Abstract
Radiation exposure to the thorax is associated with substantial risk for the subsequent development of cardiovascular disease. Thus, the increasing role of radiation therapy in the contemporary treatment of cancer, combined with improving survival rates of patients undergoing this therapy, contributes to a growing population at risk of cardiovascular morbidity and mortality. Associated cardiovascular injuries include pericardial disease, coronary artery disease, valvular disease, conduction disease, cardiomyopathy, and medium and large vessel vasculopathy—any of which can occur at varying intervals following irradiation. Higher radiation doses, younger age at the time of irradiation, longer intervals from the time of radiation, and coexisting cardiovascular risk factors all predispose to these injuries. The true incidence of radiation-related cardiovascular disease remains uncertain due to lack of large multicentre studies with a sufficient duration of cardiovascular follow-up. There are currently no consensus guidelines available to inform the optimal approach to cardiovascular surveillance of recipients of thoracic radiation. Therefore, we review the cardiovascular consequences of radiation therapy and focus on the potential role of non-invasive cardiovascular imaging in the assessment and management of radiation-related cardiovascular disease. In doing so, we highlight characteristics that can be used to identify individuals at risk for developing post-radiation cardiovascular disease and propose an imaging-based algorithm for their clinical surveillance.
Keywords: Radiation therapy, Non-invasive imaging
Introduction
Radiation therapy is established as an effective adjuvant therapy in many malignancies, and is used in the management of more than 50% of cancer patients.1 Significant improvements in disease-specific and overall survival accompany the increasing use of radiation therapy. For example, radiation following breast conserving surgery for patients with breast cancer is associated with a 50% reduction in disease recurrence and a 17% reduction in breast cancer death2; following chemoradiotherapy for Hodgkin's lymphoma, 5-year survival rates exceed 85%.3 However, radiation exposure to the thoracic region is associated with clinically significant cardiac disease that may not manifest until years after treatment.
Cardiovascular complications of thoracic radiation were originally described in the 1960s.4 These complications include pericardial disease, cardiomyopathy, coronary artery disease (CAD), valvular disease, and conduction system disease in addition to medium and large vessel vasculopathy (Figure 1). Compared with contemporary regimens, traditional regimens involved larger total radiation doses delivered over relatively wide target fields, with anterior mediastinal approaches. While avoiding toxicity to the oesophagus and spinal cord, these earlier regimens predisposed to cardiovascular toxicity.5 Recent modifications in radiation dose and delivery have reduced the incidence of cardiac complications, but the exact cardiac risks of contemporary regimens remain unknown, largely given the delayed manifestation of radiation-induced disease.
Figure 1.
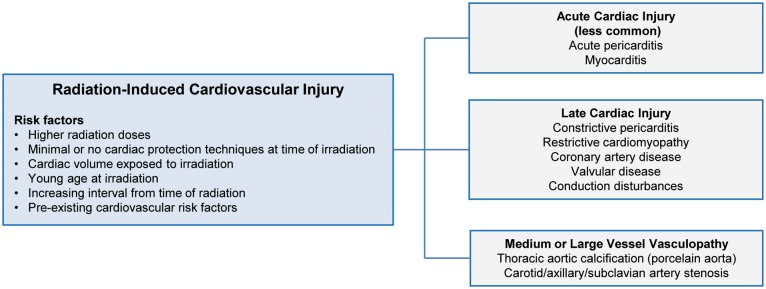
Classification of cardiovascular injury following radiation therapy.
Herein, we provide an overview of the cardiovascular risks associated with radiotherapy and discuss the role of non-invasive imaging in screening for and diagnosing radiation-induced cardiovascular injury.
Basics of radiotherapy
Radiation therapy uses high energy radiation from X-rays, gamma rays, or charged particles to induce double-stranded DNA breaks in malignant cells, thereby causing apoptosis or preventing cellular division. The total dose of radiation delivered is determined by the type and stage of the target cancer. Repeated small doses of radiation are less damaging to surrounding cells than a single fraction of an equivalent total dose, since small repeated doses permit normal tissue cells to repair damaged DNA and survive. Radiotherapy can be used as monotherapy or adjuvant therapy, and may be delivered with either palliative or curative intent.
Radiation dose is measured in Gray units; 1 gray (Gy) is the absorption of 1 J of ionizing radiation energy by 1 kg of tissue. Developments such as the use of CT planning systems, gating, intensity-modulated radiotherapy, and helical tomotherapy now serve to minimize cardiac dosing in modern-day regimens for mediastinal and thoracic irradiation.6–8 For example, cardiac doses in irradiated breast cancer patients in Sweden increased from the 1950s to the 1970s, and reduced significantly in the following two decades.9 Following breast conservation surgery for breast cancer, currently a typical dose of 45–50 Gy is delivered in 25–28 fractions (1.8–2 Gy per fraction) to the whole affected breast over a 5–7 week period,10 and a boost of approximately 10–16 Gy in 5–8 fractions is commonly added to the lumpectomy cavity. In the treatment of Hodgkin's lymphoma, 35 Gy involved field radiation therapy (IFRT) has largely replaced traditional 35 Gy extended mantle radiation therapy since the mid-1990s, reducing the median value of the mean dose to the whole heart by 29%.11 Contemporary IFRT regimens for Hodgkin's lymphoma involve total doses of approximately 35 Gy delivered in 20 daily fractions to the supra-diaphragmatic lymph node areas12; whereas a lower total dose of 20 Gy for earlier stages has been advocated.13 For any cancer type, it has long been known that radiation doses above 30 Gy are associated with cardiac damage. A recent, large population-based case–control study of 2168 women treated with radiation therapy for breast cancer over 5 decades reported an increase in rate of major coronary events of 7.4% per Gy [95% confidence interval (CI) 2.9–14.5; P < 0.001], with no apparent threshold below which there was no increased risk.14 Similarly, a retrospective analysis of patients with breast cancer suggests an increased relative risk (RR) of cardiac death of 3.1% per Gy of thoracic radiation exposure, compared with radiotherapy naïve breast cancer patients.1
Basic pathophysiology of radiation-induced heart disease
Radiation therapy causes damage to both the heart and the vasculature. With respect to the heart, histological examination of the myocardium after radiation treatment reveals diffuse fibrosis of the interstitium and narrowing of both the arterial lumen and capillaries. Fibrosis occurs both in the myocardium and pericardium with collagen deposition replacing myocytes and parietal pericardium, respectively. Fibrosis of the myocardium can lead to a restrictive cardiomyopathy and diastolic heart failure. Similarly, collagen deposition in the parietal pericardium can lead to a rigid pericardial sac resulting in increased thickness and cardiac constriction. With respect to the vasculature, radiation-induced coronary and peripheral vascular disease results from intimal proliferation of myofibroblasts with lipid-containing macrophages and subsequent atherosclerotic plaque formation. Experimental models also indicate that radiation can lead to decreased capillary density and result in chronic myocardial ischaemia and fibrosis.15
Post-radiation pericardial disease
Prior to the 1970s, acute pericarditis occurred commonly after radiotherapy, but rarely occurs today due to lower doses and modern radiation techniques designed to reduce incidental cardiac irradiation. However, 7–20% of patients may develop chronic pericarditis 10 or more years after radiation treatment.16–18 Importantly, constrictive pericarditis has a wide spectrum of clinical manifestations ranging from a few non-specific symptoms to severe congestive cardiac failure. Medical management includes diuretics (and beta-blockers) with or without surgical pericardiectomy. Constrictive pericarditis due to radiation therapy, compared with from other causes, is associated with a poorer overall survival following pericardiectomy (27% at 7 years).19 This inferior survival rate has been attributed to other associated radiation-related cardiac lesions.20 For example, post-radiation constrictive pericarditis rarely exists in isolation and is commonly accompanied by myocardial fibrosis, premature coronary artery stenosis, and valvular lesions.21,22 Associated myocardial involvement predominantly affects the right ventricle,23 presumably due to its anterior position in the chest. Thus, a strong clinical suspicion of post-radiation constrictive pericarditis should prompt a comprehensive evaluation to detect additional associated cardiac lesions.
Key features of constrictive pericarditis may be detected by non-invasive imaging. These features, shown in Figure 2 and outlined in Table 1, include pericardial thickening, tubular ventricles, dilated inferior vena cava, pericardial and pleural effusions, and interventricular septal bounce. The presence of pericardial thickening ≥4 mm on CT or MRI in the correct clinical context is highly suggestive of constrictive pericarditis.24,25 However, up to 20% of patients with surgically proven constrictive pericarditis do not have pericardial thickening,26,27 where MRI is the preferred imaging modality for characterizing pericardial anatomy.28 Transthoracic echocardiography (TTE) may demonstrate peak E′ velocities that are higher for the septal compared with the lateral mitral valve annulus. An interventricular septal bounce, on either TTE or MRI, is considered one of the most sensitive and specific signs of constrictive pericarditis, with a reported sensitivity on echocardiography of 62–81% and a specificity of 93–100%.27 In general, a multimodality approach of TTE with complementary CT or MRI can facilitate diagnosis of post-radiation constrictive pericarditis (Table 2).
