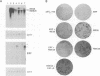
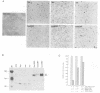
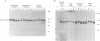Abstract
ERF (ETS2 Repressor Factor) is a novel member of the ets family of genes, which was isolated by virtue of its interaction with the ets binding site (EBS) within the ETS2 promoter. The 2.7 kb ubiquitously expressed ERF mRNA encodes a 548 amino acid phosphoprotein that exhibits strong transcriptional repressor activity on promoters that contain an EBS. The localization of the DNA-binding domain of the protein at the N-terminus and th repression domain at the C-terminus is reminiscent of the organization of ELK1-like members of the ets family; however, there is no significant homology between ERF and ELK1 or any other ets member outside the DNA-binding domain. The repressor activity of ERF can antagonize the activity of other ets genes that are known transcriptional activators. Furthermore, ERF can suppress the ets-dependent transforming activity of the gag-myb-ets fusion oncogene of ME26 virus. Although ERF protein levels remain constant throughout the cell cycle, the phosphorylation level of the protein is altered as a function of the cell cycle and after mitogenic stimulation. The ERF protein is also hyperphosphorylated in cells transformed by the activated Ha-ras and v-src genes and the transcription repressor activity of ERF is decreased after co-transfection with activated Ha-ras or the kinase domain of the c-Raf-1 gene, indicating that ERF activity is probably regulated by the ras/MAPK pathway. Consistent with the in vivo phosphorylation and inactivation by ras, ERF is efficiently phosphorylated in vitro by Erk2 and cdc2/cyclin B kinases, at sites similar to those detected in vivo. Furthermore, a single mutation at position 526 results in the loss of a specific phosphopeptide both in in vivo and in vitro (by Erk2) labeling. Substitution of Thr526 for glutamic acid also decreases the repression ability of ERF. Our data suggest a model in which modulation of ERF activity is involved in the transcriptional regulation of genes activated during entry into G1 phase. Obstruction of the ERF repressor function by the transactivating members of the ets family of genes (i.e.gag-myb-ets) may be essential for the control of genes involved in cell proliferation and may also underlie their tumorigenic effects.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Zhang X. F., Kyriakis J. M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994 Jul;19(7):279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Köhne A. C., Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992 Mar;11(3):1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear S. E., Bellacosa A., Lazo P. A., Jenkins N. A., Copeland N. G., Hanson C., Levan G., Tsichlis P. N. Provirus insertion in Tpl-1, an Ets-1-related oncogene, is associated with tumor progression in Moloney murine leukemia virus-induced rat thymic lymphomas. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7495–7499. doi: 10.1073/pnas.86.19.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. K., Thompson C. B., Lindsten T., June C. H., Fujiwara S., Koizumi S., Fisher R. J., Papas T. S. Reciprocal expression of human ETS1 and ETS2 genes during T-cell activation: regulatory role for the protooncogene ETS1. Proc Natl Acad Sci U S A. 1990 May;87(10):3723–3727. doi: 10.1073/pnas.87.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer K. J., Johnson G. L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994 Jun;19(6):236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Boulukos K. E., Pognonec P., Sariban E., Bailly M., Lagrou C., Ghysdael J. Rapid and transient expression of Ets2 in mature macrophages following stimulation with cMGF, LPS, and PKC activators. Genes Dev. 1990 Mar;4(3):401–409. doi: 10.1101/gad.4.3.401. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brickey D. A., Colbran R. J., Fong Y. L., Soderling T. R. Expression and characterization of the alpha-subunit of Ca2+/calmodulin-dependent protein kinase II using the baculovirus expression system. Biochem Biophys Res Commun. 1990 Dec 14;173(2):578–584. doi: 10.1016/s0006-291x(05)80074-9. [DOI] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Rapp U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992 Apr;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Brunner D., Dücker K., Oellers N., Hafen E., Scholz H., Klämbt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994 Aug 4;370(6488):386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Coffer P., de Jonge M., Mettouchi A., Binetruy B., Ghysdael J., Kruijer W. junB promoter regulation: Ras mediated transactivation by c-Ets-1 and c-Ets-2. Oncogene. 1994 Mar;9(3):911–921. [PubMed] [Google Scholar]
