Abstract
Generalized arterial calcification of infancy (GACI) is an autosomal recessive disorder characterized by congenital calcification of large and medium sized arteries, associated with early myocardial infarction, heart failure, and stroke, and premature death. Most cases of GACI are caused by mutations in the ENPP1 gene. We first studied two siblings with GACI from a non-consanguineous family without mutations in the ENPP1 gene. To search for disease-causing mutations, we identified genomic regions shared between the two affected siblings but not their unaffected parents or brother. The ABCC6 gene, which is mutated in pseudoxanthoma elasticum (PXE), resided within a small region of homozygosity shared by the affected siblings. Sequence analysis of ABCC6 revealed that the two affected siblings were homozygous for the missense mutation p.R1314W. Subsequently, ABCC6 mutations were identified in five additional GACI families with normal ENPP1 sequences. Genetic mutations in ABCC6 in patients with PXE are associated with ectopic tissue mineralization in the skin and arterial blood vessels. Thus, our findings provide additional evidence that the ABCC6 gene product inhibits calcification under physiologic conditions and confirm a second locus for GACI. In addition, our study emphasizes the potential utility of shared homozygosity mapping to identify genetic causes of inherited disorders.
Introduction
Generalized arterial calcification of infancy (GACI, OMIM# 208000) is an autosomal recessive disorder that is characterized by calcification of the internal elastic lamina of large- and mediumsized arteries and stenosis due to fibroproliferation of the intima of muscular arteries. Radiographs show both arterial and periarticular soft tissue calcifications. GACI usually presents with congestive cardiac failure, hypertensive disease, and/or myocardial ischemia, and the majority of children die within the first 6 months of life (Maayan et al., 1984), with only a few case reports of survivors into later childhood or adulthood (Ciana et al., 2006; Patel et al., 2004; Rutsch et al., 2003; van der Sluis et al., 2006).
Most, but not all, patients with GACI have recessive mutations in the ENPP1 gene (OMIM# 173335) located on chromosome 6q22-q23 (Rutsch et al., 2003), suggesting that GACI is genetically heterogeneous. ENPP1 encodes the ecto-nucleotide pyrophosphatase phosphodiesterase 1 (ENPP1, EC 3.6.1.9, EC 3.1.4.1) enzyme that regulates soft tissue calcification and bone mineralization by generating inorganic pyrophosphate (PPi), an essential inhibitor of hydroxyapatite deposition. Hence, GACI patients with deficiency of ENPP1 are unable to synthesize sufficient PPi to inhibit ectopic mineralization and are, therefore, prone to arterial calcification. In addition to a deficiency of PPi, recent studies indicate that some children with GACI due to ENPP1 mutations develop hypophosphatemia after the first year of life (Rutsch et al., 2008). Hypophosphatemia is assumed to be mediated by elevated plasma levels of the phosphatonin FGF23, which inhibits expression and internalization of the renal sodium-phosphate cotransporters SLC34A1 and SLC34A3 and inhibits synthesis of 1.25-dihydroxyvitamin D. In patients with GACI, hypophosphatemia appears to ameliorate soft tissue calcification (Rutsch et al., 2008). Remarkably, other patients with ENPP1 mutations develop hypophosphatemic rickets rather than GACI, a paradox that at present lacks a biochemical explanation (Levy-Litan et al., 2010; Lorenz-Depiereux et al., 2010; Rutsch et al., 2008).
Sequence analyses of ENPP1 have failed to disclose disease-causing mutations in approximately 25% of subjects with GACI (Le Boulanger et al., 2010), and linkage analyses have pointed to other chromosomal regions where other pathogenic genes may be located (Ruf et al., 2005). Recently Nitschke et al. (Nitschke et al., 2012) described a cohort of patients with GACI with mutations in ABCC6, which has previously been implicated in pseudoxanthoma elasticum (PXE) (Uitto et al., 2010) and a variant of GACI (Le Boulanger et al., 2010), suggesting that this gene plays a causative role in this disorder. Prior to these publications we investigated two siblings born to non-consanguineous parents with severe GACI and normal sequences for ENPP1 (Li et al., 2012a). We performed a genome-wide search for a genetic defect underlying GACI and searched for replication of the best candidates in a larger independent cohort of children with GACI. Here we describe additional patients with mutations in the ABCC6 gene as the second locus for GACI, emphasizing the genotypic overlap between GACI and PXE.
Results
Clinical Findings
A total of seven patients in six families with clinical manifestations consistent with GACI were examined; these families are referred to as Families A–F. The nuclear pedigrees of these families are shown in Fig. 1, and the diagnostic clinical characteristics are shown in Fig. 2 and are detailed in Supplementary Material.