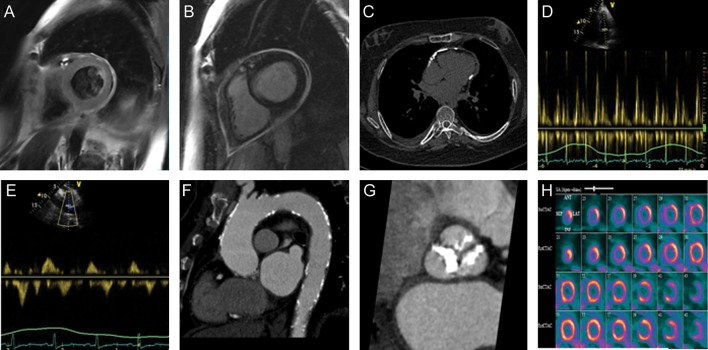
Figure 2.
Non-invasive imaging can aid in the assessment of cardiovascular complications following radiotherapy, as shown in the following examples: (A) pericardial thickening on a T2-weighted cardiac MR in a 52-year-old male found to have constrictive pericarditis 18 years following mediastinal irradiation; (B) circumferential late gadolinium enhancement of the thickened pericardium on cardiac MR in the same patient; (C) pericardial calcification seen by non-contrast CT imaging in a patient with a remote history of thoracic irradiation; (D) respiratory variation in mitral inflow peak E-wave velocities and rapid deceleration time, seen via pulse-wave Doppler echocardiography in a patient with constrictive pericarditis following radiation therapy; (E) respiratory variation in hepatic vein pulse-wave Doppler signal in the same patient as in part D; (F) diffuse calcification of the thoracic aorta on contrast-enhanced CT in another patient who received Mantle radiotherapy in childhood; (G) extensive calcification of the aortic valve on contrast-enhanced CT in a patient imaged 10 years after thoracic radiation; (H) stress and rest N13-ammonia PET perfusion in a 58-year-old female 8 years post-mediastinal radiation, demonstrating a medium-sized perfusion defect of moderate intensity in the mid-anteroseptal, mid-inferoseptal walls, the apical septum, apical inferior wall and apex that was partly reversible, consistent with ischaemia in the distribution of the mid-left anterior descending artery. In addition, there was a medium-sized defect of moderate intensity in the entire inferolateral wall that was reversible, consistent with ischaemia in the distribution of an obtuse marginal artery.
Table 1.
Features of constrictive pericarditis evident on various imaging modalities
| Echocardiography | CT | MRI |
|---|---|---|
| ||
Mitral inflow Doppler signal:
|
Pericardial calcification (CT is superior to MRI in demonstrating calcification) | |
| Diastolic flow reversal in hepatic vein and pulmonary vein Doppler signals with respiratory variation | Pericardial adhesions | |
| Myocardial tagging may demonstrate adhesions between pericardium and adjacent structures | ||
aCT or MRI are superior to echocardiography in characterizing these features.
bAn interventricular septal bounce can be readily evaluated on echo and MRI. However, it is more difficult to appreciate on CT due to inferior temporal resolution and can be evaluated only if data are acquired throughout the cardiac cycle.
Table 2.
Comparison of imaging modalities in the diagnosis of constrictive pericarditis
| Advantages | Disadvantages | |
|---|---|---|
| Echo |
|
|
| CT |
|
|
| MRI |
|
|
TTE, transthoracic echocardiography; TEE, transoesophageal echocardiography.
Post-radiation coronary disease
In the coronary arterial tree, radiation can result in macrovascular and microvascular injury. Macrovascular injury often manifests as stenoses affecting the ostia or proximal segments of the left main and/or epicardial coronary arteries.29–32 Histology demonstrates diffuse fibrosis of all arterial wall layers, with the loss of smooth muscle cells in the media and few lipid deposits.33 Microvascular injury affects the endothelial cells of myocardial capillaries and can lead to myocardial fibrosis.34 Perfusion deficits on myocardial perfusion imaging (MPI) that do not fit a classic coronary distribution in post-radiotherapy patients may be related to such microvascular injury.
Clinical manifestations of coronary disease may present several years following exposure. The relative risk (RR) of myocardial infarction or sudden death was reported at 6.7% among 352 Hodgkin's survivors followed for a mean period of 11 years post-radiation.34 In another study of 258 patients with a median follow-up of 14 years, 12% experienced ischaemic heart disease with an observed-to-expected mortality ratio of 5.3.35 In a retrospective study comparing 2232 consecutive patients with Hodgkin's lymphoma with a matched general population over a mean follow-up period of 9.5 years, the risk of myocardial infarction increased with duration from irradiation and was highest among patients irradiated at ages younger than 20 years (RR 44.1, 95% CI 17.8–91.8), reducing with increasing age at the time of treatment.36
Increased cardiovascular risk underscores the importance of identifying individuals who might benefit from primary prevention and aggressive cardiovascular risk factor modification. Exercise treadmill testing is an accessible and simple screening tool, but has limited sensitivity and specificity, particularly in women. Alternatively, stress echocardiography, nuclear MPI, CT calcium scores, and coronary CT angiography (CCTA) have been used as more sensitive screening methods (Table 3). Studies to date are small, predominantly retrospective, single centre, cross-sectional and have often excluded patients with a prior history of CAD, a likely higher-risk group for radiation-mediated coronary injury. Furthermore, radiotherapy techniques differed across studies contributing to variation in the reported prevalence of post-radiotherapy coronary disease. Additionally, many studies were based on older radiotherapy regimens/techniques and the cardiac volume included in radiation treatment field was inconsistently reported. Most studies also lacked baseline pre-radiotherapy imaging on patients, making it difficult to definitively attribute identified coronary disease to the radiation exposure. In some studies, the short interval from radiation to screening may have limited detection of delayed radiation-related cardiac injury. Some studies involving MPI are also limited by having investigated rest perfusion only, without screening for more clinically relevant stress-induced defects.
Table 3.
