Abstract
Skin exposure to ultraviolet (UV) light evokes a complex stress response in keratinocytes. Keratin filament organization provides structural stability and mechanical integrity of keratinocytes. Involucrin is a transglutaminase substrate protein contributing to the formation of insoluble cornified envelopes. However, a more complex role for keratins and involucrin has been proposed, including the regulation of cell stress response. The aim was to evaluate modulations of keratin 1, 10 and involucrin expression in HaCaT in the light of the complex response of these cells to UV-B radiation, including effects on c-Jun and matrix metalloproteinase 1 (MMP-1) gene expression and production of interleukin (IL) 6 and 8. A UV-B (300±5 nm) dose of 10 mJ/cm2 was selected since this dose resulted in a partial decrease in cell viability in contrast to higher UV-B doses, which induced complete cell death 48 h after treatment. The UV-B radiation induced significant expression of keratin 1 and 10 and decreased expression of involucrin. This was accompanied by increased expression of c-Jun and MMP-1 and IL-6 and IL-8 production. The data suggest that the expression of keratin 1, 10 and involucrin is modulated in HaCaT keratinocytes as a part of the complex stress response to UV radiation.
Keywords: involucrin, keratinocyte, keratin, ultraviolet light, inflammation
Introduction
Skin exposure to the UV component of sunlight mediates extensive detrimental effects, including a disrupted inflammatory response, premature aging (photoaging) and cancer (Fisher et al., 1997; Matsumura & Ananthaswamy, 2004; Takashima & Bergstresser, 1996). The acute response of the skin to a high dose of UV light is characterized by the stress response of skin cells, the death of damaged cells, and the induction of inflammatory response (Ichihashi et al., 2003).
The majority of UV-B rays are absorbed in the epidermis and keratinocytes are the most exposed cell type. The complex response of keratinocytes to UV exposure includes increased expression and activation of the transcriptional factor activating protein-1 (AP-1, composed of c-Jun and c-Fos) and nuclear transcriptional factor κB (NF-κB). Among other effects, AP-1 stimulates the production of matrix metalloproteinase (MMPs) in both the epidermis and dermis, leading to degradation of collagen and elastic fibers, which contributes to the photoaging process (Fisher et al., 2002). The activation of NF-κB stimulates the production of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6, and IL-8. The signaling pathways triggered by these cytokines further stimulate the action of AP-1 and NF-κB and thereby amplify the UV response in keratinocytes, promoting a multifaceted inflammatory response in the skin, involving various cell types (Heck et al., 2004).
There is evidence that the response of keratinocytes to UV light is also associated with changes in the expression of keratins and involucrin. Keratins are the basic material for filaments, whose organization provides structural stability and flexibility and ensures the mechanical integrity of keratinocytes (Coulombe & Lee, 2012; Chamcheu et al., 2012). However, keratins have been suggested to have more complex roles, which are associated with regulatory functions (Coulombe & Lee, 2012; Chamcheu et al., 2012). They form complex signaling networks, interacting with various kinases and adaptor and apoptotic proteins to regulate apoptosis, cell architecture, stress response, protein synthesis, and organelle and vesicle (re)distribution. When keratinocytes begin to stratify and differentiate, they acquire the differentiation-specific keratin 1 (K 1) and keratin 10 (K 10). Interestingly, these keratins are supposed to possess various newly-described functions related to the regulation of cell and tissue growth in the epidermis. In vitro experiments indicated a direct involvement of K10 in cell cycle control (Paramio et al., 1999; Paramio et al., 2001). Mutations in either K10 or its partner K1 can contribute to induction of hyperproliferation in the basal layer of the epidermis, hyperplasia in the basal compartment of the epidermis, and hyperkeratosis, as confirmed in K10 deficient mice (Reichelt et al., 2004; Reichelt & Magin, 2002). Furthermore, involucrin, as a protein rich in glutamines and lysines, is important for crosslinking by transglutaminase to build the cornified envelope, which is formed during the terminal maturation of keratinocytes in corneocytes (Kalinin et al., 2002). Both involucrin and keratin expression is modulated in diseases associated with alterations of the terminal differentiation of keratinocytes, e.g. psoriasis and cancer (Commandeur et al., 2009; Chen et al., 2013).
