Abstract
Molecular gradients play a significant role in regulating biological and pathological processes. Although conventional gradient-generators have been used for studying chemotaxis and axon guidance, there are still many limitations, including the inability to maintain stable tempo-spatial gradients and the lack of the cell monitoring in a real-time manner. To overcome these shortcomings, microfluidic devices have been developed. In this study, we developed a microfluidic gradient device for regulating neuron axon guidance. A microfluidic device enables the generation of Brain-derived neurotrophic factor (BDNF) gradient profiles in a temporal and spatial manner. We test the effect of the gradient profiles on axon guidance, in the BDNF concentration gradient axon towards the high concentration gradient. This microfluidic gradient device could be used as a powerful tool for cell biology research.
INTRODUCTION
During nerve system development, growing axons are directed by multiple guidance cues to locate accurately targets (Ferrario et al., 2012; Lange et al., 2012; Fuchs et al., 2012; Mortimer et al., 2010). It is critical for the formation of correct neural circuits and nervous system function. Researches show that some of the most important cues guiding the formation of connections between neurons in the developing nervous system are molecular gradients (Masuda et al., 2012; Lowery and Van Vactor, 2009). When a growth cone migrates in a guidance cue gradient, the side of the growth cone facing higher concentrations of the cue will experience higher receptor occupancy (Tojima et al., 2011), leading to asymmetric intracellular signaling events mediated by second messengers such as Ca2+ and cyclic nucleotides (Akiyama et al., 2009; Togashi et al., 2008). This leads to polarization of the growth cone and a turn toward or away from the source of the guidance cue (Forbes et al., 2012).
Although in vivo studies on axon guidance are very powerful in identifying cues relevant to neural development, it is impossible to control the microenvironment around the neurons and evaluate the role of the multitude of cues to which the growth cones are exposed (Kothapalli et al., 2011). As reported, recently many researches use molecule concentration gradient to investigate the guidance mechanism of these cues in vitro (Kennedy et al., 2006; Thompson et al., 2011). Conventional gradient-generators, such as the Boyden chamber, the Zigmond chamber, the Dunn chamber, and micropipette-based assay, have been previously developed for studying chemotaxis and axon guidance (Falasca et al., 2011; Chung and Chen, 2009; Walheim et al., 2012), but these platforms are typically limited to simple, non-quantitative gradients that cannot be actively altered or precisely controlled in a reproducible fashion (Kim et al., 2010; 2011; Chung and Choo, 2010). Therefore, there is a growing need for generating a more robust and stable molecular gradient.
To overcome the limitations of conventional gradient-generators, microfluidic-based gradient devices have been recently developed. George M. Whitesides Laboratory first use laminar flow to generate stable gradients in microfluidic chip (Dertinger et al., 2001), gradient microfluidic device consisting of a series of microchannels connected to a cell culture chamber, two or three solutions are mixed and split in a microchannel, resulting in generating stable concentration gradients in perpendicular to the flow direction. Microfluidic gradient generators can produce spatial and temporal distributions of biochemical molecules by controlling advective and diffusive transport processes. Jeon studied neutrophil chemotaxis under interleukin-8 concentration gradient in 2002 (Jeon et al., 2002). By proving its effectiveness, gradient microfluidic have been widely used for cell biology applications (Seidi et al., 2011; Nitta et al., 2009), especially on cell chemotaxis and migration research (Li et al., 2012; Brett et al., 2012; Kim et al., 2013; Meier et al., 2011; Raja et al., 2010). By providing experimental platforms that allow systematic investigation and quantitative analysis, microfluidic devices have brought a new level of understanding that was not available with the large-scale devices of the past (Kim et al., 2010).
BDNF (Brain-derived neurotrophic factor) is a member of the “neurotrophin” family of growth factors; BDNF acts on certain neurons of the central nervous system and the peripheral nervous system, helping to support the survival of existing neurons and encourage the growth and differentiation of new neurons and synapses. Researches show BDNF and its receptor, tyrosine kinase B (TrkB), expressed by pyramidal neurons in the developing hippocampus in vivo and in vitro, are key mediators of axon guidance, synapse formation, and plasticity (Lindsley et al., 2011; Kang and Christopher, 2010; Cheng et al., 2011).