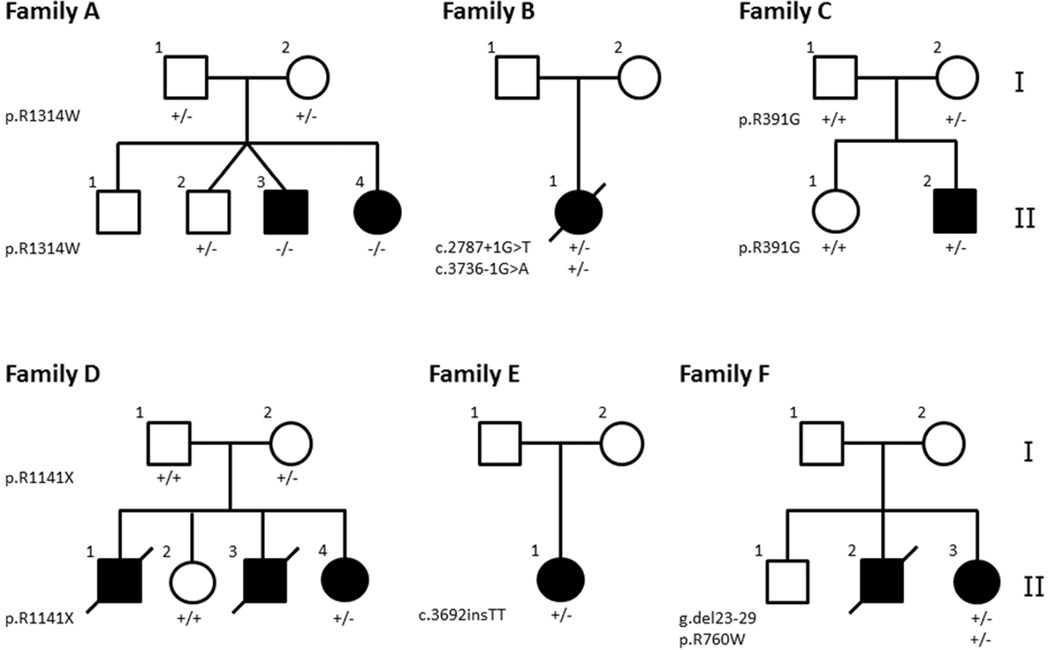
Figure 1. Nuclear pedigrees of Families A-F with GACI.
The mutations discovered in the ABCC6 gene in the individual family members are indicated below each individual: +/+, homozygous for the wild-type allele; +/−, heterozygous carrier; −/−, mutations in both alleles.
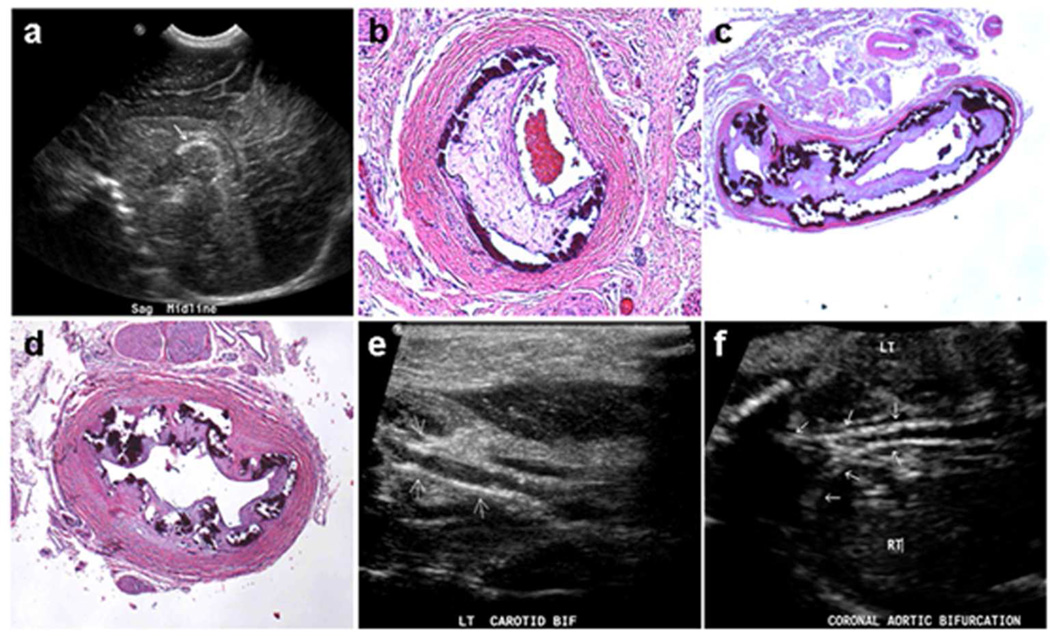
Figure 2. Ultrasound and histopathologic features in individuals with GACI.
A: Ultrasound of Patient 1 in Family A (II-3) reveals, in lateral sagittal view of head, calcifications along the cores of lenticulostriate vessels (arrow). B-D: Histopathology of Patient 3 in Family B (II-1) shows calcification of mesenteric artery (B), abdominal artery (C), and left renal artery (D); Hematoxylin-eosin stain, original magnifications ×150. E: Patient 6 in Family E (II-1) demonstrates by ultrasound imaging portions of the proximal external carotid artery with thickened walls and irregular, speckled calcification (arrows). F: Patient 7 in Family F (II-3), prenatal ultrasound of aortic bifurcation demonstrates extensive calcification (arrows).