Conflicting information is available on the effects of UV radiation on keratinocyte differentiation. Studies of the modulation of keratin and involucrin expression by UV radiation yielded incompatible results (Bernerd & Asselineau, 1997; Bosset et al., 2003; Horio et al., 1993; Lee et al., 2002; Mammone et al., 2000; Smith & Rees, 1994).
The aim of this study was to evaluate the modulation of K 1, K 10 and involucrin expression in the HaCaT human immortalized keratinocyte cell line exposed to UV-B-radiation in the context of the complex response of these cells to UV-B radiation.
Methods
Cell cultures, UV-B irradiation, and determination of cell viability
The spontaneously immortalized human keratinocyte cell line HaCaT (gift of Prof. Dr. N. Fusenig (Boukamp et al., 1988)) was grown in Dulbecco's modified Eagle's medium (DMEM, Sigma, St Louis, Missouri) supplemented with 10% fetal bovine serum (BFS, Invitrogen, Carlsbad, CA, USA), glutamine (0.29 mg/ml) (Sigma) and gentamycin (50 µg/ml) (Invitrogen) in 5% CO2 at 37 °C, as described previously (Ruszova et al., 2013; Ruszova et al., 2008).
UV-B radiation doses (5 to 50 mJ/cm2) were applied as reported previously (Hasova et al.), employing a 1000 W xenon solar UV-simulator equipped with a dichroic mirror and a 300±5 nm interference filter (Oriel Instruments, Stratford, CT, USA). Before UV-B treatment, the cell culture medium was replaced by phosphate buffer saline (pH 7.4, PBS). Immediately after UV-B radiation, PBS was replaced by the cell culture medium, and cells and/or cell media were harvested 1, 6, 24 and 48 hours after UV-B radiation. Cell viability was measured using a 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide (MTT) assay, as described previously (Vistejnova et al., 2009).
Determination of gene expression
Total RNA was isolated using Trizol® (Invitrogen) and the synthesis of cDNA was carried out using Revert-Aid H Minus MuLV-Reverse transcriptase (Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions. A quantitative real time reverse transcription polymerase chain reaction (qRT-PCR) was accomplished using Gene Expression TaqMan Assays (c-Jun: Hs_99999141_s1, MMP-1: Hs_00899658_m1, K 1: Hs00196158_m1, K 10: Hs00166289_m1, involucrin: Hs00846307_s1) on employing a Miniopticon RT-PCR system instrument (Bio-Rad, Hercules, CA, USA) at universal cycling conditions (15 minutes at 95 °C, 40 cycles for 15 s at 95 °C, and 1 minute at 60 °C). Cycle threshold values were determined from the correlation factor of the calibration curve data by means of threshold analysis using Bio-Rad Opticon software (Bio-Rad). The threshold cycle (Ct) was determined for genes of interest while actin β was used as a house keeping gene, and the relative amount of mRNA in each sample was calculated based on its Ct value normalized with the Ct value of the housekeeping gene (actin β).
Determination of cytokine production
The concentration of cytokines in the cell culture supernatant was determined by commercial Enzyme-Linked Immunosorbent Assay kits (Bender MedSystems, Austria) (Hasova et al.). The obtained values were related to the amount of viable cells.
Statistical analysis
The results show the mean ± SEM derived from at least three independent experiments. Analysis was performed with the paired Student‘s t-test, and p≤0.05 was considered statistically significant.