Summary of imaging studies used to detect cardiac injury in post-radiotherapy patient populations
| Study | N | Mean/Median age (SD/range), years | Mean radiation dose (SD), Gy | Mean/Median interval from radiation (SD/range) | Abnormality on imaging study | Study Type |
|---|---|---|---|---|---|---|
|
Stress echocardiography | ||||||
| Heidenreich et al. 200737 | 294 asymptomatic pts with HD; no known CAD | 42 (9) years | 43.5 (3.4) Gy | 15 (7) years | 16 of 292 (6%) had stress induced wall motion abnormalities | Cross-sectional cohort study; 41 deaths, 23 symptomatic CAD (including 10 AMIs) over median follow up period of 6.5 years post-screening |
|
Nuclear myocardial perfusion imaging (MPI) | ||||||
| Heidenreich et al. 200737 | 294 asymptomatic pts with HD; no known CAD | 42 (9) years | 43.5 (3.4) Gy | 15 (7) years | 32 of 274 (12%) has stress induced perfusion defect | Cross-sectional cohort study; 41 deaths, 23 symptomatic CAD (including 10 AMIs) over median follow up period of 6.5 years post-screening |
| Maunoury et al. 199299 | 31 asymptomatic pts with HD; no known CAD | 35 (23-45) years | 39.9 Gy | 7 (3-11) years | 21/25 (84%) had abnormal exercise Tl201 SPECT (defects often not fitting classical coronary distribution) | Cross-sectional study, single center |
| Gustavsson et al. 1990100 | 26 pts with HD (21 with no known CAD) | 38 (21-45) years | 40 Gy | 15 (4-20) years | 2/23 abnormal; 12/23 ambiguous (uneven isotope uptake, not classic for any pattern of CAD); 9/23 normal | Cross-sectional study, single center |
| Gyenes et al. 1994101 | 20 pts with LBC and 17 pts with RBC; non-irradiated controls; excluded pts with known IHD | 65 (43-72) years | 45 Gy in daily fractions of 1.8 Gy | ∼19 (16-21) years | Exercise 99mTc sestamibi MPI: perfusion defects in 5/20 (25%) LBC pts and 0/17 (0%) controls | Cross sectional study |
| Constine et al. 1997102 | 50 pts with HD; no known CAD | 26 (8.6) years | 35.1 (7.8) Gy | 9.1 (7.5) years | Rest/stress 99mTc sestamibi or Tl201 SPECT: stress induced ischemia in 2/38 (5%) patients | Cross-sectional, retrospective, |
| Cowen et al. (1998)103 | 19 pts with LBC; no known risk factors for CAD | 59 (42-75) years | 60 Gy (50 Gy to whole breast in 2-Gy daily fractions and 10 Gy boost irradiation) | 55 (37-90) months | Exercise stress Tl201 MPI: No perfusion defects among the 17 (0%) evaluable patients | Cross-sectional study, single center |
| Hardenbergh et al. 200138 | 20 asymptomatic pts with LBC | 54 (42-72) years | 46-50 Gy in 2-Gy daily fractions | 6 months | 99mTc sestamibi rest SPECT:New perfusion defects in: 5/10 (50%) pts treated with RT alone; 7/7 (100%) patients treated with RT and chemo | Prospective study; baseline studies; short interval from RT to imaging |
| Sioka et al. 201139 | 46 asymptomatic pts with breast ca | 59 (10) years | 60 Gy (in daily dose of 2 Gy) | 40 (6-263) months | Mean S-RSS in 28 LBC pts = 2.3 (2.4); Mean S-RSS in 18 RBC pts = 2.9 (2.9); Mean S-RSS in 85 controls= 1.5 (1.6) | Case-control study |
| Seddon et al 200234 | 24 pts with LBC, 12 pts with RBC; no known IHD | 59 years for pts with LBC; 62.5 years for pts with RBC | Median 50 Gy; IQR: 46, 54 Gy (in 2-Gy daily fractions) | 6.7 yrs for LBC pts; 8.3 yrs for RBC pts | Perfusion abnormality on 99mTc tetrofosmin in: 17/24 (71%) LBC pts and 2/12 (16.7%) RBC pts | Retrospective study |
| Lind et al 200343 | 69 pts with LBC | 53 (32-76) years | 46-50 Gy in 2-Gy daily fractions; additional 10 Gy boost post-mastectomy) | 18 months | 99mTc -tetrofosmin or sestamibi Rest SPECT: Statistically significant increase in LAD territory perfusion defects at 6-18 months post-RT compared to baseline; no difference in RCA/LCx territories | Prospective; baseline studies; short interval post-RT |
| Marks et al. 2005104 | 114 pts with LBC; 7 pts had hx of CAD; pts with pre-RT perfusion defects excluded | 57 (33-82) years | 46-50 Gy in 1.8-2Gy daily doses | Varied; up to 24 months | 99mTc -sestamibi or tetrofosmin SPECT: volume (of heart irradiated) dependent perfusion defects in 40% within 2 years of RT | Prospective study; baseline studies; short interval from RT to imaging |
| Prosnitz et al 2007105 | 44 pts with LBC | 55 (39-83) years | 47.3 Gy | Rest SPECT pre and, at varying intervals up to 6 yrs, post-RT. | Incidence of perfusion defects at 3/4/5/6 yrs post-RT was 52%/71%/67%/57%, respectively. | Prospective; baseline studies |
|
CT calcium scores | ||||||
| Rademaker et al. 20086 | 9 asymptomatic pts with HD; no known CAD | 45.6 (9.4) years | 34-45 Gy | 26 (7.5) years | Mean (SD; range) Ca Score= 361 (383; 0-1042) | Cross-sectional study |
| Andersen et al. 2010106 | 47 pts with HD; 7/47 had hx of CAD; remainder has no known CAD | 50 (7) years | 40.6 (2.3) Gy | 22 (3) years | Coronary Ca (volume score): 8/47 pts had a score of 0, 29/47 pts had a score of 1-199, and 10/47 pts had a score of ≥200. | Cross-sectional study, single center |
|
Coronary CT angiography | ||||||
| Rademaker et al. 20085 | 9 asymptomatic pts with HD; no known CAD | 45.6 (9.4) years | 34-45 Gy | 26 (7.5) years | 6/9 (67%) had diffuse CAD, 1/9 (11%) had proximal LAD disease, 1/9 (11%) had proximal RCA disease | Cross-sectional study |
| Kupeli et al 2010107 | 59 asymptomatic pts with HD | ∼19.3-23.7 years | 27.5 Gy among pts with CAD 20 Gy among pts with no CAD | ∼11-15.1 years | 14/59 (24%) patients (7 had proximal LAD disease; 1 patient had 90% ostial stenosis requiring PCI) | Cross-sectional study, single center |
LBC, left breast cancer; RBC, right breast cancer; HD, Hodgkin's disease; WMA, wall motion abnormalities; CAD, coronary artery disease; AMIs, acute myocardial infarctions; Tc, technetium; Tl, thallium; S-RSS, stress-rest summed score; IQR, interquartile range; RT, radiation therapy.
Of the studies listed in Table 3, only two studies provide follow-up data to contextualize imaging abnormalities.5,37 For example, 23 of 294 (7.8%) patients with ≥35 Gy mediastinal exposure developed symptomatic CAD over a median follow-up period of 6.5 years after screening.37 Other studies largely report prevalence of coronary defects on imaging without clinical correlations. Therefore, the clinical significance of post-radiation perfusion defects, which are often clinically silent, is uncertain.38–40 Extrapolating data from nuclear MPI in radiotherapy naïve patients would suggest that such perfusion defects may help identify patients at higher risk of adverse cardiac events,41,42 facilitating timely primary preventative strategies, but this is speculative. Risk factors for post-radiation perfusion defects include adjuvant chemotherapy and/or hormonal therapy, hypercholesterolaemia, larger LV volume within the irradiated field, and left compared with right breast radiotherapy.34,39,43 The most appropriate imaging modality to screen for CAD among post-radiotherapy patients is uncertain. The strengths and challenges of available modalities are briefly outlined in Table 4. Local access and expertise will determine modality selection for CAD diagnosis and screening.
Table 4.
Comparison of available imaging modalities used to screen for coronary disease
| Advantages | Disadvantages | |
|---|---|---|
| Stress (exercise/pharmacological) echocardiography |
|
|
| SPECT MPI (rest and exercise/pharmacological stress) |
|
|
| Cardiac PET |
|
|
| CT coronary Ca2+ Score |
|
|
| CCTA |
|
|
| Stress CMR |
|
|
| Invasive coronary angiography |
|
|
The optimal timing of initial and interval screening for CAD among radiation therapy recipients is also unknown. It has been suggested that patients older than 45 years at the time of radiation therapy should commence screening 5-year post-radiation, with screening of patients younger than 45 years commencing 10-year post-radiation.44 However, the overall mortality rate attributable to heart disease in a cohort of 972 patients with Hodgkin's lymphoma who had received mediastinal radiation was 5.5%, with 42% of deaths from myocardial infarction occurring within 10 years of radiation and 27% of coronary deaths occurring in patients under the age of 40 years.37 Such observations suggest that screening for CAD for younger patients should begin earlier than 10 years post-radiation therapy. This is reinforced by a large case–control study of 2168 women who received radiation therapy for breast cancer that demonstrated a radiation-related increased risk of major coronary events (myocardial infarction, coronary revascularization, or death from ischaemic heart disease) starting within 5 years of exposure.14
Patients with risk factors for post-radiation CAD may well benefit from earlier and/or more frequent screening. Traditional cardiovascular risk factors, such as hypercholesterolaemia, that can act synergistically with radiation exposure to promote premature CAD require careful longitudinal attention in all patients treated with radiotherapy. The RR of major coronary events in the presence of a prior history of IHD or one or more cardiovascular risk factors (other vascular disease, diabetes, chronic obstructive lung disease, smoking history, and elevated body mass index) among 2168 women treated with radiotherapy for breast cancer was 6.7 (95% CI 4.4–10.2) and 2.0 (95% CI 1.6–2.4), respectively.14 Management of post-radiation CAD may otherwise be similar to the management of non-radiotherapy associated CAD. However, in cases of heavily calcified coronary lesions, conventional approaches to intervention may be challenging. Such cases may warrant consideration of percutaneous or surgical atherectomy vs. conservative therapy.
