Abstract
Aims
Diabetes may promote myocardial extracellular matrix (ECM) expansion that increases vulnerability. We hypothesized that: (i) type 2 diabetes would be associated with quantitative cardiovascular magnetic resonance (CMR) measures of myocardial ECM expansion, i.e. extracellular volume fraction (ECV); (ii) medications blocking the renin–angiotensin–aldosterone system (RAAS) would be associated with lower ECV; and (iii) ECV in diabetic individuals would be associated with mortality and/or incident hospitalization for heart failure.
Methods and results
We enrolled 1176 consecutive patients referred for CMR without amyloidosis and computed ECV from measures of the haematocrit and myocardial and blood T1 pre- and post-contrast. Linear regression modelled ECV; Cox regression modelled mortality and/or hospitalization for heart failure. Diabetic individuals (n = 231) had higher median ECV than those without diabetes (n = 945): 30.2% (IQR: 26.9–32.7) vs. 28.1% (IQR: 25.9–31.0), respectively, P < 0.001). Diabetes remained associated with higher ECV in models adjusting for demographics, comorbidities, and medications (P < 0.001). Renin–angiotensin–aldosterone system blockade was associated with lower ECV (P = 0.028) in multivariable linear models. Over a median of 1.3 years (IQR: 0.8–1.9), 38 diabetic individuals had events (21 incident hospitalizations for heart failure; 24 deaths), and ECV was associated with these events (HR: 1.52, 95% CI: 1.21–1.89 per 3% ECV increase) in multivariable Cox regression models.
Conclusion
Diabetes is associated with increased ECV. Extracellular volume fraction detects amelioration of ECM expansion associated with RAAS blockade, and is associated with mortality and/or incident hospitalization for heart failure in diabetic individuals. Extracellular matrix expansion may be an important intermediate phenotype in diabetic individuals that is detectable and treatable.
Keywords: MRI, extracellular matrix, fibrosis, collagen, diabetes, extracellular volume fraction
See page 608 for the editorial comment on this article (doi:10.1093/eurheartj/eht245)
Introduction
Type 2 diabetes mellitus may promote changes in the myocardial extracellular matrix (ECM) that are associated with mortality and incident heart failure. Individuals with diabetes experience worse outcomes for endpoints such as heart failure,1 sudden cardiac death,2 and myocardial infarction (MI)3 (which is particularly lethal in the setting of diabetes). Mechanisms underlying this vulnerability are complex and incompletely understood, and ‘diabetic cardiomyopathy’ requires further characterization. Extracellular matrix expansion may contribute to these adverse events since it is linked with mechanical dysfunction,4 vasomotor dysfunction,5 arrhythmia,6 and mortality.6,7 Furthermore, pre-existing ECM expansion may also diminish tolerance to ischaemic insults.8 Extracellular matrix expansion may therefore indicate ‘vulnerable myocardium’. Because ECM expansion in humans is treatable [ e.g. inhibitors of the renin–angiotensin–aldosterone system (RAAS)]5,9–11 and represents a therapeutic target, quantifying ECM expansion may ultimately provide a foundation to improve care in diabetic individuals through targeted treatment. Yet, prior studies have yielded conflicting results regarding the relation between diabetes and ECM expansion.12,13
Novel cardiovascular magnetic resonance (CMR) techniques permit investigation of the relationship between diabetes, ECM expansion, and outcomes. Cardiovascular magnetic resonance employing gadolinium contrast (Gd) can quantify ECM expansion by measuring the extracellular volume fraction (ECV) based on (i) T1 measures of blood and myocardium before and after Gd and (ii) the haematocrit.14 Extracellular matrix expansion appears to result mostly from the accumulation of excess collagen in the interstitium in the absence of confounding conditions (e.g. oedema or amyloid),4,14 and Gd tracks thin strands of collagen in the ECM with high fidelity.15 Extracellular volume fraction can non-invasively detect and quantify the full spectrum of ECM expansion in humans,14 and ECV is reproducible between CMR scans.16,17
The specific aims were to examine the relationship between diabetes and ECM expansion in non-infarcted myocardium, examine whether ECM expansion was related to RAAS inhibitor use, and then examine outcomes in those with diabetes. We studied a large clinical cohort of consecutive patients referred for CMR. We hypothesized that: (i) diabetes would be associated with ECV in multivariable linear regression models; (ii) RAAS inhibitors, e.g. angiotensin-converting enzyme inhibitors or receptor blockers, and aldosterone antagonists would be associated with lower ECV in multivariable linear regression models; and (iii) ECV in diabetic individuals would be associated with subsequent mortality and/or incident heart failure admission in Cox regression models.