Since neuron growth cone guidance plays an important role in nerve development and regeneration, in order to further understand the mechanism of BDNF guidance, in this research, we constructed a gradient microfluidic chip and studied the axon guidance of dissociated primary hippocampal neurons to a diffusible gradient of BDNF in the absence of any other protein growth factors. In our research, we observed that BDNF not only affects the survival of neurons but also affects the nerve growth cone guidance. The described device can modify and extend our understanding of the complex growth cone guidance mechanisms. In future studies, by using this device, we will further reveal the BDNF guidance mechanism through signaling pathways research.
MATERIALS AND METHODS
Materials
Poly(dimethylsiloxane) (PDMS Sylgard 184), the curing agent, and Tygon tubing were purchased from Dow Corning (Midland, MI, USA). Paraformaldehyde, triton X-100, BSA (bovine serum albumin), fluorescein sodium salt, and Fluorescein isothiocyanate–dextran (FITC-dextran, average molecular weight 20 000) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Recombinant Human BDNF was obtained from Peprotech (Rocky Hill, NJ, USA). DMEM/F12 culture medium, FBS (fetal bovine serum), and B27 NeuroMix were acquired from Invitrogen. NSE (neuron-specific enolase) antibody (monoclonal mouse anti-rat), secondary goat anti-mouse biotin-conjugated IgG, avidin–biotin peroxidase complex, 3,3′-diaminobenzidine tetrahydrochloride, poly-l-Lysine were provide by Boster (Wuhan, Hubei, China). SU-8 was acquired from Microchem (Newton, MA, USA). The syringe pump (KDS-210) was acquired from Kd scientific (Holliston, MA, USA).
Chip design and fabrication
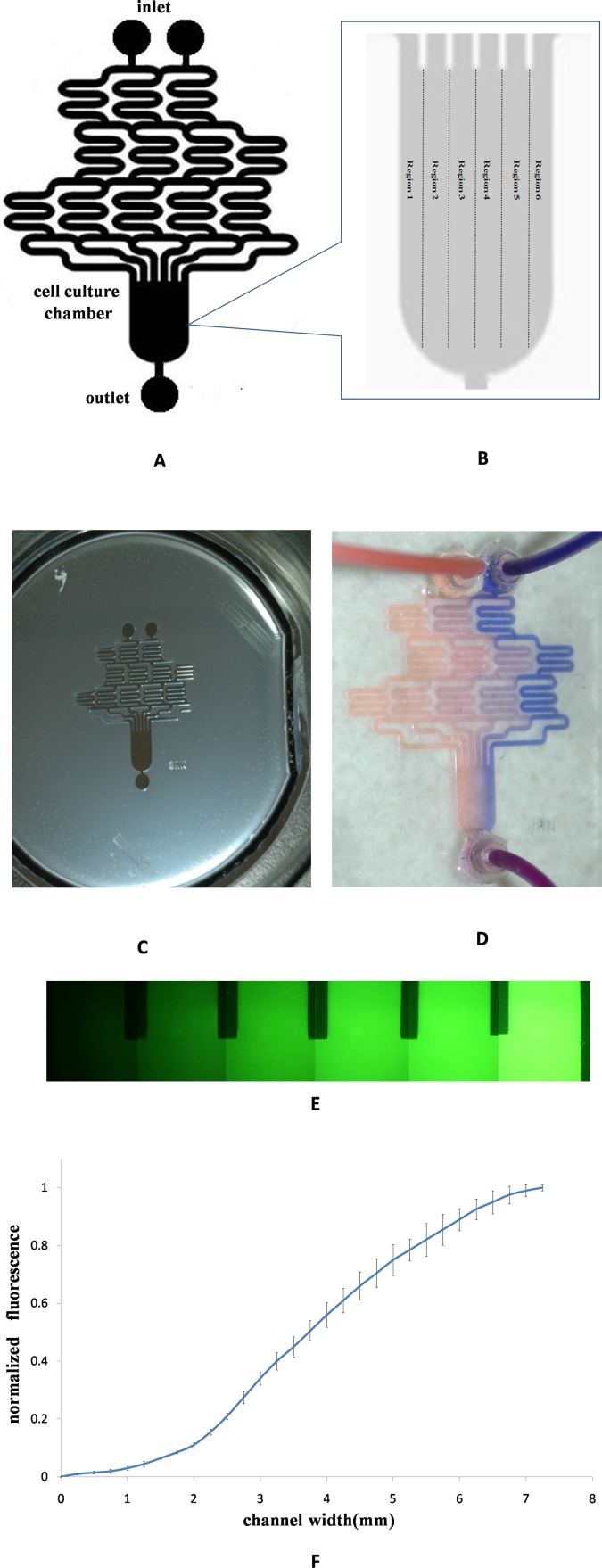
In this experiment, we use flow-based microfluidic platforms to form concentration gradients. The microfluidic was based on that originally proposed by Tirella et al. (2008) and modified, as shown in Fig. 1a. The devices consisted of the serpentine-based microchannel and cell culture chamber, two initial inputs and six final channels as affluents into the cell culture chamber, and following feature dimensions were chosen: microchannel width is 750 μm, the height is 250 μm, width of the cell culture chamber is 7250 μm, and length of the cell culture chamber is 10 000 μm.