Post-radiation valvular disease
Valvular diseases due to radiation therapy range from mild asymptomatic valvular thickening to severe, haemodynamically significant valvular thickening, and may manifest as stenosis or insufficiency.45 Aortic and mitral valves are more often affected.23 Diagnosis often occurs more than a decade following irradiation and pathology of severely diseased valves demonstrate thickened, fibrotic leaflets.7,46 A study of 112 patients who had received mediastinal radiation for Hodgkin's lymphoma reported a cumulative risk of valvular thickening on echocardiography after 30 years of follow-up of >60%, but mostly without haemodynamic disturbance.47 In another study of 415 Hodgkin's lymphoma survivors previously treated with radiation therapy, the actuarial incidence of clinically significant valvular disease was 1% at 10 years, 4% at 15 years, and 6% at 20 years following irradiation; aortic stenosis was the most common valve lesion and the observed-to-expected ratio for any valve surgery among this cohort was 8.4 over a median follow-up of 11 years.48
As long-term survival improves, surveillance for development of valvular complications may be indicated.49 Transthoracic echocardiography remains the modality of choice for screening. It has been suggested that such echocardiography surveillance should commence at 10 years after radiotherapy, and at 5 yearly intervals; for newly identified valvular lesions, management should abide by existing guidelines.44
Radiation-induced cardiomyopathy
Cardiomyopathy can occur directly due to radiation therapy exposure, as well as indirectly due to radiation-associated valvular or coronary disease. Murine models demonstrate that time- and radiation dose-dependent inflammatory changes, reduced microvascular density, impaired microvascular function, and activation of fibrogenic pathways may manifest as an initial phase of cardiac injury with evidence of impaired contractile reserve and preserved LV ejection fraction (LVEF), followed by reductions in LVEF, progressive worsening of contractile reserve, myocardial interstitial fibrosis, and sudden death.50–52 Restrictive cardiomyopathy with LV diastolic dysfunction presents more frequently than dilated cardiomyopathy with LV systolic dysfunction.53 Risk of radiation-mediated cardiomyopathy is especially high for patients who have received concomitant treatment with single- or multi-agent chemotherapy regimens with cardiotoxic potential.54 However, the synergistic interaction between local radiation therapy and contemporary, intensive multi-agent chemotherapy regimens, and the potential for subsequent cardiomyopathy are not well understood. Studies evaluating cardiotoxic potential of chemotherapeutic agents predominantly excluded patients receiving concomitant radiation therapy, and similarly studies evaluating the harmful effects of radiation therapy often excluded patients receiving adjuvant chemotherapies. Furthermore, lack of standardized TTE screening obscures the true incidence and prevalence of radiation-induced cardiomyopathies.
Given its wide availability, low cost, and high temporal resolution, TTE is the optimal imaging modality for diagnosing LV systolic and diastolic dysfunction. Myocardial strain imaging techniques may be more sensitive for detecting earlier, subclinical LV dysfunction than standard measures, such as LVEF. For example, Erven et al.55 observed in 75 women with breast cancer that myocardial strain in the anterior LV segments was significantly decreased at all post-radiation therapy time points, compared with baseline, in women with left- but not right-sided breast cancer. Such regional reductions in LV strain were subclinical, not accompanied by significant changes in conventional echocardiographic parameters and are yet of uncertain clinical significance. Thus, the optimal techniques as well as timing for echocardiographic screening remain uncertain. Furthermore, the threshold for initiating heart failure medications, such as ACE inhibitors and beta-blockers, is not yet known. However, patient-tailored strategies are likely necessary. Predisposing factors, including baseline LV dysfunction or adjuvant chemotherapies, can identify ‘at risk’ patients who may warrant increased surveillance. The varying and often long latent clinical phase requires indefinite vigilance for cardiomyopathy following local radiation therapy.
Radiation-induced vasculopathy
Radiation-induced arterial injury can involve any arteries exposed to the radiation field. Supra-aortic arch vasculopathy, resulting from head, neck, and mediastinal radiotherapy, significantly increase the risk of cerebrovascular disease related to carotid stenosis and carotid occlusion.56–60 Injuries of the subclavian artery, innominate artery, and axillary artery have also been reported.48,61,62 The incidence of significant carotid stenosis following head and neck radiotherapy is 30–50%, and the presence of traditional cardiovascular risk factors contribute to this risk.63 Up to 12% of patients screened with colour flow duplex scans demonstrated ≥70% stenosis of the internal or common carotid artery in a cross-sectional study of 240 patients at a mean interval of 6 years following head and neck radiotherapy.64
Management of radiation-induced carotid artery stenosis can be challenging and complicated. Carotid endarterectomy in these cases confers higher risk of wound complications, cranial nerve injuries, and neurological events, in part due to radiation-related sclerosis of the surgical field.65–68 On the other hand, event rates associated with carotid artery stenting are similar to those for non-radiation-induced disease.69 Following either surgery or stenting, the high risk for de novo stenoses and re-stenoses obligates continued longitudinal screening.68,70–74 Surveillance with ultrasound for early detection and possible therapeutic intervention has been advocated.64,75–79 Because post-radiation carotid stenosis progresses more rapidly than atherosclerotic carotid disease in radiation-naïve patients, some have recommended frequent carotid surveillance in this at-risk population.80 Others have argued against routine screening in asymptomatic patients due to the relatively low excess cases of cerebrovascular events.44,59 However, screening even asymptomatic patients may identify subclinical disease that may benefit from more aggressive risk factor modification and antiplatelet therapy. Prospective studies are needed to determine the overall clinical and cost effectiveness of interval carotid screening of recipients of head, neck, or mediastinal radiotherapy.
In our clinical practice, we routinely screen patients previously treated with head, neck, or mediastinal radiotherapy for symptoms and signs of extra-coronary vascular disease. Patients are also screened for cardiovascular risk factors that may predispose to post-radiation arterial disease, and receive risk factor modification as appropriate. Carotid screening with ultrasound is performed in patients with any of the following features: (i) signs or symptoms suggestive of stroke or transient ischaemic attack (TIA); (ii) carotid bruits; (iii) other vascular disease (e.g. peripheral or coronary arterial disease); or (iv) presence of at least one cardiovascular risk factor. Screening is initiated when signs of vascular occlusion manifest or with onset of signs or symptoms suggestive of a TIA or stroke. There is no clear guideline suggesting an optimal time for carotid screening post-radiotherapy in the absence of earlier cerebrovascular signs or symptoms. However, longitudinal screening may be warranted given that the median time from radiotherapy to first stroke or TIA was 17 years among 96 survivors of Hodgkin's lymphoma.59 Ultrasound is the standard screening approach, with consideration of contrast-enhanced MR or CT angiography if ultrasonography is inconclusive or identifies significant disease. Upon detecting carotid disease, annual ultrasound surveillance is preferred, particularly for stenoses of >25%, given post-radiotherapy patients' predisposition to rapid disease progression.
Pre-operative planning
Of patients who require cardiac surgery of any type [pericardiectomy, coronary artery bypass grafting (CABG), and/or valvular surgery], those who have received radiation to the chest have a higher operative risk than patients who are radiation-naïve. Post-radiation mediastinal and pericardial fibrosis are common and can prove challenging during surgery.82,83 Indeed, failure to consider previous chest radiation has been identified as a short-coming of risk algorithm scores for predicting risk from cardiac surgical procedures, such as the logistic EuroSCORE.83 Pre-operative CT imaging can demonstrate cardiovascular relationships to the chest wall as well as quantify and localize mediastinal and adjacent fibrosis; these data facilitate surgical risk assessment and inform the selection of non-traditional surgical strategies such non-midline incisions.84,85
Certain types of RT-related anatomic abnormalities deserve special mention. Radiation-related fibrosis of the internal mammary artery as a result of radiation may preclude its use as a conduit during CABG.86,87 Therefore, pre-CABG assessment of the internal mammary artery by invasive angiography or non-invasively with CT angiography is recommended. Furthermore, aortic calcification to the point of porcelain aorta can also result from radiation injury;88,89 calcification of the ascending aorta (Figure 2) is associated with increased morbidity and mortality following cardiac surgery.90–92 Pre-operative imaging with CT can also demonstrate relevant aortic calcification (Figure 2). In cases of extensive radiation-related calcification involving multiple cardiovascular structures, alternatives to high-risk surgical management may be considered, including percutaneous coronary or pericardial interventions.
Radiation dose and cardiac imaging
The carcinogenic potential of cardiac imaging-derived radiation exposure requires consideration in any screening protocol for radiation therapy-related cardiac injury. The effective radiation dose of various cardiac imaging studies is typically reported in milliSieverts (mSv). Technological advances have successfully reduced the ionizing radiation associated with CCTA, although there remains tremendous inter- and intra-site variability in associated effective radiation dose. Similarly, the effective dose of radiation from nuclear MPI is variable. Table 5 outlines the mean effective radiation doses of various cardiac imaging studies. For reference, it is useful to remember that the average annual effective dose from background radiation in the USA is 3 mSv, and that the effective dose associated with a standard chest radiograph and screening mammography is 0.1 and 0.7 mSv, respectively.93,94 Technological advances are expected to result in further reductions in the radiation dose of various cardiovascular imaging studies.
