa chronic lung infection in CF patients is indeed the co-existence of multiple phenotypes that are highly resistant to any chemotherapy treatment [23].
Although S. aureus colonization/infection usually precedes chronic colonization of the respiratory tract by P. aeruginosa, it continues into adulthood, when 51% of patients become culture positive for S. aureus [24]. Both organisms are commonly co-isolated from CF respiratory cultures and it has been shown that risk factors for initial P. aeruginosa airway infection in patients with CF include S. aureus pre-colonization [25], [26]. In addition, both species are able to shift between a planktonic (free-living) life style to surface-attached communities known as biofilms during chronic infections. In human diseases including CF, biofilm-related infections are directly correlated with dramatic increases in antibiotic resistance [27], [28], [29].
In this study, we aimed to explore the interactions between S. aureus and P. aeruginosa by using in vitro and murine models of pneumonia. During chronic infection, P. aeruginosa undergoes numerous selective pressures ranging from antibiotic treatments, host immune response and interactions with other microorganisms leading to the development of patho-adaptive lineages. The adaptation of P. aeruginosa to the CF niche selects for clones with reduced virulence in multi-hosts models [23], [30]. We focused our attention on the reciprocal influence of P. aeruginosa and S. aureus and on understanding how P. aeruginosa adaptation to the CF lung may interfere with S. aureus interaction. Using a collection of longitudinal strains isolated from CF patients, we showed that P. aeruginosa strains out-competed S. aureus. This effect was associated with P. aeruginosa early strains, which in acute infection present higher virulence. On the contrary, P. aeruginosa late adapted strains showed reduced or abolished capacity to outcompete S. aureus. This work provides key results on lung pathogenicity caused by multi-bacterial infection.
Results
P. aeruginosa early and late clonal variants differently influence growth of S. aureus
Eight lineages of P. aeruginosa strains, including 12 early (early group) and 12 late (late group) clonal isolates from different patients with CF were tested for growth inhibition of S. aureus Newman and SH1000 strains on agar surfaces [25]. In particular, late P. aeruginosa strains selected for this study were collected over a period of 16.3 years and carried several patho-adaptive traits, including mucoid and hypermutable phenotypes (Table 1) as reported previously [23], [31]. In addition, PAO1 and PA14 P. aeruginosa reference strains, which show phenotypic traits characteristic of early isolates [23], were also included.
Table 1. In vitro growth inhibition of S. aureus and P. aeruginosa.
| P. aeruginosa spot | S. aureus lawn (Newman) (inhibition halo) | S. aureus lawn (SH1000) (inhibition halo) |
| PAO1 | strong (24.5 mm) | strong (20 mm) |
| PA14 | strong (22 mm) | strong (19 mm) |
| SG1 | strong (23.5 mm) | strong ( 22.5 mm) |
| SG57* | strong (23 mm) | strong (20.5 mm) |
| SG58* | weak (14.5 mm) | weak (15 mm) |
| NN2 | weak (15 mm) | weak (14 mm) |
| NN83# * | no (9 mm) | no (9 mm) |
| BT1# | weak (15 mm) | weak (14.5 mm) |
| BT2 | weak (15 mm) | weak (15 mm) |
| BT72* | no (9 mm) | no (9 mm) |
| BT73* | weak (13.5 mm) | weak (13 mm) |
| AA2 | very strong (27 mm) | strong (20.5 mm) |
| AA43* | no (9 mm) | no (9 mm) |
| TR1 | weak (13.5 mm) | weak (13.5 mm) |
| TR2 | strong (20 mm) | strong (20 mm) |
| TR66* | weak (11.5 mm) | weak (11.5 mm) |
| TR67* | no (9 mm) | no (9 mm) |
| MF1 | weak (15 mm) | strong (21 mm) |
| MF2# | strong (21 mm) | strong (18.5 mm) |
| MF51* | no (9 mm) | no (9 mm) |
| KK1 | weak (12 mm) | no (9 mm) |
| KK2 | very strong (27 mm) | very strong (26.5 mm) |
| KK71* | no (9 mm) | no (9 mm) |
| KK72* | no (9 mm) | no (9 mm) |
| BST2 | weak (15 mm) | weak (14.5 mm) |
| BST44# * | weak (14.5 mm) | weak (12.5 mm) |
Twenty-four P. aeruginosa isolates were collected from eight individuals with CF (SG, NN, BT, AA, TR, MF, KK, BST) at the onset of chronic colonization (numbered 1-2) or after 4.5–16.3 years of colonization (numbered 43-83). PAO1 and PA14 were included as reference strains. 5 µl spots of P. aeruginosa overnight cultures, normalized to 0.5 OD, were added to S. aureus lawn (normalized to 0.5 OD) on Mueller-Hinton agar and incubated overnight at 37°C. The table summarizes the results obtained: “weak inhibition” indicates an inhibition halo ≤15 mm; “strong inhibition” indicates an inhibition halo >15 mm and ≤25 mm; “very strong inhibition” indicates an inhibition halo >25 mm; “no inhibition“ indicates absence of inhibition halo (9 mm is the diameter of the P. aeruginosa spot).
* Indicates mucoid phenotype.
Indicates hypermutable phenotype. For statistical analysis see “Results”.
As shown in Table 1, growth of S. aureus Newman and SH1000 strains was inhibited by PA14 and PAO1 P. aeruginosa reference strains and by 100% (12/12) and 91.6% (11/12) of P. aeruginosa early strains respectively in co-culture. The only exception was the strain KK1 which was previously described as different in terms of virulence potential from KK2, isolated at the same time point [23]. The strength of inhibition of S. aureus in some cases differed within clonal lineages (TR1 vs TR2; MF1 vs MF2; KK1 vs KK2).
Differently from P. aeruginosa early strains, 58.4% (7/12) of the late strains had no effect on growth of S. aureus Newman and SH1000 strains. These P. aeruginosa strains belonged to six different lineages (NN, BT, AA, TR, MF, KK) indicating presence of at least one P. aeruginosa strain unable to inhibit S. aureus growth in the majority of CF patients (75%: 6/8). The other two P. aeruginosa lineages (SG and BST) (25%: 2/8) inhibited S. aureus growth although to a lesser extent when compared to early strains. Late P. aeruginosa strains within the same lineage also differed with regard to the strength of S. aureus growth inhibition (SG57 vs SG58; BT72 vs BT73; TR66 vs TR67), indicating a diversification of the bacterial population during chronic infection as demonstrated for other virulence traits [23], [32]. The average inhibition halo of late group was 11.6 mm versus Newman and 11.3 mm versus SH1000, while the average inhibition halo of early group was 18.3 mm versus Newman and 17.4 mm versus SH1000. Late group showed a statistically significant effect in reducing levels of inhibition (regression parameter = −6.76 versus Newman and regression parameter = −6.24 versus SH1000) with p<0.01 for both settings. This data indicated that, as a group, late P. aeruginosa strains differ significantly from early strains in their capacity to inhibit S. aureus growth, suggesting a trend of P. aeruginosa patho-adaptive variants to influence the growth of S. aureus.
