Abstract
The kynurenine pathway (KP) is the main route of tryptophan degradation whose final product is NAD+. The metabolism of tryptophan can be altered in ageing and with neurodegenerative process, leading to decreased biosynthesis of nicotinamide. This fact is very relevant considering that tryptophan is the major source of body stores of the nicotinamide-containing NAD+ coenzymes, which is involved in almost all the bioenergetic and biosynthetic metabolism. Recently, it has been proposed that endogenous tryptophan and its metabolites can interact and/or produce reactive oxygen species in tissues and cells. This subject is of great importance due to the fact that oxidative stress, alterations in KP metabolites, energetic deficit, cell death, and inflammatory events may converge each other to enter into a feedback cycle where each one depends on the other to exert synergistic actions among them. It is worth mentioning that all these factors have been described in aging and in neurodegenerative processes; however, has so far no one established any direct link between alterations in KP and these factors. In this review, we describe each kynurenine remarking their redox properties, their effects in experimental models, their alterations in the aging process.
1. Kynurenine Pathway
The main route of catabolic tryptophan degradation is through kynurenine pathway (KP) which leads to production of nicotinamide adenine dinucleotide (NAD+; Figure 1) [1]. This pathway takes place mainly in the liver, kidney, and brain of humans, primates, rodents, and other small mammals [2]. It is noteworthy that humans and animals do not possess the enzymatic machinery to synthesize tryptophan by themselves, the reason why they get tryptophan from the diet. The KP is particularly modulated by the regulatory mechanisms of the immune response and by the redox status. The metabolites most widely studied are kynurenic acid (KYNA) and quinolinic acid (QUIN) due to their neuroactive capacities, while indoleamine dioxygenase-1 (IDO-1), 3-hydroxykynurenine (3-HK), and 3-hydroxyanthranilic acid (3-HA) are studied mostly due to their redox properties and modulation.
Figure 1.

Kynurenine pathway.
The first step of the KP involves the oxidative opening of the tryptophan indole ring by tryptophan 2,3-dioxygenase (TDO; in the liver) or by indoleamine 2,3-dioxygenase-I and -II (IDO-1 and IDO-2, resp., in the brain) to produce the instable metabolite, N-formylkynurenine [3–5]. The next step is the conversion of N-formylkynurenine to L-kynurenine (L-KYN), a metabolite that will serve as substrate for various enzymes: kynureninase which produces anthranilic acid (ANA), kynurenine aminotransferases (KAT I, II, and III), that catalyze the irreversible transamination from L-KYN to kynurenic acid (KYNA), and kynurenine 3-monooxygenase (KMO) that catalyzes the synthesis of 3-hydroxykynurenine (3-HK). Then 3-HK can be taken by kynurenine aminotransferase (KAT) to produce xanthurenic acid (XA) or by the kynureninase to form 3-hydroxyanthranilic acid (3-HA), which can also be produced by ANA through anthranilate 3-monooxygenase.
3-Hydroxyanthranilate dioxygenase (3-HAO) opens the ring of 3-HA to produce 2-amino-3-carboxymuconate semialdehyde, an unstable intermediate which is immediately transformed into QUIN. Finally, quinolinate phosphoribosyltransferase (QPRT) produces NAD+ from QUIN [6].
2. Enzymes Modulated by Redox Status
The flux through the KP in brain is rate limited by IDO, a cytoplasmic enzyme that converts tryptophan to the catabolism products collectively known as kynurenines [7]. IDO is a heme enzyme found in the central nervous system (CNS) which has high affinity for L-tryptophan (Km ~ 0.02 mM) and requires oxygen [8, 9] for its activity. However, IDO-1 kinetically prefers superoxides instead of oxygen [10] and can use them both as substrate and as cofactor. In fact, one of the suggested roles for IDO-1 is that it can act as scavenger of superoxide (Table 1) [11]. This function is due to the ability of superoxide to reduce inactive ferric IDO-1 to the active ferrous form [12]; then takes place the oxidation of the pyrrole ring of tryptophan to form N-formylkynurenine. IDO-1 becomes more active with increasing oxygen concentrations and, in vivo, KYN is 60% higher in brains of HBO-convulsed rats compared with rats breathing air. The intracellular reducing co-factor(s) of IDO-1 include(s) superoxide anion, dihydroflavin mononucleotide, tetrahydrobiopterin, and cytochrome reductases [12, 13]. IDO-1 can be directly activated by a number of cytokines, including IFN-γ and TNF-α. This dioxygenase is present in accessory immune cells, including macrophages and dendritic cells, and it is expressed in all organs including brain [14, 15]. Hydrogen peroxide and oxide nitric are inhibitors of IDO-1 [12, 16]. Inhibition of IDO-1 by a competitive or a noncompetitive inhibitor resulted in a dose-dependent decrease in its activity which correlated directly with the decreasing intracellular NAD+, which causes decreased cell viability and CNS functions [17].
Table 1.
Kynurenine pathway enzymes and their positive and negative regulators.
| Enzyme | Reaction catalyzed | Positive regulators | Negative regulators |
|---|---|---|---|
| Tryptophan 2,3-dioxygenase | L-Trp + O2/O2 ∙− → N-formyl-L-kynurenine |
Melatonin, H2O2 [215]. O2 ∙− [216]. |
3-HK, KYNA, XA, NADH [217]. Cu2+ [218]. Superoxide dismutase (SOD) [216]. |
|
| |||
| Indolamine 2,3-dioxygenase | L-TRYP + O2/O2 ∙− → N-formyl-L-kynurenine | O2
∙−
IFN-α/β/γ, lipopolysaccharide, hiperoxia [12, 219]. |
SOD [220]. NO [221]. H2O2, IL-4 [12]. |
|
| |||
| Formamidase | N-formyl-L-kynurenine + H2O → formate + L-KYN | H2O, ascorbic acid, arginine, L-TRYP [222]. | ANA [223]. 3-HK, Mn2+ [222]. |
|
| |||
| Kynureninase | L-KYN + H2O → ANA + L-alanine | H2O, 3-HK [224]. | |
|
| |||
| Kynurenine aminotransferases | L-KYN + 2-oxoglutarate/pyruvate → KYNA + L-glutamate | 2-Oxoglutarato, pyruvate, 2-aminoadipate, pyridoxal 5′-phosphate [225, 226]. | Glutamine, L-cysteine, 3-HK, L-phenylalanine, L-tryptophan, L-aspartate [191, 227–229]. |
|
| |||
| Kynurenine 3-monooxygenase | L-KYN + NADPH + O2 → 3-HK | NADPH, O2, FAD, NADH, inflammatory stimulus [27, 230, 231]. | ANA, XA, Cl−, pyridoxal 5′-phosphate [28, 232]. |
|
| |||
| 3-Hydroxyanthranilic acid 3,4-dioxygenase | 3-HA + O2 → 2-amino-3-carboxymuconate-6-semialdehyde | O2, Fe2+ [233]. | Zn2+ [233]. |
|
| |||
| 2-Amino-3-carboxymuconate-6-semialdehyde decarboxylase |
2-amino-3-carboxymuconate -6-semialdehyde → 2-aminomuconic-6-semialdehyde + CO2 |
KYNA, PIC, QUIN [234]. | Zn2+, Fe2+ [234, 235]. |
|
| |||
| Quinolinic acid phosphoribosyltransferase | QUIN + 5-phospho-α-D-ribose 1-diphosphate → NAD+ + diphosphate + CO2 | Mg2+ [236, 237]. | ATP, Cu2+ Fe2+, Fe3+, Zn2+ [238]. |
Another enzyme that participates in tryptophan (Trp) degradation through the kynurenine pathway is IDO-2 enzyme that is encoded by a homologous gene of IDO-1 [18, 19]. In humans, IDO-2 is expressed in placenta, brain, liver, small intestine, spleen, thymus, lung, kidney, and colon [19]. It seems that IDO-2 has lower activity than IDO-1 [18, 19] and its participation in L- Trp oxidation remains unclear since it has been shown, in some studies, that there is no detectable kynurenine formation in vivo associated with IDO-2. However, it has been related to an increase in KYN levels and IDO-2 expression, but not with IDO-1, in human carcinoma cells treated with the chemokine CXCL11 [20]. Additionally, it was described that IDO-2 showed lower Km than IDO-1 in different species (mouse: Km ≈ 29 μM and 12 mM for IDO-1 and IDO-2, resp.) and both enzymes also differ in other biochemical properties such as pH and thermal stability [21]. Thus, although that has not been found a specific physiological role for this enzyme, it is apparently quite different to IDO-1. This evidence suggests that IDO-2 is active under specific conditions; therefore it depends on the presence of specific factors and the cell type [22].
KMO is another important enzyme; it is a NADPH-dependent flavin monooxygenase. This monooxygenase is localized in the outer mitochondrial membrane in the CNS and is predominantly expressed in microglia [23–26]. KMO exists as an apoenzyme and interacting with flavin-adenine dinucleotide (FAD) forms a holoenzyme; the flavin moiety of the protein acts as an electron donor [27]. The specific function of KMO is catalyzing the incorporation of one atom of oxygen into kynurenine, in the presence of NADPH as electron donor. During the reaction, the prosthetic group FAD is reduced to FADH2 by NADPH and subsequently oxidized by oxygen to FAD. Further kinetic studies have demonstrated that the enzyme activity could be inhibited by pyridoxal phosphate and Cl− (Table 1) [28]. The relevance of KMO activity, in both physiological and pathological conditions, is that this enzyme possesses a high affinity for the substrate (Km is in the low micromolar range), thus suggesting that it metabolizes most of the available kynurenine to produce 3-HK [29]. Notably, it has been reported that KMO expression increases in inflammatory conditions or after immune stimulation [30]. Due to the alterations in the KP metabolites in various pathologies, the enzymes of this pathway represent significant targets for therapeutic intervention and KMO is one of the main enzymes studied.
Kynureninase is a pyridoxal phosphate-dependent enzyme, which is mainly located in the cytosol and catalyses the transformation of KYN into ANA as well as of 3-HK to 3-HA. It exhibits a 10-fold higher affinity for 3-HK than for KYN. The optimum pH of the enzyme is 8.25 and it displays a strong dependence on the buffer ionic strength for optimum activity [31]. Mn2+ ions activate kynureninase only in the presence of added pyridoxal phosphate, whereas Ca2+ ions activate it in presence and absence of added pyridoxal phosphate (Table 1) [32].
The enzyme that catalyzes the final aromatic ring opening reaction in the KP is the 3-HAO. In this enzymatic reaction 3-HA produces an unstable compound, α-amino-β-carboxymuconic ε-semialdehyde, which is then nonenzymatically transformed to QUIN. 3-HAO is present in small amounts, in mammalian brains [33], mainly in astrocytes surrounding glutamatergic synapses in the CNS [34]. For its activity, 3-HAO requires both nonheme Fe2+ to incorporate atoms of molecular oxygen into 3-HA and sulfhydryl groups [35, 36]. Recently, it was demonstrated that Fe2+ stimulates 4- to 6-fold 3-HAO activity, in striatal homogenates of mouse, rat, and human; this effect is prevented by ferritin [37].
On the other hand, QPRT has been identified in rat and human CNS [38]. Magnesium ions are required for QPRT activity and there is evidence that a cysteine residue at the active site is required for catalysis [39]. Interestingly, QPRT is in a P2 synaptosomal fraction particulate component [40]. This enzyme is particularly important since it catalyzes the conversion of QUIN to NAD+; changes in the amount of QPRT protein alter the intracellular ratio between NADH/NAD+ and ATP; in consequence, QUIN is accumulated, promoting the excitotoxic damage.
The kynurenines aminotransferases (KATs) are key in the KP since they produce the only endogenous antagonist of NMDA receptor, KYNA. In mammalian peripheral organs, several rather unspecific pyridoxal-5′phosphate-dependent aminotransferases are able to catalyze the conversion of KYN to KYNA [41–44]. However, in the brain of humans, rats, and mice, four proteins (KAT I, II, III, and IV) seem to be responsible for KYNA production [35, 44–49], of which KAT I and KAT II are the most studied. KAT I prefers pyruvate as co-substrate [50] and it is strongly inhibited by the competing substrates such as tryptophan, phenylalanine and glutamine. Immunohistochemical studies in rat brain have demonstrated that this enzyme is located preferentially in astrocytes. KAT II has a slight preference for oxoglutarate as a cosubstrate and also displays L-aminoadipate aminotransferase activity. This enzyme is inhibited by α-aminoadipate and quisqualate. 3-HK inhibits both KAT I and KAT III activity but is more active against KAT II [44]. Currently, there are different crystallographic structures of KATs deposited in the Protein Data Bank (PDB), which allows us to give a structural interpretation into catalysis and inhibition mechanism of these enzymes.
3. Metabolites with Redox and Neuroactive Properties
3.1. Tryptophan
Trp is an essential amino acid, and its structure contains a ring that can stabilize radicals through resonance or delocalization, thus enabling it to break radical chain reactions [51]. Trp is able to react with hydroxyl radicals and to trap tert-butoxyl radical (CH3)3CO•, with rate constant values of κ = 1010 M/s and 2.8 × 109 M/s, respectively [52]. Analyses performed with other indolic structures have shown that ONOO− reacts preferentially with 3 substituted indoles such as Trp derivatives rather than with unsubstituted indoles; and the most important products observed at physiological pH are 1-nitrosotryptophan derivatives kynurenines/kynuramines obtained by opening of the pyrrole ring [53]. Moreover, the administration of Trp decreased the lipid peroxidation induced in rats under experimental endotoxic shock, suggesting antioxidant properties of this amino acid [54]. This finding is consistent with the report of Pazos and coworkers [55], who showed that Trp is the amino acid with the highest antiradical activity. In addition, tryptophan turned out to be a potent scavenger of radicals induced by chloramine T or hydrogen peroxide, which was detected by a chemiluminescence assay [56].