- Cowell I. G., Hurst H. C. Transcriptional repression by the human bZIP factor E4BP4: definition of a minimal repression domain. Nucleic Acids Res. 1994 Jan 11;22(1):59–65. doi: 10.1093/nar/22.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell I. G. Repression versus activation in the control of gene transcription. Trends Biochem Sci. 1994 Jan;19(1):38–42. doi: 10.1016/0968-0004(94)90172-4. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Feng S., Chen J. K., Yu H., Simon J. A., Schreiber S. L. Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science. 1994 Nov 18;266(5188):1241–1247. doi: 10.1126/science.7526465. [DOI] [PubMed] [Google Scholar]
- Fleischman L. F., Pilaro A. M., Murakami K., Kondoh A., Fisher R. J., Papas T. S. c-Ets-1 protein is hyperphosphorylated during mitosis. Oncogene. 1993 Mar;8(3):771–780. [PubMed] [Google Scholar]
- Fujiwara S., Fisher R. J., Bhat N. K., Diaz de la Espina S. M., Papas T. S. A short-lived nuclear phosphoprotein encoded by the human ets-2 proto-oncogene is stabilized by activation of protein kinase C. Mol Cell Biol. 1988 Nov;8(11):4700–4706. doi: 10.1128/mcb.8.11.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Koizumi S., Fisher R. J., Bhat N. K., Papas T. S. Phosphorylation of the ETS-2 protein: regulation by the T-cell antigen receptor-CD3 complex. Mol Cell Biol. 1990 Mar;10(3):1249–1253. doi: 10.1128/mcb.10.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Golub T. R., Barker G. F., Lovett M., Gilliland D. G. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell. 1994 Apr 22;77(2):307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., McNagny K., Brady G., Frampton J. Chicken "erythroid" cells transformed by the Gag-Myb-Ets-encoding E26 leukemia virus are multipotent. Cell. 1992 Jul 24;70(2):201–213. doi: 10.1016/0092-8674(92)90096-u. [DOI] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Han K., Manley J. L. Transcriptional repression by the Drosophila even-skipped protein: definition of a minimal repression domain. Genes Dev. 1993 Mar;7(3):491–503. doi: 10.1101/gad.7.3.491. [DOI] [PubMed] [Google Scholar]
- Hannon R., Evans T., Felsenfeld G., Gould H. Structure and promoter activity of the gene for the erythroid transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3004–3008. doi: 10.1073/pnas.88.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach B. M., Johnson A. D. Transcriptional repression in eukaryotes. Annu Rev Cell Biol. 1993;9:479–509. doi: 10.1146/annurev.cb.09.110193.002403. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Marais R., John S., Wynne J., Dalton S., Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta. 1993 Dec 23;1155(3):346–356. doi: 10.1016/0304-419x(93)90014-4. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., O'Farrell P. H. Active repression of transcription by the engrailed homeodomain protein. EMBO J. 1991 Jun;10(6):1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcyk C. L., Watson D. K., Mavrothalassitis G. J., Papas T. S. The human ETS1 gene: genomic structure, promoter characterization and alternative splicing. Oncogene. 1991 Apr;6(4):523–532. [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Karin M. Signal transduction from the cell surface to the nucleus through the phosphorylation of transcription factors. Curr Opin Cell Biol. 1994 Jun;6(3):415–424. doi: 10.1016/0955-0674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Lai Z. C., Rubin G. M. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992 Aug 21;70(4):609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Licht J. D., Grossel M. J., Figge J., Hansen U. M. Drosophila Krüppel protein is a transcriptional repressor. Nature. 1990 Jul 5;346(6279):76–79. doi: 10.1038/346076a0. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lim W. A., Richards F. M., Fox R. O. Structural determinants of peptide-binding orientation and of sequence specificity in SH3 domains. Nature. 1994 Nov 24;372(6504):375–379. doi: 10.1038/372375a0. [DOI] [PubMed] [Google Scholar]
- Lu Y. Y., Blair D. G., Segal S., Shih T. Y., Clanton D. J. Tumorigenicity, metastasis and suppression of MHC class-I expression in murine fibroblasts transformed by mutant v-ras deficient in GTP binding. Int J Cancer Suppl. 1991;6:45–53. doi: 10.1002/ijc.2910470712. [DOI] [PubMed] [Google Scholar]
- Macleod K., Leprince D., Stehelin D. The ets gene family. Trends Biochem Sci. 1992 Jul;17(7):251–256. doi: 10.1016/0968-0004(92)90404-w. [DOI] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Rauscher F. J., 3rd A structure-function analysis of transcriptional repression mediated by the WT1, Wilms' tumor suppressor protein. Oncogene. 1993 Jul;8(7):1713–1720. [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Mavrothalassitis G. J., Papas T. S. Positive and negative factors regulate the transcription of the ETS2 gene via an oncogene-responsive-like unit within the ETS2 promoter region. Cell Growth Differ. 1991 May;2(5):215–224. [PubMed] [Google Scholar]