Table 5.
Radiation exposure associated with various cardiac imaging studies
| Mean effective dose, mSv | |
|---|---|
| Stress echocardiography | 0 |
| Stress CMR | 0 |
| CT coronary calcium score | 3.1108 |
| Coronary CT angiogram109 | |
| Prospective ECG-gating | 3.5 |
| Retrospective ECG-gating | 16.3 |
| Tube current modulation | 8.8 |
| Myocardial Perfusion Imaging108–112 | |
| Stress only 99mTc-sestimibi | 4 |
| Rest/stress 99mTc-sestimibi | 9 |
| Ammonia-13 stress/rest PET | 2 |
| MUGA (99mTc-labeled red blood cells) | 7.8112 |
| Invasive diagnostic coronary angiography | 7112 |
Conclusion
The growing population of cancer survivors who have received radiation field exposure to the heart, thoracic aorta, and/or great vessels is at increased risk for cardiovascular morbidity and mortality. Cardiovascular complications are probably under-reported due to the long delay between radiotherapy exposure and manifest injury.95 The evidence base that informs risk estimation is based on outdated radiation therapy regimens and studies with methodological limitations. Adequate exposure of target tissues during radiation therapy often necessitates the inclusion of certain volume of the heart; however, technological advances have facilitated reductions in incidental cardiac dosing associated with contemporary protocols. For these reasons, it is difficult to quantify the exact cardiac risk for patients treated with either traditional or contemporary radiation regimens. Furthermore, radiation therapy as the aetiology of cardiovascular disease in cancer survivors treated with radiation therapy may be overlooked in the presence of other cardiovascular risk factors, or only considered a plausible but uncertain aetiologic factor in the absence of other risk factors.
Minimizing risk of radiation-induced cardiovascular disease should begin with pre-treatment assessment of patients' individualized risk of cardiovascular morbidity. Similar to the pre-operative cardiovascular assessment, patients may benefit from screening for cardiovascular symptoms and coexisting traditional cardiovascular risk factors, such as hypertension and diabetes mellitus, prior to initiation of radiation therapy. The pre-treatment screen should also consider cancer-specific radiation protocols, including the proposed cumulative dose of radiation and volume of heart included in the radiation field, and the use of adjuvant chemotherapies. Patients at higher risk for cardiovascular events can be identified prior to treatment and, where appropriate, benefit from baseline assessment of cardiac function and therapeutic interventions to minimize cardiovascular comorbidities starting before radiation treatment and continuing throughout survivorship96,14. Large, prospective studies designed with adequate longitudinal follow-up for detecting cardiovascular sequelae of radiation therapy could facilitate design of a risk-prediction score to optimally risk stratify patients before, as well as after, initiation of radiation therapy. The health economic impact of such pre-treatment cardiovascular assessment is uncertain, but development of risk prediction models could enable providers to focus the use of resources for higher-risk patients.
For patients who have already received thoracic radiation, the available evidence suggests that routine screening for radiation-related cardiac injury is warranted. Early detection of such complications can prompt timely interventions that may improve cardiovascular prognosis. Consensus guidelines for cardiovascular screening of the ‘at risk’ population are lacking; a deficit highlighted by recent European Society of Medical Oncology clinical practice guidelines.97 The National Comprehensive Cancer Network guidelines for survivors of Hodgkin's lymphoma recommend aggressive cardiovascular risk factor management and a surveillance stress test or echocardiogram at 10 years following treatment, even in asymptomatic patients.98 Such guidelines are based on a limited evidence base and, because information gained from an echocardiogram and a stress test is not equivalent, the goals for recommending one vs. the other remain uncertain.
Any algorithm for the evaluation and treatment of post-radiotherapy patients will require validation in prospective large studies. Prospective studies can better define the role of the various imaging modalities in screening this population, while determining appropriate timing and frequency of screening, with respect to risk for outcomes. One of the challenges facing such studies will be the need to recruit large number of patients for detection of a statistically significant difference in the ratio of observed to expected events. This large number of patients will need to be followed for many years, given the often delayed manifestation of radiation-related cardiac events. The requirement for baseline imaging for study patients will need to be considered to determine the true incidence of radiation-mediated cardiac injury. Furthermore, the cost effectiveness of screening protocols for this population will need to be evaluated. Clarifying risk factors for cardiac injury will help identify patients who may warrant increased surveillance; the role of cardiac biomarkers in identifying such higher-risk patients may help focus screening protocols.
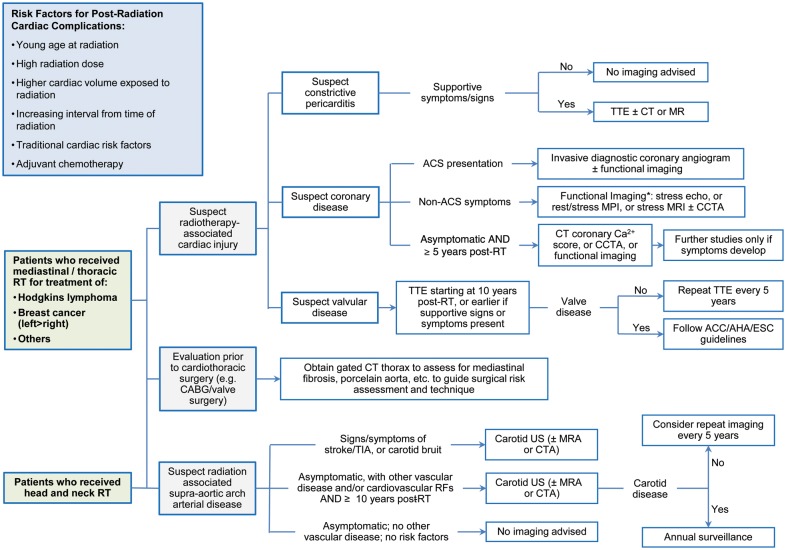
The diversity of cardiovascular injury that can follow radiation challenges a single imaging modality-based screening tool. Screening protocols will require complementary information obtained from a multimodality imaging approach. The algorithm that is followed in clinical practice by our group is outlined in Figure 3. Although initial diagnosis and treatment of patients with cancer are increasingly focused at larger centres with ready access to the multitude of cardiac imaging modalities, longer term follow-up of survivors can occur in practices with more restricted access to cardiac imaging. Thus, local availability and expertise will dictate modality selection.97
Figure 3.
A proposed clinical algorithm for screening and diagnosis of radiation-induced cardiovascular disease. *Choice of functional imaging modality to be guided by local availability and expertise.
Ultimately, physicians must be aware of post-radiation cardiac complications, recognize at-risk patients, and screen such patients for symptoms and signs of cardiac disease. Regular comprehensive symptoms review and clinical examinations are the cornerstones of any cardiovascular screening algorithm for radiation patients. Maintaining a low threshold for screening with non-invasive imaging may help identify injury at a stage where timely intervention may reduce cardiovascular morbidity and mortality in cancer survivors.
Funding
No funding was obtained for completing this work. Dr. Moslehi is a recipient of a NIH K-grant (K08-HL097031) and the Watkins Discovery Award Program and Cardiovascular Leadership Council Investigator Award, Brigham and Women's Hospital.
Conflict of interest: none declared.