Figure 1.
Chip fabrication and concentration gradients generation. (a) Schematic of gradient microfluidic chip. (b) Divided the cell culture chamber into six regions. (c) The SU-8 mold of gradients microfluidic chip. (d) Using blue and orange dyes to make the gradient formation visualization. (e) Using fluorescein to quantitatively analyse gradient profile. (f) The relationship between fluorescent intensity and the width of cell culture chamber.
The microfluidic device was fabricated by using a conventional soft lithographic techniques.
First, the master was fabricated by standard photolithography, which performed on the 24th Institute of Ministry of Electronics Industry (Chongqing, China). Briefly, SU-8 photoresist was spin-coated onto a silicon wafer to make microchannels. In experiment, the photoresist was spun in two stages: the first cycle at 800 rpm for 5 s and the second cycle of spinning at 1100 rpm for 25 s to produce the required thickness (250 μm). After soft-baking, the wafer was exposed to UV light through the mask for 12 min and then post-baked and developed. Then PDMS prepolymer was mixed in a 10:1 ratio with curing agent, the mixture was poured over the master mold and degassed in a vacuum oven, then cured at 70 °C for 1.5 h. After cooling, the PDMS replica containing microchannels was peeled off the master, cut to desired size, and access holes were punched for tubing. Finally, the PDMS structure is exposed to oxygen plasma (PDL-GC, Henye Co., Chengdu, Sichuan, China) and bonded onto glass at 250 W and 80 mTorr for 50 s. The device along with the inlet and outlet tubing was ethylene oxide sterilized overnight prior to microfluidic cell culture.
Gradient imaging
In order to characterize the concentration gradient profiles in the microfluidic device, orange and blue dyes, PBS buffer solution and 1.5 × 10−4M FITC-dextran (10 mM, MW 20 000), which have a similar molecular weight to BDNF (MW 27 000) were withdrawn from the left and right inlet reservoirs of the microchannel using a syringe pump. Images were taken using an inverted fluorescent microscope (AE31 EF-INV, Motic, Xiamen, Fujian, China) and confocal laser-scanning microscope (TCSTIV, Leica, Nussloch, Germany). The normalized fluorescent intensity profiles of concentration gradients were obtained from fluorescent images of fluorescein sodium.
Hippocampus neurons isolation
Animal experiments were carried out in compliance with the National Laws related to the conduct of animal experimentation. Primary neuronal cultures of hippocampus were prepared from postnatal day 1 (P1) of Wistar rats (Animal Center, Third Military Medical University, China) according to Brewer (1995). Rats were decapitated in ice-cold, oxygenated artificial cerebrospinal fluid (ACSF; in mM: NaCl 119, KCl 2.5, MgCl2 1.3, CaCl2 2.5, NaH2PO4 1, NaHCO3 26.2, and glucose 11). Meninges were removed, the hippocampi were dissected, and tissue was incubated for 15 min in trypsin/EDTA; the hippocampi were rinsed with PBS and dissociation medium (DMEM/F12 culture medium with B27 NeuroMix, 100 U/ml penicillin, and 100 μg/ml streptomycin), dissociated by Pasteur pipette, pelleted by centrifugation, and redissociated in medium for culture in microfluidic chip.