Results
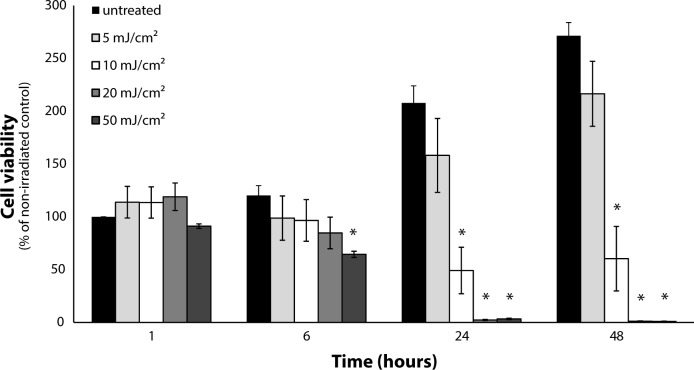
The modulation of HaCaT viability by a range of UV-B doses was screened (Figure 1). Doses of 20 and 50 mJ/cm2 UV-B radiation induced complete cell death of keratinocytes after 24 hours (Figure 1). A 10 mJ/cm2 UV-B dose affected the viability, which however did not fall lower than by approximately 40% even after 48 hours, providing enough surviving cells for further analyses (Figure 1). In contrast, a dose of 5 mJ/cm2 did not significantly affect cell numbers compared to non-irradiated control. Thus, the 10 mJ/cm2 dose was selected as a model dose for further evaluation of keratinocyte stress response to UV-B radiation.
Figure 1.
Dose-dependent reduction in human keratinocyte viability induced by UV-B radiation. Keratinocytes were irradiated by UV-B (5, 10, 20, 50 mJ/cm2) and cell viability was determined by the MTT assay 1 to 48 hours after UV radiation. Data are expressed as mean ± SEM (n=4). Statistically significant differences (Student's t-test, p≤0.05) to the respective non-radiated control group incubated for 1 hour are marked with an asterisk.
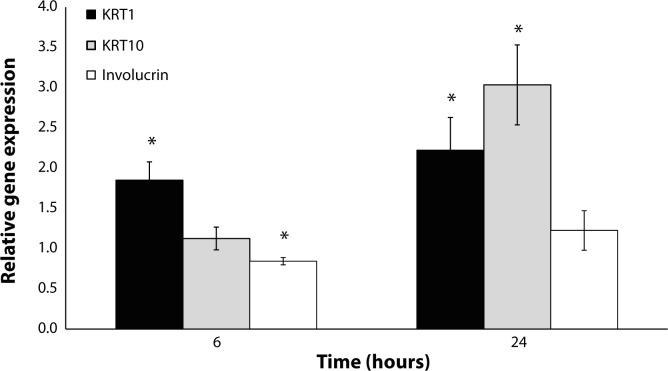
Employing this optimized protocol of the UV-B radiation of HaCaT, the expressions of K 1, K 10 and involucrin were evaluated. Interestingly, UV-B radiation increased the expression of K 1 both 6 and 24 hours after the irradiation (Figure 2). The expression of K 10 was increased significantly only 24 hours after irradiation (Figure 2). In contrast, involucrin expression was significantly decreased 6 hours after irradiation and unchanged after the longer time interval (Figure 2).
Figure 2.
Modulation of the gene expressions of K 1, K10 and involucrin in HaCaT induced 6 and 24 hours after UV-B irradiation (10 mJ/cm2). Data are expressed as a percentage of non-irradiated control mean ± SEM (n=6). Statistically significant differences (Student's t-test, p≤0.05) to the respective non-radiated control group are marked with an asterisk.