Methods
Patient population
After ethics committee protocol approval that complied with the Declaration of Helsinki, we recruited 1386 adult patients referred for clinical CMR at the UPMC CMR Center at the time of CMR (representing ∼95% of all patients scanned.) This cohort was formed to examine whether novel CMR measures of ECM expansion are associated with patient outcomes. Inclusion criteria were written informed consent and completion of a Gd enhanced CMR scan which required a glomerular filtration rate ≥30 mL/min/1.7 m2, and no other contraindications to CMR. Exclusion criteria were: (i) known or suspected cardiac amyloidosis (n = 18), a unique disorder that markedly expands the interstitium independent of myocardial fibrosis (unpublished data), (ii) hypertrophic cardiomyopathy (n = 61), a genetic disorder with distinct clinical characteristics, (iii) type 1 diabetes (n = 4) as defined in the clinical chart, and (iv) adult congenital heart disease (n = 127). To maximize generalizability, we included those with MI since MI size can vary greatly and since we measured ECV specifically in remote non-infarcted myocardium avoiding even the area at risk. Extracellular matrix expansion in the myocardium remote from the infarction is an important feature of ischaemic cardiomyopathy.12,18
The final cohort included 1176 patients. Diabetic status and other comorbidity data were determined at the time of CMR scanning according to the medical record, as these were used for medical decision making. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Pittsburgh.19 Vital status was ascertained by Social Security Death Index queries and medical record review. Incident heart failure admission was identified by medical record review which used the same definition as prior epidemiological studies.20 Incident heart failure admission required physician documentation and: (i) documented symptoms (e.g. shortness of breath, fatigue, orthopnoea) and physical signs (e.g. oedema, rales) consistent with HF; (ii) supporting clinical findings (e.g. pulmonary oedema on chest X-ray); or (iii) therapy for HF, including diuretics, digitalis, angiotensin-converting enzyme inhibitors, or beta-blockers.20
Cardiovascular magnetic resonance scans
Cine cardiovascular magnetic resonance
Patients received clinical CMR scans by dedicated CMR technologists with a 1.5 Tesla Siemens Magnetom Espree (Siemens Medical Solutions, Erlangen, Germany) and a 32 channel phased array cardiovascular coil. The exam included standard breath held segmented cine imaging with steady-state free precession (SSFP). Left ventricular dimensions, myocardial mass (indexed to body surface area), left ventricular volume indices, and ejection fraction (EF) were measured without geometric assumptions from short-axis stacks of end-diastolic and end-systolic cine frames by experienced readers (two cardiologists and three radiologists).
Late gadolinium enhancement
Late gadolinium enhancement (LGE) imaging was performed 10 min after a 0.2 mmol/kg i.v. gadoteridol bolus (Prohance, Bracco Diagnostics, Princeton, NJ, USA). To optimize LGE, we used a phase sensitive inversion recovery pulse sequence to increase signal-to-noise ratios, correct for surface coil intensity variation, and render signal intensity proportional to T1 recovery.21 When patients could not breath hold or had arrhythmia, single-shot SSFP, motion corrected, averaged PSIR images were acquired.22 Myocardial infarction size was measured blinded to clinical data as described previously.23
Quantification of the extracellular volume fraction
We employed methods described previously that yield reproducible ECV measures of ECM expansion in non-infarcted myocardium after a gadolinium bolus with minimal variation related to heart rate or time elapsed following the bolus.7,16,24,25 We validated T1 measures using an ECG-gated single-shot-modified Look Locker inversion recovery sequence against CuSO4 phantoms with physiological T1 and T2 values for the myocardium and blood.7,16 We did not exclude foci of non-infarcted scar on LGE images (i.e. atypical of MI) from quantitative ECV measures acquired in non-infarcted myocardium7 which would bias ECV measures. We did not want spatial variation of ECM expansion (the key feature which renders it detectable on LGE images) to confound its quantification, especially since ECM expansion exists as a continuum, from focal to diffuse.7 Moreover LGE for quantifying fibrosis/ECM expansion in non-infarcted myocardium is not well validated, and established LGE methodologies are lacking. Extracellular volume fraction has more rigorous validation for that purpose.14,17 For ECV measures, we excluded the myocardium in the vicinity of infarcted, edematous,26 or stunned myocardium and traced the middle third of the myocardium to avoid partial volume effects. We identified MI when LGE involving the subendocardium in a coronary distribution, a strategy that yields sensitivities and specificities >90% for MI detection.