References
- 1.Cutter DJ, Darby SC, Yusuf SW. Risks of heart disease after radiotherapy. Tex Heart Inst J. 2011;38:257–258. [PMC free article] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R Early Breast Cancer Trialists’ Collaborative G. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boice JD., Jr An affair of the heart. J Natl Cancer Inst. 2007;99:186–187. doi: 10.1093/jnci/djk058. [DOI] [PubMed] [Google Scholar]
- 4.Cohn KE, Stewart JR, Fajardo LF, Hancock EW. Heart disease following radiation. Medicine (Baltimore) 1967;46:281–298. doi: 10.1097/00005792-196705000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rademaker J, Schoder H, Ariaratnam NS, Strauss HW, Yahalom J, Steingart R, Oeffinger KC. Coronary artery disease after radiation therapy for Hodgkin's lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol. 2008;191:32–37. doi: 10.2214/AJR.07.3112. [DOI] [PubMed] [Google Scholar]
- 6.Magne N, Chargari C, MacDermed D, Conforti R, Vedrine L, Spano JP, Khayat D. Tomorrow's targeted therapies in breast cancer patients: what is the risk for increased radiation-induced cardiac toxicity? Crit Rev Oncol Hematol. 2010;76:186–195. doi: 10.1016/j.critrevonc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Tamura A, Takahara Y, Mogi K, Katsumata M. Radiation-induced valvular disease is the logical consequence of irradiation. Gen Thorac Cardiovasc Surg. 2007;55:53–56. doi: 10.1007/s11748-006-0070-x. [DOI] [PubMed] [Google Scholar]
- 8.Nieder C, Schill S, Kneschaurek P, Molls M. Influence of different treatment techniques on radiation dose to the LAD coronary artery. Radiat Oncol. 2007;2:20. doi: 10.1186/1748-717X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor CW, Nisbet A, McGale P, Goldman U, Darby SC, Hall P, Gagliardi G. Cardiac doses from Swedish breast cancer radiotherapy since the 1950s. Radiother Oncol. 2009;90:127–135. doi: 10.1016/j.radonc.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Edwards-Bennett SM, Correa CR, Harris EE. Optimization of adjuvant radiation in breast conservation therapy: can we minimize without compromise? Int J Breast Cancer. 2011;2011:321304. doi: 10.4061/2011/321304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh ES, Tran TH, Heydarian M, Sachs RK, Tsang RW, Brenner DJ, Pintilie M, Xu T, Chung J, Paul N, Hodgson DC. A comparison of mantle versus involved-field radiotherapy for Hodgkin's lymphoma: reduction in normal tissue dose and second cancer risk. Radiat Oncol. 2007;2:13. doi: 10.1186/1748-717X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, Horning SJ, Dar AR, Shustik C, Stewart DA, Crump M, Djurfeldt MS, Chen BE, Shepherd LE, Group NCT Eastern Cooperative Oncology G. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eich HT, Kriz J, Muller RP. Evolution of radiation therapy within the German Hodgkin Study Group trials. J Natl Compr Canc Netw. 2011;9:1073–1080. doi: 10.6004/jnccn.2011.0088. [DOI] [PubMed] [Google Scholar]
- 14.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 15.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce LJ, Trott KR, Yeh ET, Shore RE. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Matteo J, Vacheron A, Heulin A, Meeus L, Di Matteo G, Gilles R, Delage F, de Ratuld A. Cardiac complications of thoracic radiotherapy. Arch Mal Coeur Vaiss. 1978;71:447–452. [PubMed] [Google Scholar]
- 17.Barbetakis N, Xenikakis T, Paliouras D, Asteriou C, Samanidis G, Kleontas A, Lafaras C, Platogiannis D, Bischiniotis T, Tsilikas C. Pericardiectomy for radiation-induced constrictive pericarditis. Hellenic J Cardiol. 2010;51:214–218. [PubMed] [Google Scholar]
- 18.Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, Seward JB, Tajik AJ. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380–1386. doi: 10.1161/01.cir.100.13.1380. [DOI] [PubMed] [Google Scholar]
- 19.Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, Lytle BW, Blackstone EH, Lauer MS, Klein AL. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445–1452. doi: 10.1016/j.jacc.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Ni Y, von Segesser LK, Turina M. Futility of pericardiectomy for postirradiation constrictive pericarditis? Ann Thorac Surg. 1990;49:445–448. doi: 10.1016/0003-4975(90)90252-2. [DOI] [PubMed] [Google Scholar]
- 21.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 22.Orzan F, Brusca A. Radiation-induced constrictive pericarditis. Associated cardiac lesions, therapy and follow-up. G Ital Cardiol. 1994;24:817–823. [PubMed] [Google Scholar]
- 23.Veeragandham RS, Goldin MD. Surgical management of radiation-induced heart disease. Ann Thorac Surg. 1998;65:1014–1019. doi: 10.1016/s0003-4975(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 24.Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J. Multimodality imaging of pericardial diseases. JACC Cardiovasc Imaging. 2010;3:650–660. doi: 10.1016/j.jcmg.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369–373. doi: 10.1148/radiology.182.2.1732952. [DOI] [PubMed] [Google Scholar]
- 26.Khandaker MH, Espinosa RE, Nishimura RA, Sinak LJ, Hayes SN, Melduni RM, Oh JK. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85:572–593. doi: 10.4065/mcp.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Napolitano G, Pressacco J, Paquet E. Imaging features of constrictive pericarditis: beyond pericardial thickening. Can Assoc Radiol J. 2009;60:40–46. doi: 10.1016/j.carj.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Oyama N, Oyama N, Komuro K, Nambu T, Manning WJ, Miyasaka K. Computed tomography and magnetic resonance imaging of the pericardium: anatomy and pathology. Magn Reson Med Sci. 2004;3:145–152. doi: 10.2463/mrms.3.145. [DOI] [PubMed] [Google Scholar]
- 29.Chinnasami BR, Schwartz RC, Pink SB, Skotnicki RA. Isolated left main coronary stenosis and mediastinal irradiation. Clin Cardiol. 1992;15:459–461. doi: 10.1002/clc.4960150614. [DOI] [PubMed] [Google Scholar]
- 30.Pilliere R, Luquel L, Brun D, Jault F, Gandjbakhch I, Bourdarias JP. Ostial stenosis of the left main coronary artery after mediastinal radiotherapy. Apropos of a case. Arch Mal Coeur Vaiss. 1991;84:869–872. [PubMed] [Google Scholar]
- 31.Radwaner BA, Geringer R, Goldmann AM, Schwartz MJ, Kemp HG., Jr Left main coronary artery stenosis following mediastinal irradiation. Am J Med. 1987;82:1017–1020. doi: 10.1016/0002-9343(87)90167-7. [DOI] [PubMed] [Google Scholar]
- 32.Wan SK, Babb JD. Radiation-induced stenosis of the left main coronary artery. Cathet Cardiovasc Diagn. 1993;28:225–227. doi: 10.1002/ccd.1810280307. [DOI] [PubMed] [Google Scholar]
- 33.Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. 1993;69:496–500. doi: 10.1136/hrt.69.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon B, Cook A, Gothard L, Salmon E, Latus K, Underwood SR, Yarnold J. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002;64:53–63. doi: 10.1016/s0167-8140(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 35.Reinders JG, Heijmen BJ, Olofsen-van Acht MJ, van Putten WL, Levendag PC. Ischemic heart disease after mantlefield irradiation for Hodgkin's disease in long-term follow-up. Radiother Oncol. 1999;51:35–42. doi: 10.1016/s0167-8140(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 36.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 37.Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee BK, Mariscal CS, Tate DJ, Horning SJ, Hoppe RT, Hancock SL. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25:43–49. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 38.Hardenbergh PH, Munley MT, Bentel GC, Kedem R, Borges-Neto S, Hollis D, Prosnitz LR, Marks LB. Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys. 2001;49:1023–1028. doi: 10.1016/s0360-3016(00)01531-5. [DOI] [PubMed] [Google Scholar]
- 39.Sioka C, Exarchopoulos T, Tasiou I, Tzima E, Fotou N, Capizzello A, Ragos V, Tsekeris P, Fotopoulos A. Myocardial perfusion imaging with (99 m)Tc-tetrofosmin SPECT in breast cancer patients that received postoperative radiotherapy: a case-control study. Radiat Oncol. 2011;6:151. doi: 10.1186/1748-717X-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Prosnitz RR, Zhou S, Hardenberg PH, Tisch A, Blazing MA, Borges-Neto S, Hollis D, Wong T, Marks LB. Symptomatic cardiac events following radiation therapy for left-sided breast cancer: possible association with radiation therapy-induced changes in regional perfusion. Clin Breast Cancer. 2003;4:193–197. [PubMed] [Google Scholar]