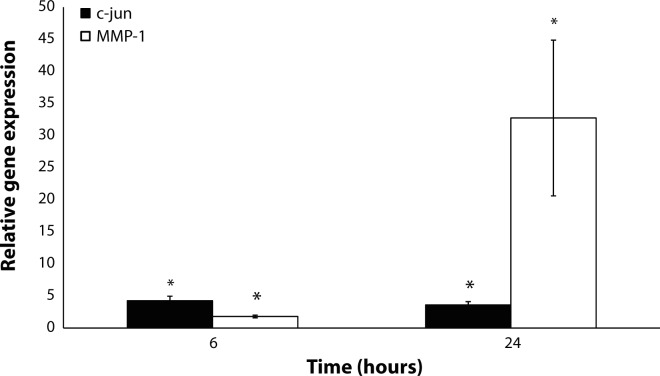
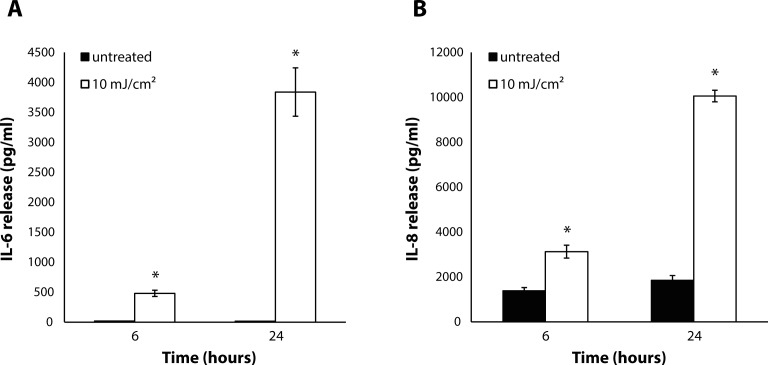
These UV-B radiation-modulated changes in the expressions of K 1, K 10 and involucrin were accompanied by a significant increase in the expression of c-Jun compared to control 6 and 24 hours after the irradiation (Figure 3). Moreover, we observed a significant increase in MMP-1 expression after 6 and 24 hours (Figure 3). In addition, a UV-B dose of 10 mJ/cm2 stimulated the release of IL-6 and IL-8 after 6 and 24 hours, with more profound effects 24 hours after irradiation (Figure 4).
Figure 3.
Increases in the gene expressions of c-Jun and MMP-1 in HaCaT induced 6 and 24 hours after UV radiation (10 mJ/cm2). Data are expressed as a percentage of non-irradiated control mean ± SEM (n=6). Statistically significant differences (Student's t-test, p≤0.05) to the respective non-irradiated control group are marked with an asterisk.
Figure 4.
Increases in IL-6 (A) and IL-8 (B) production by keratinocytes into the medium 6 and 24 after UV radiation (10 mJ/cm2). Data are expressed as mean ± SEM (n=3). Statistically significant differences (Student's t-test, p≤0.05) to the respective non-irradiated control group are marked with an asterisk.
Discussion
The activation of a range of protective and reparative intracellular mechanisms and the production of various mediators stimulating the development of local inflammatory response are features of the complex stress response of keratinocytes to sublethal doses of UV radiation. In this study, we showed that a dose of UV radiation which did not induce complete cell death in human keratinocytes in vitro modulated the gene expressions of K 1, K 10 and involucrin. These effects were part of a complex reaction to UV radiation, which also included increased expression of c-Jun and MMP-1, and the production of IL-6 and IL-8.
To evaluate the mechanisms of keratinocyte response to UV radiation, a single dose of 10 mJ/cm2 of UV-B radiation (300±5 nm) was selected as the smallest dose with detectable biological effects. This dose did not induce complete death of keratinocytes under the conditions applied in our study, thus providing enough surviving cells for further analyses of the modulation of cell physiology. Similar keratinocyte sensitivity to UV-B radiation was also observed by other authors (Hunt et al., 2006; Ishida & Sakaguchi, 2007).