We quantified ECM expansion with the ECV as described previously:7,12,14,27
where λ = [ΔR1myocardium] / [ΔR1bloodpool] pre- and post-Gd (where R1 = 1/T1). Each ECV measurement for a short-axis slice location was derived from a single pre-contrast and post-contrast T1 occurring after clinical LGE images (usually 20 min after the contrast bolus). Haematocrit measures were acquired on the day of scanning. We averaged ECV measures from basal and mid-ventricular short-axis slices to yield the final measurement. Apical slices were avoided due to concerns of error related to partial volume averaging.28
Statistical analysis
Categorical variables were summarized as percentages, and continuous variables were summarized as median and inter-quartile range. Statistical tests were two-sided, and P < 0.05 was considered significant. Chi square (χ2) tests (or Fisher exact tests) compared categorical variables. Wilcoxon rank sum tests compared continuous variables, since some continuous variables exhibited skewed distributions on visual inspection, and the Shapiro Wilk test indicated non-normal distributions. Linear regression models measured the association between quantitative ECV and other variables; variance inflation factors <2 excluded collinearity. Survival analysis employed the log-rank test and Cox regression where χ2 data indicated strength of association between baseline covariates and outcomes. The number of events limited the number of covariates to permit roughly 10 events per covariate variable to prevent model over-fitting, and stratification permitted further adjustment without increasing degrees of freedom. Proportional hazards assumptions were verified by non-significant time interaction terms for ECV. Statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Results
Patient characteristics
Baseline characteristics are summarized in Table 1. Diabetic individuals (n = 231) were older and had a higher burden of comorbidity and disease severity than those without diabetes (n = 945), including greater weight, lower haematocrit, and worse renal function. Among those with diabetes, 97 (41%) were treated with oral hypoglycaemic medication, and 125 (54%) treated with insulin. We did not measure glycosylated haemoglobin (HbA1C) at baseline; in those with available HbA1C, median HbA1C was 7.1% (IQR: 6.5–8.5%, n = 118).
Table 1.
Patient characteristics (n = 1176)
| Variable | Diabetes (n = 231) frequency, n (%) or median (inter-quartile range) | No diabetes (n = 945) frequency, n (%) or median (inter-quartile range) | P-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 61 (54–70) | 53 (40–64) | <0.001 |
| Female (%) | 81 (35) | 401 (42) | 0.041 |
| White race (%) | 197 (85) | 837 (89) | 0.17 |
| Black race (%) | 28 (12) | 74 (8) | 0.038 |
| General indication for CMR exam (%) | |||
| Known or suspected cardiomyopathy | 107 (46) | 386 (41) | 0.13 |
| Possible coronary disease/viability/vasodilator stress testing | 128 (55) | 331 (35) | <0.001 |
| Evaluation for arrhythmia substrate | 46 (20) | 287 (30) | 0.002 |
| Known or suspected mass or thrombus | 7 (3) | 47 (5) | 0.21 |