On the contrary S. aureus did not exert any effect on the growth of P. aeruginosa (Table S1).
Competition between S. aureus and P. aeruginosa in planktonic co-cultures
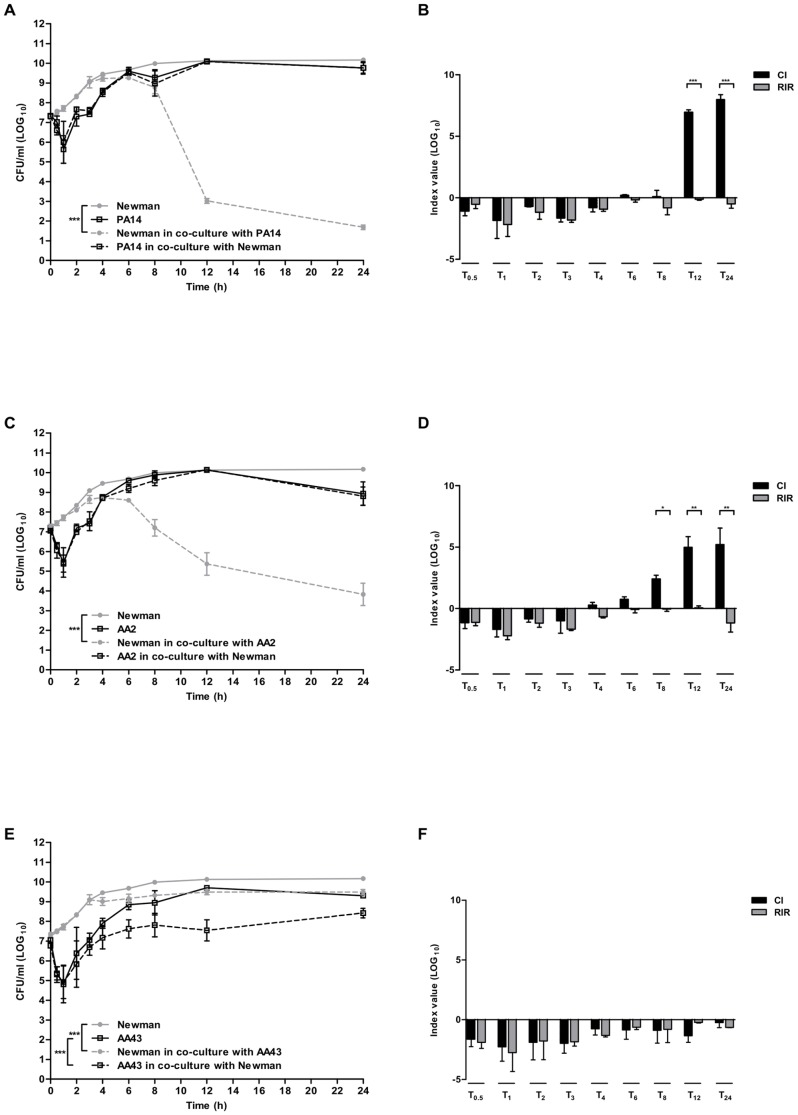
Next, we investigated the interactions between S. aureus and P. aeruginosa in planktonic growth by comparing the growth kinetics of the two organisms in co-culture to those obtained in pure culture. One reference P. aeruginosa strain PA14 and a pair of sequential strains from patient AA were selected. Figure 1A shows the growth curves of the reference S. aureus Newman and P. aeruginosa PA14 strains in single and dual cultures. PA14 maintained the same growth rate in pure culture and in co-culture, and had a significant negative effect on the overall trend of the growth of Newman (p<0.001). In order to have a clear comprehension of the differences in growth between S. aureus and P. aeruginosa, we calculated the Competition Index (CI), that allows to compare the differences in growth curve of mixed cultures, and the CI-like index, the Relative Increase Ratio (RIR), that compares the growth curves of the two species in pure culture (see Materials and Methods). As shown in Figure 1B, the CI of PA14 versus Newman was significantly different from the RIR in late exponential phase (12 h, p<0.001) and stationary phase (24 h, p<0.001) of growth, suggesting an inhibitory effect of P. aeruginosa on S. aureus.
Figure 1. Single and dual species batch growth curves and competition index values.
S. aureus strain (Newman) and P. aeruginosa strains (PA14 and two clinical early and late isolates from a CF patient AA2 and AA43) were grown for 24 hours in BHI in single culture and in co-culture after inoculation at equal ratio from mid-exponential phase pure cultures. Growth rate was monitored by colony count after plating on selective media for both species. Results are represented as the mean of values obtained from three independent experiments. The error bars indicate the standard deviations. A nonlinear mixed-effect model was fitted, using a four-parameters logistic regression function. Panel A: growth curves of Newman in pure culture and in co-culture with PA14; Panel B: Competition index (CI) and Relative Increase Ratio (RIR) calculated from single and dual cultures of Newman and PA14; Panel C: growth curves of Newman in pure culture and in co-culture with AA2; Panel D: CI and RIR calculated from single and dual cultures of Newman and AA2; Panel E: growth curves of Newman in pure culture and in co-culture with AA43; Panel F: CI and RIR calculated from single and dual cultures of Newman and AA43. Each value represents the mean of CI and RIR values from three independent experiments and the bars indicate standard deviation. Statistically significant differences in Student's t test and in nonlinear mixed-effect model are indicated by symbols when present: *: p<0.05; **: p<0.01; ***: p<0.001.
Next, we explored the effect of P. aeruginosa strains isolated at the onset of chronic colonization (early strains) or several years after acquisition (late strains) from CF patients on growth of S. aureus. A pair of well characterized P. aeruginosa clonal strains isolated from CF patient were selected: the AA2 early strain and AA43 late adapted strain carrying several phenotypic changes in virulence factor production, and patho-adaptive mutations within the genome temporally associated with CF lung infection [23], [30], [33]. The growth of Newman was significantly inhibited by the presence of the early AA2 strain (p<0.001), while AA2 strain was not affected from the presence of S. aureus (Figure 1C). The CI of AA2 versus Newman was significantly higher than the RIR in late exponential phase (8 h, p<0.05 and 12 h, p<0.01, Figure 1D) and in stationary phase (24 h, p<0.01). On the other hand, Newman and the late strain AA43 interfered each other in co-culture, slightly but significantly reducing their growth rate compared to pure culture (p<0.001, Figure 1E). It is worth noting that AA43 inhibited the growth of Newman to a lower extent compared to AA2: while AA2 determined a reduction of 3, 5 and 6 log at 8, 12 and 24 h respectively, AA43 determined a reduction of less than 1 log at the same time points (Figure 1C and E). Being the competition reciprocal between the two species and considering their different growth rate in pure culture, the CI did not differ from the RIR (Figure 1F). Similar results were obtained using the same isolates of P. aeruginosa, AA2 and AA43, in co-culture with the reference S. aureus SH1000 (Figure S1) strengthening the results obtained with Newman. Taken together these data indicate that P. aeruginosa strains, including reference or those isolated at the early stage of chronic infection, can outcompete S. aureus in planktonic cultures. On the other hand, P. aeruginosa patho-adaptive strains lose this capacity over time.