The expressions of selected keratins and involucrin are recognized as markers of keratinocyte differentiation and maturation. To this date, inconsistent information exists about the modulation of keratin and involucrin expression by human keratinocytes in response to UV radiation. This study showed that the expression of K 1 and K 10 were increased after UV-B irradiation, suggesting that this acute response of keratinocytes to UV-B radiation is followed by induction of a higher level of keratinocyte differentiation status in the case of filament formation. Currently, the results for keratin expressions after UV-B irradiation of the skin are inconsistent, since one study found an increase in K 1 and K 10 in suprabasal located keratinocytes (Smith & Rees, 1994), some studies reported decreased K 1 and K 10 expression in keratinocytes (Bernerd & Asselineau, 1997; Horio et al., 1993), and one study reported that UV-B radiation had no effect on K 1 gene expression (Lee et al., 2002). The increase in K 10 could have an impact on the regulation of HaCaT response to UV radiation, particularly a decrease in cell proliferation, since K10 was shown to contribute to the inhibition of cell cycle entry (Paramio et al., 1999). Accordingly, a loss of K 10 was reported to be connected with increased proliferation of keratinocytes (Reichelt et al., 2004). K 1 has been suggested to participate in an inflammatory network in keratinocytes, since the absence of K 1 caused an increase in keratinocyte-autonomous IL-18 expression and release (Roth et al., 2012).
In this study, involucrin expression was decreased but at the time of the most robust decrease in keratin expression, i.e.24 hours after irradiation, involucrin was unchanged. These results indicate distinct responses to UV irradiation of proteins involved in keratinocyte differentiation. In general, involucrin expression is known to be increased after UV irradiation and/or in sun-exposed skin (Bertrand-Vallery et al., 2010; Bosset et al., 2003; Gambichler et al., 2008; Kwon et al., 2008; Lee et al., 2002), but there are also studies reporting decreased involucrin expression (Mammone et al., 2000) or no effect of UV irrradiation on involucrin (Bernerd & Asselineau, 1997). These significant differences between the results obtained depend on the UVB source, the dose, and the biological model. It was also reported that other factors which produced a response to UV radiation, such as IL-1, TNF-α and c-JUN, were involved in involucrin induction in keratinocytes (Han et al., 2012; Yano et al., 2008), a finding that we did not observe.
The acute reaction of keratinocytes to UV radiation has been suggested to be mediated by early response transcription factor c-Jun, which is a part of the AP-1 complex stimulating inflammatory and other processes. In agreement with this, we observed increased gene expression of this transcriptional factor. It has been shown that c-Jun transcription factors participate in the UV-B-induced breakdown of the dermal extracellular matrix by inducing the expressions of a series of MMP responsible for collagen degradation (Fisher et al., 1999; Soriani et al., 2000). This reaction further continues with the activation of the MMP-1 gene, a direct downstream target of the AP-1 factor. In the present study, we observed increased MMP-1 expression 6 and 24 hours after single UV-B irradiation of keratinocytes. Increased MMP-1 expression after solar-simulated UV irradiation was reported in human skin in vivo, suggesting the importance of these in vitro observations (Lahmann et al., 2001).
The response of keratinocytes to UV radiation comprises a strong positive feedback loop, which is responsible for the amplification of the reaction and leads to the undesirable manifestations of acute UV damage to the skin. In the present work, a UV-B-induced production of IL-6 and IL-8 was observed, which was in accordance with other authors presenting findings on various pro-inflammatory cytokines (Schwarz & Luger, 1989; Takashima & Bergstresser, 1996), including IL-1,IL-6 (Kirnbauer et al., 1989) and IL-8 (Kondo et al., 1993; Pernet et al., 1999). The secretion of these cytokines may augment local immunological and inflammatory reactions following UV irradiation (Schwarz & Luger, 1989).