3.2. Kynurenine
A central compound of the KP is KYN, given that it is a substrate for different enzymes to produce KYNA, 3-HK, or ANA. Some reports have shown a protective effect of KYN in toxic experimental models. However, this effect has been attributed mainly to the production of KYNA, which has an antagonist effect on both NMDA and α7-nicotinic receptors. Nevertheless, KYN per se has scavenging properties that should be considered to explain the effects of this metabolite in the toxic models in which has been tested.
Zsizsik and Hardeland observed KYNA formation from KYN in light-exposed homogenates of the dinoflagellate Lingulodinium polyedrum, which was under a prooxidant environment induced by paraquat and CCCP, suggesting that oxidative kynurenine deamination leads to KYNA production; furthermore, in this process KYN could be acting as an antioxidant [57]. This finding correlates with the fact that L-KYN reduces the chemiluminescence induced by hydrogen peroxide or chloramine T [56] and also with its ability to trap hydroxyl radical (K r 1.4 × 1010 M−1s−1; determined by EPR-spin trapping and pulse radiolysis method) Table 2 [58, 59]. Recently, it has been showed that L-KYN was able to abolish ROS production induced by 3-nitropropionic acid and ONOO−; this effect was independent of KYNA formation since the samples were obtained from brain homogenates of KAT II knockout mice (which lack the major enzyme for the biosynthesis of KYNA) [60]. Altogether, this evidence strongly suggests that KYN can be considered as a potential endogenous antioxidant, which can donate an electron and protect macromolecules in vivo and in vitro against oxidative modifications [53, 61]. These properties can be independent of the KYNA formation.
Table 2.
Kynurenines with redox behavior.

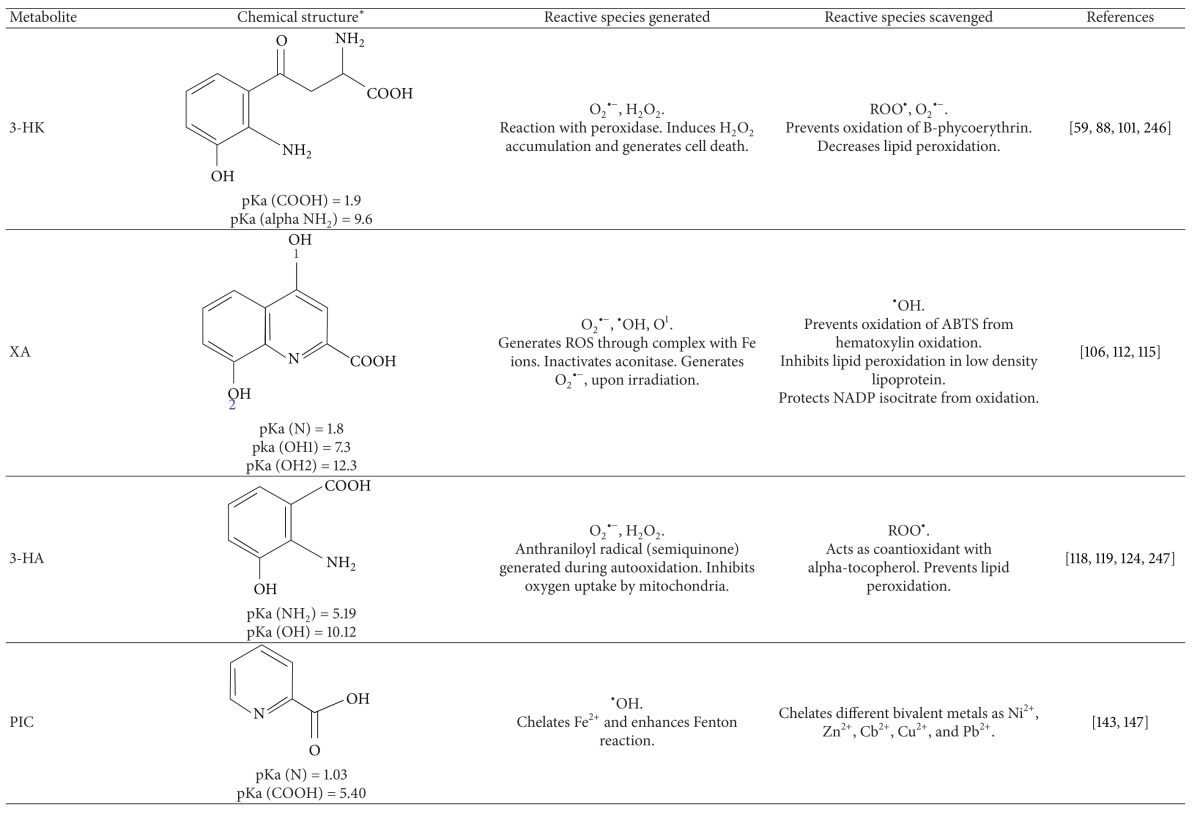

|
*The chemical structures were built with the program ACD/ChemSketch Freeware (http://www.acdlabs.com/resources/freeware/chemsketch/).
However, KYN has also shown prooxidant effects. It has been described that aerobic irradiation of KYN produces superoxide radicals and leads to reduction of cytochrome c [62, 63]. Additionally, in vitro studies show that KYN is able to photooxidize cysteine, NADH, and ascorbic acid and this capacity may be directly relevant to photobiological processes occurring in the lens in vivo. In particular, these photooxidation processes can be responsible for the age-related depletion of reduced glutathione and/or formation of hydrogen peroxide in lens [64]. On the other hand, KYN can also cause cell death through ROS pathway in NK cells [65].
3.3. Kynurenic Acid
The major KP metabolite considered as neuroinhibitor is KYNA, which is synthesized and released by astrocytes and antagonizes NMDAr [66] and α7-nicotinic acetylcholine receptor (α7nAChR) [67]. KYNA synthesis is mediated by KATs. Studies in rodents have shown that modest increases or reductions in KYNA levels decrease or facilitate extracellular dopamine and glutamate release, respectively [68–72]. Accordingly, dysregulation of endogenous KYNA may contribute to the physiopathology of several disorders [73–75].
Recently, KYNA was identified as an endogenous ligand of GPR35 [76]. This fact highlighted the importance of KP in regulating immune functions because the activation of GPR35 inhibits TNF-α release by macrophages under inflammatory conditions induced by LPS. Upon this context, KYNA might exert an anti-inflammatory effect [76]. Additionally, GPR35 decreases intracellular Ca2+ probably by inhibiting its entrance [77]; thus, KYNA most likely exerts an effect on the release of inflammatory mediators and excitatory amino acids from glial cells. The ligand-activated transcription factor aryl hydrocarbon (AHR), a nuclear protein involved in the regulation of gene transcription, is also activated by KYNA and is able to cause immunosuppression [78].
On the other hand, various groups have studied the redox properties of KYNA. This kynurenine is a reducing agent that might even be able to act as (electron transfer) redox catalyst in vivo. KYNA has been shown to scavenge hydroxyl radicals; it is able to prevent radicals-induced malondialdehyde formation from 2-deoxyribose [79–82]. However, KYNA can behave, under certain circumstances, as a prooxidant since it has been shown to have a strong potentiation of the prooxidants properties of δ-aminolevulinic acid [83]. The putative mechanism by which KYNA scavenges free radicals was proposed by Zsizsik and Hardeland [82]; the reaction is initiated by the hydroxyl radical, and then a decarboxylation should be the favored process. The resulting decarboxylated cation radical interacts with another hydroxyl radical, and the next intermediate interacts with superoxide, leading to the nitric oxide release. The resulting 2-hydroxychromanone then may be in equilibrium with its tautomer, 2,4-dihydrochromene. The balance of the radical scavenging is that three radicals would be scavenged (two of OH• and one of superoxide) and one would be formed (•NO) [82]. Additionally, another study showed that KYNA can also scavenge peroxynitrite. It also can prevent the lipid peroxidation and ROS production in rat forebrain homogenates and in Xenopus laevis oocytes (preparation free of NMDA receptors) induced by FeSO4, suggesting that the protective effect of KYNA is independent of its activity over receptors. An in vivo study also showed that KYNA decreases the hydroxyl radical formation induced by the acute infusion of FeSO4 in the rat striatum [84]. Furthermore, it has been shown that KYNA significantly increased oxygen consumption during state IV respiration leading to an impaired respiratory control index and ADP/oxygen ratio [85, 86].
All these evidences show that KYNA is an important neuromodulator but also is an endogenous antioxidant and its protective effect showed in divers toxic models may be due to its redox character in addition to its activity on receptors.
3.4. 3-Hydroxykynurenine (3-HK)
3-HK is a controversial kynurenine since it has shown prooxidants and antioxidants activities. The structure-toxicity relationship shows that the o-aminophenol structure common to 3-HK is required to exert its toxicity. o-Aminophenol compounds are considered to be subject to several steps of oxidation reactions initiated by their oxidative conversion to quinoneimines, which is accompanied by concomitant production of ROS, generating mostly superoxide anion and H2O2 (Table 2) [87].
The neurotoxicity of 3-HK in primary neuronal cultures prepared from rat striatum is blocked by catalase and desferrioxamine but not by superoxide dismutase, indicating that the generation of H2O2 is involved in the toxicity. The protective effect of desferrioxamine suggests a role for iron in 3-HK toxicity, either in catalyzing the oxidation of 3-HK or in promoting the reduction of H2O2 to the highly reactive hydroxyl radical. Additionally, it has been proposed that the ROS generation by low concentration of 3-HK (1–10 μM) occurs intracellularly and depends on the 3-HK uptake activity which is variable in the different brain regions [88]. This is one of the possible mechanisms by which 3-HK induces cell death [89]. It has been showed that the endogenous xanthine oxidase activity is involved in the H2O2 generation produced by 3-HK and also exacerbates cell damage generated by this kynurenine. However, the precise mechanism by which this enzyme is acting in this process is not clear [89]. 3-HK, besides being considered as cytotoxic for neuronal cells [90], has also been shown to cause bladder cancer [91]. Moreover, 3-HK modifies the respiratory parameters, decreasing respiratory control index as well as ADP/oxygen ratio of glutamate/malate-respiring heart mitochondria [87].
On the other hand, it has been demonstrated that 3-HK and 3-HA reduce Cu(II) and both generate superoxide and H2O2 in a Cu-dependent manner [92]. The incubation of bovine α-crystallins with low concentrations of 3-HK causes protein cross-linking and oxidation of methionine and tryptophan residues [93], indicating that the protein damage likely results from generation of reactive oxygen species. In the human lens, these reactions have been associated with both aging [94] and cataractous processes [95]. Also, it was shown that 3-HK and 3-HA provoke protein oxidative damage and induce apoptosis characterized by chromatin condensation and internucleosomal DNA cleavage in PC12, GT1-7, and SK-N-SH cells [92, 96–98]. In vivo experiments have demonstrated that injection of 3-HK into the striatum causes tissue damage that is prevented by N-acetyl-L-cysteine coapplication [99].
Conversely, 3-HK has also been proposed to be an antioxidant, peroxy radicals scavenger in inflammatory diseases [100], and superoxide scavenger in the Malpighian tubes of insects [101]. Since 3-HK is an o-aminophenol, it might be expected to undergo complex oxidative processes. In fact, under severe oxidative stress induced via the hydrogen peroxide-horseradish peroxidase system, 3-HK forms hydroxanthommatin and xanthommatin, products of six- and eight-electron oxidations, respectively [87]. The initial stable product of autooxidation of 3-HK does react with O2 •− (lower limit for k is 5.6 × 106 M−1 s−1), and it is possible that this autooxidation product could be responsible for protection from the deleterious effects of O2 •− [59]. The amount of 3-HK is abundant in Malpighian tubes of insects and was reported to work as a major antioxidant in the tubes [101, 102].
Besides, 3-HK and 3-HA, like vitamin C and Trolox, belong to the class of small molecules that react very rapidly with peroxyl radicals and hence are potentially important biological antioxidants. In particular, 3-HK and 3-HA protected B-phycoerythrin from peroxyl radical-mediated oxidative damage more effectively than equimolar amounts of either ascorbate or Trolox [100]. 3-HK was more reactive with the ferryl complex than glutathione, suggesting that the antioxidative efficiency is better than glutathione. Additionally, the C6 glioma cells exposed to 3-HK increased its total antioxidant reactivity values and the TBA-RS levels were decreased without changing the morphology of the cells [103].
This redox behavior of 3-HK can be explained by Giles and coworkers, who propose that 3-HK can initially act as two-electron donors (antioxidant) but the ortho-quinone-imine formed oxidatively and the ROS produced in this process are responsible for its prooxidant effects [104]. Therefore the behavior of 3-HK depends on the redox status of the cell.
3.5. Xanthurenic Acid
Xanthurenic acid (XA), a metabolite of the KP is synthesized through 3-HK transamination, and it is closely related structurally to KYNA but possesses different biological roles; actually the biological function of XA remains obscure. Gobaille and coworkers proposed that XA can have a role in the neurotransmission/neuromodulation since it is actively taken up by synaptic vesicles from rat brain, effect that is inhibited in absence of ATP [105].
Some groups have focused on the study of the redox properties of this metabolite, which have showed metal-chelating activities and antioxidant properties [106, 107]. Zsizsik and Hardeland showed that XA turned out to be an efficient scavenger of hydroxyl radicals and ABTS•+ produced in the ABTS system. XA was able to inhibit the lipid peroxidation induced by iron and copper oxidation in low density lipoprotein, and this metabolite also prevents the inactivation of NADP-isocitrate dehydrogenase produced by the oxidation of these metals [106]. XA scavenges superoxide in a hematoxylin autooxidation system [108]. XA has also been shown to act as a peroxyl radical scavenger in vitro [100]. A recent study evaluated the antioxidant action of XA using heme and iron as promoters of radical formation. In this model, XA proved to be a powerful antioxidant, inhibiting lipid peroxidation in a pH-dependent manner [109]. The antioxidant properties of XA could be related to the fact that all phenolic metabolites show antioxidant activities points toward the importance of the phenolic moiety as the active entity [100].