Identification of ABCC6 as a candidate gene
We initially focused on Family A with two affected siblings with characteristic features of GACI (Fig. 2A). Sequencing of ENPP1 as one of the candidate genes for mutations (Table 1), which is known to cause GACI, did not reveal the presence of pathogenic mutations. To facilitate the identification of the molecular basis of GACI in this family, we performed genome-wide (610K) SNP array analysis on all individuals. We analyzed the data from the three siblings and the parents to phase informative SNP markers and determine shared haplotype segments between the affected siblings that were not also shared with the unaffected sibling. As expected, this analysis confirmed the exclusion of ENPP1 as a candidate gene, as well as the MGP and ADIPOQ genes, each considered as candidates based on known association to mineralization (Table 1). Further analysis of the genotypes within the region containing the ABCC6 gene revealed that the shared haplotypes between the affected siblings reside in a relatively small, 799 kb region of homozygosity, thus identifying ABCC6 as the primary candidate gene for mutations in this family.
Table 1.
Candidate genes for GACI identified by shared homozygosity analysis in Family A with two affected patients with an unaffected sibling.
| Gene | Reason assessed | Excluded from affected siblings |
Excluded using affected and unaffected |
Comments on haplotype analysis |
|---|---|---|---|---|
| ENPP1 | Common cause of GACI | No | Yes | Same haplotypes in all three children |
| MGP | Anti-mineralization factor | Yes | Yes | Different in affected siblings |
| ADIPOQ | adiponectin involved in mineralization | Yes | Yes | Different in affected siblings |
| ADIPOR1 | Adiponectin, type 1 receptor | No | No | Possible candidate gene |
| ABCC6 | Common cause of PXE | No | No | All SNPs over a region of homozygosity |
We subsequently used Sanger sequencing to assess the ABCC6 gene for mutations in affected members of this family and other unrelated individuals in additional families with GACI.
Mutations in the ABCC6 gene underlie GACI
We used PCR to amplify and directly sequence all 31 exons and the flanking intronic sequences of the ABCC6 gene in patients with GACI with normal ENPP1 sequences, as well as their unaffected family members. Affected members of Family A (Patients 1 and 2), who shared a 799 kb region of homozygosity on chromosome 16, were found to have homozygous missense mutations (c.3940C>T, p.R1314W) in exon 28 of ABCC6 (Fig. 3). This missense mutation replaces a conserved arginine residue with tryptophan in the second nucleotide binding fold of ABCC6. The proband in Family B (Patient 3) was found to have compound heterozygous mutations (c.2787+1G>T and c.3736-1G>A) in introns 21 and 26 of ABCC6. The proband in Family D (Patient 5) had compound heterozygous mutations c.346-6G>A and p.R1141× in intron 3 and exon 24 of ABCC6. In Family F, the proband (Patient 7) was found to carry compound heterozygous mutations consisting of a large deletion (g.del 23–29) and a missense mutation (p.R760W). The arginine-to-tryptophan substitution affects the first nucleotide binding fold of ABCC6 protein. Only one ABCC6 mutation has been identified for the proband of Family C (Patient 4; p.R391G) and the proband of Family E (Patient 6; c.3692insTT). The p.R391G mutation resides in the fourth intracellular loop corresponding to the second transmembrane domain of ABCC6 protein, while the c.3692insTT mutation causes a frameshift and predicts truncation of the protein as a result of a premature termination codon 125 bp downstream of the mutation and likely leads to nonsense-mediated mRNA decay (Table 2). Multiplex ligation-dependent probe amplification (MLPA) failed to identify additional deletion mutations in Families C and E. All the identified mutations, with the exception of the mutation c.3692insTT, have previously been reported in subjects with PXE as biallelic mutations, and are predicted to result in reduction or loss of ABCC6 activity (Pfendner et al., 2007; Uitto et al., 2010).