In conclusion, increases in the expression of the keratins K 1 and K 10 and a decrease in the expression of involucrin were described in UV-B-irradiated human keratinocytes. These changes are presented in the context of the typical keratinocyte response to UV-B radiation, which includes an increase in the expression of the stress-related transcriptional factor c-Jun and a consequent increase in MMP-1 expression, as well as the production of pro-inflammatory cytokines IL-6 and IL-8. All these changes were time dependent and more evident 24 hours after irradiation, when cell viability significantly decreased. On balance, these responses could be interrelated. Keratins may affect the ability of cells to proliferate or affect interleukin networks (Paramio et al., 2001; Roth et al., 2012). Conversely, inflammation response, e.g. the production of IL-1, was found to have a strong effect on the creation of hyperkeratosis, which included changes in keratin and involucrin levels (O'Shaughnessy et al., 2010). Transcription factor AP-1 and its component c-Jun are induced by various stimuli, e.g. cytokines or stress stimuli, which in turn lead to the regulation of cell proliferation and differentiation (Shaulian & Karin, 2002).
Acknowledgements
The authors are grateful to J. Stejskalova, K. Becickova and M. Felgrova for technical assistance with cell cultivation and protein assays. Lukas Kubala was supported by the European Regional Development Fund – Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
REFERENCES
- 1.Bernerd F, Asselineau D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev Biol. 1997;183(2):123–138. doi: 10.1006/dbio.1996.8465. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand-Vallery V, Boilan E, Ninane N, Demazy C, Friguet B, Toussaint O, Poumay Y, Debacq-Chainiaux F. Repeated exposures to UVB induce differentiation rather than senescence of human keratinocytes lacking p16(INK-4A) Biogerontology. 2010;11(2):167–181. doi: 10.1007/s10522-009-9238-y. [DOI] [PubMed] [Google Scholar]
- 3.Bosset S, Bonnet-Duquennoy M, Barre P, Chalon A, Lazou K, Kurfurst R, Bonte F, Schnebert S, Disant F, Le Varlet B, Nicolas JF. Decreased expression of keratinocyte beta1 integrins in chronically sun-exposed skin in vivo. Br J Dermatol. 2003;148(4):770–778. doi: 10.1046/j.1365-2133.2003.05159.x. [DOI] [PubMed] [Google Scholar]
- 4.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Commandeur S, de Gruijl FR, Willemze R, Tensen CP, El Ghalbzouri A. An in vitro three-dimensional model of primary human cutaneous squamous cell carcinoma. Exp Dermatol. 2009;18(10):849–856. doi: 10.1111/j.1600-0625.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- 6.Coulombe PA, Lee CH. Defining keratin protein function in skin epithelia: epidermolysis bullosa simplex and its aftermath. J Invest Dermatol. 2012;132(3 Pt 2):763–775. doi: 10.1038/jid.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GJ, Talwar HS, Lin J, Voorhees JJ. Molecular mechanisms of photoaging in human skin in vivo and their prevention by all-trans retinoic acid. Photochem Photobiol. 1999;69(2):154–157. doi: 10.1562/0031-8655(1999)069<0154:mmopih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 10.Gambichler T, Rotterdam S, Tigges C, Altmeyer P, Bechara FG. Impact of ultraviolet radiation on the expression of marker proteins of gap and adhesion junctions in human epidermis. Photodermatol Photoimmunol Photomed. 2008;24(6):318–321. doi: 10.1111/j.1600-0781.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 11.Han B, Rorke EA, Adhikary G, Chew YC, Xu W, Eckert RL. Suppression of AP1 transcription factor function in keratinocyte suppresses differentiation. PLoS One. 2012;7(5):36941. doi: 10.1371/journal.pone.0036941. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Hasova M, Crhak T, Safrankova B, Dvorakova J, Muthny T, Velebny V, Kubala L. Hyaluronan minimizes effects of UV irradiation on human keratinocytes. Arch Dermatol Res. 2011;303(4):277–284. doi: 10.1007/s00403-011-1146-8. [DOI] [PubMed] [Google Scholar]