S. aureus and P. aeruginosa interaction in biofilm
In order to understand if the reciprocal interaction among the two species could affect their capacity to produce biofilm, we quantified the biofilm biomass of individually cultured or co-cultured at a ratio 1∶1 S. aureus and P. aeruginosa by staining with crystal violet. As shown in Figure 2, the results obtained from co-cultured pair of strains formed by Newman and PA14 revealed significantly lower level of biomass compared to Newman only (Newman vs Newman+PA14 p<0.01), but similar to that corresponding to PA14 alone. This data suggests an inhibitory effect exerted by P. aeruginosa on S. aureus biofilm formation. For clinical P. aeruginosa strains, while the OD value detected in the mixed biofilm formed by Newman and AA2 was not significantly different from both Newman and AA2 individually cultured, the OD value associated to the mixed biofilm formed by Newman and AA43 revealed significantly lower levels of biomass compared to both Newman (Newman vs Newman+AA43 p<0.001) and AA43 (AA43 vs Newman+AA43 p<0.001) individually cultured. This finding suggests a reciprocal interference of the two species, confirming the results of batch co-culture experiments.
Figure 2. Biofilm formation by S. aureus and P. aeruginosa strains in single and dual cultures.
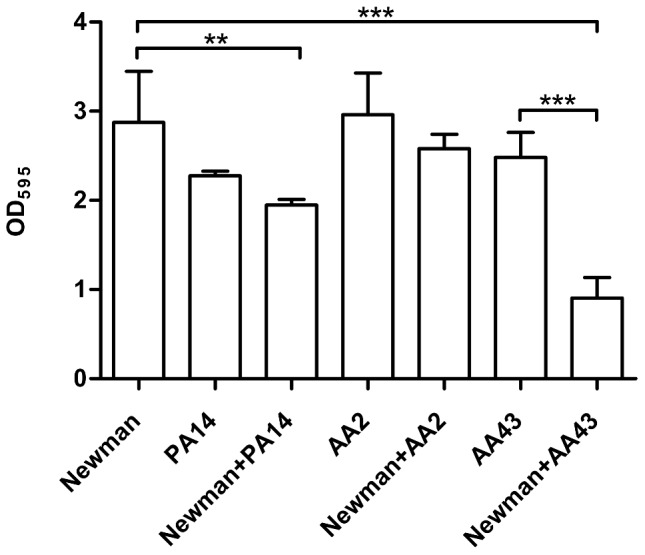
Bacteria were grown overnight in 96-well flat-bottom microtiter plates in NB medium at 37°C either individually cultured or co-cultured at a 1∶1 ratio. Biofilm biomass was quantified by staining with crystal violet and absorbance measurements at OD 595 nm. The values represent the means of three independent experiments, and the bars indicate standard deviation. Statistically significant differences in Student's t test are indicated by symbols when present: **: p<0.01; ***: p<0.001.
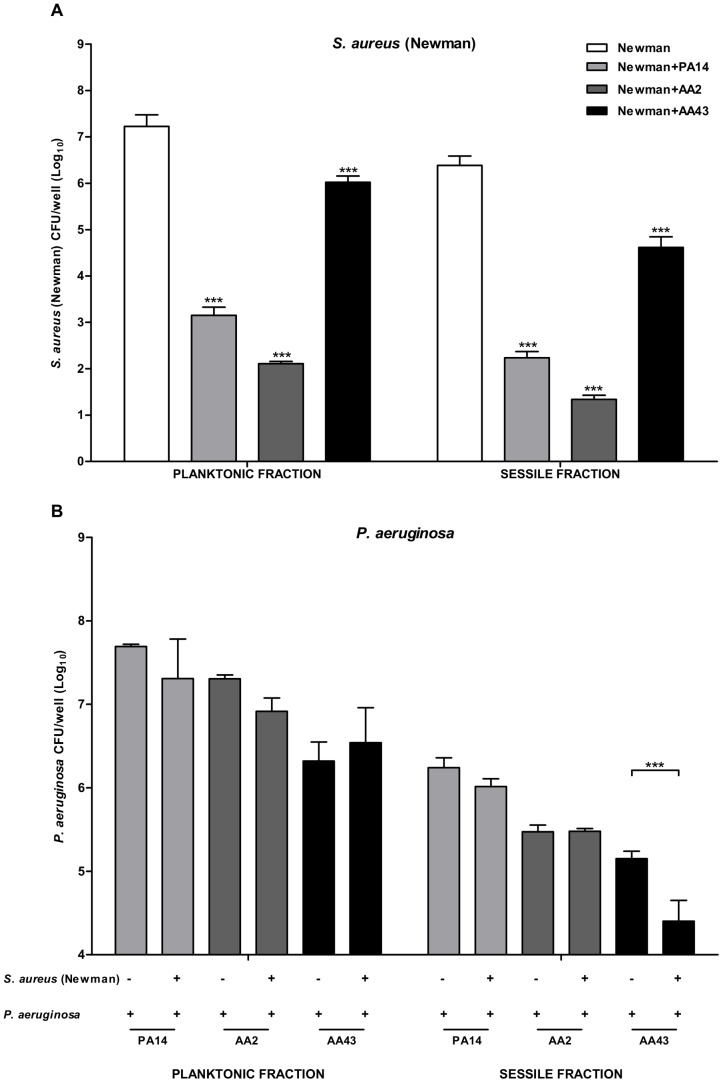
We also determined the amount of viable bacteria of each species in both planktonic and sessile fractions in single and dual cultures. In co-culture, we noticed that all strains of P. aeruginosa tested determined a reduction of the number of both sessile and planktonic Newman cells (p<0.001) (Figure 3A). In particular, the bacterial load of Newman in sessile fraction, when co-cultured with clinical early P. aeruginosa AA2, decreased of five log compared to pure culture, while the clonal late strain AA43 caused a lower (two log) reduction. A similar effect was observed also in planktonic fraction (Figure 3A) in agreement with batch co-culture data.
Figure 3. S. aureus and P. aeruginosa planktonic and sessile cells in single and dual cultures.
Bacteria were grown overnight in 96-well flat-bottom microtiter plates in NB medium at 37°C either individually cultured or co-cultured at a 1∶1 ratio. CFU counts were determined in both planktonic and sessile fractions. Panel A: planktonic (left) and sessile (right) cells of S. aureus strain Newman in pure culture and in co-culture with P. aeruginosa strains PA14, AA2 and AA43. Statistically significant differences are referred to Newman in pure culture. Panel B: planktonic (left) and sessile (right) cells of P. aeruginosa strains PA14, AA2 and AA43 in pure culture and in co-culture with S. aureus strain Newman. The values represent the means of three independent experiments, and the bars indicate standard deviation. Statistically significant differences in non-parametric Mann–Whitney test are indicated by symbols when present: **: p<0.01; ***: p<0.001.