On the contrary, XA sometimes acts as a prooxidant due to its chelating effect. Recent studies revealed that XA binds ferric ion at the 8-hydroxyl group and the nitrogen atom of the quinoline moiety, resulting in the enhancement of the autooxidation of ferrous ion to ferric ion [110]. The formation of metal-chelate complex modifying the oxidation-reduction potential of metal ion is responsible for the generation of reactive oxygen species (ROS) [111]. Oxygen molecules accept one electron from ferrous ion to form superoxide radical, which can also produce another ROS. Once that XA forms the metal complex, inactivates aconitase through ROS generation mainly hydroxyl radical [112]. Furthermore, XA was demonstrated to act as an apoptosis-inducing metabolite in vascular smooth muscle and lens epithelial cells [113, 114]. Additionally, XA acts as a photosensitizer and generates superoxide and singlet oxygen upon irradiation [115]. The photooxidation and polymerization by XA of lens proteins are related to the age-dependent cataractogenesis [116, 117]. All these studies suggest that the cytotoxic action of XA may be explained by the prooxidant properties of chelate complexes with metals.
3.6. 3-Hydroxyanthranilic Acid
Many studies considered 3-HA as free radicals generator [28, 29] because in its autooxidation it is able to generate free radicals. This autooxidation of 3-HA involves first, the generation of semiquinoneimine (anthraniloyl radical) which oxidizes to the quinoneimine, followed by condensation and oxidation reactions to yield a cinnabarinic acid. 3-HA auto-oxidation to cinnabarinate requires molecular oxygen and generates superoxide radicals and H2O2. Superoxide dismutase (SOD) accelerates 3-HA auto-oxidation, probably by preventing back reactions between superoxide and either the anthraniloyl radical or the quinoneimine formed during the initial step of auto-oxidation. Mn2+, Mn3+, and Fe3+-EDTA catalyze cinnabarinate formation under aerobic conditions [118].
In experimental models, the pattern of 3-HA in mitochondrial processes involves the inhibition of oxygen uptake by mitochondrial respiring with NAD-dependent substrates, uncoupling the respiratory chain and the oxidative phosphorylation [87, 119]. A marked inhibition (79%) of oxygen uptake by 1 mM 3-HA was observed in an oxoglutarate-respiring rat liver or rat heart mitochondria [119]. Furthermore, it has been shown that 3-HA induces apoptosis in monocyte/macrophage cell lines, and the apoptosis response was enhanced by ferrous or manganese ions, according to a mechanism that presumably involves production of hydrogen peroxide, since the effect was attenuated by catalase [120]. Fallarino and coworkers [121] showed that both 3-HA and QUIN can induce apoptosis of thymocytes of terminally differentiated T helper cells, in particular, Th1 but not Th2 clones, through Fas-independent mechanisms involving the activation of caspase-8 and the release of cytochrome c from mitochondria. It has also been suggested that 3-HA inhibits NF-κB activation upon T cell antigen receptor engagement by specifically targeting PDK1 [122]. Additionally, it was demonstrated that 3-HA induced depletion of intracellular glutathione in activated T cells without increased ROS formation [123].
On the contrary, there are also reports that show that 3-HA is a potent antioxidant [124] and downregulates the inducible nitric oxide synthase expression [125, 126] by enhancing OH-1 expression in macrophages stimulated with IFN-γ and lipopolysaccharide, thereby resulting in a further increase in IDO expression and activity [127]. Additionally, 3-HA reduces α-tocopheroxyl radical restoring the levels of α-tocopherol and preventing LDL lipid peroxidation [124, 128].
Furthermore, 3-HA and 3-HK inhibited the spontaneous lipid peroxidation in the brain and this inhibitory property remained even in the presence of Fe3+, protecting cerebral cortex against oxidative stress [129]. The GSH spontaneous oxidation and the peroxyl radicals were significantly prevented by 3-HA [103].
Electrochemical studies suggest that 3-HA can initially act as antioxidant and next as a prooxidant [104] since the ortho-quinone-imine formed possesses oxidant properties. The most likely explanation for the dual effect in vitro of 3-HA is a concentration-dependent action.
3.7. Anthranilic Acid
Although ANA is generally accepted to be biologically inactive, it can interact with copper to form an anti-inflammatory complex. This complex acts as a hydroxyl radical-inactivating ligand able to remove the highly injurious hydroxyl radicals at inflammatory sites. However, the ANA-Cu2+ complex increases the Fenton reactivity of copper, producing more hydroxyl radicals, which are quickly removed by the same complex [130, 131]. Due to this property, the synthetic derivative of ANA, 3-methoxyanthranilate, has been proposed as a potential anti-inflammatory drug [132].
Nevertheless, in a study in vitro using organotypic cultures of rat hippocampus it was demonstrated that ANA (at high mM concentration) may cause neurodegeneration [133]. However, the mechanism of this finding has not been elicited yet, but it is known that alterations in the metabolite levels have been observed in some degenerative diseases [134]. Additionally, the anthranilate was found to have more pronounced effect on active than on resting rate of respiration. This metabolite (1.25–5 mM) has an effect, in a dose-dependent way, on the respiratory parameters: it dropped state III and respiratory control index using 5 mM glutamate/malate as respiratory substrates. On the other hand, no effect was seen in the presence of succinate or NADH as substrates [86, 135]. These contradictory effects found for ANA can be due to its capability to produce hydroxyl radicals to the 3-HA metabolite, considering that ANA is a substrate to produce it.
3.8. Picolinic Acid
Picolinic acid (PIC) is a six-member ring structure and isomer of nicotinic acid, containing five carbon atoms, a nitrogen, and a carboxyl group at position 2. The most widely studied physical characteristic of PIC is its efficient chelator properties; it was first described that this metabolite was an efficient chelating agent for both copper and iron. Later, Suzuki and coworkers described that PIC was also able to chelate other bivalent metals including Ni2+, Zn2+, Cd2+, Pb2+, and Cu2+ [136]. Therefore, picolinate is an unselective metal ion chelator [137] and also activates macrophages via induction of macrophage inhibitory protein- (MIP-) 1α and MIP-1β, which is potentiated by simultaneous IFN-γ treatment [138]. It also possesses both extracellular and intramacrophage antimicrobial activity against Mycobacterium avium [139] and Candida albicans [140] and antiviral/apoptotic activity against HVI-1 and Herpes simplex virus-2-infected cells [141]. Additionally, PIC is able to induce synergistically with IFN-γ, the expression of nitric oxide synthase in macrophages [142].
Moreover, PIC also has been shown to protect the cholinergic neurons of the nucleus basalis magnocellularis and the nicotinamide adenine dinucleotide diaphorase containing neurons of rat striatum against QUIN-induced neurotoxicity [143, 144]. This protection can be related to the fact that PIC significantly decreases glutamic acid release, evoked by exposure of striatal slices to 1 mM kainate in the presence of calcium. In the absence of external calcium, PIC (100 μM) failed to influence kainic acid-induced release [145]. Additionally, it has been proposed that PIC may act as a glycine agonist at strychnine-sensitive receptors since it was able to reduce the inhibition of firing by glycine in these receptors [146].
However, α-PIC chelates Fe2+ ions and enhances the hydroxyl radical formation. This effect is attributed to its structure; the two adjacent atoms in the 2-pyridinecarboxylic acid moiety, that is, the nitrogen atom in pyridine ring and the oxygen atom in the carboxyl group, seem to be participating in the chelation of Fe2+ ion [147]. Some reports also show the toxic effect of this metabolite since its systemic administration produces alterations in neuronal cell bodies. These alterations developed within selected regions of the brain, as was demonstrated within the hippocampus, substantia nigra, and striatum [148]. Additionally, results indicate that PIC alters cell shape by changing the pattern of distribution of cytoskeletal elements in culture normal rat kidney (NRK) and SV40-transformated NRK cells [149]. All these toxic effects may be related to the hydroxyl radical produced by PIC.
3.9. Quinolinic Acid
Quinolinic acid (QUIN), a neuroactive metabolite of the kynurenine pathway, is an agonist of N-methyl-D-aspartate (NMDA) receptor; it has a high in vivo potency as an excitotoxin [150]. Free radical generation and oxidative stress are involved in the toxicity induced by QUIN; however it is necessary to have in mind that these mechanisms can be or not dependent of QUIN activity on NMDA receptors. The ROS NMDA receptor-dependent production is promoted by Ca2+ entry, which induces the NOS activity and decreases the SOD activity, leading to excess of nitric oxide and superoxide. The interaction between these radicals quickly produces peroxynitrite [151, 152]. Additionally, it has been shown that QUIN can reduce glutathione as well as copper and zinc-dependent superoxide dismutase activity (Cu, Zn-SOD) [153] and induce ROS production, lipid peroxidation, and cell death [154, 155]. Other toxic effects of QUIN through NMDA receptors have been observed like inflammatory events, energetic deficit, behavioral and morphological alterations [150, 156, 157]. It has been shown that depending QUIN levels it can change its activity and toxicity. Several authors have demonstrated the QUIN participation in apoptosis of different cells like oligodendrocytes, neurons, and astrocytes via NMDA-dependent ROS formation. Braidy and coworkers observed that QUIN can act as a substrate for NAD+ synthesis at very low concentrations (<50 nM) but can also be a cytotoxic agent at subphysiological concentrations (>150 nM) through the NMDA overactivation, NOS induction, and nitric oxide increase conducing to free-radical damage in astrocytes and neurons. Also, the increased PARP activity leads to NAD+ depletion and consequently to cell death [158, 159].
Additionally, Stipek and coworkers (1997) showed that QUIN is able to form complexes with Fe2+ and modulate the lipid peroxidation [160]. In phosphate buffer, the QUIN-Fe2+ complex enhances the formation of hydroxyl radical via the Fenton reaction, compared to Fe2+ ions alone, and also inhibits the auto-oxidation of Fe2+ [161]. Further investigation has suggested that the QUIN-Fe2+ complex is relatively stable at physiological pH, and although this initiates the generation of hydroxyl radicals, a further QUIN derivative is formed, which enables redox cycling of Fe2+ and Fe3+ ions, thus maintaining hydroxyl radical formation [162]. The QUIN-Fe2+ complex was shown to be also responsible for in vitro DNA chain breakage and lipid peroxidation mediated by hydroxyl radicals [79]. Therefore, the generation of reactive oxygen species by QUIN is secondary to the formation and slow pH-dependent autooxidation of QUIN-Fe2+ complexes and can be readily prevented by iron chelation [162, 163]. All these evidences suggest that QUIN-Fe2+ complexes display significant prooxidant characteristics that could be of concern for QUIN neurotoxicity.
Different ROS scavengers, molecules with antioxidant properties, inducers of activity of antioxidant enzymes, and some pharmacological substances have been tested successfully against QUIN toxicity, showing protection of nervous tissue from oxidative damage induced by QUIN in vitro and in vivo [164–172].
4. Kynurenines Disturbances in Aging and Neurological Diseases
Alterations at the level of kynurenine pathway metabolites and enzymes have been observed in the aging (Table 3) [173, 174] and in several age-associated neuropathological conditions and diseases involving immune activation [175]. However, few studies have investigated changes in tryptophan metabolism with aging. Upregulation of tryptophan-KYN metabolism has been reported in older individuals (72–93 years of age) as compared with younger adults (34–60 years of age) [176]. A study concerned with the formation of UV filters in the human lens, which are formed from L-tryptophan through the KP, observed the highest levels of kynurenine in lenses (postmortem) from young people, below the age of 20 years, and lowest levels were detected in lenses of 80 years of age or older, suggesting that the protective effect of the metabolite against UV damage is reduced with the advancing of age [177]. In a study in rats was found a significant decrease in liver TDO activity with age [178], while another showed anomalous tryptophan catabolism, partly because of vitamin B6 and nicotinamide deficiency [179]. In this context, Braidy and coworkers [180] showed a significant decrease in TDO activity with age progression in the brain, liver, and kidney of female rats. Additionally, it was observed a significant increase in IDO brain activity with age, which is consistent with the observed that there is age-dependent increase of KYN in brain. This raising in available KYN is probably enough to explain the described age-dependent increase in KYNA, PIC, and QUIN. These observations may reflect adaptive changes related to the aging process in immune activity within the brain [178]. Under this perspective, aging is associated with the chronic, low grade, Th-1 type inflammation, in which IFN-γ, a potent proinflammatory cytokine and an inducer of IDO, is involved [181].
Table 3.
| Metabolite/enzyme | Brain | Liver | Kidney |
|---|---|---|---|
| TRP | ↓ | ↓ | ↓ |
|
| |||
| TDO | ↓ | ↓ | ↓ |
|
| |||
| IDO | ↑ | ↓ | ↓ |
|
| |||
| KYN | ↑ | ↓ at 12 months No changes at 24 months |
↓ at 12 months ↑ at 24 months |
|
| |||
| KATs | ↑ | No changes | ↑ |
|
| |||
| KYNA | ↑ | No changes | ↑ |
|
| |||
| KMO | ↓ | ↓ | |
|
| |||
| Kynureninase | ↓ | ↓ | |
|
| |||
| 3-Hydroxyanthranilate 3,4-dioxygenase | ↑ | ↑ | |
|
| |||
| Aminocarboxymuconate-semialdehyde decarboxylase (ACMSD) | ↑ | ↑ | |
|
| |||
| QPRT | ↓ | ↓ | ↑ |
|
| |||
| QUIN | ↑ | ↑ | ↓ at 12 months No changes at 24 months |
|
| |||
| PIC | ↑ | ↑ | ↓ at 12 months No changes at 24 months |
In another study related to enzymatic variations with age, IDO activity was measured. In the group of rats aged 2-3 months, the highest specific activity was observed in the small intestine and the lowest in the lungs and kidneys, whereas at 12 months of age the highest IDO activity was found in the brain, and kidneys presented the lowest activity. At 18 months, IDO returned to be more elevated in the small intestine. At 12 months old the values of IDO in tissues varied slightly, while at 18 months similar activities were found between lungs and brain and between the small intestine and kidneys. In relation to age, IDO specific activity declined in the small intestine, after 2-3 months of age [182].