- 41.Schinkel AF, Elhendy A, van Domburg RT, Bax JJ, Vourvouri EC, Bountioukos M, Rizzello V, Agricola E, Valkema R, Roelandt JR, Poldermans D. Incremental value of exercise technetium-99m tetrofosmin myocardial perfusion single-photon emission computed tomography for the prediction of cardiac events. Am J Cardiol. 2003;91:408–411. doi: 10.1016/s0002-9149(02)03234-4. [DOI] [PubMed] [Google Scholar]
- 42.Jain D, Lessig H, Patel R, Sandler L, Weiland F, Edell SL, Elizabeth Oates M, O'Malley-Tysko E, Khutoryansky N, Jacobson AF. Influence of 99mTc-tetrofosmin SPECT myocardial perfusion imaging on the prediction of future adverse cardiac events. J Nucl Cardiol. 2009;16:540–548. doi: 10.1007/s12350-009-9080-2. [DOI] [PubMed] [Google Scholar]
- 43.Lind PA, Pagnanelli R, Marks LB, Borges-Neto S, Hu C, Zhou SM, Light K, Hardenbergh PH. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys. 2003;55:914–920. doi: 10.1016/s0360-3016(02)04156-1. [DOI] [PubMed] [Google Scholar]
- 44.van Leeuwen-Segarceanu EM, Bos WJ, Dorresteijn LD, Rensing BJ, der Heyden JA, Vogels OJ, Biesma DH. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat Rev. 2011;37:391–403. doi: 10.1016/j.ctrv.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-associated valvular disease. Chest. 1991;99:538–545. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- 46.Reber D, Birnbaum DE, Tollenaere P. Heart diseases following mediastinal irradiation: surgical management. Eur J Cardiothorac Surg. 1995;9:202–205. doi: 10.1016/s1010-7940(05)80145-9. [DOI] [PubMed] [Google Scholar]
- 47.Glanzmann C, Kaufmann P, Jenni R, Hess OM, Huguenin P. Cardiac risk after mediastinal irradiation for Hodgkin's disease. Radiother Oncol. 1998;46:51–62. doi: 10.1016/s0167-8140(97)00125-4. [DOI] [PubMed] [Google Scholar]
- 48.Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 49.Jahangiri M, Edmondson SJ, Rees GM. Surgery for radiation-induced valvular disease. J Heart Valve Dis. 1995;4:288–290. [PubMed] [Google Scholar]
- 50.Mezzaroma E, Di X, Graves P, Toldo S, Van Tassell BW, Kannan H, Baumgarten C, Voelkel N, Gewirtz DA, Abbate A. A mouse model of radiation-induced cardiomyopathy. Int J Cardiol. 2012;156:231–233. doi: 10.1016/j.ijcard.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monceau V, Pasinetti N, Schupp C, Pouzoulet F, Opolon P, Vozenin MC. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug Targets. 2010;11:1395–1404. doi: 10.2174/1389450111009011395. [DOI] [PubMed] [Google Scholar]
- 52.Seemann I, Gabriels K, Visser NL, Hoving S, te Poele JA, Pol JF, Gijbels MJ, Janssen BJ, van Leeuwen FW, Daemen MJ, Heeneman S, Stewart FA. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 2012;103:143–150. doi: 10.1016/j.radonc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–1281. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 54.Velensek V, Mazic U, Krzisnik C, Demsar D, Jazbec J, Jereb B. Cardiac damage after treatment of childhood cancer: a long-term follow-up. BMC Cancer. 2008;8:141. doi: 10.1186/1471-2407-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erven K, Florian A, Slagmolen P, Sweldens C, Jurcut R, Wildiers H, Voigt JU, Weltens C. Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1172–1178. doi: 10.1016/j.ijrobp.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 57.Li CS, Schminke U, Tan TY. Extracranial carotid artery disease in nasopharyngeal carcinoma patients with post-irradiation ischemic stroke. Clin Neurol Neurosurg. 2010;112:682–686. doi: 10.1016/j.clineuro.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Scott AS, Parr LA, Johnstone PA. Risk of cerebrovascular events after neck and supraclavicular radiotherapy: a systematic review. Radiother Oncol. 2009;90:163–165. doi: 10.1016/j.radonc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 59.De Bruin ML, Dorresteijn LD, van't Veer MB, Krol AD, van der Pal HJ, Kappelle AC, Boogerd W, Aleman BM, van Leeuwen FE. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 60.Bowers DC, McNeil DE, Liu Y, Yasui Y, Stovall M, Gurney JG, Hudson MM, Donaldson SS, Packer RJ, Mitby PA, Kasper CE, Robison LL, Oeffinger KC. Stroke as a late treatment effect of Hodgkin's Disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 61.Hassen-Khodja R, Kieffer E University Association for Research in Vascular S. Radiotherapy-induced supra-aortic trunk disease: early and long-term results of surgical and endovascular reconstruction. J Vasc Surg. 2004;40:254–261. doi: 10.1016/j.jvs.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 62.Hashmonai M, Elami A, Kuten A, Lichtig C, Torem S. Subclavian artery occlusion after radiotherapy for carcinoma of the breast. Cancer. 1988;61:2015–2018. doi: 10.1002/1097-0142(19880515)61:10<2015::aid-cncr2820611014>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 63.Abayomi OK. Neck irradiation, carotid injury and its consequences. Oral Oncol. 2004;40:872–878. doi: 10.1016/j.oraloncology.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Cheng SW, Wu LL, Ting AC, Lau H, Lam LK, Wei WI. Irradiation-induced extracranial carotid stenosis in patients with head and neck malignancies. Am J Surg. 1999;178:323–328. doi: 10.1016/s0002-9610(99)00184-1. [DOI] [PubMed] [Google Scholar]
- 65.Tallarita T, Oderich GS, Lanzino G, Cloft H, Kallmes D, Bower TC, Duncan AA, Gloviczki P. Outcomes of carotid artery stenting versus historical surgical controls for radiation-induced carotid stenosis. J Vasc Surg. 2011;53 doi: 10.1016/j.jvs.2010.09.056. 629–636 e1–e5. [DOI] [PubMed] [Google Scholar]
- 66.Benitez RP, Armonda RA, Harrop J, Thomas JE, Rosenwasser RH. Carotid angioplasty and stenting for recurrent and radiation-induced stenosis: preliminary experience. Neurosurg Focus. 1998;5:e14. doi: 10.3171/foc.1998.5.4.15. [DOI] [PubMed] [Google Scholar]
- 67.Cohen JE, Rajz G, Lylyk P, Ben-Hur T, Gomori JM, Umansky F. Protected stent-assisted angioplasty in radiation-induced carotid artery stenosis. Neurol Res. 2005;27(Suppl. 1):S69–S72. doi: 10.1179/016164105X25333. [DOI] [PubMed] [Google Scholar]
- 68.Melliere D, Becquemin JP, Berrahal D, Desgranges P, Cavillon A. Management of radiation-induced occlusive arterial disease: a reassessment. J Cardiovasc Surg (Torino) 1997;38:261–269. [PubMed] [Google Scholar]
- 69.White RA, Sicard GA, Zwolak RM, Sidawy AN, Schermerhorn ML, Shackelton RJ, Siami FS, Committee SVSO. Society of vascular surgery vascular registry comparison of carotid artery stenting outcomes for atherosclerotic vs nonatherosclerotic carotid artery disease. J Vasc Surg. 2010;51:1116–1123. doi: 10.1016/j.jvs.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dorresteijn LD, Vogels OJ, de Leeuw FE, Vos JA, Christiaans MH, Ackerstaff RG, Kappelle AC. Outcome of carotid artery stenting for radiation-induced stenosis. Int J Radiat Oncol Biol Phys. 2010;77:1386–1390. doi: 10.1016/j.ijrobp.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 71.Sadek M, Cayne NS, Shin HJ, Turnbull IC, Marin ML, Faries PL. Safety and efficacy of carotid angioplasty and stenting for radiation-associated carotid artery stenosis. J Vasc Surg. 2009;50:1308–1313. doi: 10.1016/j.jvs.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Shin SH, Stout CL, Richardson AI, DeMasi RJ, Shah RM, Panneton JM. Carotid angioplasty and stenting in anatomically high-risk patients: Safe and durable except for radiation-induced stenosis. J Vasc Surg. 2009;50:762–767. doi: 10.1016/j.jvs.2009.04.066. discussion 767–768. [DOI] [PubMed] [Google Scholar]
- 73.Favre JP, Nourissat A, Duprey A, Nourissat G, Albertini JN, Becquemin JP Association Universitaire de Recherche en Chirurgie V. Endovascular treatment for carotid artery stenosis after neck irradiation. J Vasc Surg. 2008;48:852–858. doi: 10.1016/j.jvs.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 74.Leseche G, Castier Y, Chataigner O, Francis F, Besnard M, Thabut G, Abdalla E, Cerceau O. Carotid artery revascularization through a radiated field. J Vasc Surg. 2003;38:244–250. doi: 10.1016/s0741-5214(03)00320-3. [DOI] [PubMed] [Google Scholar]
- 75.Lam WW, Leung SF, So NM, Wong KS, Liu KH, Ku PK, Yuen HY, Metreweli C. Incidence of carotid stenosis in nasopharyngeal carcinoma patients after radiotherapy. Cancer. 2001;92:2357–2363. doi: 10.1002/1097-0142(20011101)92:9<2357::aid-cncr1583>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 76.Bootz F, Diener HC. Radiation-induced damage to the large arteries. HNO. 1987;35:24–26. [PubMed] [Google Scholar]