On the contrary, the presence of Newman had no effect on PA14 and AA2 growth in both planktonic and biofilm fractions, while it moderately inhibited the attachment to polystyrene and biofilm formation of the late P. aeruginosa strain AA43, confirming a reciprocal interaction between Newman and AA43 (Figure 3B, p<0.001).
Figure 4 shows the percentage of planktonic and sessile cells of the two species in single and dual cultures. While Newman in pure culture presented the highest percentage of sessile cells, in dual culture was negatively affected by the presence of PA14 and AA2, and the biofilm composition of the co-culture reflected that of P. aeruginosa in pure culture (Figure 4A and 4B). A reciprocal influence was evident only for the pair represented by Newman and the late strain AA43 (Figure 4C). It is worth noting that in single species biofilm, the mucoid AA43 strain, even if apparently displaying a lower biofilm biomass compared to AA2 after staining with crystal violet, presented a higher percentage of sessile cells compared to AA2 (5.7% vs 3.4% respectively).
Figure 4. Percentage of planktonic and sessile cells in single and dual cultures.
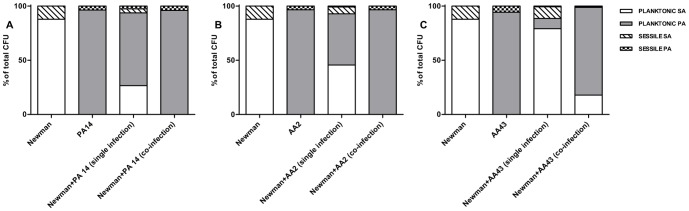
Bacteria were grown overnight in 96-well flat-bottom microtiter plates in NB medium at 37°C either individually cultured or co-cultured at a 1∶1 ratio. CFU counts were determined in both planktonic and sessile fractions and the percentage of S. aureus and P. aeruginosa in the two fractions of single and dual cultures was calculated. Panel A: percentages of planktonic and sessile cells of Newman in single culture (first histogram), PA14 in single culture (second histogram), Newman and PA14 in ideal co-culture if the 2 species would not interfere each other (third histogram, percentages have been calculated considering the values of the first and second histograms), and Newman and PA14 in co-culture (fourth histogram). Panel B: percentages of planktonic and sessile cells of Newman in single culture (first histogram), AA2 in single culture (second histogram), Newman and AA2 in ideal co-culture if the 2 species would not interfere each other (third histogram, percentages have been calculated considering the values of the first and second histograms), and Newman and AA2 in co-culture (fourth histogram). Panel C: percentages of planktonic and sessile cells of Newman in single culture (first histogram), AA43 in single culture (second histogram), Newman and AA43 in ideal co-culture if the 2 species would not interfere each other (third histogram, percentages have been calculated considering the values of the first and second histograms), and Newman and AA43 in co-culture (fourth histogram). SA: S. aureus; PA: P. aeruginosa.
Competition between P. aeruginosa and S. aureus in a mouse model of acute lung infection
To test whether the observed differences in planktonic growth and biofilm formation in vitro would be relevant in vivo, a mouse model of acute pneumonia was used. Thus, we set up in vivo competition between P. aeruginosa and S. aureus in C57Bl/6NCrlBR mice challenged with 1×106 CFU of S. aureus and P. aeruginosa strains mixed together at a 1∶1 ratio. Eighteen hours after infection, murine lungs were homogenized and plated. Differential CFU counting was performed to calculate the CI. Results show that P. aeruginosa reference strain PA14 and the early isolate AA2 outcompeted S. aureus strain Newman, as the CI, being significantly different from 1, indicated a competitive advantage of P. aeruginosa over S. aureus (PA14/Newman average CI = 5.0, p<0.01; AA2/Newman average CI = 3.3, p<0.05). Different results were obtained for the P. aeruginosa late isolate AA43 and Newman as the CI 18 hours after challenge was not significantly different from 1 (AA43/Newman CI = 0.9), indicating no competition in this case (Figure 5 and Table 2).
Figure 5. Competition between P. aeruginosa and S. aureus strains in a murine model of pneumoniae.
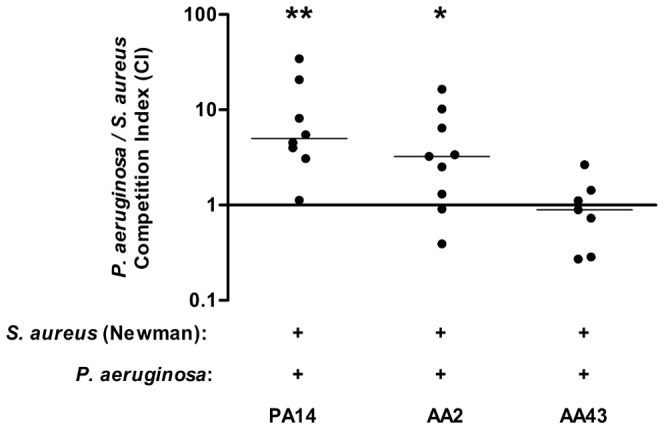
Planktonic S. aureus strain Newman and P. aeruginosa clinical isolates AA2 and AA43 and reference strain PA14 were used to infect C57BL/6NCrlBR mice at a ratio of 1∶1. After 18 hours of acute infection lungs homogenates were plated on selective plates to determine S. aureus and P. aeruginosa CFU. Each circle represents the CI for a single animal in each group. A CI value equal to 1 indicates equal competition of the two species; a CI value significantly <1 indicates a competitive advantage of S. aureus that outcompetes P. aeruginosa; a CI value significantly >1 indicates a competitive advantage of P. aeruginosa that outcompetes S. aureus. Wilcoxon signed rank test of the null hypothesis that the distribution of CI is symmetric about 1 was performed. Statistically significant differences are indicated by symbols when present: *: p<0.05; **: p<0.01. The data are pooled from two or three independent experiments.
Table 2. Colonization of murine lungs with S. aureus and P. aeruginosa reference and clinical strains in competition experiments.
| PA14/Newman (n = 9a) | AA2/Newman (n = 9a) | AA43/Newman (n = 9a) | |
| Mortality, % (no. of dead/total mice) | 0 (0/9) | 0 (0/9) | 0 (0/9) |
| Co-infected b , % (no. of co-infected/surviving mice) | 89 (8/9) | 100 (9/9) | 78 (7/9) |
| P. aeruginosa infected c , % (no. of infected/surviving mice) | 100 (9/9) | 100 (9/9) | 78 (7/9) |
| S. aureus infected d , % (no. of infected/surviving mice) | 89 (8/9) | 100 (9/9) | 78 (7/9) |
| Total cfu/lung e | 3.3×104 | 6.7×103 | 5.8×103 |
| P. aeruginosa cfu/lung e | 2.9×104 | 4.5×103 | 2.7×103 |
| S. aureus cfu/lung e | 4.2×103 | 2.2×103 | 3.1×103 |
| CI f | 5.0 | 3.3 | 0.9 |
Pooled mice, analyzed in two independent experiments.
Co-infected mice, surviving after 18 hours from challenge.