| Syncope evaluation | 7 (3) | 41 (4) | 0.37 |
| Comorbidity (%) | |||
| Hypertension | 197 (85) | 365 (39) | <0.001 |
| Dyslipidaemia | 153 (66) | 273 (29) | <0.001 |
| Current cigarette smoking | 42 (18) | 134 (14) | 0.13 |
| Atrial fibrillation or flutter | 20 (9) | 78 (8) | 0.84 |
| Hospitalized status | 101 (44) | 240 (25) | <0.001 |
| Coronary vessels with known stenosis>70% (>50% for left main) documented by angiography (%) | |||
| 0 | 161 (70) | 836 (88) | <0.001 for trend |
| 1 | 25 (11) | 49 (5) | |
| 2 | 11 (5) | 30 (3) | |
| 3 or left main involvement | 34 (15) | 30 (3) | |
| Prior coronary revascularization | 76 (33) | 129 (14) | <0.001 |
| Prior stroke | 17 (7) | 38 (4) | 0.031 |
| Acute myocardial infarction | 25 (11) | 42 (4) | <0.001 |
| Weight (kg) | 97 (82–115) | 83 (69–98) | <0.001 |
| Body mass index (kg/m2) | 31 (28–38) | 27. (24–32) | <0.001 |
| Medications (%) | |||
| ACEI, ARB, or aldosterone antagonists | 146 (63) | 335 (35) | <0.001 |
| Beta-blockers | 157 (68) | 402 (43) | <0.001 |
| Aspirin or other antiplatelet | 162 (70) | 384 (41) | <0.001 |
| Loop diuretics | 76 (33) | 165 (17) | <0.001 |
| Statin medication | 151 (65) | 282 (30) | <0.001 |
| Laboratory and CMR characteristics | |||
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 0.9 (0.8–1.1) | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 75 (58–92) | 90 (74–94) | <0.001 |
| Haematocrit (%) | 37 (32–40) | 40 (36–43) | <0.001 |
| Ejection fraction (%) | 54 (39–63) | 58 (47–64) | <0.001 |
| Left ventricular mass index (g/m2) | 63 (54–76) | 56 (46–70) | <0.001 |
| End-diastolic volume index (mL/m2) | 80 (65–104) | 81 (68–97) | 0.91 |
| Non-ischaemic scar evident on LGE images (%) | 60 (26) | 203 (21) | 0.14 |
| Myocardial infarction evident on LGE images | 83 (36) | 153 (16) | <0.001 |
| Myocardial infarction size (% of left ventricular mass in those with MI; n = 71 for diabetes; n = 98 for no diabetes) (%) | 12% (5–23) | 13% (4–27) | 0.90 |
Associations between diabetes and extracellular volume fraction
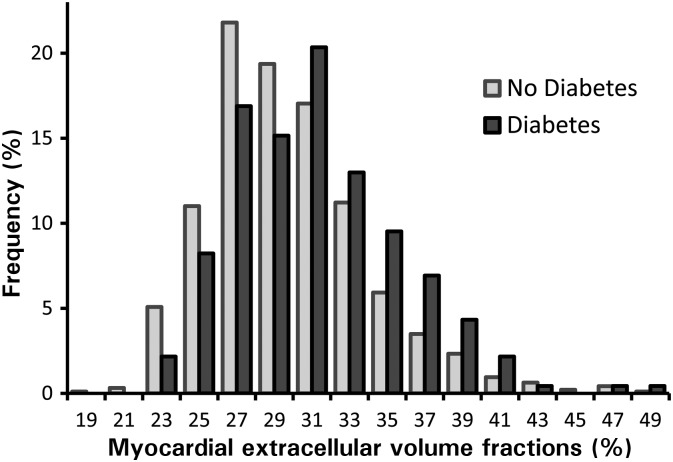
Comorbidities and treatment did not explain the relationship between diabetes and ECV. Diabetic individuals had higher median ECV than those without diabetes: 30.2% (IQR: 26.9–32.7) vs. 28.1% (IQR: 25.9–31.0), respectively, P < 0.001) as shown in Figure 1. Diabetes remained significantly associated with ECV in multivariable linear regression models with extensive risk adjustment (Table 2). This relationship persisted even when limiting the analysis to those with preserved EF, and those without MI, known obstructive coronary artery disease documented by prior angiography, or LGE (Table 2). We found no relation between HbA1C and ECV in those in whom HbA1C data were available.
Figure 1.
Frequency histograms of myocardial extracellular volume fraction indicate higher extracellular volume fraction in those with diabetes. The shapes of the distributions are similar, but the histogram for diabetic individuals is shifted rightward towards higher extracellular volume fraction.
Table 2.