- 13.Heck DE, Gerecke DR, Vetrano AM, Laskin JD. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol Appl Pharmacol. 2004;195(3):288–297. doi: 10.1016/j.taap.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Horio T, Miyauchi H, Sindhvananda J, Soh H, Kurokawa I, Asada Y. The effect of ultraviolet (UVB and PUVA) radiation on the expression of epidermal keratins. Brit J Dermatol. 1993;128(1):10–15. doi: 10.1111/j.1365-2133.1993.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 15.Hunt DW, Boivin WA, Fairley LA, Jovanovic MM, King DE, Salmon RA, Utting OB. Ultraviolet B light stimulates interleukin-20 expression by human epithelial keratinocytes. Photochem Photobiol. 2006;82(5):1292–1300. doi: 10.1562/2005-08-31-RA-668. [DOI] [PubMed] [Google Scholar]
- 16.Chamcheu JC, Wood GS, Siddiqui IA, Syed DN, Adhami VM, Teng JM, Mukhtar H. Progress towards genetic and pharmacological therapies for keratin genodermatoses: current perspective and future promise. Exp Dermatol. 2012;21(7):481–489. doi: 10.1111/j.1600-0625.2012.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JQ, Man XY, Li W, Zhou J, Landeck L, Cai SQ, Zheng M. Regulation of Involucrin in Psoriatic Epidermal Keratinocytes: The Roles of ERK1/2 and GSK-3beta. Cell Biochem Biophys. 2013;66(3):523–528. doi: 10.1007/s12013-012-9499-y. [DOI] [PubMed] [Google Scholar]
- 18.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189(1–2):21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 19.Ishida T, Sakaguchi I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biol Pharm Bull. 2007;30(5):928–934. doi: 10.1248/bpb.30.928. [DOI] [PubMed] [Google Scholar]
- 20.Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002;24(9):789–800. doi: 10.1002/bies.10144. [DOI] [PubMed] [Google Scholar]
- 21.Kirnbauer R, Kock A, Schwarz T, Urbanski A, Krutmann J, Borth W, Damm D, Shipley G, Ansel JC, Luger TA. IFN-beta 2, B cell differentiation factor 2, or hybridoma growth factor (IL-6) is expressed and released by human epidermal cells and epidermoid carcinoma cell lines. J Immunol. 1989;142(6):1922–1928. [PubMed] [Google Scholar]
- 22.Kondo S, Kono T, Sauder DN, McKenzie RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. The Journal of investigative dermatology. 1993;101(5):690–694. doi: 10.1111/1523-1747.ep12371677. [DOI] [PubMed] [Google Scholar]
- 23.Kwon OS, Yoo HG, Han JH, Lee SR, Chung JH, Eun HC. Photoaging-associated changes in epidermal proliferative cell fractions in vivo. Arch Dermatol Res. 2008;300(1):47–52. doi: 10.1007/s00403-007-0812-3. [DOI] [PubMed] [Google Scholar]
- 24.Lahmann C, Young AR, Wittern KP, Bergemann J. Induction of mRNA for matrix metalloproteinase 1 and tissue inhibitor of metalloproteinases 1 in human skin in vivo by solar simulated radiation. Photochem Photobiol. 2001;73(6):657–663. doi: 10.1562/0031-8655(2001)073<0657:IOMFMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, An HT, Chung JH, Kim KH, Eun HC, Cho KH. Acute effects of UVB radiation on the proliferation and differentiation of keratinocytes. Photodermatol Photoimmunol Photomed. 2002;18(5):253–261. doi: 10.1034/j.1600-0781.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 26.Mammone T, Gan D, Collins D, Lockshin RA, Marenus K, Maes D. Successful separation of apoptosis and necrosis pathways in HaCaT keratinocyte cells induced by UVB irradiation. Cell Biol Toxicol. 2000;16(5):293–302. doi: 10.1023/a:1026746330146. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195(3):298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 28.O'Shaughnessy RF, Choudhary I, Harper JI. Interleukin-1 alpha blockade prevents hyperkeratosis in an in vitro model of lamellar ichthyosis. Hum Mol Genet. 2010;19(13):2594–2605. doi: 10.1093/hmg/ddq145. [DOI] [PubMed] [Google Scholar]