Number of pooled mice infected with P. aeruginosa after 18 hours.
Number of pooled mice infected with S. aureus after 18 hours.
Median values are reported.
Competition Index.
Discussion
The goal of this study was to investigate the influence of P. aeruginosa adaptation to the CF lung on interaction with S. aureus in co-culture, during biofilm formation and mouse lung infection, in order to gain important insight on the interplay occurring between the two main pathogens of CF airways, which is still largely unknown. For this purpose, we used a panel of deeply genetically and phenotypically characterized P. aeruginosa clonal strains isolated from CF patients at different time points during CF chronic lung infection [33], [34].
We evaluated the inhibitory effect of eight P. aeruginosa lineages on S. aureus, including strains isolated both at early and late stage of chronic infection. A negative effect on S. aureus growth significantly associated with early-infecting P. aeruginosa strains was observed, while clonal late-infecting P. aeruginosa strains presented a significantly reduced or abolished virulence when co-cultivated with S. aureus. During chronic infection, P. aeruginosa undergoes adaptation to the CF lung, leading to patho-adaptive lineages that differ genotypically and phenotypically from the originally infecting strain. Such microevolution usually determines loss of motility, acquisition of mucoidy, antibiotic resistance and loss-of-function mutations in virulence genes, suggesting attenuation of virulence for CF adapted strains [30], [35], [36], [37]. Here we demonstrated for the first time that P. aeruginosa virulence traits affect also the interaction with other CF-related pathogen as S. aureus. As described for other traits, intra-clonal variation was observed both in clonal P. aeruginosa early strains and late strains isolated at the same time from the CF patients. One of the most striking characteristics of P. aeruginosa chronic lung infection in CF patients is the intense diversification of the bacterial population, leading to the co-existence of multiple phenotypes that may colonize different airways niches. Thus, the intra-clonal variation that we have observed is most likely the result of this process of genetic adaptation.
Under planktonic growth conditions, we have shown that both the reference P. aeruginosa strain PA14 and the clinical early strain AA2 strongly inhibited the growth of S. aureus during late logarithmic phase and stationary phase, without being influenced in their growth rate. Antagonism between microorganisms within a community could be attributed to simple competition for limited resources or to direct antagonistic effects [36]. There is evidence supporting antagonism between P. aeruginosa and S. aureus. Mashburn et al. demonstrated that P. aeruginosa can lyse S. aureus to use the iron released for its own growth [38]. Moreover, it has been reported that S. aureus is susceptible to an arsenal of respiratory inhibitors generated by P. aeruginosa, such as pyocyanin, hydrogen cyanide or alkyl-hydroxyquinoline N-oxides (HQNO), which are able to suppress the aerobic metabolism and growth of S. aureus [25], [39]. Interestingly, the late P. aeruginosa strain AA43, clonal to AA2, inhibited the growth of S. aureus at a lower extent, compared to AA2 and was not able to outcompete it. Besides being less virulent, AA43 was also negatively affected by the presence of S. aureus as its growth rate was significantly slowed down by S. aureus cells.
Despite the increasing interest on the crucial role of biofilm in CF infections, interspecies interactions of different organisms in mixed species biofilms are still poorly understood [27]. Here we have shown that in co-culture biofilms all P. aeruginosa strains were able to outcompete S. aureus in both sessile and planktonic fractions and the composition of the population in mixed biofilms was determined by P. aeruginosa, albeit to different extent. Also under biofilm growth conditions, the clonal late P. aeruginosa strains AA43 presented a different behavior in the presence of S. aureus compared to the early AA2 strain. In single species biofilm, the mucoid AA43 strain, even if apparently displaying a lower biofilm biomass compared to AA2 after staining with crystal violet, presented a higher percentage of sessile cells compared to AA2. This difference in biofilm production reflects the well documented phenotypic changes occurring in P. aeruginosa during the establishment of chronic infection. Indeed, P. aeruginosa strains isolated from CF patients at early stage of chronic infection are generally non-encapsulated and express a variety of virulence factors, whereas P. aeruginosa isolates from late stage typically lack virulence factors and convert to a mucoid phenotype, associated with greater biofilm formation and resistance to phagocytosis [37]. In apparent contradiction, also the early strain AA2 was able to produce biofilm. This could be explained considering the complexity of the microbial interactions in the CF lung, the presence of a diverse community of P. aeruginosa strains, and the many factors contributing to the formation of the biofilm matrix of P. aeruginosa, besides alginate production. In addition not all adapted isolates are mucoid and also early not adapted strains could produce biofilm exploiting other biofilm matrix molecules [40]. In agreement with data obtained in planktonic co-cultures, AA2 strongly inhibited the growth of S. aureus in mixed biofilm, without being affected. Qazi et al. demonstrated that factors related to biofilm formation are down-regulated in S. aureus in response to P. aeruginosa presence, consistently with our results [41]. Compared to AA2, P. aeruginosa AA43 inhibited S. aureus growth at lower extent, determining a reduction of S. aureus CFU count of about 1 and 2 log in planktonic and sessile fractions respectively, when measured against S. aureus individual biofilm. Moreover, the capacity to produce biofilm of AA43 was negatively affected by the presence of S. aureus, confirming its attenuated virulence and susceptibility to competitor organism.
Although several studies using in vitro models demonstrated an inhibitory effect of P. aeruginosa on the growth of also highly virulent S. aureus strains such as USA 300, in line with our results [27], [42], [43], in vivo models show contradictory results [42], [44]. We further investigated S. aureus/P. aeruginosa reciprocal interaction setting up a murine model of acute lung co-infection. In agreement with in vitro data, the reference strain PA14 and the early CF clinical isolate AA2, after 18 hours of co-infection, inhibited S. aureus, while the late CF clinical isolate AA43 did not outcompete S. aureus.
It is known that environmental and early clinical isolates of P. aeruginosa are equipped with a repertoire of virulence factors and, among them, also substances with anti-bacterial activity, these factors are selected against during the adaptation process to the CF airways environment. The results obtained in the acute pneumonia model, in which an early isolate is able to inhibit the growth of another pathogen, while its clonal adapted strain is no longer able to do so, strengthen the loss of anti-bacterial factors during adaptation.
Our data underline the importance of bacterial interactions in lung infection and in particular of the complexity of the interactions of different pathogens that coexist in the CF airways. However, given the genetic adaptation process of P. aeruginosa that leads to the selection of different patho-adaptive variants, descending from the initial infecting clone, further combinations of clonal lineages of early and late isolates should be tested to strengthen our in vivo data. Moreover, considering that the adaptation process during chronic infection involves also S. aureus, other experiments using clinical early and late S. aureus strains as well as adapted phenotypes such as small colony variants should be performed. Our results showing the influence of adaptation on the reciprocal interactions between S. aureus and P. aeruginosa deserve further investigations including the host response and the effect of environmental conditions, such as microaerobic and anaerobic conditions, on pathogens interactions, using both in vitro and in vivo models of chronic infection that better mirror the progression of CF lung disease.