Cell loading to microfluidic chip and for axon guidance research
Prior to culture neurons in the chamber, 0.1 mg/ml solution of poly-l-lysine was injected into the chamber from the outlet and incubated for a minimum of 3 h to enhance cell adhesion in culture chamber, and then washed with PBS; after wash, the microchannel and culture chamber were filled with DMEM/F12 culture medium. The neurons were seeded into the devices immediately after dissociation through the outlet at the cell density of 5 × 106 cells/ml; all cells were cultured with DMEM/F12 medium plus B27 NeuroMix and maintained in a humidified incubator at 5% CO2 and 37 °C; neurons were allowed to adhere for 24 h before the gradient of BDNF medium starting perfusion. After 24 h, a digital syringe pump was connected to the two inlet tubes which in turn were connected to two reservoirs, one containing the 100 mg/l BDNF in serum free DMEM/F12 medium and one with only serum-free medium. The flow rate, controlled by the pump, was set at 1 μl/min. When BDNF concentration gradient was introduced to cell culture chamber, neurons were cultured for 5 days to observe the axon guidance effect of BDNF. In the control group, both two inlets were filled with 100 mg/l BDNF in serum free DMEM/F12 medium at 1 μl/min, and other conditions were the same as the BDNF gradient group.
Neuron-specific enolase immunostaining
To identify neuronal cells and make axon more clear, NSE immunostaining was performed at the end point of guidance experiment. The cells in microfluidic chamber were fixed in 4% paraformaldehyde and were subsequently permeabilized with 0.1% Triton X-100 in PBS for 15 min. They were also blocked using 1% BSA in PBS for 30 min at room temperature. Then the cells were incubated with a monoclonal NSE antibody (diluted 1:400) for 24 h at 4 °C and then incubated with secondary anti-mouse biotin-conjugated IgG (diluted 1:100). After being washed with PBS, neurons were incubated with an avidin–biotin peroxidase complex and visualized with 3,3′-diaminobenzidine tetrahydrochloride according to the manufacturer's instructions.
Microscopy, cell imaging, and data analysis
After neurons in microfluidic chip were fixed and NSE immunostaining, neurons were observed under an inverted optical microscope and photographed by NIS-elements BR 3.2 system (Nikon, Japan). The number of neurons and the direction of axon in each region of cell culture chamber were calculated and analysed.
RESULTS AND DISCUSSION
Chip fabrication and concentration gradients generation
The gradient generator is a network of microchannels that can create a concentration profile by controlled diffusive mixing from two input streams. As seen in the schematic (Fig. 1a), the microfluidic chip has long serpentine channels to allow complete diffusive mixing inside the microchannels. In order to quantitative analysis the guidance effect of BDNF, we divided the cell culture chamber into six regions (Fig. 1b), the width of each region was near 1210 μm. Chip was fabricated by using a conventional soft lithographic techniques, Fig. 1c showed the SU-8 mold of gradient microfluidic chip.
We characterized the stability and profile of gradient formation in cell culture chamber using two trace dyes solution and fluorescein. By using blue and orange dyes, we made the gradient formation visualization (Fig. 1d), as the streams of dye flow in the microchannel, they were repeatedly split at the junction, combined with neighboring streams, and allowed to mix by diffusion in the serpentine channels. At the end of the network, all streams carried different concentrations of blue and orange dyes combined into cell culture chamber; it took around 30 min for the gradient to reach steady state when the flow speed is set to be 5 μl/min. By using fluorescein sodium (Fig. 1e), we quantitatively analysed gradient profile in the cell culture chamber; Fig. 1f shows a linear gradient of fluorescent across the width of cell culture chamber. If keeping the fluid flow, we can get a long-time stable gradient for neuronal axon guidance studying in our experiment. By adjusting the concentration and flow speed of inlet fluid, we could generate multiple gradient profiles inside a single microfluidic device in a temporal and spatial manner.
Use microfluidic chip culture neurons
Microfluidic technology has emerged in the past few years as an attractive strategy for providing a simplified, controlled environment in cell research. Conveniently, PDMS is a biocompatible, optically transparent, gas-permeable polymer. In our experiment, first we used the chip culture neuron cells, dissociated hippocampus neurons were seeded through the outlet at the cell density of 5 × 106 cells/ml, cells were maintained with DMEM/F12 medium plus B27 NeuroMix, in about 1 h after plating the cells formed an uniform distribution across the surface, hippocampus neurons require 1 day of recovery in microfluidic chip after isolation prior to establishing the gradient. At the 2nd day, we changed the culture medium once a day; at the 3rd day of culture, cells were viable and extended axons, and the axon prolonged as time progressed, the morphology of the neurons in culture chambers is comparable to the bright-periphery somas seen in phase-contrast images that are a hallmark of thriving cultures. Neurons can be maintained in culture for up to 4 weeks without any adverse effects on the viability of the neurons, neurons were in physiological conditions. This give us a long time-window for studying the response of neurons to molecular and physical guidance cues.