In univariable and multivariable linear regression models, diabetes is consistently associated with ECV (the myocardial extracellular volume fraction), a quantitative measure of myocardial extracellular matrix expansion
| β | P-value | Model | n | Exclusions |
|---|---|---|---|---|
| 1.37 | <0.001 | Univariable | 1176 | — |
| 1.26 | <0.001 | Multivariable | 1179 | — |
| 1.25 | 0.002 | Multivariable | 818 | EF < 50% |
| 1.29 | 0.004 | Multivariable | 728 | EF < 50%, myocardial infarction |
| 1.61 | <0.001 | Multivariable | 693 | EF < 50%, myocardial infarction, coronary disease shown by prior angiography (stenosis ≥70, or ≥50% for left main) |
| 1.40 | <0.006 | Multivariable | 574 | EF < 50%, myocardial infarction, coronary disease shown by prior angiography (stenosis ≥70, or ≥50% for left main), late gadolinium enhancement |
Multivariable models adjusted for age, gender, race, current smoking, prior smoking, hypertension, glomerular filtration rate, left ventricular ejection fraction (EF), myocardial infarction size (per cent of left ventricular mass), left ventricular mass index, number of known diseased coronary arteries, known beta-blocker use, and use of any medications that block the renin–angiotensin–aldosterone system (RAAS). Separate models were also created after serially excluding those with EF <50%, myocardial infarction by CMR, or known coronary disease by prior coronary angiography
Diabetes, extracellular volume fraction, and outcomes
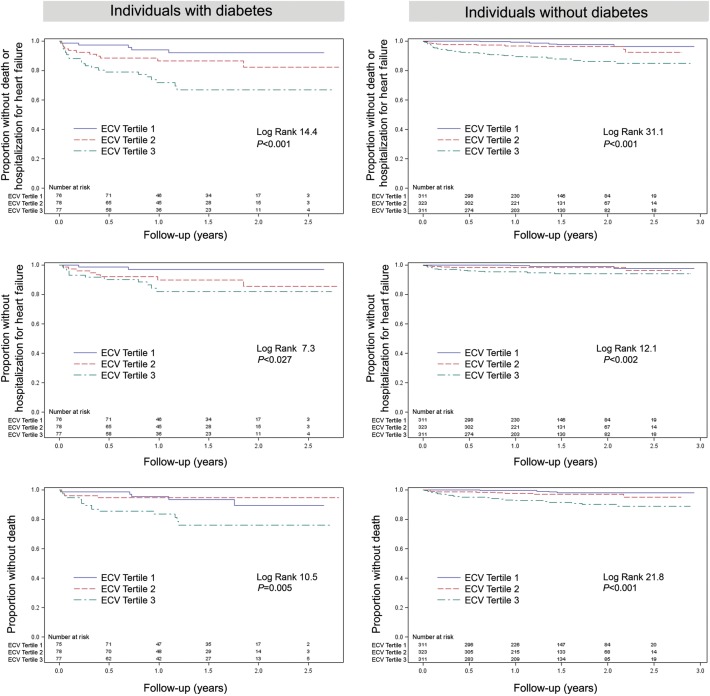
Extracellular volume fraction was associated with adverse outcomes in diabetic individuals. Over a median of 1.3 years (IQR: 0.8–1.9), there were 24 deaths and 21 incident heart failure admissions among diabetic individuals (38 events total since some experienced both). Extracellular volume fraction was significantly associated with; (i) the combined endpoint of death or incident heart failure admission; (ii) incident heart failure admission while censoring for death; or (iii) all-cause mortality. The Kaplan–Meier curves are shown in Figure 2. Extracellular volume fraction remained associated with events in diabetic individuals in multivariable Cox regression models as shown in Table 3. We obtained similar survival analysis results when excluding the 35 surviving individuals (15%) who did not follow-up at our institution after CMR scanning. We found similar associations in those without diabetes.
Figure 2.
In 231 individuals with diabetes and 945 individuals without diabetes, extracellular matrix expansion in myocardium quantified by extracellular volume fraction is associated with increased risks of: death or heart failure admission (top panel); heart failure admission ignoring or censoring for death (middle panel); or all-cause mortality (lower panel). Event rates were higher for those with diabetes. The numbers of events are shown in Table 3.
Table 3.
Myocardial extracellular volume fraction is similarly associated with adverse outcomes in those with and without diabetes in multivariable Cox regression models
| Individuals with diabetes (n = 231) |
Individuals without diabetes (n = 945) |
||||
|---|---|---|---|---|---|
| Outcome | Events (n) | Hazard ratio for every 3% increase in ECV (95% CI) | Events (n) | Hazard ratio for every 3% increase in ECV (95% CI) | Risk adjustment (after stratification by gender and hospitalization status) |
| Mortality or incident hospitalization for heart failure | 38 | 1.52 (1.21–1.89) | 56 | 1.46 (1.25–1.71) | Ejection fraction, age, MI size |
| Incident hospitalization for heart failure (censoring for death) | 21 | 1.35 (1.01–1.80) | 25 | 1.48 (1.19–1.85) | Ejection fraction |
| Mortality | 24 | 1.56 (1.19–2.06) | 39 | 1.54 (1.30–1.82) | Ejection fraction, MI size |
To prevent over-fitting of the models while employing identical risk adjustment, the number of covariates was constrained by the number of events for the smaller subgroup with diabetes. Models stratified by gender and hospitalization status. ECV hazard ratios reflect 3% increments, where ECV ranged from 22–47% for those with diabetes and 18–48% for those without diabetes.