- Mavrothalassitis G. J., Watson D. K., Papas T. S. Molecular and functional characterization of the promoter of ETS2, the human c-ets-2 gene. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1047–1051. doi: 10.1073/pnas.87.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrothalassitis G. J., Watson D. K., Papas T. S. The human ETS-2 gene promoter: molecular dissection and nuclease hypersensitivity. Oncogene. 1990 Sep;5(9):1337–1342. [PubMed] [Google Scholar]
- Mavrothalassitis G., Fisher R. J., Smyth F., Watson D. K., Papas T. S. Structural inferences of the ETS1 DNA-binding domain determined by mutational analysis. Oncogene. 1994 Feb;9(2):425–435. [PubMed] [Google Scholar]
- May W. A., Gishizky M. L., Lessnick S. L., Lunsford L. B., Lewis B. C., Delattre O., Zucman J., Thomas G., Denny C. T. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5752–5756. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- O'Neill E. M., Rebay I., Tjian R., Rubin G. M. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994 Jul 15;78(1):137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Paules R. S., Resnick J., Kasenally A. B., Ernst M. K., Donovan P., Vande Woude G. F. Characterization of activated and normal mouse Mos gene in murine 3T3 cells. Oncogene. 1992 Dec;7(12):2489–2498. [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. elk-1 proteins interact with MAP kinases. Oncogene. 1994 Jul;9(7):1855–1860. [PubMed] [Google Scholar]
- Roussel M. F., Davis J. N., Cleveland J. L., Ghysdael J., Hiebert S. W. Dual control of myc expression through a single DNA binding site targeted by ets family proteins and E2F-1. Oncogene. 1994 Feb;9(2):405–415. [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989 Sep 25;17(18):7539–7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoysky E., Mizuno T., Sowa Y., Watanabe H., Sawada J., Nomura H., Ohsugi Y., Handa H., Sakai T. The retinoblastoma binding factor 1 (RBF-1) site in RB gene promoter binds preferentially E4TF1, a member of the Ets transcription factors family. Oncogene. 1994 Jul;9(7):1839–1846. [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Ascione R., Fisher R. J., Mavrothalassitis G. J., Bhat N. K., Papas T. S. The ets gene family. Cell Growth Differ. 1992 May;3(5):327–334. [PubMed] [Google Scholar]
- Seth A., Hodge D. R., Thompson D. M., Robinson L., Panayiotakis A., Watson D. K., Papas T. S. ETS family proteins activate transcription from HIV-1 long terminal repeat. AIDS Res Hum Retroviruses. 1993 Oct;9(10):1017–1023. doi: 10.1089/aid.1993.9.1017. [DOI] [PubMed] [Google Scholar]
- Seth A., Papas T. S. The c-ets-1 proto-oncogene has oncogenic activity and is positively autoregulated. Oncogene. 1990 Dec;5(12):1761–1767. [PubMed] [Google Scholar]
- Seth A., Robinson L., Thompson D. M., Watson D. K., Papas T. S. Transactivation of GATA-1 promoter with ETS1, ETS2 and ERGB/Hu-FLI-1 proteins: stabilization of the ETS1 protein binding on GATA-1 promoter sequences by monoclonal antibody. Oncogene. 1993 Jul;8(7):1783–1790. [PubMed] [Google Scholar]
- Seth A., Watson D. K., Blair D. G., Papas T. S. c-ets-2 protooncogene has mitogenic and oncogenic activity. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7833–7837. doi: 10.1073/pnas.86.20.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei H., Nihonmatsu I., Yokokura T., Ueda R., Sano Y., Okuda T., Sato K., Hirata K., Fujita S. C., Yamamoto D. pokkuri, a Drosophila gene encoding an E-26-specific (Ets) domain protein, prevents overproduction of the R7 photoreceptor. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6856–6860. doi: 10.1073/pnas.89.15.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994 Feb;4(1):96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Thompson C. B., Kaelin W. G., Leiden J. M. Regulation of the Ets-related transcription factor Elf-1 by binding to the retinoblastoma protein. Science. 1993 May 28;260(5112):1330–1335. doi: 10.1126/science.8493578. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Maira S. M., Sobieszczuk P., Wasylyk B. Reversion of Ras transformed cells by Ets transdominant mutants. Oncogene. 1994 Dec;9(12):3665–3673. [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- Yuan C. C., Kan N., Dunn K. J., Papas T. S., Blair D. G. Properties of a murine retroviral recombinant of avian acute leukemia virus E26: a murine fibroblast assay for v-ets function. J Virol. 1989 Jan;63(1):205–215. doi: 10.1128/jvi.63.1.205-215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]