Response of primary neurons to BDNF gradient in the device
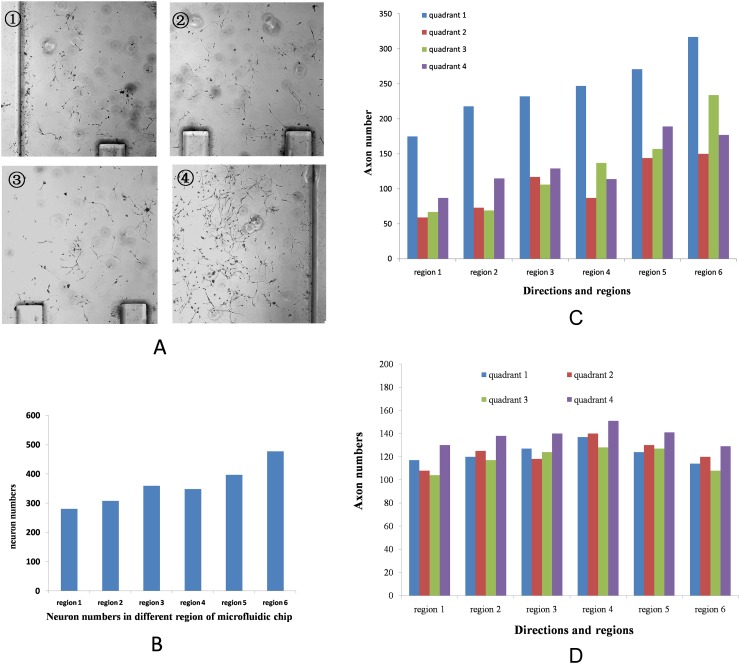
For studying the effect of BDNF concentration gradient on axon guidance, neurons were cultured under DMEM/F12 medium with 10% FBS in microfluidic chip for 1 day. At the 2nd day, we maintained the BDNF concentration gradient (form 0 to 100 mg/l) in cell culture chamber; at the 6th day, we stopped culture, paraformaldehyde fixed, and NSE stained. For experimenting neurons in every region of cell culture chamber, these parameters were measured: (1) neuron numbers in each region and (2) the direction of axon growth. In order to evaluate the neuron guidance angle, we divided into four quadrants from each neuron soma center, among the four quadrants, values in 1st quadrant (−45° to 45°) reflect growth towards guidance cue and in 3rd quadrant (135° to 225°) reflect axon turning away from the guidance cue (Fig. 2). The number of axons in each quadrant was counted for devices cultured under similar conditions. Figs. 3a, 3b show the population view of the neurons at the end of the experimental time period under gradient concentration. With increasing BDNF concentration, cell numbers progressively increased. Fig. 3c shows the directions and the number of axon in different chamber area of BDNF gradient microfluidic chip; we can see more axons turning towards the high concentration gradient. In control group, the number of axons towards to quadrant 4 was slightly higher than other quadrants, which may be due to the fluidic mechanical stress (Polacheck et al., 2013).
Figure 2.
The method to evaluate the neuron guidance angle.
Figure 3.
Neurons response in the BDNF gradient and BDNF uniform microfluidic chip. (a) Population view of the neurons in the BDNF gradient microfluidic chip. From ① to ④, the concentration of BDNF concentration is increased, cell numbers increased too. (b) The number of neurons in different regions of BDNF gradient chip. (c) The direction of axon in the gradient microfluidic chip. Most of axons towards the high concentration gradient. (d) The direction of axon in the BDNF uniform microfluidic chip.
BDNF acts on certain neurons of the central nervous system and the peripheral nervous system, helping to support the survival of existing neurons and encourage the growth and differentiation of new neurons and synapses. Researches show BDNF and its receptor, TrkB, expressed by pyramidal neurons in the developing hippocampus in vivo and in vitro, are key mediators of axon guidance, synapse formation, and plasticity. The axonal elongation guidance was tested on the fabricated microfluidic device. We observed the growth and elongation of the hippocampus neurons which were stimulated by releasing BDNF gradient.