- 29.Paramio JM, Casanova ML, Segrelles C, Mittnacht S, Lane EB, Jorcano JL. Modulation of cell proliferation by cytokeratins K10 and K16. Mol Cell Biol. 1999;19(4):3086–3094. doi: 10.1128/mcb.19.4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol. 2001;21(21):7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pernet I, Sagot V, Schmitt D, Viac J. UVA1 and UVB radiation but not PGE2 stimulate IL-8 release in normal human keratinocytes. Arch Dermatol Res. 1999;291(9):527–529. doi: 10.1007/s004030050448. [DOI] [PubMed] [Google Scholar]
- 32.Reichelt J, Furstenberger G, Magin TM. Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice. J Invest Dermatol. 2004;123(5):973–981. doi: 10.1111/j.0022-202X.2004.23426.x. [DOI] [PubMed] [Google Scholar]
- 33.Reichelt J, Magin TM. Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J Cell Sci. 2002;115(Pt 13):2639–2650. doi: 10.1242/jcs.115.13.2639. [DOI] [PubMed] [Google Scholar]
- 34.Roth W, Kumar V, Beer HD, Richter M, Wohlenberg C, Reuter U, Thiering S, Staratschek-Jox A, Hofmann A, Kreusch F, Schultze JL, Vogl T, Roth J, Reichelt J, Hausser I, Magin TM. Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J Cell Sci. 2012;125(Pt 22):5269–5279. doi: 10.1242/jcs.116574. [DOI] [PubMed] [Google Scholar]
- 35.Ruszova E, Cheel J, Pavek S, Moravcova M, Hermannova M, Matejkova I, Spilkova J, Velebny V, Kubala L. Epilobium angustifolium extract demonstrates multiple effects on dermal fibroblasts in vitro and skin photo-protection in vivo. Gen Physiol Biophys. 2013;32(3):347–359. doi: 10.4149/gpb_2013031. [DOI] [PubMed] [Google Scholar]
- 36.Ruszova E, Pavek S, Hajkova V, Jandova S, Velebny V, Papezikova I, Kubala L. Photoprotective effects of glucomannan isolated from Candida utilis. Carbohydr Res. 2008;343(3):501–511. doi: 10.1016/j.carres.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4(5):E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz T, Luger TA. Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B. 1989;4(1):1–13. doi: 10.1016/1011-1344(89)80097-1. [DOI] [PubMed] [Google Scholar]
- 39.Smith MD, Rees JL. Wavelength-specific upregulation of keratin mRNA expression in response to ultraviolet radiation. J Invest Dermatol. 1994;102(4):433–439. doi: 10.1111/1523-1747.ep12372958. [DOI] [PubMed] [Google Scholar]
- 40.Soriani M, Hejmadi V, Tyrrell RM. Modulation of c-jun and c-fos transcription by UVB and UVA radiations in human dermal fibroblasts and KB cells. Photochem Photobiol. 2000;71(5):551–558. doi: 10.1562/0031-8655(2000)071<0551:mocjac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Takashima A, Bergstresser PR. Impact of UVB radiation on the epidermal cytokine network. Photochem Photobiol. 1996;63(4):397–400. doi: 10.1111/j.1751-1097.1996.tb03054.x. [DOI] [PubMed] [Google Scholar]
- 42.Vistejnova L, Dvorakova J, Hasova M, Muthny T, Velebny V, Soucek K, Kubala L. The comparison of impedance-based method of cell proliferation monitoring with commonly used metabolic-based techniques. Neuro Endocrinol Lett. 2009;30(Suppl 1):121–127. [PubMed] [Google Scholar]
- 43.Yano S, Banno T, Walsh R, Blumenberg M. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin-1. J Cell Physiol. 2008;214(1):1–13. doi: 10.1002/jcp.21300. [DOI] [PubMed] [Google Scholar]