Additionally, Moroni and coworkers [183] described a similar increase of KYNA levels in the aging rat brain. The brain concentration of KYNA was extremely low during the first week of life; then it increased at 3 months and a high raise was observed at 18 months of age, in accordance with the data of Finn and coworkers [184] and Gramsbergen and coworkers [185]. A positive relationship between CSF KYNA levels in humans and ageing has also been reported [186]. Elevated KYNA metabolism may be involved in the hypofunction of the glutamatergic and/or nicotinic cholinergic neurotransmission in the CNS of ageing humans [186]. Additionally, the increases of KYNA levels could underlie cognitive decline found in the aging.
Moreover, QPRTase activity in the brain is reduced with ageing, in parallel with an age-related increase in QUIN [180, 183]. An excessive accumulation of QUIN in brain tissue can induce a cytotoxic cascade within the CNS [187]. Increased QUIN content in the aging rat brain also suggests that the activity of the enzyme leading to the synthesis of QUIN (3-HAO) may also increase in the brain with advancing age [178].
These changes in metabolites and enzymes of KP are related to reports that show a decline in NAD+ levels and an increase in oxidative markers [188], suggesting a strong link between these factors in longevity which allow to propose KP as a therapeutic target to modulate free radicals and restore NAD+ levels.
On the other hand, neurodegenerative diseases are related to disturbances of the mitochondrial function, oxidative stress, and alterations in kynurenines levels [189, 190]. In this study we have described the redox activity of the kynurenines and how the KP can be modulated by the environment; however, their production in several pathologies can be more difficult to clarify since many factors converge and can change the cellular environment.
Alterations in the kynurenines metabolism can be due to alteration of energetic metabolism, oxidative damage, and inflammation, affecting the cellular function. Its relevance can be viewed under pathological conditions [86, 134, 135, 189, 191–194]. Table 4 summarizes changes in the KP metabolites found in different neuropathologies.
Table 4.
Alterations in kynurenines levels in neurodegenerative diseases.
| Disease | Metabolite | Sample | Reference |
|---|---|---|---|
| Alzheimer disease | (i) ↑ TRP/KYN ratio | Plasma | [249] |
| (ii) ↑ KYNA levels and KAT I activity | Putamen and caudate nucleus | [192] | |
| (iii) ↓ KYNA levels | CSF and plasma | [250, 251] | |
| (iv) ↑ 3-HK | Serum | [252] | |
|
| |||
| Huntington disease | (i) ↑KYNA and 3-HK levels | Neostriatum and cortex in early-stage HD patients | [253] |
| (ii) ↓ 3-HK and 3-HA | [254] | ||
|
| |||
| Parkinson disease | (i) ↓ KYNA | Frontal cortex, putamen, and SNpc of patients with PD, CFS |
[255, 256] |
| (ii) ↑ 3-HK | |||
|
| |||
| Schizophrenia | (i) ↓ KMO and 3-HAO | Prefrontal cortex | [257, 258] |
| (ii) ↑ L-KYN and KYNA | |||
|
| |||
| Depression | (i) ↓ TRP | Plasma | [259–262] |
| (ii) ↑ KYN | |||
| (iii) ↑ IDO | |||
5. Modulation of KP and Its Implications in the Intracellular NAD+ Levels
Recent studies have focused on the possible effects modulating the KP. The main strategies to follow are (1) tryptophan supplementation, (2) the use of inhibitors of the KP's enzymes, and (3) the use of analogues of KP metabolites, as KYNA. The first strategy has been widely studied considering that Trp, besides from being a precursor of kynurenines, is also of serotonin and melatonin. Trp supplementation has been used as a helpful therapy to treat behavior problems in animals since a low-protein diet supplemented with Trp helped in managing canine aggression problems [195]. Nevertheless the specific mechanism by which Trp acts in this way is still unclear but might be also due to the neuroactive metabolites of KP. In this context, Ciji et al. found increased serum cortisol levels and decreased serum testosterone and estradiol levels in fish exposed to nitrite; these effects were prevented by vitamin E and tryptophan diet; however, the benefits effect of vitamin E were due to its antioxidant characteristics, but the effect of Trp was unclear. Nonetheless, its protective effect may be not only the result of its own redox properties but also due to the metabolites with antioxidant properties produced by Trp degradation [196]. Moreover, a study showed that in healthy women under a rich diet in Trp increased the urinary excretion of KYN, ANA, KYNA, 3-HK, 3-HA, and QUIN [197], which under certain circumstances can be toxic. In fact, it has been shown that the excessive Trp supplementation would aggravate or would induce autoimmune diseases [198] due to the metabolites produced during its metabolism. The Trp supplementation can also affect intracellular NAD+ levels.
Braidy and coworkers have recently shown that 3-HA, 3-HK, and QUIN can promote NAD+ synthesis at concentrations below 100 nM in human primary astrocytes and neurons. However, these metabolites at concentrations >100 nM decreased intracellular NAD+ levels and increased extracellular LDH activity in both primary human astrocytes and neurons [158]. The vulnerability showed in human cerebral neurons may be due to the fact that the neurons can take up exogenous QUIN but can only catabolize a small amount [199] since QPRT is rapidly saturated. These events, depending on the kynurenines concentration, need to be taken into consideration since the biosynthesis of NAD+ is vital to the maintenance and ongoing cell viability of all of them.
The inhibition of IDO, KMO, and QPRT represents an important pharmacological target, since the kynurenines are involved in many neurodegenerative diseases. Several experimental models have been used to test some inhibitors of specific KP's enzymes. The inhibition of IDO and QPRT activities with 1-methyl-L-tryptophan and phthalic acid, respectively, resulted in reduction of intracellular NAD+ and cell viability, in both astrocytes and neurons; however, these effects are higher in neurons than astrocytes, suggesting that changes in KP metabolism have a greater effect on the neuronal population compared to glial cells. It is noteworthy that in a mouse model for multiple sclerosis the IDO inhibition aggravated the disease progression, denoting that IDO inhibition exacerbated the disease [200]. This could be related to the fact that IDO inhibition reduces NAD+ synthesis and therefore promoting cell death.
Following the same line, Blight and coworkers observed that treatment with 4-chloro-3-hydroxyanthranilate, a synthetic inhibitor of 3-hydroxyanthranilic acid oxidase, was able to reduce QUIN production and functional deficits following experimental spinal cord injury in guinea pigs [201]. More recently, it has been showed that the KMO inhibitor, 3,4-dimethoxy-N-[4-(3-nitrophenyl)-thiazol-2-yl]-benzenesulfonamide (Ro61-8048) [202] prevents ataxia and death in mice infected with the malaria parasite Plasmodium. This protection was associated with the elevated levels of KYNA and ANA [203]. Additionally, Campesan and coworkers showed the first evidence that inhibition of KMO and TDO activity protects against a transgenic Drosophila melanogaster model of Huntington disease [204, 205]. According to this subject, the oral administration of JM6, a novel prodrug inhibitor of KMO, avoided behavioral deficits and synaptic loss and raised KYNA levels in well-established genetic mouse models of Alzheimer [206]. Actually, whereas KMO inhibition leads to brain 3-HK and QUIN reduction, this may provide benefits in neurodegenerative diseases [203, 207–212]; the blockade of KAT II brings about a decrease in brain KYNA but can be related to cognition-enhancing effects [213, 214].
Further studies are necessary to explore whether prolonged manipulations of both KP metabolism arms have diverse consequences and which experimental models could be the best strategy because KYNA promotion can not only be an effective target just in some neurotoxic models, those that display a great excitotoxic damage, but can also promote NAD+ depletion and in a prolonged time could lead to cell death.
6. Concluding Remarks
In recent years, different groups and researchers have investigated the redox properties of KP metabolites; however, due to the dual effects of these metabolites and the high degree of modulation of the KP (inflammatory cytokines, metals, pH, and redox environment), is complex it try to establish a precise mechanism by which cellular alterations can be produced. What we do know is that these metabolites must have a physiological activity and a great impact on aging and especially in pathological conditions, processes in which are also altered factors that regulate the production of these kynurenines. The precise degree of involvement of these events constitutes a fertile line of research to explore in the next years.
Acknowledgment
This work was supported by CONACYT Grant no. 183867.
Conflict of Interests
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
References
- 1.Beadle GW, Mitchell HK, Nyc JF. Kynurenine as an intermediate in the formation of nicotinic acid from tryptophane by neurospora. Proceedings of the National Academy of Sciences of the United States of America. 1947;33(6):155–158. doi: 10.1073/pnas.33.6.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moroni F, Russi P, Carla V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neuroscience Letters. 1988;94(1-2):145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- 3.Hayaishi O, Rothberg S, Mehler AH, Saito Y. Studies on oxygenases; enzymatic formation of kynurenine from tryptophan. The Journal of Biological Chemistry. 1957;229(2):889–896. [PubMed] [Google Scholar]
- 4.Hayaishi O. Properties and function of indoleamine 2,3 dioxygenase. Journal of Biochemistry. 1976;79(4):13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [DOI] [PubMed] [Google Scholar]
- 5.Hirata F, Hayaishi O. Possible participation of superoxide anion in the intestinal tryptophan 2,3-dioxygenase reaction. The Journal of Biological Chemistry. 1971;246(24):7825–7826. [PubMed] [Google Scholar]
- 6.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacological Reviews. 1993;45(3):309–379. [PubMed] [Google Scholar]
- 7.Tone S, Takikawa O, Habara-Ohkubo A, Kadoya A, Yoshida R, Kido R. Primary structure of human indoleamine 2,3-dioxygenase deduced from the nucleotide sequence of its cDNA. Nucleic Acids Research. 1990;18(2, article 367) doi: 10.1093/nar/18.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito K, Heyes MP. Kynurenine pathway enzymes in brain: properties of enzymes and regulation of quinolinic acid synthesis. Advances in Experimental Medicine and Biology. 1996;398:485–492. [PubMed] [Google Scholar]
- 9.King NJC, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. International Journal of Biochemistry & Cell Biology. 2007;39(12):2167–2172. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Hayaishi O, Hirata F, Ohnishi T. Indoleamine 2,3 dioxygenase. Incorporation of 18O2- and 18O2 into the reaction products. The Journal of Biological Chemistry. 1977;252(10):3548–3550. [PubMed] [Google Scholar]
- 11.Taniguchi T, Hirata F, Hayaishi O. Intracellular utilization of superoxide anion by indoleamine 2,3 dioxygenase of rabbit enterocytes. The Journal of Biological Chemistry. 1977;252(8):2774–2776. [PubMed] [Google Scholar]
- 12.Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Report. 1999;4(5):199–220. doi: 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- 13.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase- initiated L-tryptophan metabolism. Biochemical and Biophysical Research Communications. 2005;338(1):12–19. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Wirleitner B, Neurauter G, Schröcksnadel K, Frick B, Fuchs D. Interferon-γ-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Current Medicinal Chemistry. 2003;10(16):1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- 15.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Reviews Immunology. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 16.Poljak A, Grant R, Austin CJD, et al. Inhibition of indoleamine 2,3 dioxygenase activity by H2O2 . Archives of Biochemistry and Biophysics. 2006;450(1):9–19. doi: 10.1016/j.abb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Grant R, Kapoor V. Inhibition of indoleamine 2,3-dioxygenase activity in IFN-γ stimulated astroglioma cells decreases intracellular NAD levels. Biochemical Pharmacology. 2003;66(6):1033–1036. doi: 10.1016/s0006-2952(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 18.Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Metz R, DuHadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Research. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 20.Lo BKK, Jalili RB, Zloty D, et al. CXCR3 ligands promote expression of functional indoleamine 2,3-dioxygenase in basal cell carcinoma keratinocytes. The British Journal of Dermatology. 2011;165(5):1030–1036. doi: 10.1111/j.1365-2133.2011.10489.x. [DOI] [PubMed] [Google Scholar]
- 21.Austin CJD, Mailu BM, Maghzal GJ, et al. Biochemical characteristics and inhibitor selectivity of mouse indoleamine 2,3-dioxygenase-2. Amino Acids. 2010;39(2):565–578. doi: 10.1007/s00726-010-0475-9. [DOI] [PubMed] [Google Scholar]
- 22.Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2, 3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45(6):1319–1329. doi: 10.1007/s00726-013-1602-1. [DOI] [PubMed] [Google Scholar]