To illustrate the prognostic ability of ECV, we compared it separately against (i) EF, or (ii) non-ischaemic scar on LGE images in simple Cox regression models, with only ECV and EF/non-ischaemic scar as covariates. Using χ2 values to quantify strength of association, ECV surpassed EF or non-ischaemic scar in prognostic ability (Supplementary material online, Table 1–3). There was no interaction between ECV and EF or between ECV and non-ischaemic scar. In the full cohort, there was no interaction between diabetes and ECV for death or incident heart failure admission (P = 0.77).
Blockade of the renin–angiotensin–aldosterone system and extracellular volume fraction
In the entire cohort, there was no significant difference in the median ECV between those taking agents to block the RAAS and those who did not (28.6 vs. 28.2%, P = 0.07). Since use of agents to suppress RAAS is reserved for those with higher disease severity where ECM expansion is expected to be higher, we adjusted for important baseline differences in potential confounders to address this bias. RAAS blockade was then significantly associated with lower ECV (β −0.6, P = 0.028) in the multivariable linear regression model adjusting for baseline differences: age, gender, race, smoking, diabetes, hypertension, dyslipidaemia, glomerular filtration rate, left ventricular EF, MI size (per cent of left ventricular mass), coronary disease severity (the number of coronary arteries with stenoses) left ventricular mass index, and beta-blocker use.
Discussion
Our data from a large, consecutive cohort of patients referred for CMR indicate that ECM expansion quantified by ECV may play a role in the vulnerability associated with diabetes. The principal findings demonstrate for the first time that: (i) diabetes is associated with higher ECV; (ii) increased ECV is associated with mortality and/or incident heart failure admission in individuals with diabetes after adjusting for baseline differences in EF and other parameters; (iii) ECM expansion measured by ECV appeared to be ameliorated with inhibition of the RAAS. Extracellular matrix expansion may be an important intermediate phenotype in diabetic individuals that is detectable by ECV and treatable.
Several studies support the concept that ECM expansion may contribute to adverse outcomes in the setting of diabetes. First, glucose stimulates human cardiac myofibroblast proliferation in vitro, therefore linking diabetes to cells responsible for ECM expansion and myocardial fibrosis.29 Second, subclinical cardiac dysfunction in diabetic people also appears associated with ECM expansion8,13,30 and mortality.31 Third, RAAS inhibition reduces myocardial fibrosis in serial biopsies of human myocardium culminating in improved vasomotor5 and diastolic function.9–11 Fourth, large trials of RAAS inhibition yield improved survival in those with diabetes, irrespective of blood pressure effects even in the absence of heart failure.32 Those with heart failure appear to benefit most from RAAS inhibition if there is underlying ECM expansion from myocardial fibrosis.33 The myocardium may have less tolerance of vascular or ischaemic insults in the presence of myocardial fibrosis and ECM expansion.8 Given prior associations of ECM expansion with mechanical dysfunction,9,18,34 vasomotor dysfunction,5 arrhythmia,6 and mortality6,7 our data further support the concept that ECM expansion represents an important intermediate phenotype in diabetes.
Importantly, the adverse effects of increased collagen are modifiable with regression of the collagen volume fraction after treatment with ACE inhibitors,5,9 angiotensin receptor blockers,11 and mineralocorticoid antagonists10 in humans, as assessed by serial myocardial biopsy specimens. Yet, the degree of regression of the collagen volume fraction with these agents appears quite modest in these studies, limited to ∼20% relative change and 1% absolute change over 10 months of treatment on average. Our data showing modestly lower ECV in those with RAAS inhibition agree well with prior biopsy data. Together, these findings suggest an opportunity for more potent agents currently under development to be more efficacious for both regressing ECM expansion and improving outcomes. Still, the association between RAAS inhibition and lower ECV should be confirmed in a prospective trial.
Despite the median follow-up of 1.3 years and limited events, we still obtained significant results suggesting that ECV functions well as a risk stratifier. Similar associations were obtained for those without diabetes. From the viewpoint of the clinician, stratifying short-term risks even if the events are limited remains important.