Materials and Methods
Animals and ethics statement
Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute (Milan, Italy) Institutional Animal Care and Use Committee (IACUC, Number 444) and adhered strictly to the Italian Ministry of Health guidelines for the use and care of experimental animals. All efforts were made to minimize the number of animals used and their suffering.
Research on P. aeruginosa bacterial isolates from the individuals with CF has been approved by the responsible physician at the CF center at Hannover Medical School, Germany. All patients gave informed consent before the sample collection. Approval for storing of biological materials was obtained by the Hannover Medical School, Germany.
Bacterial strains
For S. aureus, Newman and SH1000 reference strains, were used in the study [45], [46]. Two P. aeruginosa reference strains, PA14 [47] and PAO1 [48], and 8 clonal lineages of P. aeruginosa clinical strains from CF patients (AA, SG, NN, BT, TR, MF, KK, BST), including strains isolated at the onset of chronic colonization (early: AA2, SG1, NN2, BT1, BT2, TR1, TR2, MF1, MF2, KK1, KK2, BST2) or several years after acquisition and before patient's death (late: AA43, SG57, SG58, NN83, BT72, BT73, TR66, TR67, MF51, KK71, KK72, BST44) were used in this study [23]. Clonality of strains, assessed by Pulsed Field Gel Electrophoresis and multiple phenotypic traits, including motility, mucoid phenotype, LasR phenotype, and pyocyanin secretion, have been determined and previously reported [23], [32].
S. aureus growth inhibition on agar surface
S. aureus cultures (Newman and SH1000) grown overnight in Luria-Bertani broth (LB, Difco™) were normalized to 0.5 OD600 and uniformly spread on Mueller-Hinton agar plate (MH, Difco™) by using a cotton swab. Then 5 µl of P. aeruginosa culture, grown overnight in LB broth and normalized to 0.5 OD600, were added to the S. aureus lawn followed by incubation overnight at 37°C [25]. The same procedure was performed spotting S. aureus culture on P. aeruginosa lawn. The following P. aeruginosa clonal lineages, including early and late clinical strains, were tested: AA, SG, NN, BT, TR, MF, KK and BST (for details see paragraph “Bacterial strains”). As P. aeruginosa reference strains we used PA14 and PAO1. The inhibition score was defined as follows: “no inhibition” when no halo was observed around the spot of P. aeruginosa that measures 9 mm; “weak inhibition” indicated an inhibition halo ≤15 mm; “strong inhibition” indicated an inhibition halo >15 mm and ≤25 mm; “very strong inhibition” indicated an inhibition halo >25 mm. The choice for inhibition strength ranges was based on preliminary assays performed using the lawn of about 30 S. aureus strains (including both reference and clinical strains of different origin) and spotting about 60 P. aeruginosa strains (both reference and clinical strains) on the different lawns.
Planktonic mono-culture and co-culture growth curves
All growth curves were performed in 30 ml of nutrient-rich not selective medium, Brain-Heart Infusion broth (BHI, Difco™), at 37°C with shaking (180 rpm). The following strains were tested: S. aureus (Newman, SH1000), P. aeruginosa (PA14, AA2 and AA43). Strains were grown overnight in BHI and subcultured in fresh medium for 2.5 hours to reach the mid-exponential phase of growth. Bacteria were centrifuged, pellet was resuspended in fresh medium and the OD600 was measured to adjust the concentration of bacteria. For co-cultures each pair of S. aureus and P. aeruginosa strains were inoculated at equal ratio (1 OD600, optical density) from mid-exponential phase pure cultures and incubated at 37°C for 24 hours. Pure cultures of each organism were used for comparative purposes. At different time points (0, 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 hours), samples were taken, serially diluted in sterile phosphate-buffered saline (PBS) and plated onto Mannitol Salt agar (MSA, Difco™) and Pseudomonas Isolation agar (PIA, Difco™) to discriminate the two bacterial species. The agar plates were incubated for 24 hours at 37°C and colony forming units (CFU) were enumerated. Each experiment was repeated three times independently. The competition index (CI) for mixed culture was calculated as P. aeruginosa-to-S. aureus ratio within the output sample, divided by the corresponding ratio for the input (inoculum at time t = 0), as described by Macho and colleagues [49]. To allow an easier comparison between the variations observed in single versus mixed cultures a CI-like index, the Relative Increase Ratio (RIR) was calculated as P. aeruginosa-to-S. aureus ratio within the output sample, divided by the corresponding ratio in the inoculum, using growth results from pure cultures [49]. As the RIR is calculated on the results obtained from single growth curves, only a CI that differs statistically from the RIR of the same time-point can be considered a result of a significant competition between the species [49].
Biofilm production
Biofilm production in static conditions was visualized by crystal violet (CV) staining as previously described [34]. The following S. aureus and P. aeruginosa strains were tested: Newman, PA14, AA2 and AA43. Strains were grown overnight in Nutrient Broth (NB, Difco™) and subcultured in fresh medium for 2.5 hours to reach the mid-exponential phase of growth. Bacteria were centrifuged, pellet was washed with PBS, resuspended in fresh medium and the OD600 was measured to adjust the concentration of bacteria [34]. Experiments were performed in triplicate and repeated three times independently. The data were then averaged and the standard deviation was calculated.
To correlate the growth in the planktonic fraction with biofilm formation, the planktonic cell fractions, which were transferred to new microtiter plates, were quantified by plating serial dilutions on MSA and PIA agar plates. To enumerate the sessile cells of S. aureus and P. aeruginosa, the wells were rinsed three times with 200 µl of PBS to remove non-adherent and weakly adherent bacteria. Then, the biofilm was removed by scraping the surface of each well with 1 ml PBS and the recovered cells were suspended by vortexing for 30 sec. The number of sessile cells was determined by plating serial dilutions on MSA and PIA agar plates. To ensure the complete detachment of the bacteria, CV (1%) assay was performed on each of the wells scraped, and absorbance determined at 595 nm.
Mouse model of acute lung single and co-infection
Experiment of acute infection with S. aureus and P. aeruginosa strains were performed using C57Bl/6NCrlBR male mice (20–22 gr), purchased by Charles River, with minor modification to previous published protocols [20], [30]. For the co-infections, P. aeruginosa referent strain PA14 and clinical isolates AA2 and AA43, and S. aureus referent strain Newman, grown at middle exponential phase, were recovered by centrifugation and resuspended in PBS to the desired sub-lethal dose for infection of 1×106 CFU both for P. aeruginosa and S. aureus and mixed together at a ratio of 1∶1.
C57Bl/6NCrlBR mice were anesthetized by an intraperitoneal injection of a solution of 2.5% Avertin (2,2,2-tribromethanol, 97%; Sigma Aldrich) in 0.9% NaCl and administered in a volume of 0.015 mlg−1 body weight. Trachea was directly visualized by a ventral midline incision, exposed and intubated with a sterile, flexible 22-g cannula (Becton, Dickinson, Italy) attached to a 1 ml syringe. Co-infection was established with a 60 µl inoculum implanted via the cannula into the lung, with both lobes inoculated. Mice were also infected with 1×106 CFU of planktonic P. aeruginosa or S. aureus for comparative purposes.