- 23.Schwarcz R, Guidetti P, Sathyasaikumar KV, Muchowski PJ. Of mice, rats and men: revisiting the quinolinic acid hypothesis of Huntington’s disease. Progress in Neurobiology. 2010;90(2):230–245. doi: 10.1016/j.pneurobio.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. Journal of Neurochemistry. 2001;78(4):842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 25.Giorgini F, Möller T, Kwan W, et al. Histone deacetylase inhibition modulates kynurenine pathway activation in yeast, microglia, and mice expressing a mutant huntingtin fragment. The Journal of Biological Chemistry. 2008;283(12):7390–7400. doi: 10.1074/jbc.M708192200. [DOI] [PubMed] [Google Scholar]
- 26.Thevandavakkam MA, Schwarcz R, Muchowski PJ, Giorgini F. Targeting kynurenine 3-monooxygenase (kmo): implications for therapy in Huntington’s disease. CNS & Neurological Disorders. 2010;9(6):791–800. doi: 10.2174/187152710793237430. [DOI] [PubMed] [Google Scholar]
- 27.Nisimoto Y, Takeuchi F, Shibata Y. Molecular properties of L kynurenine 3 hydroxylase from rat liver mitochondria. Journal of Biochemistry. 1977;81(5):1413–1425. [PubMed] [Google Scholar]
- 28.Breton J, Avanzi N, Magagnin S, et al. Functional characterization and mechanism of action of recombinant human kynurenine 3-hydroxylase. European Journal of Biochemistry. 2000;267(4):1092–1099. doi: 10.1046/j.1432-1327.2000.01104.x. [DOI] [PubMed] [Google Scholar]
- 29.Bender DA, McCreanor GM. The preferred route of kynurenine metabolism in the rat. Biochimica et Biophysica Acta. 1982;717(1):56–60. doi: 10.1016/0304-4165(82)90379-8. [DOI] [PubMed] [Google Scholar]
- 30.Saito K, Chen CY, Masana M, Crowley JS, Markey SP, Heyes MP. 4-chloro-3-hydroxyanthranilate, 6-chlorotryptophan and norharmane attenuate quinolinic acid formation by interferon-γ-stimulated monocytes (THP-1 cells) The Biochemical Journal. 1993;291(1):11–14. doi: 10.1042/bj2910011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh HA, Botting NP. Purification and biochemical characterization of some of the properties of recombinant human kynureninase. European Journal of Biochemistry. 2002;269(8):2069–2074. doi: 10.1046/j.1432-1033.2002.02854.x. [DOI] [PubMed] [Google Scholar]
- 32.Saran A. Properties and partial purification of kynureninase. The Biochemical Journal. 1958;70(2):182–188. doi: 10.1042/bj0700182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster AC, White RJ, Schwarcz R. Synthesis of quinolinic acid by 3-hydroxyanthranilic acid oxygenase in rat brain tissue in vitro. Journal of Neurochemistry. 1986;47(1):23–30. doi: 10.1111/j.1471-4159.1986.tb02826.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberts RC, McCarthy KE, Du F, Ottersen P, Okuno E, Schwarcz R. 3-hydroxyanthranilic acid oxygenase-containing astrocytic processes surround glutamate-containing axon terminals in the rat striatum. The Journal of Neuroscience. 1995;15(2):1150–1161. doi: 10.1523/JNEUROSCI.15-02-01150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. Journal of Pharmacology and Experimental Therapeutics. 2002;303(1):1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 36.Nandi D, Lightcap ES, Koo YK, Lu X, Quancard J, Silverman RB. Purification and inactivation of 3-hydroxyanthranilic acid 3,4-dioxygenase from beef liver. International Journal of Biochemistry & Cell Biology. 2003;35(7):1085–1097. doi: 10.1016/s1357-2725(02)00347-3. [DOI] [PubMed] [Google Scholar]
- 37.Stachowski EK, Schwarcz R. Regulation of quinolinic acid neosynthesis in mouse, rat and human brain by iron and iron chelators in vitro. Journal of Neural Transmission. 2012;119(2):123–131. doi: 10.1007/s00702-011-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster AC, Whetsell WO, Jr., Bird ED, Schwarcz R. Quinolinic acid phosphoribosyltransferase in human and rat brain: activity in Huntington’s disease and in quinolinate-lesioned rat striatum. Brain Research. 1985;336(2):207–214. doi: 10.1016/0006-8993(85)90647-x. [DOI] [PubMed] [Google Scholar]
- 39.Okuno E, White RJ, Schwarcz R. Quinolinic acid phosphoribosyltransferase: purification and partial characterization from human liver and brain. Journal of Biochemistry. 1988;103(6):1054–1059. doi: 10.1093/oxfordjournals.jbchem.a122379. [DOI] [PubMed] [Google Scholar]
- 40.McDaniel HG, Reddy WJ, Boshell BR. The mechanism of inhibition of phosphoenolpyruvate carboxylase by quinolinic acid. Biochimica et Biophysica Acta. 1972;276(2):543–550. doi: 10.1016/0005-2744(72)91015-7. [DOI] [PubMed] [Google Scholar]
- 41.Noguchi T, Nakamura J, Kido R. Kynurenine pyruvate transaminase and its inhibitor in rat intestine. Life Sciences. 1973;13(7):1001–1010. doi: 10.1016/0024-3205(73)90091-x. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi T, Nakatani M, Minatogawa Y. Cerebral aromatic aminotransferase. Journal of Neurochemistry. 1975;25(5):579–582. doi: 10.1111/j.1471-4159.1975.tb04371.x. [DOI] [PubMed] [Google Scholar]
- 43.Okuno E, Minatogawa Y, Nakamura M, et al. Crystallization and characterization of human liver kynurenine—glyoxylate aminotransferase. Identity with alanine—glyoxylate aminotransferase and serine—pyruvate aminotransferase. The Biochemical Journal. 1980;189(3):581–590. doi: 10.1042/bj1890581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. Journal of Neuroscience Research. 1997;50(3):457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Okuno E, Schmidt W, Parks DA, Nakamura M, Schwarcz R. Measurement of rat brain kynurenine aminotransferase at physiological kynurenine concentrations. Journal of Neurochemistry. 1991;57(2):533–540. doi: 10.1111/j.1471-4159.1991.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 46.Han Q, Robinson H, Cai T, Tagle DA, Li J. Biochemical and structural properties of mouse kynurenine aminotransferase III. Molecular and Cellular Biology. 2009;29(3):784–793. doi: 10.1128/MCB.01272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. Journal of Neurochemistry. 2007;102(1):103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- 48.Han Q, Li J, Li J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. European Journal of Biochemistry. 2004;271(23-24):4804–4814. doi: 10.1111/j.1432-1033.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- 49.Yu P, Li Z, Zhang L, Tagle DA, Cai T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006;365(1-2):111–118. doi: 10.1016/j.gene.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Okuno E, Du F, Ishikawa T, et al. Purification and characterization of kynurenine-pyruvate aminotransferase from rat kidney and brain. Brain Research. 1990;534(1-2):37–44. doi: 10.1016/0006-8993(90)90109-o. [DOI] [PubMed] [Google Scholar]
- 51.Tsopmo A, Diehl-Jones BW, Aluko RE, Kitts DD, Elisia I, Friel JK. Tryptophan released from mother’s milk has antioxidant properties. Pediatric Research. 2009;66(6):614–618. doi: 10.1203/PDR.0b013e3181be9e7e. [DOI] [PubMed] [Google Scholar]
- 52.Mahal HS, Sharma HS, Mukherjee T. Antioxidant properties of melatonin: a pulse radiolysis study. Free Radical Biology and Medicine. 1999;26(5-6):557–565. doi: 10.1016/s0891-5849(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 53.Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. Journal of Pineal Research. 2008;45(3):235–246. doi: 10.1111/j.1600-079X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 54.Bitzer-Quintero OK, Dávalos-Marín AJ, Ortiz GG, et al. Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomedicine & Pharmacotherapy. 2010;64(1):77–81. doi: 10.1016/j.biopha.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Pazos M, Andersen ML, Skibsted LH. Amino acid and protein scavenging of radicals generated by iron/hydroperoxide system: an electron spin resonance spin trapping study. Journal of Agricultural and Food Chemistry. 2006;54(26):10215–10221. doi: 10.1021/jf062134n. [DOI] [PubMed] [Google Scholar]
- 56.Weiss G, Diez-Ruiz A, Murr C, Theur I, Fuchs D. Tryptophan metabolites as scavengers of reactive oxygen and chlorine species. Pteridines. 2002;13(4):140–144. [Google Scholar]
- 57.Zsizsik BK, Hardeland R. Formation of kynurenic and xanthurenic acids from kynurenine and 3-hydroxykynurenine in the dinoflagellate Lingulodinium polyedrum: role of a novel, oxidative pathway. Comparative Biochemistry and Physiology C. 2002;133(3):383–392. doi: 10.1016/s1532-0456(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 58.Matuszak Z, Reszka KJ, Chignell CF. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radical Biology and Medicine. 1997;23(3):367–372. doi: 10.1016/s0891-5849(96)00614-4. [DOI] [PubMed] [Google Scholar]
- 59.Atherton SJ, Dillon J, Gaillard ER. A pulse radiolysis study of the reactions of 3-hydroxykynurenine and kynurenine with oxidizing and reducing radicals. Biochimica et Biophysica Acta. 1993;1158(1):75–82. doi: 10.1016/0304-4165(93)90099-t. [DOI] [PubMed] [Google Scholar]
- 60.Muñiz PU, Blanco-Ayala T, Pineda B, Pedraza-Chaverrí J, Santmaría A, Pérez-de la Cruz V. On the scavenging properties of L-kynurenine and its anti-oxidant effect in various pro-oxidants models. Proceedings of the Neuroscience Meeting; October 2012; New Orleans, La, USA. Neuroscience Meeting Planner; [Google Scholar]
- 61.Tan DX, Manchester LC, Burkhardt S, et al. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. The FASEB Journal. 2001;15(12):2294–2296. doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- 62.Goda K, Hisaoka M, Ueda T. Photoinduced electron transfer reaction from N-formyl-L-kynurenine and L-kynurenine to cytochrome C. Biochemistry International. 1987;15(3):635–643. [PubMed] [Google Scholar]
- 63.Luthra M, Balasubramanian D. 3-hydroxykynurenine and 3-hydroxyanthranilic acid may act as endogenous antioxidants in the eye lens. Experimental Eye Research. 1992;55(4):641–643. doi: 10.1016/s0014-4835(05)80177-0. [DOI] [PubMed] [Google Scholar]
- 64.Reszka KJ, Bilski P, Chignell CF, Dillon J. Free radical reactions photosensitized by the human lens component, kynurenine: an EPR and spin trapping investigation. Free Radical Biology and Medicine. 1996;20(1):23–34. doi: 10.1016/0891-5849(95)02018-7. [DOI] [PubMed] [Google Scholar]
- 65.Song H, Park H, Kim Y-S, et al. L-Kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. International Immunopharmacology. 2011;11(8):932–938. doi: 10.1016/j.intimp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. Journal of Neurochemistry. 1989;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 67.Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. The Journal of Neuroscience. 2001;21(19):7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rassoulpour A, Wu H-Q, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. Journal of Neurochemistry. 2005;93(3):762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 69.Kaiser S, Wonnacott S. α-bungarotoxin-sensitive nicotinic receptors indirectly modulate [3H]dopamine release in rat striatal slices via glutamate release. Molecular Pharmacology. 2000;58(2):312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 70.Wu H-Q, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? Journal of Neural Transmission. 2007;114(1):33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 71.Carpenedo R, Pittaluga A, Cozzi A, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. European Journal of Neuroscience. 2001;13(11):2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 72.Alkondon M, Pereira EFR, Yu P, et al. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via α7 nicotinic receptors in the hippocampus. The Journal of Neuroscience. 2004;24(19):4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Konradsson-Geuken Å, Wu HQ, Gash CR, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169(4):1848–1859. doi: 10.1016/j.neuroscience.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H-Q, Pereira EFR, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived α7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. Journal of Molecular Neuroscience. 2010;40(1-2):204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zmarowski A, Wu H-Q, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. European Journal of Neuroscience. 2009;29(3):529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. The Journal of Biological Chemistry. 2006;281(31):22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 77.Ohshiro H, Tonai-Kachi H, Ichikawa K. GPR35 is a functional receptor in rat dorsal root ganglion neurons. Biochemical and Biophysical Research Communications. 2008;365(2):344–348. doi: 10.1016/j.bbrc.2007.10.197. [DOI] [PubMed] [Google Scholar]
- 78.Moroni F, Cozzi A, Sili M, Mannaioni G. Kynurenic acid: a metabolite with multiple actions and multiple targets in brain and periphery. Journal of Neural Transmission. 2012;119(2):133–139. doi: 10.1007/s00702-011-0763-x. [DOI] [PubMed] [Google Scholar]
- 79.Goda K, Kishimoto R, Shimizu S, Hamane Y, Ueda M. Quinolinic acid and active oxygens: possible contribution of active oxygens during cell death in the brain. Advances in Experimental Medicine and Biology. 1996;398:247–254. [PubMed] [Google Scholar]
- 80.Hardeland R, Zsizsik BK, Poeggeler B, Fuhrberg B, Holst S, Coto-Montes A. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Advances in Experimental Medicine and Biology. 2000;467:389–395. doi: 10.1007/978-1-4615-4709-9_49. [DOI] [PubMed] [Google Scholar]
- 81.Zsizsik BK, Hardeland R. Kynurenic acid inhibits hydroxyl radical-induced destruction of 2-deoxyribose. In: Hardeland R, editor. Studies on Antioxidants and Their Metabolites. Göttingen, Germany: Cuvillier; 1999. pp. 92–94. [Google Scholar]
- 82.Zsizsik BK, Hardeland R. A putative mechanism of kynurenic acid oxidation by free radicals: scavenging of two hydroxyl radicals and a superoxide anion, release of NO an CO2 . In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen, Germany: Cuvillier; 2001. pp. 164–167. [Google Scholar]
- 83.Coto-Montes A, Zsizsik BK, Hardeland R. Kynurenic acid—not only an antioxidant: strong prooxidative interactions between kynurenic and aminolevulinic acids under light exposure. In: Hardeland R, editor. Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites. Göttingen, Germany: Cuvillier; 2001. pp. 148–155. [Google Scholar]