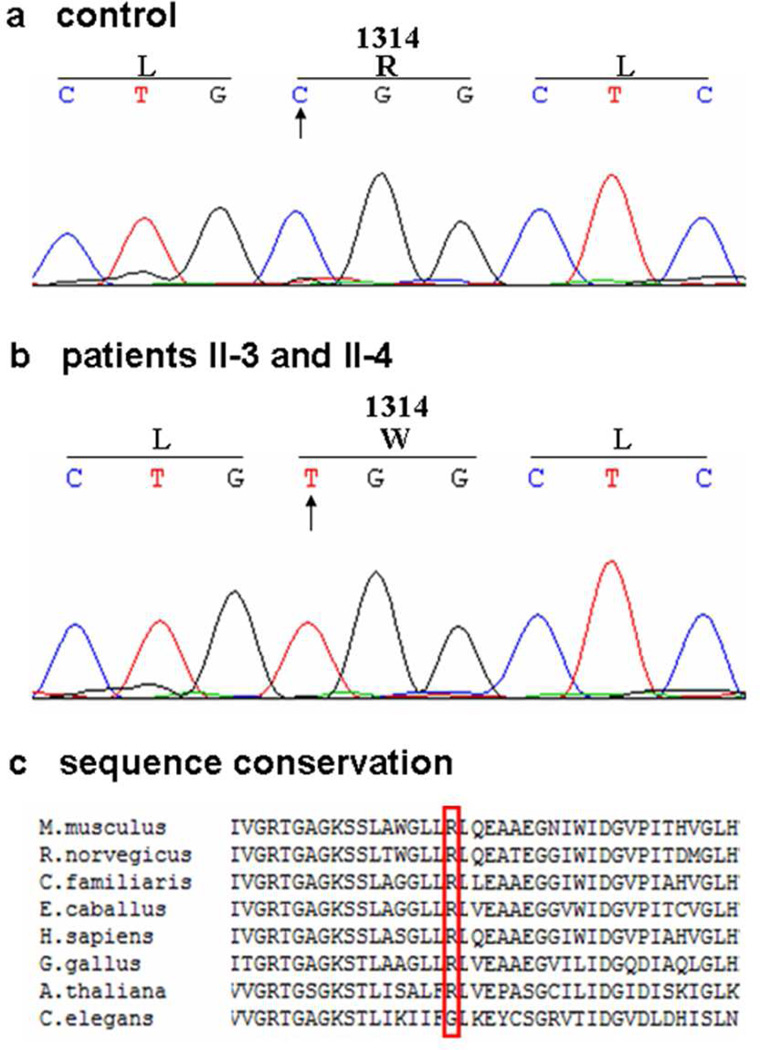
Figure 3. Mutation analysis of the ABCC6 gene in Family A with GACI.
Sequencing of the ABCC6 gene in Patients 1 and 2 (II-3 and II-4 in Figure 1) revealed a homozygous nucleotide T substitution (B) replacing nucleotide C in the control DNA (A). This mutation results in substitution of arginine (R) by tryptophan (W) at amino acid position 1314. The arginine residue is well conserved through evolution (C).
Table 2.
Molecular and biochemical features of GACI patients
| Patient | Family | Age | Mutation 1 | Mutation 2 | Mutation type2 | Plasma [FGF23], RU/mL3 |
|---|---|---|---|---|---|---|
| 1 | A | 3 yrs | p.R1314W | p.R1314W | MS/MS | 59 |
| 2 | A | 6 yrs | p.R1314W | p.R1314W | MS/MS | 97 |
| 3 | B | 1 mo | c.2787+1G>T | c.3736−1G>A | SS/SS | NT5 |
| 4 | C | 5 yrs | p.R391G | ND1 | MS/ND | 83 |
| 5 | D | 1 mo | p.R1141* | c.346-6G>A | NS/SS4 | NT |
| 6 | E | 1 mo | c.3692 insTT | ND | FS/ND | 374 |
| 7 | F | 1 mo | p.R760W | del23-29 | MS/del | 1430 |
ND, not detected.
MS, missense; SS, splice site; NS, nonsense; FS, frame shift
FGF23 normal range is < 230 RU/mL for ages 3 months to 17 years. Values greater than 900 RU/mL are present in infants.
The effects of the c.346-6G>A mutation on splicing have not been experimentally confirmed
NT, not tested as serum sample was not available
Biochemical findings
Serum levels of calcium, phosphorus, and alkaline phosphatase were normal in all subjects (data not shown). In addition, because circulating FGF23 is elevated in patients with GACI due to ENPP1 mutations, we measured plasma levels of FGF23 using an immunoassay that detects both the intact form and carboxy-terminal fragments of FGF23 protein. We found that circulating levels of FGF23 were normal in all affected subjects from whom serum could be obtained (Table 2).
Immunofluorescence findings in arterial blood vessels
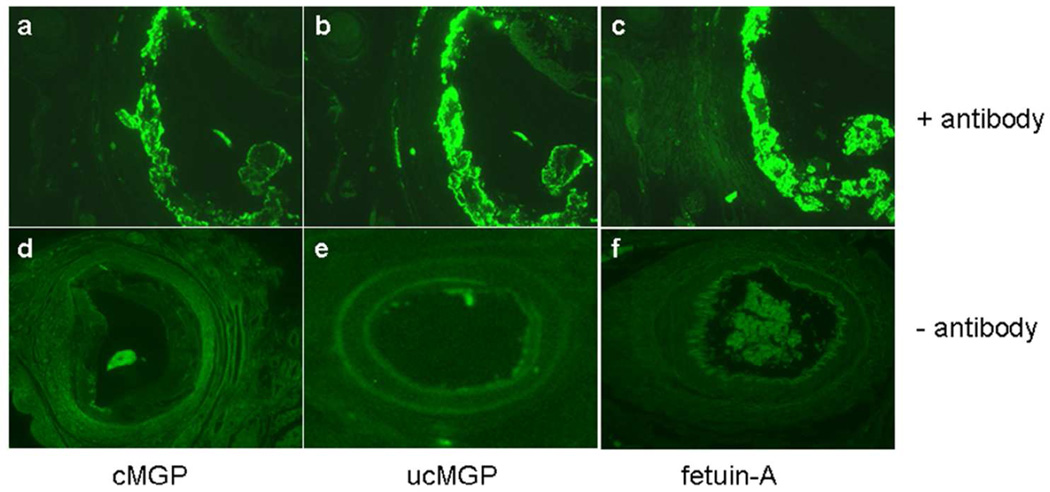
To further characterize the consequences of the ABCC6 mutations, we performed histopathologic examination of arteries in patients with GACI, investigating the activation status of matrix gla protein (MGP), a powerful, local anti-mineralization factor when activated by γ-glutamyl carboxylation and which has been implicated in the pathomechanism of PXE (Berkner, 2008; Uitto et al., 2010). We also examined for the presence of fetuin-A, a systemic anti-mineralization factor on the lesional area of mineralized blood vessels (Brylka and Jahnen-Dechent, 2013). Histopathology of the affected vessels revealed extensive mineralization. Because MGP has been shown to participate in the arterial mineralization processes, the presence of both carboxylated (cMGP, active) and uncarboxylated (ucMGP, inactive) forms of this protein was investigated by immunofluorescence with conformation-specific monoclonal antibodies (Schurgers et al., 2005). While the active form (cMGP) was detectable in association with the mineral deposits (Fig. 4, frame A), a similar, but stronger staining pattern was noted with the antibody recognizing ucMGP (Fig. 4, frame B), suggesting that MGP was predominantly in the inactive form. Immunostaining with an antibody recognizing fetuin-A revealed co-localization of the protein with the mineral deposits (Fig. 4, frame C). The controls without the primary antibody were negative (Fig. 4, frames D–F).