- 77.Chang YJ, Chang TC, Lee TH, Ryu SJ. Predictors of carotid artery stenosis after radiotherapy for head and neck cancers. J Vasc Surg. 2009;50:280–285. doi: 10.1016/j.jvs.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 78.Steele SR, Martin MJ, Mullenix PS, Crawford JV, Cuadrado DS, Andersen CA. Focused high-risk population screening for carotid arterial stenosis after radiation therapy for head and neck cancer. Am J Surg. 2004;187:594–598. doi: 10.1016/j.amjsurg.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 79.Carmody BJ, Arora S, Avena R, Curry KM, Simpkins J, Cosby K, Sidawy AN. Accelerated carotid artery disease after high-dose head and neck radiotherapy: is there a role for routine carotid duplex surveillance? J Vasc Surg. 1999;30:1045–1051. doi: 10.1016/s0741-5214(99)70042-x. [DOI] [PubMed] [Google Scholar]
- 80.Cheng SW, Ting AC, Ho P, Wu LL. Accelerated progression of carotid stenosis in patients with previous external neck irradiation. J Vasc Surg. 2004;39:409–415. doi: 10.1016/j.jvs.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 81.McEniery PT, Dorosti K, Schiavone WA, Pedrick TJ, Sheldon WC. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol. 1987;60:1020–1024. doi: 10.1016/0002-9149(87)90345-6. [DOI] [PubMed] [Google Scholar]
- 82.Kleikamp G, Schnepper U, Korfer R. Coronary artery and aortic valve disease as a long-term sequel of mediastinal and thoracic irradiation. Thorac Cardiovasc Surg. 1997;45:27–31. doi: 10.1055/s-2007-1013679. [DOI] [PubMed] [Google Scholar]
- 83.Mack MJ. Risk scores for predicting outcomes in valvular heart disease: how useful? Curr Cardiol Rep. 2011;13:107–112. doi: 10.1007/s11886-010-0167-9. [DOI] [PubMed] [Google Scholar]
- 84.Kamdar AR, Meadows TA, Roselli EE, Gorodeski EZ, Curtin RJ, Sabik JF, Schoenhagen P, White RD, Lytle BW, Flamm SD, Desai MY. Multidetector computed tomographic angiography in planning of reoperative cardiothoracic surgery. Ann Thorac Surg. 2008;85:1239–1245. doi: 10.1016/j.athoracsur.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 85.Khan NU, Yonan N. Does preoperative computed tomography reduce the risks associated with re-do cardiac surgery? Interact Cardiovasc Thorac Surg. 2009;9:119–123. doi: 10.1510/icvts.2008.189506. [DOI] [PubMed] [Google Scholar]
- 86.van Son JA, Noyez L, van Asten WN. Use of internal mammary artery in myocardial revascularization after mediastinal irradiation. J Thorac Cardiovasc Surg. 1992;104:1539–1544. [PubMed] [Google Scholar]
- 87.Hicks GL., Jr Coronary artery operation in radiation-associated atherosclerosis: long-term follow-up. Ann Thorac Surg. 1992;53:670–674. doi: 10.1016/0003-4975(92)90331-w. [DOI] [PubMed] [Google Scholar]
- 88.Apter S, Shemesh J, Raanani P, Portnoy O, Thaler M, Zissin R, Ezra D, Rozenman J, Pfeffer R, Hertz M. Cardiovascular calcifications after radiation therapy for Hodgkin lymphoma: computed tomography detection and clinical correlation. Coron Artery Dis. 2006;17:145–151. doi: 10.1097/00019501-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 89.Daitoku K, Fukui K, Ichinoseki I, Munakata M, Takahashi S, Fukuda I. Radiotherapy-induced aortic valve disease associated with porcelain aorta. Jpn J Thorac Cardiovasc Surg. 2004;52:349–352. doi: 10.1007/s11748-004-0069-0. [DOI] [PubMed] [Google Scholar]
- 90.Buz S, Pasic M, Unbehaun A, Drews T, Dreysse S, Kukucka M, Mladenow A, Hetzer R. Trans-apical aortic valve implantation in patients with severe calcification of the ascending aorta. Eur J Cardiothorac Surg. 2011;40:463–468. doi: 10.1016/j.ejcts.2010.11.075. [DOI] [PubMed] [Google Scholar]
- 91.Kempfert J, Van Linden A, Linke A, Schuler G, Rastan A, Lehmann S, Lehmkuhl L, Mohr FW, Walther T. Transapical aortic valve implantation: therapy of choice for patients with aortic stenosis and porcelain aorta? Ann Thorac Surg. 2010;90:1457–1461. doi: 10.1016/j.athoracsur.2010.06.080. [DOI] [PubMed] [Google Scholar]
- 92.Kolettis TN, Spargias K, Stavridis GT. Combined transapical aortic valve implantation with coronary artery bypass grafting in a young patient with porcelain aorta. Hellenic J Cardiol. 2009;50:79–82. [PubMed] [Google Scholar]
- 93.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 94.Marti JL, Dauer LT, Stempel M, Patil S, Kaplan JB, Montgomery LL. Cumulative imaging radiation exposure following breast-conservation therapy. Ann Surg Oncol. 2011;18:104–108. doi: 10.1245/s10434-010-1279-6. [DOI] [PubMed] [Google Scholar]
- 95.Gaya AM, Ashford RF. Cardiac complications of radiation therapy. Clin Oncol (R Coll Radiol) 2005;17:153–159. doi: 10.1016/j.clon.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 96.Moslehi J. The cardiovascular perils of cancer survivorship. N Engl J Med. 2013;368:1055–1056. doi: 10.1056/NEJMe1215300. [DOI] [PubMed] [Google Scholar]
- 97.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F, Group EGW. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl. 7):vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 98. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Hodgkins Lymphoma Version 2.2012 http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. 19 November 2012.
- 99.Maunoury C, Pierga JY, Valette H, Tchernia G, Cosset JM, Desgrez A. Myocardial perfusion damage after mediastinal irradiation for Hodgkin's disease: a thallium-201 single photon emission tomography study. Eur J Nucl Med. 1992;19:871–873. doi: 10.1007/BF00168163. [DOI] [PubMed] [Google Scholar]
- 100.Gustavsson A, Eskilsson J, Landberg T, Svahn-Tapper G, White T, Wollmer P, Akerman M. Late cardiac effects after mantle radiotherapy in patients with Hodgkin's disease. Ann Oncol. 1990;1:355–363. doi: 10.1093/oxfordjournals.annonc.a057774. [DOI] [PubMed] [Google Scholar]
- 101.Gyenes G, Fornander T, Carlens P, Rutqvist LE. Morbidity of ischemic heart disease in early breast cancer 15–20 years after adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:1235–1241. doi: 10.1016/0360-3016(94)90500-2. [DOI] [PubMed] [Google Scholar]
- 102.Constine LS, Schwartz RG, Savage DE, King V, Muhs A. Cardiac function, perfusion, and morbidity in irradiated long-term survivors of Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1997;39:897–906. doi: 10.1016/s0360-3016(97)00467-7. [DOI] [PubMed] [Google Scholar]
- 103.Cowen D, Gonzague-Casabianca L, Brenot-Rossi I, Viens P, Mace L, Hannoun-Levi JM, Alzieu C, Resbeut M. Thallium-201 perfusion scintigraphy in the evaluation of late myocardial damage in left-side breast cancer treated with adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:809–815. doi: 10.1016/s0360-3016(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 104.Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, Hollis D, Lind P, Tisch A, Wong TZ, Borges-Neto S. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 105.Prosnitz RG, Hubbs JL, Evans ES, Zhou SM, Yu X, Blazing MA, Hollis DR, Tisch A, Wong TZ, Borges-Neto S, Hardenbergh PH, Marks LB. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 106.Andersen R, Wethal T, Gunther A, Fossa A, Edvardsen T, Fossa SD, Kjekshus J. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin's lymphoma. Am J Cardiol. 2010;105:149–152. doi: 10.1016/j.amjcard.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Kupeli S, Hazirolan T, Varan A, Akata D, Alehan D, Hayran M, Besim A, Buyukpamukcu M. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma. J Clin Oncol. 2010;28:1025–1030. doi: 10.1200/JCO.2009.25.2627. [DOI] [PubMed] [Google Scholar]
- 108.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188–1194. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun Z, Ng KH. Coronary computed tomography angiography in coronary artery disease. World J Cardiol. 2011;3:303–310. doi: 10.4330/wjc.v3.i9.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Einstein AJ. Effects of radiation exposure from cardiac imaging how good are the data? J Am Coll Cardiol. 2012;59:553–565. doi: 10.1016/j.jacc.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berrington de Gonzalez A, Kim KP, Smith-Bindman R, McAreavey D. Myocardial perfusion scans: projected population cancer risks from current levels of use in the United States. Circulation. 2010;122:2403–2410. doi: 10.1161/CIRCULATIONAHA.110.941625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]