- 84.Lugo-Huitrón R, Blanco-Ayala T, Ugalde-Muñiz P, et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicology and Teratology. 2011;33(5):538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Baran H, Staniek K, Kepplinger B, Gille L, Stolze K, Nohl H. Kynurenic acid influences the respiratory parameters of rat heart mitochondria. Pharmacology. 2001;62(2):119–123. doi: 10.1159/000056082. [DOI] [PubMed] [Google Scholar]
- 86.Baran H, Staniek K, Kepplinger B, Stur J, Draxler M, Nohl H. Kynurenines and the respiratory parameters on rat heart mitochondria. Life Sciences. 2003;72(10):1103–1115. doi: 10.1016/s0024-3205(02)02365-2. [DOI] [PubMed] [Google Scholar]
- 87.Ishii T, Iwahashi H, Sugata R, Kido R. Formation of hydroxanthommatin-derived radical in the oxidation of 3-hydroxykynurenine. Archives of Biochemistry and Biophysics. 1992;294(2):616–622. doi: 10.1016/0003-9861(92)90733-d. [DOI] [PubMed] [Google Scholar]
- 88.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. Journal of Neurochemistry. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 89.Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(22):12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Research. 1989;495(2):225–231. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- 91.Bryan GT, Brown RR, Price JM. Mouse bladder carcinogenicity of certain tryptophan metabolites and other aromatic nitrogen compounds suspended in cholesterol. Cancer Research. 1964;24:596–602. [PubMed] [Google Scholar]
- 92.Goldstein LE, Leopold MC, Huang X, et al. 3-hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote α-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39(24):7266–7275. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- 93.Korlimbinis A, Hains PG, Truscott RJW, Aquilina JA. 3-hydroxykynurenine oxidizes α-crystallin: potential role in cataractogenesis. Biochemistry. 2006;45(6):1852–1860. doi: 10.1021/bi051737+. [DOI] [PubMed] [Google Scholar]
- 94.Dillon J, Castineiras SG, Santiago MA, Spector A. The endopeptidase-resistant protein fraction from human cataractous lenses. Experimental Eye Research. 1984;39(1):95–106. doi: 10.1016/0014-4835(84)90118-0. [DOI] [PubMed] [Google Scholar]
- 95.Tomoda A, Yoneyama Y, Yamaguchi T, Kakinuma K, Kawasaki K, Yonemura D. Spectroscopic studies of brunescent cataractous lenses. FEBS Letters. 1987;219(2):472–476. doi: 10.1016/0014-5793(87)80275-2. [DOI] [PubMed] [Google Scholar]
- 96.Wei H, Leeds P, Chen R-W, et al. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. Journal of Neurochemistry. 2000;75(1):81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- 97.Lee M-W, Park SC, Chae H-S, et al. The protective role of HSP90 against 3-hydroxykynurenine-induced neuronal apoptosis. Biochemical and Biophysical Research Communications. 2001;284(2):261–267. doi: 10.1006/bbrc.2001.4938. [DOI] [PubMed] [Google Scholar]
- 98.Lee HJ, Bach J-H, Chae H-S, et al. Mitogen-activated protein kinase/extracellular signal-regulated kinase attenuates 3-hydroxykynurenine-induced neuronal cell death. Journal of Neurochemistry. 2004;88(3):647–656. doi: 10.1111/j.1471-4159.2004.02191.x. [DOI] [PubMed] [Google Scholar]
- 99.Nakagami Y, Saito H, Katsuki H. 3-hydroxykynurenine toxicity on the rat striatum in vivo. Japanese Journal of Pharmacology. 1996;71(2):183–186. doi: 10.1254/jjp.71.183. [DOI] [PubMed] [Google Scholar]
- 100.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(7):2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goshima N, Wadano A, Miura K. 3-hydroxykynurenine as O2 • − scavenger in the blowfly, Aldrichina grahami. Biochemical and Biophysical Research Communications. 1986;139(2):666–672. doi: 10.1016/s0006-291x(86)80042-0. [DOI] [PubMed] [Google Scholar]
- 102.Ishii T, Iwahashi H, Sugata R, Kido R. Oxidation of 3-hydroxykynurenine catalyzed by methemoglobin with hydrogen peroxide. Free Radical Biology and Medicine. 1992;13(1):17–20. doi: 10.1016/0891-5849(92)90160-i. [DOI] [PubMed] [Google Scholar]
- 103.Leipnitz G, Schumacher C, Dalcin KB, et al. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochemistry International. 2007;50(1):83–94. doi: 10.1016/j.neuint.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 104.Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochemical and Biophysical Research Communications. 2003;300(3):719–724. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- 105.Gobaille S, Kemmel V, Brumaru D, Dugave C, Aunis D, Maitre M. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. Journal of Neurochemistry. 2008;105(3):982–993. doi: 10.1111/j.1471-4159.2008.05219.x. [DOI] [PubMed] [Google Scholar]
- 106.Murakami K, Ito M, Yoshino M. Xanthurenic acid inhibits metal ion-induced lipid peroxidation and protects NADP-isocitrate dehydrogenase from oxidative inactivation. Journal of Nutritional Science and Vitaminology. 2001;47(4):306–310. doi: 10.3177/jnsv.47.306. [DOI] [PubMed] [Google Scholar]
- 107.Hirai T, Fukushima K, Kumamoto K, Iwahashi H. Effects of some naturally occurring iron ion chelators on in vitro superoxide radical formation. Biological Trace Element Research. 2005;108(1–3):77–85. doi: 10.1385/BTER:108:1-3:077. [DOI] [PubMed] [Google Scholar]
- 108.Zsizsik BK, Hardeland R. Comparative studies on kynurenic, xanthurenic and quinaldic acids as scavengers of hydroxyl and ABTS cation radicals. In: Hardeland R, editor. Studies on Antioxidants and Their Metabolites. Göttingen, Germany: Cuvillier; 1999. pp. 82–91. [Google Scholar]
- 109.Lima VL, Dias F, Nunes RD, et al. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038349.e38349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murakami K, Haneda M, Yoshino M. Prooxidant action of xanthurenic acid and quinoline compounds: role of transition metals in the generation of reactive oxygen species and enhanced formation of 8-hydroxy-2′-deoxyguanosine in DNA. BioMetals. 2006;19(4):429–435. doi: 10.1007/s10534-005-4528-6. [DOI] [PubMed] [Google Scholar]
- 111.Welch KD, Davis TZ, Aust SD. Iron autoxidation and free radical generation: effects of buffers, ligands, and chelators. Archives of Biochemistry and Biophysics. 2002;397(2):360–369. doi: 10.1006/abbi.2001.2694. [DOI] [PubMed] [Google Scholar]
- 112.Vásquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. The Journal of Biological Chemistry. 2000;275(19):14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 113.Malina H, Richter C, Frueh B, Hess OM. Lens epithelial cell apoptosis and intracellular Ca2+ increasein the presence of xanthurenic acid. BMC Ophthalmology. 2002;2, article 1 doi: 10.1186/1471-2415-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Malina HZ, Richter C, Mehl M, Hess OM. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown. BMC Physiology. 2001;1, article 7 doi: 10.1186/1472-6793-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roberts JE, Wishart JF, Martinez L, Chignell CF. Photochemical studies on xanthurenic acid. Photochemistry and Photobiology. 2000;72(4):467–471. doi: 10.1562/0031-8655(2000)072<0467:psoxa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 116.Roberts JE, Finley EL, Patat SA, Schey KL. Photooxidation of lens proteins with xanthurenic acid: a putative chromophore for cataractogenesis. Photochemistry and Photobiology. 2001;74(5):740–744. doi: 10.1562/0031-8655(2001)074<0740:polpwx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 117.Thiagarajan G, Shirao E, Ando K, Inoue A, Balasubramanian D. Role of xanthurenic acid 8-O-beta-D-glucoside, a novel fluorophore that accumulates in the brunescent human eye lens. Photochemistry and Photobiology. 2002;76(3):368–372. doi: 10.1562/0031-8655(2002)076<0368:roxaod>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 118.Dykens JA, Sullivan SG, Stern A. Oxidative reactivity of the tryptophan metabolites 3-hydroxyanthranilate, cinnabarinate, quinolinate and picolinate. Biochemical Pharmacology. 1987;36(2):211–217. doi: 10.1016/0006-2952(87)90691-5. [DOI] [PubMed] [Google Scholar]
- 119.Quagliariello E, Papa S, Saccone C, Alifano A. Effect of 3-hydroxyanthranilic acid on the mitochondrial respiratory system. The Biochemical Journal. 1964;91(1):137–146. doi: 10.1042/bj0910137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morita T, Saito K, Takemura M, et al. 3-hydroxyanthranilic acid, an L-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-γ . Annals of Clinical Biochemistry. 2001;38(part 3):242–251. doi: 10.1258/0004563011900461. [DOI] [PubMed] [Google Scholar]
- 121.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death and Differentiation. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 122.Hayashi T, Mo J-H, Gong X, et al. 3-hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee S-M, Lee Y-S, Choi J-H, et al. Tryptophan metabolite 3-hydroxyanthranilic acid selectively induces activated T cell death via intracellular GSH depletion. Immunology Letters. 2010;132(1-2):53–60. doi: 10.1016/j.imlet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 124.Thomas SR, Witting PK, Stocker R. 3-Hydroxyanthranilic acid is an efficient, cell-derived co-antioxidant for α-tocopherol, inhibiting human low density lipoprotein and plasma lipid peroxidation. The Journal of Biological Chemistry. 1996;271(51):32714–32721. doi: 10.1074/jbc.271.51.32714. [DOI] [PubMed] [Google Scholar]
- 125.Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Köhler C, Denis-Donini S, Cesura AM. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-γ-activated murine macrophages and microglial cells. Journal of Immunology. 1997;159(1):419–426. [PubMed] [Google Scholar]
- 126.Sekkaï D, Guittet O, Lemaire G, Tenu J-P, Lepoivre M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Archives of Biochemistry and Biophysics. 1997;340(1):117–123. doi: 10.1006/abbi.1997.9913. [DOI] [PubMed] [Google Scholar]
- 127.Oh G-S, Pae H-O, Choi B-M, et al. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochemical and Biophysical Research Communications. 2004;320(4):1156–1162. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 128.Christen S, Thomas SR, Garner B, Stocker R. Inhibition by interferon-γ of human mononuclear cell-mediated low density lipoprotein oxidation. Participation of tryptophan metabolism along the kynurenine pathway. Journal of Clinical Investigation. 1994;93(5):2149–2158. doi: 10.1172/JCI117211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Latini A, Rodriguez M, Rosa RB, et al. 3-hydroxyglutaric acid moderately impairs energy metabolism in brain of young rats. Neuroscience. 2005;135(1):111–120. doi: 10.1016/j.neuroscience.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 130.Gaubert S, Bouchaut M, Brumas V, Berthon G. Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radical Research. 2000;32(5):451–461. doi: 10.1080/10715760000300451. [DOI] [PubMed] [Google Scholar]
- 131.Miche H, Brumas V, Berthon G. Copper(II) interactions with nonsteroidal antiinflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. Journal of Inorganic Biochemistry. 1997;68(1):27–38. doi: 10.1016/s0162-0134(97)00005-6. [DOI] [PubMed] [Google Scholar]
- 132.Halova-Lajoie B, Brumas V, Fiallo MML, Berthon G. Copper(II) interactions with non-steroidal anti-inflammatory agents. III—3-methoxyanthranilic acid as a potential •OH-inactivating ligand: a quantitative investigation of its copper handling role in vivo. Journal of Inorganic Biochemistry. 2006;100(3):362–373. doi: 10.1016/j.jinorgbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 133.Whetsell WO, Jr., Shapira NA. Neuroexcitation, excitotoxicity and human neurological disease. Laboratory Investigation. 1993;68(4):372–387. [PubMed] [Google Scholar]
- 134.Guidetti P, Walsh JL, Schwarcz R. A fluorimetric assay for the determination of anthranilic acid in biological materials. Analytical Biochemistry. 1994;220(1):181–184. doi: 10.1006/abio.1994.1316. [DOI] [PubMed] [Google Scholar]
- 135.Baran H, Schwarcz R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. Journal of Neurochemistry. 1990;55(3):738–744. doi: 10.1111/j.1471-4159.1990.tb04553.x. [DOI] [PubMed] [Google Scholar]
- 136.Suzuki K, Yasuda M, Yamasaki K. Stability constants of picolinic and quinaldic acid chelates of bivalent metals. Journal of Physical Chemistry. 1957;61(2):229–231. [Google Scholar]
- 137.Aggett PJ, Fenwick PK, Kirk H. An in vitro study of the effect of picolinic acid on metal translocation across lipid bilayers. The Journal of Nutrition. 1989;119(10):1432–1437. doi: 10.1093/jn/119.10.1432. [DOI] [PubMed] [Google Scholar]
- 138.Bosco MC, Rapisarda A, Massazza S, Melillo G, Young H, Varesio L. The tryptophan catabolite picolinic acid selectively induces the chemokines macrophage inflammatory protein-1α and -1β in macrophages. Journal of Immunology. 2000;164(6):3283–3291. doi: 10.4049/jimmunol.164.6.3283. [DOI] [PubMed] [Google Scholar]
- 139.Cai S, Sato K, Shimizu T, et al. Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin and fluoroquinolones. Journal of Antimicrobial Chemotherapy. 2006;57(1):85–93. doi: 10.1093/jac/dki418. [DOI] [PubMed] [Google Scholar]
- 140.Abe S, Hu W, Ishibashi H, Hasumi K, Yamaguchi H. Augmented inhibition of Candida albicans growth by murine neutrophils in the presence of a tryptophan metabolite, picolinic acid. Journal of Infection and Chemotherapy. 2004;10(3):181–184. doi: 10.1007/s10156-004-0311-9. [DOI] [PubMed] [Google Scholar]
- 141.Fernandez-Pol JA, Klos DJ, Hamilton PD. Antiviral, cytotoxic and apoptotic activities of picolinic acid on human immunodeficiency virus-1 and human herpes simplex virus-2 infected cells. Anticancer Research. 2001;21(6):3773–3776. [PubMed] [Google Scholar]
- 142.Melillo G, Cox GW, Biragyn A, Sheffler LA, Varesio L. Regulation of nitric-oxide synthase mRNA expression by interferon-γ and picolinic acid. The Journal of Biological Chemistry. 1994;269(11):8128–8133. [PubMed] [Google Scholar]