CONCLUSION
Microfluidic technology has become an attractive platform for analysis of biological phenomena. Flow-based microfluidic systems have been utilized for neuron axon guidance studies, given their ability to generate versatile and precisely defined chemical gradients. In some studies, researchers trace the neurons’ axon trajectories under biomolecule gradient during the whole experiment, which may make the cells under microscopy light beam to not be in physiological status conditions. In order to eliminate the influence, in our study we observed the axon guidance result at the end-point of the experiment. We observed more than 2000 neuron axon guidance instead of analysing single neuron axon guidance, in which cell-cell interaction may be lost during the experiment. In our experiment, we developed a microfluidic gradient device for regulating neuron axon guidance. A microfluidic device provided the generation of BDNF gradient profiles. We demonstrated the long term stability of a gradient of the diffusible guidance molecule BDNF. We tested the effect of the gradient profiles on axon guidance, in the BDNF concentration gradient axon towards the high concentration gradient. Therefore, this microfluidic gradient device could be a powerful tool for controlling the function of neuron cells and understanding cell biology. However, it is clear that further investigations will emerge from ongoing and future collaborations between neurobiologists and bioengineers.
ACKNOWLEDGMENTS
The authors of this paper would like to acknowledge support for this work from National Natural Science Foundation of China (No. 31371016) and Natural Science Foundation Project of CQ CSTC (CSTC, 2009BB5335). We also thank the 24th Institute of Ministry of Electronics Industry of China for helping us in chip fabrication.
References
- Akiyama, H., Matsu-ura, T., Mikoshiba, K., and Kamiguchi, H., Sci. Signal. 2, ra34 (2009). 10.1126/scisignal.2000196 [DOI] [PubMed] [Google Scholar]
- Brett, M. E., Deflorio. R., Stone, D. E., and Eddington, D. T., Lab Chip 12, 3127–3134 (2012). 10.1039/c2lc40398f [DOI] [PubMed] [Google Scholar]
- Brewer, G. J., J. Neurosci. Res. 42, 674–768 (1995). 10.1002/jnr.490420510 [DOI] [PubMed] [Google Scholar]
- Cheng, P. L., Song, A. H., Wong, Y. H., Wang, S., Zhang, X., and Poo, M. M., Proc. Natl. Acad. Sci. U.S.A. 108, 18430–18435 (2011). 10.1073/pnas.1115907108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, B. G. and Choo, J., Electrophoresis 31, 3014–3027 (2010). 10.1002/elps.201000137 [DOI] [PubMed] [Google Scholar]
- Chung, C. A. and Chen, C. Y., J. Theor. Biol. 261, 610–625 (2009). 10.1016/j.jtbi.2009.08.030 [DOI] [PubMed] [Google Scholar]
- Dertinger, S. K. W., Chiu, D. T., Jeon, N. L., and Whitesides, G. M., Anal. Chem. 73, 1240–1246 (2001). 10.1021/ac001132d [DOI] [Google Scholar]
- Falasca, M., Raimondi, C., and Maffucci, T., Methods Mol. Biol. 769, 87–95 (2011). 10.1007/978-1-61779-207-6_7 [DOI] [PubMed] [Google Scholar]
- Ferrario, J. E., Baskaran, P., Clark, C., Hendry, A., Lerner, O., Hintze, M., Allen, J., Chilton, J. K., and Guthrie, S., Proc. Natl. Acad. Sci. U.S.A. 109, 14669–14674 (2012). 10.1073/pnas.1116481109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E. M., Thompson, A. W., Yuan, J., and Goodhill, G. J., Neuron 74, 490–503 (2012). 10.1016/j.neuron.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Fuchs, J., Stettler, O., Alvarez-Fischer, D., Prochiantz, A., Moya, K. L., and Joshi, R. L., Eur. J. Neurosci. 35, 1837–1845 (2012). 10.1111/j.1460-9568.2012.08139.x [DOI] [PubMed] [Google Scholar]
- Jeon, N. L., Baskaran, H., Dertinger, S. K. W., Whitesides, G. M., Van de Water, L., and Toner, M., Nat. Biotechnol. 20, 826–830 (2002). 10.1038/nbt712 [DOI] [PubMed] [Google Scholar]