Figure 4. Immunofluorescence of the presence of carboxylated (active) and uncarboxylated (inactive) forms of MGP and fetuin-A in Patient 3 in Family B (II-1).
Immunofluorescence with antibodies recognizing carboxylated (A) and uncarboxylated (B) forms of MGP, and fetuin-A (C). Frames D, E and F represent the corresponding negative controls in which the primary antibodies were omitted.
Discussion
In this study we used a strategy of shared haplotype mapping to identify potential candidate genes for GACI in a family with two affected siblings and one unaffected sibling, confirmed by Sanger sequencing. This mapping led to the identification of ABCC6, which has been previously shown to be mutated in patients with PXE (Pfendner et al., 2007; Uitto et al., 2010) or associated in heterozygous state with premature coronary artery disease (Köblös, 2010; Trip et al., 2002), as the candidate gene. These studies indicate the usefulness of both shared haplotype and homozygosity analysis for identification of genetic causes of rare inherited disorders in patients even from apparently non-consanguineous families. Our results confirm the successful use of individuals from outbred populations in homozygosity mapping (Hildebrandt et al., 2009), which together with rapid next generation sequencing, should greatly accelerate gene discovery.
We subsequently used Sanger sequencing to confirm the association between ABCC6 and GACI in five additional unrelated families where the clinically affected subjects had normal ENPP1 gene sequences. We identified mutations in both alleles of ABCC6 in three subjects, but we found only a single mutant allele in two patients with GACI. Our results are similar to those noted in other studies in which mutations in both ABCC6 alleles were disclosed in only ~80% of patients with PXE (Pfendner et al., 2007; Uitto et al., 2010). Because GACI, similar to PXE, is an autosomal recessive disease, it is likely that individuals with a detectable mutation in only one allele harbor a second mutation in the other ABCC6 allele in trans. In this context, it should be noted that the mutation detection strategy we used to analyze the ABCC6 gene employs PCR amplification of all exons and flanking intronic sequences, combined with multiplex ligation-dependent probe amplification. This combined approach should detect most point mutations, insertion/deletion mutations and copy number variants, but would not detect small mutations in the promoter region or imbedded in the introns of ABCC6 that might affect transcription or splicing. An alternate, less likely possibility is that the affected individuals in Families C and E demonstrate digenic inheritance due to one mutation in the ABCC6 gene and a second heterozygous mutation in another, genetically distinct, yet functionally overlapping gene involved in ectopic tissue mineralization. This possibility is raised in light of our recent demonstration that heterozygosity for one mutation in the ABCC6 gene and one mutation in the GGCX gene can result in PXE-like cutaneous findings (Li et al., 2009a).