- 143.Cockhill J, Jhamandas K, Boegman RJ, Beninger RJ. Action of picolinic acid and structurally related pyridine carboxylic acids on quinolinic acid-induced cortical cholinergic damage. Brain Research. 1992;599(1):57–63. doi: 10.1016/0006-8993(92)90852-z. [DOI] [PubMed] [Google Scholar]
- 144.Kalisch BE, Jhamandas K, Boegman RJ, Beninger RJ. Picolinic acid protects against quinolinic acid-induced depletion of NADPH diaphorase containing neurons in the rat striatum. Brain Research. 1994;668(1-2):1–8. doi: 10.1016/0006-8993(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 145.Vrooman L, Jhamandas K, Boegmaqn RJ, Beninger RJ. Picolinic acid modulates kainic acid-evoked glutamate release from the striatum in vitro. Brain Research. 1993;627(2):193–198. doi: 10.1016/0006-8993(93)90320-m. [DOI] [PubMed] [Google Scholar]
- 146.Tonohiro T, Tanabe M, Kaneko T, Iwata N. Is picolinic acid a glycine agonist at strychnine-sensitive receptors? Brain Research. 1990;516(2):332–334. doi: 10.1016/0006-8993(90)90937-7. [DOI] [PubMed] [Google Scholar]
- 147.Minakata K, Fukushima K, Nakamura M, Iwahashi H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe3+ and NADPH. Journal of Clinical Biochemistry and Nutrition. 2011;49(3):207–215. doi: 10.3164/jcbn.11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beskid M, Jachimowicz J, Taraszewska A, Kukulska D. Histological and ultrastructural changes in the rat brain following systemic administration of picolinic acid. Experimental and Toxicologic Pathology. 1995;47(1):25–30. doi: 10.1016/S0940-2993(11)80278-2. [DOI] [PubMed] [Google Scholar]
- 149.Fernandez-Pol JA. Morphological changes induced by picolinic acid in cultured mammalian cells. Experimental and Molecular Pathology. 1978;29(3):348–357. doi: 10.1016/0014-4800(78)90077-1. [DOI] [PubMed] [Google Scholar]
- 150.Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. The FEBS Journal. 2012;279(8):1356–1365. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 151.Pérez-de la Cruz V, González-Cortés C, Galván-Arzate S, et al. Excitotoxic brain damage involves early peroxynitrite formation in a model of Huntington’s disease in rats: protective role of iron porphyrinate 5,10,15,20-tetrakis (4-sulfonatophenyl)porphyrinate iron (III) Neuroscience. 2005;135(2):463–474. doi: 10.1016/j.neuroscience.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 152.Santiago-López D, Vázquez-Román B, Pérez-de la Cruz V, et al. Peroxynitrite decomposition catalyst, iron metalloporphyrin, reduces quinolinate-induced neurotoxicity in rats. Synapse. 2004;54(4):233–238. doi: 10.1002/syn.20084. [DOI] [PubMed] [Google Scholar]
- 153.Rodríguez E, Méndez-Armenta M, Villeda-Hernández J, et al. Dapsone prevents morphological lesions and lipid peroxidation induced by quinolinic acid in rat corpus striatum. Toxicology. 1999;139(1-2):111–118. doi: 10.1016/s0300-483x(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 154.Santamaría A, Galván-Arzate S, Lisý V, et al. Quinolinic acid induces oxidative stress in rat brain synaptosomes. NeuroReport. 2001;12(4):871–874. doi: 10.1097/00001756-200103260-00049. [DOI] [PubMed] [Google Scholar]
- 155.Leipnitz G, Schumacher C, Scussiato K, et al. Quinolinic acid reduces the antioxidant defenses in cerebral cortex of young rats. International Journal of Developmental Neuroscience. 2005;23(8):695–701. doi: 10.1016/j.ijdevneu.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 156.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature Reviews Neuroscience. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lugo-Huitrón R, Muñiz PU, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V. Quinolinic acid an endogenous neurotoxin with multiple targets. Oxidative Medicine and Cellular Longevity. 2013;2013:14 pages. doi: 10.1155/2013/104024.104024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ. Effects of kynurenine pathway metabolites on intracellular NAD+ synthesis and cell death in human primary astrocytes and neurons. International Journal of Tryptophan Research. 2009;2(1):61–69. doi: 10.4137/ijtr.s2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotoxicity Research. 2009;16(1):77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 160.Stipek S, Stastny F, Platenik J, Crkovska J, Zima T. The effect of quinolinate on rat brain lipid peroxidation is dependent on iron. Neurochemistry International. 1997;30(2):233–237. [PubMed] [Google Scholar]
- 161.Iwahashi H, Kawamori H, Fukushima K. Quinolinic acid, α-picolinic acid, fusaric acid, and 2,6-pyridinedicarboxylic acid enhance the Fenton reaction in phosphate buffer. Chemico-Biological Interactions. 1999;118(3):201–215. doi: 10.1016/s0009-2797(99)00080-0. [DOI] [PubMed] [Google Scholar]
- 162.Pláteník J, Stopka P, Vejrazka M, Stípek S. Quinolinic acid—iron(II) complexes: slow autoxidation, but enhanced hydroxyl radical production in the fenton reaction. Free Radical Research. 2001;34(5):445–459. doi: 10.1080/10715760100300391. [DOI] [PubMed] [Google Scholar]
- 163.Dairam A, Fogel R, Daya S, Limson JL. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. Journal of Agricultural and Food Chemistry. 2008;56(9):3350–3356. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 164.Lapin IP, Mirzaev SM, Ryzov IV, Oxenkrug GF. Anticonvulsant activity of melatonin against seizures induced by quinolinate, kainate, glutamate, NMDA, and pentylenetetrazole in mice. Journal of Pineal Research. 1998;24(4):215–218. doi: 10.1111/j.1600-079x.1998.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 165.Southgate GS, Daya S, Potgieter B. Melatonin plays a protective role in quinolinic acid-induced neurotoxicity in the rat hippocampus. Journal of Chemical Neuroanatomy. 1998;14(3-4):151–156. doi: 10.1016/s0891-0618(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 166.Behan WMH, McDonald M, Darlington LG, Stone TW. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. British Journal of Pharmacology. 1999;128(8):1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rossato JI, Ketzer LA, Centurião FB, et al. Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochemical Research. 2002;27(4):297–303. doi: 10.1023/a:1014907228580. [DOI] [PubMed] [Google Scholar]
- 168.Swathy SS, Panicker S, Nithya RS, Anuja MM, Rejitha S, Indira M. Antiperoxidative and antiinflammatory effect of sida cordifolia linn. on quinolinic acid induced neurotoxicity. Neurochemical Research. 2010;35(9):1361–1367. doi: 10.1007/s11064-010-0192-5. [DOI] [PubMed] [Google Scholar]
- 169.Kalonia H, Mishra J, Kumar A. Targeting neuro-inflammatory cytokines and oxidative stress by minocycline attenuates quinolinic-acid-induced Huntington’s disease-like symptoms in rats. Neurotoxicity Research. 2012;22(4):310–320. doi: 10.1007/s12640-012-9315-x. [DOI] [PubMed] [Google Scholar]
- 170.Sreekala S, Indira M. Impact of co administration of selenium and quinolinic acid in the rat’s brain. Brain Research. 2009;1281:101–107. doi: 10.1016/j.brainres.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 171.Silva-Adaya D, Pérez-de la Cruz V, Herrera-Mundo MN, et al. Excitotoxic damage, disrupted energy metabolism, and oxidative stress in the rat brain: antioxidant and neuroprotective effects of L-carnitine. Journal of Neurochemistry. 2008;105(3):677–689. doi: 10.1111/j.1471-4159.2007.05174.x. [DOI] [PubMed] [Google Scholar]
- 172.Dairam A, Chetty P, Daya S. Non-steroidal anti-inflammatory agents, tolmetin and sulindac, attenuate oxidative stress in rat brain homogenate and reduce quinolinic acid-induced neurodegeneration in rat hippocampal neurons. Metabolic Brain Disease. 2006;21(2-3):221–233. doi: 10.1007/s11011-006-9014-5. [DOI] [PubMed] [Google Scholar]
- 173.Beal MF, Ferrante RJ, Swartz KJ, Kowall NW. Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. The Journal of Neuroscience. 1991;11(6):1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer’s disease hippocampus. Neuropathology and Applied Neurobiology. 2005;31(4):395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 175.Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in aids dementia complex. Neurotoxicity Research. 2005;7(1-2):103–123. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- 176.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clinical Biochemistry. 2004;37(8):684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 177.Bova LM, Sweeney MHJ, Jamie JE, Truscott RJW. Major changes in human ocular UV protection with age. Investigative Ophthalmology & Visual Science. 2001;42(1):200–205. [PubMed] [Google Scholar]
- 178.Comai S, Costa CVL, Ragazzi E, Bertazzo A, Allegri G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clinica Chimica Acta. 2005;360(1-2):67–80. doi: 10.1016/j.cccn.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 179.Crepaldi G, Allegri G, de Antoni A. Relationship between tryptophan metabolism and vitamin B6 and nicotinamide in aged subjects. Acta Vitaminologica et Enzymologica. 1975;29(1–6):140–144. [PubMed] [Google Scholar]
- 180.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. The FEBS Journal. 2011;278(22):4425–4434. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- 181.Oxenkrug G. Interferon-gamma—inducible inflammation: contribution to aging and aging-associated psychiatric disorders. Aging and Disease. 2011;2(6):474–486. [PMC free article] [PubMed] [Google Scholar]
- 182.Comai S, Bertazzo A, Ragazzi E, Caparrotta L, Costa CVL, Allegri G. Influence of age on Cu/Zn-superoxide dismutase and indole 2,3-dioxygenase activities in rat tissues. The Italian Journal of Biochemistry. 2005;54(3-4):232–239. [PubMed] [Google Scholar]
- 183.Moroni F, Lombardi G, Moneti G, Aldinio C. The excitotoxin quinolinic acid is present in the brain of several mammals and its cortical content increases during the aging process. Neuroscience Letters. 1984;47(1):51–55. doi: 10.1016/0304-3940(84)90385-9. [DOI] [PubMed] [Google Scholar]
- 184.Finn SF, Hyman BT, Storey E, Miller JM, Beal MF. Effects of aging on quinolinic acid lesions in rat striatum. Brain Research. 1991;562(2):276–280. doi: 10.1016/0006-8993(91)90631-5. [DOI] [PubMed] [Google Scholar]
- 185.Gramsbergen JBP, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Research. 1992;588(1):1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- 186.Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P. Age-related increase of kynurenic acid in human cerebrospinal fluid—IgG and β2-microglobulin changes. NeuroSignals. 2005;14(3):126–135. doi: 10.1159/000086295. [DOI] [PubMed] [Google Scholar]
- 187.Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006344.e6344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0042357.e42357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Beal MF. Mitochondria, free radicals, and neurodegeneration. Current Opinion in Neurobiology. 1996;6(5):661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 190.Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. Journal of Neurochemistry. 2005;92(3):494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 191.Baran H, Cairns N, Lubec B, Lubec G. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sciences. 1996;58(21):1891–1899. doi: 10.1016/0024-3205(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 192.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. Journal of Neural Transmission. 1999;106(2):165–181. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- 193.Iwahashi H, Ishii T, Sugata R, Kido R. The effects of caffeic acid and its related catechols on hydroxyl radical formation by 3-hydroxyanthranilic acid, ferric chloride, and hydrogen peroxide. Archives of Biochemistry and Biophysics. 1990;276(1):242–247. doi: 10.1016/0003-9861(90)90033-u. [DOI] [PubMed] [Google Scholar]
- 194.Martinsons A, Rudzite V, Jurika E, Silava A. The relationship between kynurenine, catecholamines, and arterial hypertension in mesangioproliferative glomerulonephritis. Advances in Experimental Medicine and Biology. 1996;398:417–419. doi: 10.1007/978-1-4613-0381-7_64. [DOI] [PubMed] [Google Scholar]
- 195.DeNapoli JS, Dodman NH, Shuster L, Rand WM, Gross KL. Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggression, and hyperactivity in dogs. Journal of the American Veterinary Medical Association. 2000;217(4):504–508. doi: 10.2460/javma.2000.217.504. [DOI] [PubMed] [Google Scholar]
- 196.Ciji A, Sahu NP, Pal AK, Akhtar MS. Nitrite-induced alterations in sex steroids and thyroid hormones of Labeo rohita juveniles: effects of dietary vitamin E and L-tryptophan. Fish Physiology and Biochemistry. 2013;39(5):1297–1307. doi: 10.1007/s10695-013-9784-8. [DOI] [PubMed] [Google Scholar]
- 197.Hiratsuka C, Fukuwatari T, Sano M, Saito K, Sasaki S, Shibata K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. The Journal of Nutrition. 2013;143(6):859–866. doi: 10.3945/jn.112.173823. [DOI] [PubMed] [Google Scholar]
- 198.Penberthy WT. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Current Drug Metabolism. 2007;8(3):245–266. doi: 10.2174/138920007780362545. [DOI] [PubMed] [Google Scholar]
- 199.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 200.Sakurai K, Zou J-P, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. Journal of Neuroimmunology. 2002;129(1-2):186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 201.Blight RT, Cohen TI, Saito K, Heyes MP. Quinolinic acid accumulation and functional deficits following experimental spinal cord injury. Brain. 1995;118(part 3):735–752. doi: 10.1093/brain/118.3.735. [DOI] [PubMed] [Google Scholar]
- 202.Röver S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2- yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. Journal of Medicinal Chemistry. 1997;40(26):4378–4385. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- 203.Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infection and Immunity. 2005;73(8):5249–5251. doi: 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Campesan S, Green EW, Breda C, et al. The kynurenine pathway modulates neurodegeneration in a drosophila model of Huntington’s disease. Current Biology. 2011;21(11):961–966. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Green EW, Campesan S, Breda C, et al. Drosophila eye color mutants as therapeutic tools for Huntington disease. Fly. 2012;6(2):117–120. doi: 10.4161/fly.19999. [DOI] [PubMed] [Google Scholar]