- Shen, K. and Cowan, C. W., Cold Spring Harb. Perspect. Biol. 2, a001842 (2010). 10.1101/cshperspect.a001842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, T. E., Wang, H., Marshall, W., and Tessier-Lavigne, M., J. Neurosci. 26, 8866–8874 (2006). 10.1523/JNEUROSCI.5191-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. J., Hannanta-anan, P., Chau, M., Kim, Y. S., Swartz, M. A., and Wu, M., PLoS One 8, e68422 (2013). 10.1371/journal.pone.0068422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S., Kim, H. J., and Jeon, N. L., Integr. Biol. 2, 584–603 (2010). 10.1039/c0ib00055h [DOI] [PubMed] [Google Scholar]
- Kim, S. H., Kang, J. H., Chung, I. Y., and Chung, B. G., Electrophoresis 32, 254–260 (2011). 10.1002/elps.201000501 [DOI] [PubMed] [Google Scholar]
- Kothapalli, C. R., van Veen, E., de Valence, S., Chung, S., Zervantonakis, I. K., Gertler, F. B., and Kamm, R. D., Lab Chip 11, 497–507 (2011). 10.1039/c0lc00240b [DOI] [PubMed] [Google Scholar]
- Lange, J., Yafai, Y., Noack, A., Yang, X. M., Munk, A. B., Krohn, S., Iandiev, I., Wiedemann, P., Reichenbach, A., and Eichler, W., Glia 60, 1567–1578 (2012). 10.1002/glia.22376 [DOI] [PubMed] [Google Scholar]
- Li, J., Zhu, L., Zhang, M., and Lin, F., Biomicrofluidics 6, 24121 (2012). 10.1063/1.4718721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, T. A., Shah, S. N., and Ruggiero, E. A., Alcohol Clin. Exp. Res. 35, 1321–1330 (2011). 10.1111/j.1530-0277.2011.01468.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery, L. A. and Van Vactor, D., Nat. Rev. Mol. Cell Biol. 10, 332–343 (2009). 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T., Sakuma, C., Taniguchi, M., Kanemoto, A., Yoshizawa, M., Satomi, K., Tanaka, H., Takeuchi, K., Ueda, S., Yaginuma, H., and Shiga, T., Brain Res. 1480, 30–40 (2012). 10.1016/j.brainres.2012.08.055 [DOI] [PubMed] [Google Scholar]
- Meier, B., Zielinski, A., Weber, C., Arcizet, D., Youssef, S., Franosch, T., Rädler, J. O., and Heinrich, D., Proc. Natl. Acad. Sci. U.S.A. 108, 11417–11422 (2011). 10.1073/pnas.1014853108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer, D., Pujic, Z., Vaughan, T., Thompson, A. W., Feldner, J., Vetter, I., and Goodhill, G. J., Proc. Natl. Acad. Sci. U.S.A. 107, 5202–5207 (2010). 10.1073/pnas.0909254107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta, N., Aoki, Y., Isogawa, Y., Tsuchiya, T., Tsuchiya, T., and Kanegasaki, S., Eur. J. Cell Biol. 88, 541–549 (2009). 10.1016/j.ejcb.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Polacheck, W. J., Li, R., Uzel, S. G. M., and Kamm, R., Lab Chip 13, 2252–2267 (2013). 10.1039/c3lc41393d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja, W. K., Gligorijevic, B., Wyckoff, J., Condeelis, J. S., and Castracane, J., Integr. Biol. 2, 696–706 (2010). 10.1039/c0ib00044b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidi, A., Kaji, H., Annabi, N., Ostrovidov, S., Ramalingam, M., and Khademhosseini, A., Biomicrofluidics 5, 22214 (2011). 10.1063/1.3580756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, A. W., Pujic, Z., Richards, L. J., and Goodhill, G. J., Mol. Cell Neurosci. 47, 45–52 (2011). 10.1016/j.mcn.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Tirella, A., Marano, M., Vozzi, F., and Ahluwalia, A., Toxicol. In Vitro 22, 1957–1964 (2008). 10.1016/j.tiv.2008.09.016 [DOI] [PubMed] [Google Scholar]
- Togashi, K., von Schimmelmann, M. J., Nishiyama, M., Lim, C. S., Yoshida, N., Yun, B., Molday, R. S., Goshima, Y., and Hong, K., Neuron 58, 694–707 (2008). 10.1016/j.neuron.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Tojima, T., Hines, J. H., Henley, J. R., and Kamiguchi, H., Nat. Rev. Neurosci. 12, 191–203 (2011). 10.1038/nrn2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walheim, C. C., Zanin, J. P., and de Bellard, M. E., J. Vis. Exp. 59, e3330 (2012). 10.3791/3330 [DOI] [PMC free article] [PubMed] [Google Scholar]