Like many imaging parameters, further work with larger multicentre cohorts is needed to demonstrate the extent to which quantitative CMR measures of ECM expansion stratify risk in individuals and guide clinical care as a therapeutic target to initiate treatment and track response. Trials and effectiveness data showing the benefits of CMR guided care are limited. Nonetheless, early results showing robust diagnostic and prognostic ability of ECV are promising. Myocardial tissue characterization appears to be a relevant pursuit. Given the strong prognostic ability of ECV on par with EF, ECV merits further investigation (Supplementary material online, Table 1–3).
Limitations
Our study has limitations. First, our observational data come from a referred sample in a single centre, not a population study, so our results may not generalize, and we may not have adjusted for all confounders. Still, ECM expansion cannot otherwise be identified clinically and ECV measures were not available at the time of referral to bias referring clinicians. To maximize generalizability, we prospectively enrolled large numbers of consecutive patients and minimized exclusions. Still, certain subgroups such as acute MI patients were not well represented [e.g. 6% (67/1176)], and generalizability to this subgroup is uncertain. Second, we did not quantify ECV in all myocardial segments. Nonetheless, we typically measured fibrosis in 12 of 17 myocardial segments provided they did not contain MI. Despite any imperfections in ECV measurement which would likely obscure relationships with outcomes, we still obtained significant results. Third, the effect of the RAAS blockade on ECV was analysed in a cross-sectional evaluation but not in a longitudinal follow-up study, but our results are consistent with serial human myocardial biopsy data.5,9,10 Fourth, risk adjustment was limited with potentially unmeasured confounders. Yet, we obtained consistent relationships between ECV and outcomes in Cox regression models. Finally, the mechanism of death was not clear in sufficient numbers to permit inference about how ECM expansion may have affected cause of death (e.g. heart failure or malignant arrhythmia). Still, adjudication for cause of death can be challenging, controversial, and biased, whereas all-cause mortality remains objective and inherently relevant. We believe cardiac causes are likely in our sample that was enriched with heart disease which also remains prevalent in the general population. We also did not examine other relevant outcomes such as coronary events (infarction and revascularization).
Conclusion
Diabetes is associated with myocardial ECM expansion quantified by the ECV. Extracellular volume fraction detects reductions in ECM expansion associated with RAAS inhibition. Extracellular matrix expansion in diabetic individuals is associated with mortality and incident heart failure admission. Extracellular matrix expansion may be an important intermediate phenotype in diabetic individuals that is treatable and detectable non-invasively. Further work is needed to advance understanding of the causes, consequences, and treatment of ECM expansion and understand how measurement of ECV may improve care.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
E.B.S. is supported by a grant from The Pittsburgh Foundation, Grant M2009-0068, and an American Heart Association Scientist Development grant (09SDG2180083) including a T. Franklin Williams Scholarship Award; funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Association of Specialty Professors, and the American Heart Association. Dr. T.C.W. is supported by a grant K12 HS19461-01 from the Agency for Healthcare Research and Quality. S.G.S.'s research efforts are partially supported by the McGinnis Endowed Chair funds. This work was also supported by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Conflict of interest: E.B.S. has served as an unpaid scientific advisor to Siemens Cardiovascular MR Research and Development.
Supplementary Material
Acknowledgements
We acknowledge the support of Drs Joan Lacomis, Christopher Deible, and Ferenc Czeyda-Pommersheim from the Department of Radiology and the contributions of Kathy Puntil, Deborah Yasko, Jim Zheng, and Elizabeth Ruhl. We thank the patients who volunteered to participate.