Our molecular results confirm and extend previous studies that have indicated a significant overlap between the clinical features of PXE and GACI (Nitschke and Rutsch, 2012). PXE predominantly affects the elastic tissues of the skin, eyes, and cardiovascular system (Neldner, 1988). In the skin, histopathology demonstrates accumulation of pleiomorphic elastic structures in the mid- and upper reticular dermis with profound, progressive mineralization. The eye manifestations characteristically consist of angioid streaks due to mineralization of an elastinrich Bruch’s membrane behind the pigmented retina (Georgalas et al., 2009). Mineralization of this membrane results in blood vessel rupture and subsequent neovascularization, leading to loss of visual acuity and occasionally to blindness. The cardiovascular manifestations include arterial calcification resulting in intermittent claudication, decreased peripheral pulses, hypertension, hemorrhage, and early myocardial infarcts. A recent report described two siblings with identical ABCC6 gene mutations that led to classical PXE in the older brother and a phenotype similar to GACI in the younger brother (Le Boulanger et al., 2010). The authors demonstrated that MGP and fetuin-A, involved in the mineralization process in PXE, were also expressed in the affected younger sibling with GACI. These proteins act physiologically as local and systemic inhibitors of mineralization. Additionally, a patient with GACI due to homozygous missense mutation in the ENPP1 gene has recently been reported to have PXE-like cutaneous manifestations consisting of yellowish papules coalescing into plaques of inelastic skin (Li et al., 2012b). Skin histopathology of this patient demonstrated accumulation of pleiomorphic elastotic structures with progressive mineralization, a diagnostic feature of PXE. Hence, GACI may be an atypical and severe end of the vascular phenotype spectrum of PXE. A comparison of clinical features of PXE and GACI is provided in Table 3. It should be noted that all the ABCC6 mutations discovered in this study in patients with GACI, with the exception of a mutation c.3692ins TT, have been previously reported in patients with PXE (Li et al., 2009b; Pfendner et al., 2007). The reasons for the development of such divergent phenotypes as a result of the same mutation in ABCC6 remain unclear. One could postulate, however, that genetic modifier genes or epigenetic factors profoundly alter the phenotypic expression of the ABCC6 mutations. This notion is supported by recent mouse studies indicating that a non-synonymous single nucleotide polymorphism in the Abcc6 gene, which results in aberrant mRNA splicing, can result in highly different degrees of mineralization in four inbred mouse strains (Berndt et al., 2013). Finally, diet, lifestyle variables and environmental factors can modify the phenotypic presentations of patients with PXE, possibly extending to GACI (Uitto et al., 2013).
Table 3.
Genotypic and phenotypic overlaps of PXE and GACI
| PXE | GACI |
|---|---|
| Vascular mineralization | Vascular mineralization |
| Skin and eye findings | Rare skin findings |
| Late onset | Pre- and perinatal diagnosis |
| Mostly normal life span | Demise usually <6 months |
| ABCC6 > ENPP1 | ENPP1 > ABCC6 |
The basis for the ectopic mineralization in GACI is only partially understood. Loss of function mutations in ENPP1 result in decreased extracellular levels of inorganic pyrophosphate (PPi), an inhibitor of tissue mineralization. As a result, ectopic deposition of hydroxyapatite occurs in large and medium-sized arteries leading to intimal proliferation, arterial stenosis, and visceral ischemia. Recently, biallelic loss of function mutations in ENPP1 have also been identified as a cause of autosomal recessive hypophosphatemic rickets (Levy-Litan et al., 2010; Lorenz-Depiereux et al., 2010; Mehta et al., 2012; Saito et al., 2011). As in other forms of hypophosphatemic rickets, serum levels of the phosphatonin FGF23 are elevated in rickets patients with ENPP1 mutations. Hence, we sought to determine whether loss of ABCC6 might also be associated with elevated serum concentrations of FGF23. Using an immunoassay we found that serum concentrations of FGF23 were essentially normal in all affected patients that we assessed. Thus, it is unlikely that FGF23 plays a significant role in the development of intraarterial calcification in patients with GACI.
The mechanism for vascular and soft tissue calcification in patients with GACI or PXE due to mutations in ABCC6 is unknown (Uitto et al., 2013). The mineral deposits in the affected tissues of patients with PXE have been shown by Alizarin Red and von Kossa staining to consist of calcium and phosphate, and this composition has been confirmed by energy dispersive X-ray analysis (Kavukcuoglu et al.). ABCC6 encodes a putative transmembrane transporter protein, ABCC6, expressed primarily in the baso-lateral surface of hepatocytes, and previously shown by in vitro studies to serve as an efflux pump that transports anionic small molecular weight conjugates (Ilias et al., 2002). Interestingly, in vitro, ABCC6 does not transport calcium or phosphate.
In conclusion, our findings confirm that loss of function mutations in the ABCC6 gene provide the second genetic basis for GACI. In addition to ABCC6 and ENPP1, a number of additional genes can harbor mutations causing heritable diseases manifesting with ectopic mineralization of the skin and/or vascular tissues due to mutations in different genes. These include familial tumoral calcinosis, the normophosphatemic type being due to mutations in the SAMD9 gene, and the hyperphosphatemic types due to mutations in FGF23, GALNT3, and KL genes (Sprecher, 2010). In addition, CD73 deficiency due to mutations in the NT5E gene manifests with vascular mineralization similar, but distinct from that in PXE (Markello et al., 2011). At the same time, a number of different gene products, such as MGP, α-fetuin, and osteopontin can serve as powerful anti-mineralization factors. Thus, there is an intricate network of factors which either promote or antagonize mineralization processes, and a delicate balance between these factors is required under normal physiologic conditions to maintain tissue homeostasis and prevent ectopic mineralization (Li and Uitto, 2013; Rutsch et al., 2011). Collectively, these observations underscore the role of aberrant expression of such regulatory factors in ectopic mineralization in humans, providing potential opportunities for pharmacologic intervention to prevent the development and progression of these disorders.