After 18 hours from infection, mice were euthanized and murine lungs were aseptically excised, homogenized and plated onto MSA and PIA plates for differential CFU counting. The competition index (CI) was calculated as the ratio of P. aeruginosa to S. aureus bacteria recovered from the murine lungs after 18 hours from infection adjusted by the input ratio that was inoculated in each animal (in vivo CI). A CI value equal to 1 indicates equal competition of the two species; a CI value significantly <1 indicates a competitive advantage of S. aureus that outcompetes P. aeruginosa; a CI value significantly >1 indicates a competitive advantage of P. aeruginosa that outcompetes S. aureus.
Statistical analysis
In vitro agar growth inhibition data were analyzed by means of a LME (Linear Mixed effect model) separated for Newman and SH1000. Response variable was inhibition and covariates were groups (early versus late) and a random effect on patient to account for lineages heterogeneity. To analyze batch co-culture data reported in Figure 1A, 1C, 1E the CFU/ml values were transformed using a log10 function. Data retrieved from single and co-culture experiments showed a similar starting point (estimated by intercept parameter A) and different behavior in some settings over time , leading to different plateau values (estimated by parameter B) at the end of the follow-up period. This suggested to use a nonlinear mixed-effect model (ref), (with the non-linearity described by a four-parameters logistic regression function) to estimate the log10(CFU/ml) trend, modelled as it follows: A+B/{1+exp[(C−x)/exp(D)]}.
This kind of model is widely used for growth curve modeling. Since the parameter A represents horizontal asymptote relative to the starting point, we assign a random effect (representing the heterogeneity among experiments) on this parameter to include heterogeneity among experiments. Parameter B represents the horizontal asymptote relative to the final plateau; we studied the possible influence of single/co-culture (described by its indicator variable), in order to test the hypothesis of different plateau at the end of the follow up. This represents the main effect of interest and its significance was tested comparing likelihood with and without it. Parameter C is the inflection point and has been estimated using a maximum likelihood principle. Parameter D is strictly connected to the so called scale parameter and represents the growth rate of the logistic function. A fixed effect common for single single and co-culture was estimated. RIR and CI indexes were analyzed using Student's t-test and the null hypothesis: mean CI was not significantly different from mean RIR [49]. In vitro biofilm data (OD values) were analyzed using Two-tailed Student's t-test [34]. CFU biofilm data were analyzed by means of Mann–Whitney test, a non-parametric procedure to evaluate a null hypothesis that two populations are the same against an alternative that one population tends to have larger values than the other. Competition index (CI) of in vivo experiments was calculated adapting the methods previously published [20]. To assess bacterial competition Wilcoxon signed rank test of the null hypothesis that the distribution of CI is symmetric about 1 was used. Significance was set at the usual level 0.05. All statistical analyses were performed using R 2.15.2 (http://www.R-project.org/).
Supporting Information
In vitro growth inhibition of P. aeruginosa .
(DOCX)
Single and dual species batch growth curves and competition index values. S. aureus strain (SH1000) and P. aeruginosa strains (PA14 and two clinical early and late isolates from a CF patient AA2 and AA43) were grown for 24 hours in BHI in single culture and in co-culture after inoculation at equal ratio from mid-exponential phase pure cultures. Growth rate was monitored by colony count after plating on selective media for both species. Results are represented as the mean of values obtained from three independent experiments. The error bars indicate the standard deviations. A nonlinear mixed-effect model was fitted, using a four-parameters logistic regression function. Panel A: growth curves of SH1000 in pure culture and in co-culture with PA14; Panel B: Competition index (CI) and Relative Increase Ratio (RIR) calculated from single and dual cultures of SH1000 and PA14; Panel C: growth curves of SH1000 in pure culture and in co-culture with AA2; Panel D: CI and RIR calculated from single and dual cultures of SH1000 and AA2; Panel E: growth curves of SH1000 in pure culture and in co-culture with AA43; Panel F: CI and RIR calculated from single and dual cultures of SH1000 and AA43. Each value represents the mean of CI and RIR values from three independent experiments and the bars indicate standard deviation. Statistically significant differences in Student's t test and in nonlinear mixed-effect model are indicated by symbols when present: *: p<0.05; ***: p<0.001.
(TIF)
Acknowledgments
The authors thank Barbara Sipione for her technical support. The authors thank B. Tummler, Klinische Forschergruppe, OE 6710, Medizinische Hochschule Hannover, Hannover, Germany, for supplying the P. aeruginosa strains from CF patients and Prof. BC. Kahl, Institut für Medizinische Mikrobiologie Universitätskliniken Muenster, Germany for supplying S. aureus strains.
Funding Statement
This study was supported to DMC and AB by Fondazione per la ricerca sulla Fibrosi Cistica (project FFC#9/2010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sibley CD, Rabin H, Surette MG (2006) Cystic fibrosis: a polymicrobial infectious disease. Future Microbiol 1: 53–61. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry: Annual Data Report 2008. Available: http://www.cff.org. Accessed 2014 Feb 12.