- 206.Zwilling D, Huang S-Y, Sathyasaikumar KV, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Carpenedo R. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience. 1994;61(2):237–244. doi: 10.1016/0306-4522(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 208.Miranda AF, Boegman RJ, Beninger RJ, Jhamandas K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience. 1997;78(4):967–975. doi: 10.1016/s0306-4522(96)00655-0. [DOI] [PubMed] [Google Scholar]
- 209.Moroni F, Cozzi A, Peruginelli F, Carpenedo R, Pellegrini-Giampietro DE. Neuroprotective effects of kynurenine-3-hydroxylase inhibitors in models of brain ischemia. Advances in Experimental Medicine and Biology. 2000;467:199–206. doi: 10.1007/978-1-4615-4709-9_26. [DOI] [PubMed] [Google Scholar]
- 210.Richter A, Hamann M. The kynurenine 3-hydroxylase inhibitor Ro 61-8048 improves dystonia in a genetic model of paroxysmal dyskinesia. European Journal of Pharmacology. 2003;478(1):47–52. doi: 10.1016/j.ejphar.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 211.Ceresoli-Borroni G, Guidetti P, Amori L, Pellicciari R, Schwarcz R. Perinatal kynurenine 3-hydroxylase inhibition in rodents: pathophysiological implications. Journal of Neuroscience Research. 2007;85(4):845–854. doi: 10.1002/jnr.21183. [DOI] [PubMed] [Google Scholar]
- 212.Grégoire L, Rassoulpour A, Guidetti P, et al. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behavioural Brain Research. 2008;186(2):161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 213.Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. Journal of Neurochemistry. 2009;109(2):316–325. doi: 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pellicciari R, Rizzo RC, Costantino G, et al. Modulators of the kynurenine pathway of tryptophan metabolism: synthesis and preliminary biological evaluation of (S)-4-(Ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. ChemMedChem. 2006;1(5):528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- 215.Ferry G, Ubeaud C, Lambert P-H, et al. Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: ivestigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. The Biochemical Journal. 2005;388(part 1):205–215. doi: 10.1042/BJ20042075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yoshida R, Hayaishi O. Overview: superoxygenase. Methods in Enzymology. 1984;105:61–70. doi: 10.1016/s0076-6879(84)05009-6. [DOI] [PubMed] [Google Scholar]
- 217.Paglino A, Lombardo F, Arcà B, Rizzi M, Rossi F. Purification and biochemical characterization of a recombinant Anopheles gambiae tryptophan 2,3-dioxygenase expressed in Escherichia coli . Insect Biochemistry and Molecular Biology. 2008;38(9):871–876. doi: 10.1016/j.ibmb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 218.Schartau W, Linzen B. The tryptophan 2,3 dioxygenase of the blowfly, Protophormia terrae novae: partial purification and characterization. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1976;357(1):41–49. doi: 10.1515/bchm2.1976.357.1.41. [DOI] [PubMed] [Google Scholar]
- 219.Yasui H, Takai K, Yoshida R, Hayaishi O. Interferon enhances tryptophan metabolism by inducing pulmonary indoleamine 2,3-dioxygenase: its possible occurrence in cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(17):6622–6626. doi: 10.1073/pnas.83.17.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sono M. The roles of superoxide anion and methylene blue in the reductive activation of indoleamine 2,3-dioxygenase by ascorbic acid or by xanthine oxidase-hypoxanthine. The Journal of Biological Chemistry. 1989;264(3):1616–1622. [PubMed] [Google Scholar]
- 221.Samelson-Jones BJ, Yeh S-R. Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry. 2006;45(28):8527–8538. doi: 10.1021/bi060143j. [DOI] [PubMed] [Google Scholar]
- 222.Serrano AE, Jr., Nagayama F. Inhibition studies of liver arylformamidases of rainbow trout and cattle. Comparative Biochemistry and Physiology B. 1991;99(2):281–285. doi: 10.1016/0305-0491(91)90042-c. [DOI] [PubMed] [Google Scholar]
- 223.Menge U. Formamidase—microheterogeneity, catalytic properties and inhibitors (author’s transl) Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1979;360(2):185–196. [PubMed] [Google Scholar]
- 224.Walsh HA, O’Shea KC, Botting NP. Comparative inhibition by substrate analogues 3-methoxy- and 3-hydroxydesaminokynurenine and an improved 3 step purification of recombinant human kynureninase. BMC Biochemistry. 2003;4, article 13 doi: 10.1186/1471-2091-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Okuno E, Tsujimoto M, Nakamura M, Kido R. 2-aminoadipate-2-oxoglutarate aminotransferase isoenzymes in human liver: a plausible physiological role in lysine and tryptophan metabolism. Enzyme & Protein. 1993;47(3):136–148. doi: 10.1159/000468669. [DOI] [PubMed] [Google Scholar]
- 226.Goh DLM, Patel A, Thomas GH, et al. Characterization of the human gene encoding α-aminoadipate aminotransferase (AADAT) Molecular Genetics and Metabolism. 2002;76(3):172–180. doi: 10.1016/s1096-7192(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 227.Takeuchi F, Otsuka H, Shibata Y. Purification, characterization and identification of rat liver mitochondrial kynurenine aminotransferase with α-aminiadipate aminotransferase. Biochimica et Biophysica Acta. 1983;743(3):323–330. doi: 10.1016/0167-4838(83)90389-8. [DOI] [PubMed] [Google Scholar]
- 228.Kocki T, Luchowski P, Luchowska E, Wielosz M, Turski WA, Urbanska EM. L-Cysteine sulphinate, endogenous sulphur-containing amino acid, inhibits rat brain kynurenic acid production via selective interference with kynurenine aminotransferase II. Neuroscience Letters. 2003;346(1-2):97–100. doi: 10.1016/s0304-3940(03)00579-2. [DOI] [PubMed] [Google Scholar]
- 229.Han Q, Cai T, Tagle DA, Robinson H, Li J. Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II. Bioscience Reports. 2008;28(4):205–215. doi: 10.1042/BSR20080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Saito Y, Hayaishi O, Rothberg S. Studies on oxygenases; enzymatic formation of 3-hydroxy-L-kynurenine from L-kynurenine. The Journal of Biological Chemistry. 1957;229(2):921–934. [PubMed] [Google Scholar]
- 231.Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-γ? Neuroscience Letters. 2008;441(1):29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 232.Saito K, Quearry BJ, Saito M, Nowak TS, Jr., Markey SP, Heyes MP. Kynurenine 3-hydroxylase in brain: species activity differences and effect of gerbil cerebral ischemia. Archives of Biochemistry and Biophysics. 1993;307(1):104–109. doi: 10.1006/abbi.1993.1567. [DOI] [PubMed] [Google Scholar]
- 233.Calderone V, Trabucco M, Menin V, Negro A, Zanotti G. Cloning of human 3-hydroxyanthranilic acid dioxygenase in Escherichia coli: characterisation of the purified enzyme and its in vitro inhibition by Zn2+ . Biochimica et Biophysica Acta. 2002;1596(2):283–292. doi: 10.1016/s0167-4838(02)00216-9. [DOI] [PubMed] [Google Scholar]
- 234.Pucci L, Perozzi S, Cimadamore F, Orsomando G, Raffaelli N. Tissue expression and biochemical characterization of human 2-amino 3-carboxymuconate 6-semialdehyde decarboxylase, a key enzyme in tryptophan catabolism. The FEBS Journal. 2007;274(3):827–840. doi: 10.1111/j.1742-4658.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 235.Garavaglia S, Perozzi S, Galeazzi L, Raffaelli N, Rizzi M. The crystal structure of human α-amino-β-carboxymuconate-ℰ- semialdehyde decarboxylase in complex with 1,3-dihydroxyacetonephosphate suggests a regulatory link between NAD synthesis and glycolysis. The FEBS Journal. 2009;276(22):6615–6623. doi: 10.1111/j.1742-4658.2009.07372.x. [DOI] [PubMed] [Google Scholar]
- 236.Liu H, Woznica K, Catton G, Crawford A, Botting N, Naismith JH. Structural and kinetic characterization of quinolinate phosphoribosyltransferase (hQPRTase) from Homo sapiens. Journal of Molecular Biology. 2007;373(3):755–763. doi: 10.1016/j.jmb.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shibata K, Iwai K. Isolation and properties of crystalline quinolinate phosphoribosyltransferase from hog kidney. Biochimica et Biophysica Acta. 1980;611(2):280–288. doi: 10.1016/0005-2744(80)90063-7. [DOI] [PubMed] [Google Scholar]
- 238.Iwai K, Taguchi H. Crystallization and properties of quinolinate phosphoribosyltransferase from hog liver. Methods in Enzymology. 1980;66:96–101. doi: 10.1016/0076-6879(80)66444-1. [DOI] [PubMed] [Google Scholar]
- 239.Feksa LR, Latini A, Rech VC, et al. Tryptophan administration induces oxidative stress in brain cortex of rats. Metabolic Brain Disease. 2008;23(2):221–233. doi: 10.1007/s11011-008-9087-4. [DOI] [PubMed] [Google Scholar]
- 240.Harasimowicz J, Marques KL, Silva AFT, et al. Chemiluminometric evaluation of melatonin and selected melatonin precursors’ interaction with reactive oxygen and nitrogen species. Analytical Biochemistry. 2012;420(1):1–6. doi: 10.1016/j.ab.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 241.Lide DR. Handbook of Chemistry and Physics. A Ready-Reference Book of Chemical and Physical Data. 83rd edition. Boca Raton, Fla, USA: CRC Press; 2003. [Google Scholar]
- 242.Tsentalovich YP, Snytnikova OA, Sherin PS, Forbes MDE. Photochemistry of kynurenine, a tryptophan metabolite: properties of the triplet state. Journal of Physical Chemistry A. 2005;109(16):3565–3568. doi: 10.1021/jp045142k. [DOI] [PubMed] [Google Scholar]
- 243.Zsizsik BK, Hardeland R. Kynurenic acid inhibits hydroxyl radical-induced destruction of 2-deoxyribose. In: Hardeland R, editor. Studies on Antioxidants and Their Metabolites. Göttingen, Germany: Cuvillier; 1999. [Google Scholar]
- 244.Hardeland R, Zsizsik BK. Kynurenic acid as a free radical scavenger: measurements of educt and product fluorescence and of light emission from an excited intermediate state. In: Hardeland R, editor. Biological Rhythms and Antioxidative Protection. Göttingen, Germany: Cuvillier; 1997. pp. 153–160. [Google Scholar]
- 245.Bag SP, Fernando Q, Freiser H. The influence of metal chelation on the structure of certain hydroxyquinaldinic acids. Inorganic Chemistry. 1964;3(1):93–96. [Google Scholar]
- 246.Cardi ER, Vallone JJ. Primary carcinoma of the liver presenting itself as an acute abdominal condition. The American Journal of Surgery. 1954;87(1):149–151. doi: 10.1016/0002-9610(54)90060-4. [DOI] [PubMed] [Google Scholar]
- 247.Armarego W, Chai CLL. Purification of organic chemicals-aromatic compounds. In: Armarego W, Chai CLL, editors. Purification of Laboratory Chemicals. 7th edition. Oxford, UK: Bulterworth-Heinemann; 2013. p. p. 339. [Google Scholar]
- 248.Grant RS, Coggan SE, Smythe GA. The physiological action of picolinic acid in the human brain. International Journal of Tryptophan Research. 2009;2(1):71–79. doi: 10.4137/ijtr.s2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. Journal of Neural Transmission. 2000;107(3):343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 250.Heyes MP, Saito K, Crowley JS, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(part 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 251.Hartai Z, Juhász A, Rimanóczy Á, et al. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochemistry International. 2007;50(2):308–313. doi: 10.1016/j.neuint.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 252.Schwarz MJ, Guillemin GJ, Teipel SJ, Buerger K, Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. European Archives of Psychiatry and Clinical Neuroscience. 2013;263(4):345–352. doi: 10.1007/s00406-012-0384-x. [DOI] [PubMed] [Google Scholar]
- 253.Guidetti P, Luthi-Carter RE, Augood SJ, Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiology of Disease. 2004;17(3):455–461. doi: 10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 254.Stoy N, Mackay GM, Forrest CM, et al. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. Journal of Neurochemistry. 2005;93(3):611–623. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- 255.Ogawa T, Matson WR, Beal MF, et al. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992;42(9):1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 256.Lewitt PA, Li J, Lu M, Beach TG, Adler CH, Guo L. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Movement Disorders. 2013;28(12):1653–1660. doi: 10.1002/mds.25555. [DOI] [PubMed] [Google Scholar]
- 257.Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophrenia Bulletin. 2011;37(6):1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biological Psychiatry. 2001;50(7):521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 259.DeMyer MK, Shea PA, Hendrie HC, Yoshimura NN. Plasma tryptophan and five other amino acids in depressed and normal subjects. Archives of General Psychiatry. 1981;38(6):642–646. doi: 10.1001/archpsyc.1981.01780310042003. [DOI] [PubMed] [Google Scholar]
- 260.Joseph MS, Brewerton TD, Reus VI, Stebbins GT. Plasma L-tryptophan/neutral amino acid ratio and dexamethasone suppression in depression. Psychiatry Research. 1984;11(3):185–192. doi: 10.1016/0165-1781(84)90067-2. [DOI] [PubMed] [Google Scholar]
- 261.Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpé S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sciences. 2002;71(16):1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- 262.Maes M, Ombelet W, Verkerk R, Bosmans E, Scharpé S. Effects of pregnancy and delivery on the availability of plasma tryptophan to the brain: relationships to delivery-induced immune activation and early post-partum anxiety and depression. Psychological Medicine. 2001;31(5):847–858. doi: 10.1017/s0033291701004007. [DOI] [PubMed] [Google Scholar]