Materials and Methods
Patients
We studied seven affected individuals from six unrelated families with clinical features of GACI and normal sequences of the ENPP1 gene (Fig. 1). In all patients the serum levels of calcium, phosphorus, alkaline phosphatase and vitamin D metabolites were normal. The clinical details of these patients are presented in the Supplementary Material.
All patients were enrolled with written informed consent or assent into an institutional review board-approved study at either The Children’s Hospital of Philadelphia or the National Institutes of Health. All studies adhered to the Helsinki Guidelines.
Genome-wide arrays and analyses
DNA samples from Family A were analyzed using Illumina HapMap Quad610 arrays per manufacturer’s protocol (Illumina, San Diego, CA), and copy number analysis was performed as described previously (Conlin et al., 2010; Shaikh et al., 2009). The Homozygosity/LOH Detector v.1.0.3 Auto-Bookmark plug-in within Illumina Beadstudio 3.1.3 Genotyping Module 3.3.4 (Illumina, San Diego, CA) was used for detection of regions of homozygosity. We excluded the X chromosome from our homozygosity analyses because the male and female siblings were similarly affected.
Shared haplotypes were determined using parental genotypes to phase parental alleles for each of the three siblings in Family A. Subsequently, inconsistent and uninformative genotypes were removed from the analysis and regions were identified where both maternal and paternal haplotypes were shared among the affected siblings but not with the unaffected sibling. Candidate genes were assessed regarding their exclusion or inclusion from approximate shared regions (Table 1).
Mutation analysis in the ABCC6 and ENPP1 genes
Genomic DNA was isolated from peripheral blood (QIAamp Blood Maxi kit; Qiagen Inc., Valencia, CA). PCR was performed using Taq polymerase (Qiagen) according to the manufacturers’ instructions. The entire coding region and intron/exon boundaries of the ABCC6 gene were amplified using PCR primers as described previously (Pfendner et al., 2007). For the detection of the genomic deletion of exons 23–29, the primers previously described (Le Saux et al., 2001) were used. The PCR products were analyzed with direct sequencing using an Applied Biosystems 3730 Sequencer (Applied Biosystems, Foster City, CA). The +1 in the ABCC6 gene corresponds to the A nucleotide in the ATG translation initiation codon (GenBank accession no. AF076622). We subjected DNA from patients in whom only a single ABCC6 mutation was identified to direct sequencing analysis by multiplex ligation-dependent probe amplification (SALSA MLPA P092 ABCC6 probemix, MRC-Holland, Amsterdam) according to the manufacturer’s recommendations. Sequencing of the ENPP1 gene was performed as described previously (Nitschke et al., 2012).
Immunofluorescence
Immunofluorescence of the human arterial sections was performed on tissues embedded in paraffin. Sections were stained with moAb-cMGP (4.5 mg/ml; monoclonal antibody towards the carboxylated forms of human MGP), moAb-ucMGP (4.5 mg/ml; monoclonal antibody towards the uncarboxylated forms of human MGP) (Schurgers et al., 2005), and goat anti-human fetuin-A (10 Pg/ml; R&D Systems, Minneapolis, MN), respectively. The secondary antibody, Alexa Fluor 488 goat anti-mouse IgG (1:800) (Invitrogen, Carlsbad, CA) or Alexa Fluor 488 donkey anti-goat IgG (1:800) (Invitrogen, Carlsbad, CA), was applied. Controls for the immunoreactions were performed by incubating the sections with blocking buffer instead of the primary antibody.
Supplementary Material
Acknowledgement
We thank Dr. Steven Mumm at Shriner’s Hospital, St Louis, for sequencing the ENPP1 gene in Patient 4 in Family C. Carol Kelly assisted in manuscript preparation. This work was supported by National Institutes of Health Grants K08HD055488 (NICHD; MAD) and R01AR28450 (NIAMS; JU), by Institutional funds from the Children’s Hospital of Philadelphia, and by the Division of Intramural Research, NIDCR, NIH, DHHS. QL is recipient of a Dermatology Foundation Research Career Development Award.