- 3. Rogers GB, Hoffman LR, Whiteley M, Daniels TW, Carroll MP, et al. (2010) Revealing the dynamics of polymicrobial infections: implications for antibiotic therapy. Trends Microbiol 18 (8) 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8 (1) 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan RP, Fouhy Y, Garcia BF, Watt SA, Niehaus K, et al. (2008) Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa . Mol Microbiol 68: 75–86. [DOI] [PubMed] [Google Scholar]
- 6. Nocker A, Burr M, Camper AK (2007) Genotypic microbial community profiling: a critical technical review. Microb Ecol 54: 276–289. [DOI] [PubMed] [Google Scholar]
- 7. Rogers GB, Carroll MP, Bruce KD (2009) Studying bacterial infections through culture-independent approaches. J Med Microbiol 58: 1401–1418. [DOI] [PubMed] [Google Scholar]
- 8. Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, et al. (2008) A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A 105: 15070–15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bittar F, Richet H, Dubus JC, Reynaud-Gaubert M, Stremler N, et al. (2008) Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One 3: e2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouchara JP, Hsieh HY, Croquefer S, Barton R, Marchais V, et al. (2009) Development of an oligonucleotide array for direct detection of fungi in sputum samples from patients with cystic fibrosis. J Clin Microbiol 47: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armougom F, Bittar F, Stremler N, Rolain JM, Robert C, et al. (2009) Microbial diversity in the sputum of a cystic fibrosis patient studied with 16S rDNA pyrosequencing. Eur J Clin Microbiol Infect Dis 28: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 12. Ecker DJ, Sampath R, Massire C, Blyn LB, Hall TA, et al. (2008) Ibis T5000: a universal biosensor approach for microbiology. Nat Rev Microbiol 6: 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers GB, Stressmann FA, Walker AW, Carroll MP, Bruce KD (2010) Lung infections in cystic fibrosis; deriving clinical insight from microbial complexity. Exp Rev Mol Diag 10: 187–196. [DOI] [PubMed] [Google Scholar]
- 14. Brogden KA, Guthmiller JM, Taylor CE (2005) Human polymicrobial infections. Lancet 365 (9455) 253–255. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME (2012) Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 25 (1) 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Döring G (1997) Staphylococcus aureus in cystic fibrosis: implications for prognosis and treatment. Pediatr Pulmonol Suppl 16: 235–6. [DOI] [PubMed] [Google Scholar]
- 17. Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, et al. (2009) Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 136 (6) 1554–60. [DOI] [PubMed] [Google Scholar]
- 18. Goss CH, Muhlebach MS (2011) Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros 10 (5) 298–306. [DOI] [PubMed] [Google Scholar]
- 19. Johnson AP (2011) Methicillin-resistant Staphylococcus aureus: the European landscape. J Antimicrob Chemother 66 Suppl 4: iv43–iv48. [DOI] [PubMed] [Google Scholar]
- 20. Baldan R, Testa F, Lorè NI, Bragonzi A, Cichero P, et al. (2012) Factors contributing to epidemic MRSA clones replacement in a hospital setting. PLoS One 7 (8) e43153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ratjen F, McColley SA (2012) Update in cystic fibrosis 2011. Am J Respir Crit Care Med 1 185 (9) 933–6. [DOI] [PubMed] [Google Scholar]
- 22. Döring G, Hoiby N (2004) Consensus Study Group (2004) Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros 3 (2) 67–91. [DOI] [PubMed] [Google Scholar]
- 23. Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, et al. (2009) Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 15 180 (2) 138–45. [DOI] [PubMed] [Google Scholar]
- 24. Jarry TM, Cheung AL (2006) Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect Immun 74 (5) 2568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffman LR, Deziel E, D'Argenio DA, Lepine F, Emerson J, et al. (2006) Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa . Proc Natl Acad Sci USA 103 (52) 19890–19895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maselli J, Sontag MK, Norris JM, MacKenzie T, Wagener JS, et al. (2003) Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol 35 (4) 257–262. [DOI] [PubMed] [Google Scholar]
- 27. Yang L, Liu Y, Markussen T, Høiby N, Tolker-Nielsen T, et al. (2011) Pattern differentiation in co-culture biofilms formed by Staphylococcus aureus and Pseudomonas aeruginosa . FEMS Immunol Med Microbiol 62 (3) 339–47. [DOI] [PubMed] [Google Scholar]
- 28. Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35 (4) 322–32. [DOI] [PubMed] [Google Scholar]
- 29. Lipuma JJ (2010) The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 23: 299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorè NI, Cigana C, De Fino I, Riva C, Juhas M, et al. (2012) Cystic fibrosis-niche adaptation of Pseudomonas aeruginosa reduces virulence in multiple infection hosts. PLoS One 7 (4) e35648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Montanari S, Oliver A, Salerno P, Mena A, Bertoni G, et al. (2007) Biological cost of hypermutation in Pseudomonas aeruginosa strains from patients with cystic fibrosis. Microbiology 153 (Pt 5) 1445–54. [DOI] [PubMed] [Google Scholar]
- 32. Bragonzi A, Wiehlmann L, Klockgether J, Cramer N, Worlitzsch D, et al. (2006) Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 152 (Pt 11) 3261–9. [DOI] [PubMed] [Google Scholar]
- 33. Cigana C, Curcurù L, Leone MR, Ieranò T, Lorè NI, et al. (2009) Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One 23;4 (12) e8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bragonzi A, Farulla I, Paroni M, Twomey KB, Pirone L, et al. (2012) Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One 7 (12) e52330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bianconi I, Milani A, Cigana C, Paroni M, Levesque RC, et al. (2011) Positive signature-tagged mutagenesis in Pseudomonas aeruginosa: tracking patho-adaptive mutations promoting airways chronic infection. PLoS Pathog 3;7 (2) e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harrison F (2007) Microbial ecology of the cystic fibrosis lung. Microbiology 153 (Pt 4) 917–23. [DOI] [PubMed] [Google Scholar]
- 37. Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, et al. (2012) Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol 10 (12) 841–51. [DOI] [PubMed] [Google Scholar]
- 38. Mashburn LM, Jett AM, Akins DR, Whiteley M (2005) Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J Bacteriol 187 (2) 554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biswas L, Biswas R, Schlag M, Bertram R, Götz F (2009) Small-colony variant selection as a survival strategy for Staphylococcus aureus in the presence of Pseudomonas aeruginosa . Appl Environ Microbiol 75 (21) 6910–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mann EE, Wozniak DJ (2012) Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev 36 (4) 893–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qazi S, Middleton B, Muharram SH, Cockayne A, Hill P, et al. (2006) N-acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus . Infect Immun 74 (2) 910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pastar I, Nusbaum AG, Gil J, Patel SB, Chen J, et al. (2013) Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 8 (2) e56846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qin Z, Yang L, Qu D, Molin S, Tolker-Nielsen T (2009) Pseudomonas aeruginosa extracellular products inhibit staphylococcal growth, and disrupt established biofilms produced by Staphylococcus epidermidis . Microbiology 155 (Pt 7) 2148–56. [DOI] [PubMed] [Google Scholar]
- 44. Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, et al. (2011) An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One 6 (11) e27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K (2008) Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J Bacteriol 190 (1) 300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, et al. (2002) SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184 (19) 5457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, et al. (2006) An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 21 103 (8) 2833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, et al. (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406 (6799) 959–64. [DOI] [PubMed] [Google Scholar]
- 49. Macho AP, Zumaquero A, Ortiz-Martín I, Beuzón CR (2007) Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae-plant interactions. Mol Plant Pathol 8 (4) 437–50.pone.0089614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro growth inhibition of P. aeruginosa .
(DOCX)
Single and dual species batch growth curves and competition index values. S. aureus strain (SH1000) and P. aeruginosa strains (PA14 and two clinical early and late isolates from a CF patient AA2 and AA43) were grown for 24 hours in BHI in single culture and in co-culture after inoculation at equal ratio from mid-exponential phase pure cultures. Growth rate was monitored by colony count after plating on selective media for both species. Results are represented as the mean of values obtained from three independent experiments. The error bars indicate the standard deviations. A nonlinear mixed-effect model was fitted, using a four-parameters logistic regression function. Panel A: growth curves of SH1000 in pure culture and in co-culture with PA14; Panel B: Competition index (CI) and Relative Increase Ratio (RIR) calculated from single and dual cultures of SH1000 and PA14; Panel C: growth curves of SH1000 in pure culture and in co-culture with AA2; Panel D: CI and RIR calculated from single and dual cultures of SH1000 and AA2; Panel E: growth curves of SH1000 in pure culture and in co-culture with AA43; Panel F: CI and RIR calculated from single and dual cultures of SH1000 and AA43. Each value represents the mean of CI and RIR values from three independent experiments and the bars indicate standard deviation. Statistically significant differences in Student's t test and in nonlinear mixed-effect model are indicated by symbols when present: *: p<0.05; ***: p<0.001.
(TIF)