References
- 1.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. doi:10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 2.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142–2147. doi: 10.1093/eurheartj/ehi376. doi:10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 3.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–896. doi: 10.1001/2012.jama.11089. doi:10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. doi:10.1161/01.CIR.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36:220–225. doi: 10.1161/01.hyp.36.2.220. doi:10.1161/01.HYP.36.2.220. [DOI] [PubMed] [Google Scholar]
- 6.Tamarappoo BK, John BT, Reinier K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Vulnerable myocardial interstitium in patients with isolated left ventricular hypertrophy and sudden cardiac death: a postmortem histological evaluation. J Am Heart Assoc. 2012;1:e001511. doi: 10.1161/JAHA.112.001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. doi:10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, Heagerty AM, Malik RA. Diabetic cardiomyopathy—a distinct disease? Best Pract Res Clin Endocrinol Metab. 2009;23:347–360. doi: 10.1016/j.beem.2008.10.016. doi:10.1016/j.beem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. doi:10.1161/01.CIR.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 10.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–2945. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 11.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. doi:10.1161/01.CIR.0000017264.66561.3D. [DOI] [PubMed] [Google Scholar]
- 12.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. doi: 10.1093/eurheartj/ehr481. doi:10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng ACT, Auger D, Delgado V, van Elderen SGC, Bertini M, Siebelink H-M, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JWA, Leung DY, Bax JJ, Lamb HJ. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced t1 mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ Cardiovasc Imaging. 2011;5:51–59. doi: 10.1161/CIRCIMAGING.111.965608. doi:10.1161/CIRCIMAGING.111.965608. [DOI] [PubMed] [Google Scholar]
- 14.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. doi:10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 15.Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE. Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010;3:743–752. doi: 10.1161/CIRCIMAGING.108.835793. doi:10.1161/CIRCIMAGING.108.835793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. doi:10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana M, White SK, Banypersad SM, Sado DM, Maestrini V, Flett AS, Piechnik SK, Neubauer S, Roberts N, Moon JC. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson. 2012;14:88. doi: 10.1186/1532-429X-14-88. doi:10.1186/1532-429X-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. doi:10.1161/01.CIR.89.1.151. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Kritchevsky SB, Wilson PW, Newman AB, Harris TB, Butler J. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circ Heart Fail. 2010;3:495–502. doi: 10.1161/CIRCHEARTFAILURE.109.904300. doi:10.1161/CIRCHEARTFAILURE.109.904300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. doi: 10.1002/mrm.10051. doi:10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledesma-Carbayo MJ, Kellman P, Hsu LY, Arai AE, McVeigh ER. Motion corrected free-breathing delayed-enhancement imaging of myocardial infarction using nonrigid registration. J Magn Reson Imaging. 2007;26:184–190. doi: 10.1002/jmri.20957. doi:10.1002/jmri.20957. [DOI] [PubMed] [Google Scholar]
- 23.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–28. doi: 10.1016/S0140-6736(00)03567-4. doi:10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 24.Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67:1644–1655. doi: 10.1002/mrm.23153. doi:10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. doi: 10.1002/jmri.21119. doi:10.1002/jmri.21119. [DOI] [PubMed] [Google Scholar]
- 26.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. doi:10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, Sibley CT, Kellman P, Arai AE, Bluemke DA. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011;13:75. doi: 10.1186/1532-429X-13-75. doi:10.1186/1532-429X-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. doi:10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann S, Huse K, Semrau R, Diegeler A, Gebhardt R, Buniatian GH, Scholz GH. Aldosterone and D-glucose stimulate the proliferation of human cardiac myofibroblasts in vitro. Hypertension. 2002;39:756–760. doi: 10.1161/hy0302.105295. doi:10.1161/hy0302.105295. [DOI] [PubMed] [Google Scholar]
- 30.Jellis C, Wright J, Kennedy D, Sacre J, Jenkins C, Haluska B, Martin J, Fenwick J, Marwick TH. Association of imaging markers of myocardial fibrosis with metabolic and functional disturbances in early diabetic cardiomyopathy. Circ Cardiovasc Imaging. 2011;4:693–702. doi: 10.1161/CIRCIMAGING.111.963587. doi:10.1161/CIRCIMAGING.111.963587. [DOI] [PubMed] [Google Scholar]
- 31.Kuller LH, Velentgas P, Barzilay J, Beauchamp NJ, O'Leary DH, Savage PJ. Diabetes mellitus: subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arterioscler Thromb Vasc Biol. 2000;20:823–829. doi: 10.1161/01.atv.20.3.823. doi:10.1161/01.ATV.20.3.823. [DOI] [PubMed] [Google Scholar]
- 32.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. doi:10.1016/S0140-6736(99)12323-7. [PubMed] [Google Scholar]
- 33.Zannad F, Alla F, Dousset B, Perez A, Pitt B Rales investigators. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES) Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. doi:10.1161/01.CIR.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 34.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Sonnenblick EH, Olivetti G, Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27:291–305. doi: 10.1016/s0022-2828(08)80028-4. doi:10.1016/S0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.