Abbreviations
- GACI
generalized arterial calcification of infancy
- PXE
pseudoxanthoma elasticum
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008;78:131–156. doi: 10.1016/S0083-6729(07)00007-6. [DOI] [PubMed] [Google Scholar]
- Berndt A, Li Q, Potter CS, Liang Y, Silva KA, Kennedy V, et al. A single-nucleotide polymorphism in the Abcc6 gene associates with connective tissue mineralization in mice similar to targeted models for pseudoxanthoma elasticum. J Invest Dermatol. 2013;133:833–836. doi: 10.1038/jid.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brylka L, Jahnen-Dechent W. The role of fetuin-A in physiological and pathological mineralization. Calcif Tissue Int. 2013 doi: 10.1007/s00223-012-9690-6. (in press). [DOI] [PubMed] [Google Scholar]
- Ciana G, Trappan A, Bembi B, Benettoni A, Maso G, Zennaro F, et al. Generalized arterial calcification of infancy: two siblings with prolonged survival. Eur J Pediatr. 2006;165:258–263. doi: 10.1007/s00431-005-0035-6. [DOI] [PubMed] [Google Scholar]
- Conlin LK, Thiel BD, Bonnemann CG, Medne L, Ernst LM, Zackai EH, et al. Mechanisms of mosaicism, chimerism and uniparental disomy identified by single nucleotide polymorphism array analysis. Hum Mol Genet. 2010;19:1263–1275. doi: 10.1093/hmg/ddq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgalas I, Papaconstantinou D, Koutsandrea C, Kalantzis G, Karagiannis D, Georgopoulos G, et al. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag. 2009;5:81–89. [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Heeringa SF, Ruschendorf F, Attanasio M, Nurnberg G, Becker C, et al. A systematic approach to mapping recessive disease genes in individuals from outbred populations. PLoS genetics. 2009;5:e1000353. doi: 10.1371/journal.pgen.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- Kavukcuoglu NB, Li Q, Pleshko N, Uitto J. Connective tissue mineralization in Abcc6(−/−) mice, a model for Pseudoxanthoma elasticum. Matrix Biol. 2012;31:246–252. doi: 10.1016/j.matbio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köblös G, Andrikovics H, Prohászka Z, Tordai A, Váradi A, Arányi T. The R1141× loss-of-function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers. 2010;14:75–78. doi: 10.1089/gtmb.2009.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boulanger G, Labreze C, Croue A, Schurgers LJ, Chassaing N, Wittkampf T, et al. An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am J Med Genet A. 2010;152A:118–123. doi: 10.1002/ajmg.a.33162. [DOI] [PubMed] [Google Scholar]
- Le Saux O, Beck K, Sachsinger C, Silvestri C, Treiber C, Goring HH, et al. A spectrum of ABCC6 mutations is responsible for pseudoxanthoma elasticum. Am J Hum Gene. 2001;69:749–764. doi: 10.1086/323704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Gene. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brodsky JL, Conlin L, Bradfield J, Pawel B, Glatz A, et al. Mutations in the ABCC6 gene can cause generalized arterial calcification of infancy in addition to pseudoxanthoma elasticum. J Invest Dermatol. 2012a;132:S91. doi: 10.1038/jid.2013.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Grange DK, Armstrong NL, Whelan AJ, Hurley MY, Rishavy MA, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009a;129:553–563. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Pfendner E, Varadi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009b;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Schumacher W, Siegel D, Jablonski D, Uitto J. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br J Dermatol. 2012b;166:1107–1111. doi: 10.1111/j.1365-2133.2012.10811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Uitto J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol. 2013;183:10–18. doi: 10.1016/j.ajpath.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Gene. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan C, Peleg O, Eyal F, Mogle P, Rosenmann E, Bar Ziv J. Idiopathic infantile arterial calcification: a case report and review of the literature. Eur J Pediatr. 1984;142:211–215. doi: 10.1007/BF00442452. [DOI] [PubMed] [Google Scholar]
- Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Mitchell A, Tysoe C, Caswell R, Owens M, Vincent T. Novel compound heterozygous mutations in ENPP1 cause hypophosphataemic rickets with anterior spinal ligament ossification. Rheumatology (Oxford) 2012;51:1919–1921. doi: 10.1093/rheumatology/kes089. [DOI] [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front Genet. 2012;3:302. doi: 10.3389/fgene.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Andronikou S, Solomon R, Sinclair P, McCulloch M. Idiopathic arterial calcification in childhood. Pediatr Radiol. 2004;34:652–655. doi: 10.1007/s00247-004-1166-z. [DOI] [PubMed] [Google Scholar]
- Pfendner EG, Vanakker OM, Terry SF, Vourthis S, McAndrew PE, McClain MR, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P, Rutsch F. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI) Hum Mutat. 2005;25:98. doi: 10.1002/humu.9297. [DOI] [PubMed] [Google Scholar]
- Rutsch F, Boyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, et al. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nature Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- Saito T, Shimizu Y, Hori M, Taguchi M, Igarashi T, Fukumoto S, et al. A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone. 2011;49:913–916. doi: 10.1016/j.bone.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, et al. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–1633. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, et al. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res. 2009;19:1682–1690. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130:652–660. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trip MD, Smulders YM, Wegman JJ, Hu X, Boer JM, ten Brink JB, et al. Frequent mutation in the ABCC6 gene (R1141×) is associated with a strong increase in the prevalence of coronary artery disease. Circulation. 2002;106:773–775. doi: 10.1161/01.cir.0000028420.27813.c0. [DOI] [PubMed] [Google Scholar]
- Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Varadi A, Bercovitch L, Terry PF, Terry SF. Pseudoxanthoma elasticum: progress in research toward treatment: summary of the 2012 PXE International Research Meeting. J Invest Dermatol. 2013;133:1444–1449. doi: 10.1038/jid.2013.20. [DOI] [PubMed] [Google Scholar]
- van der Sluis IM, Boot AM, Vernooij M, Meradji M, Kroon AA. Idiopathic infantile arterial calcification: clinical presentation, therapy and long-term follow-up. Eur J Pediatr. 2006;165:590–593. doi: 10.1007/s00431-006-0146-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.