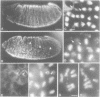
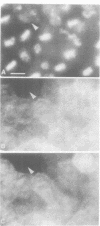
Abstract
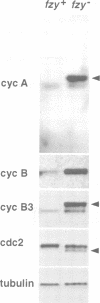
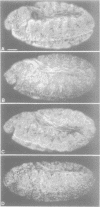
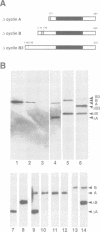
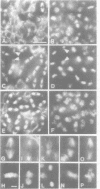
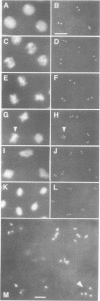
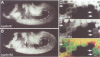
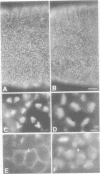
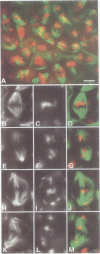
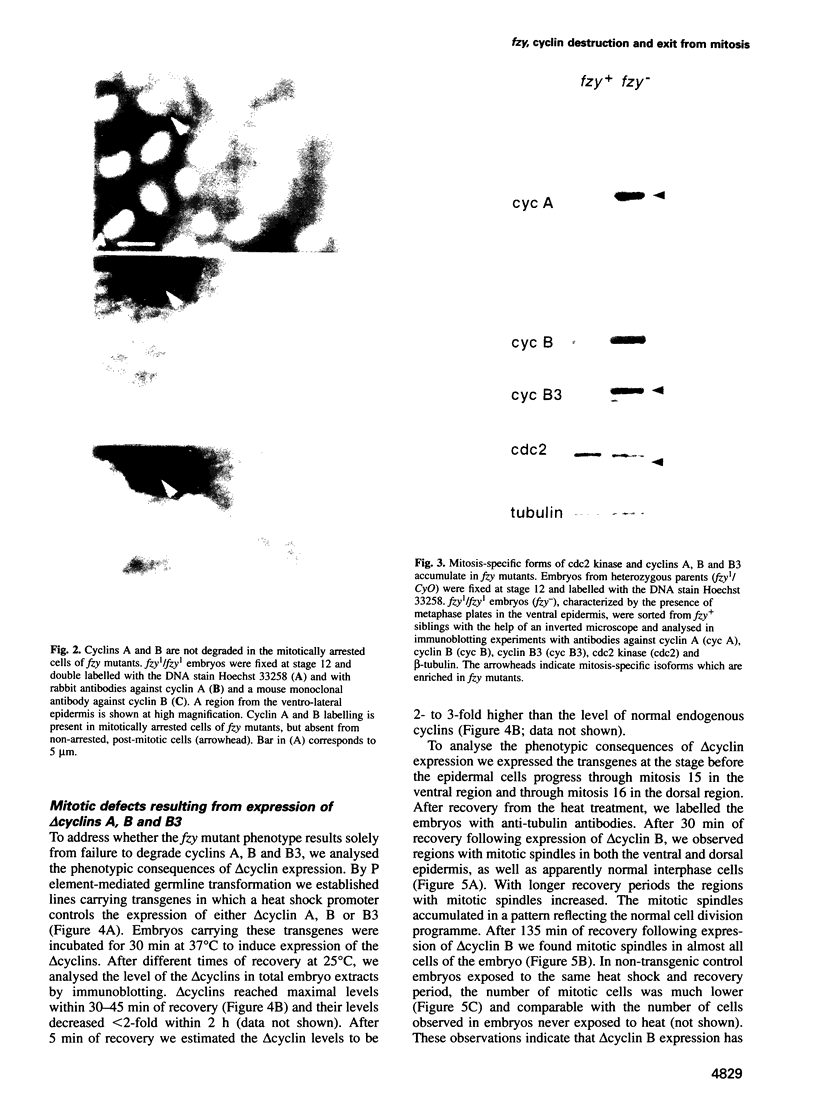
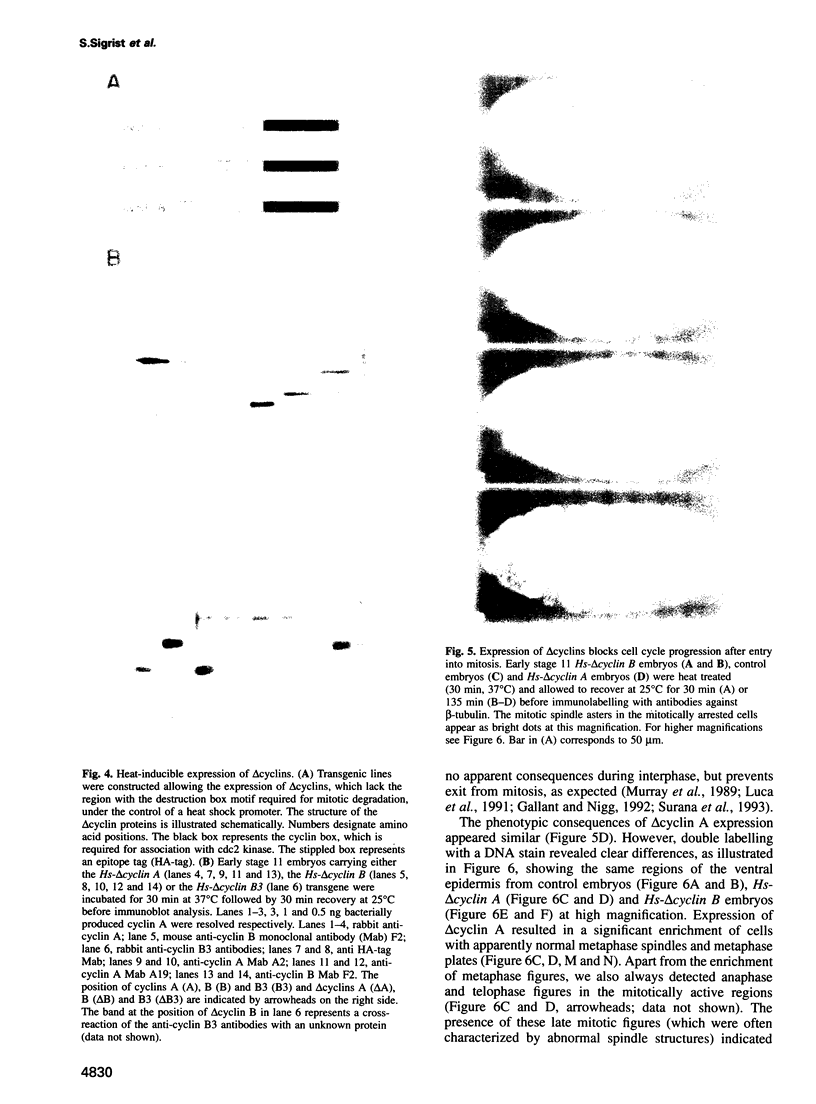
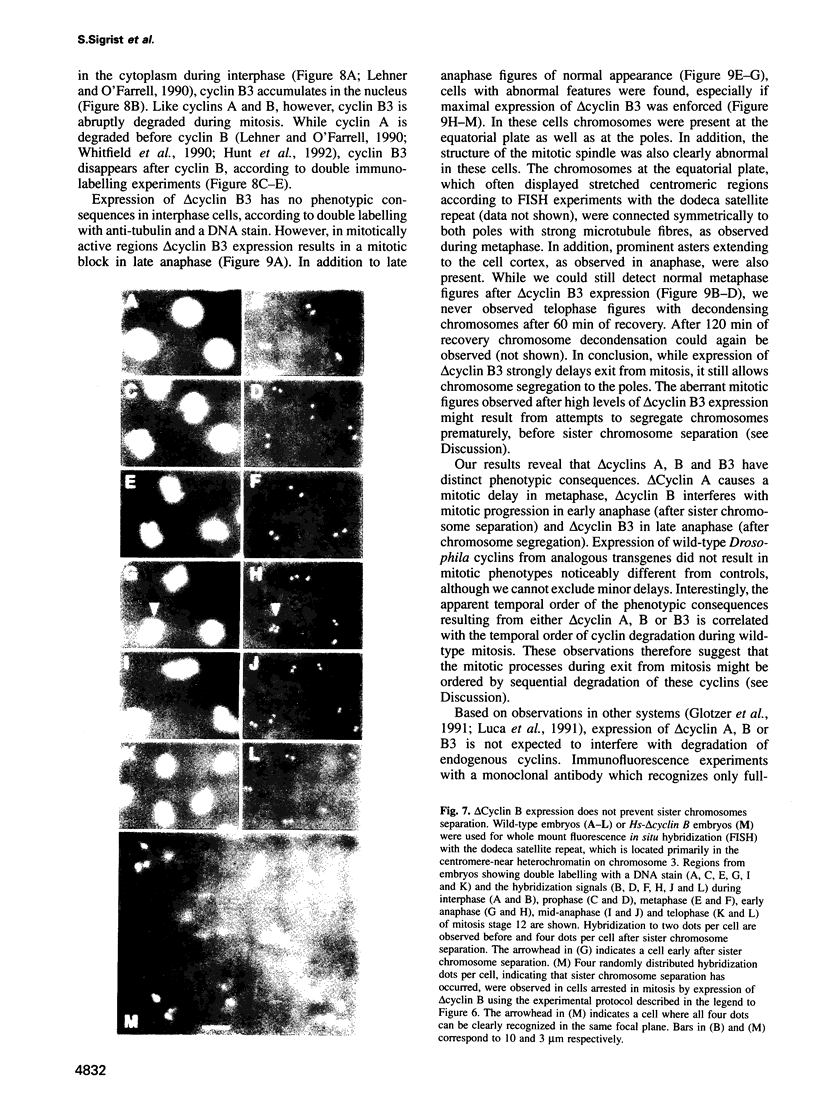
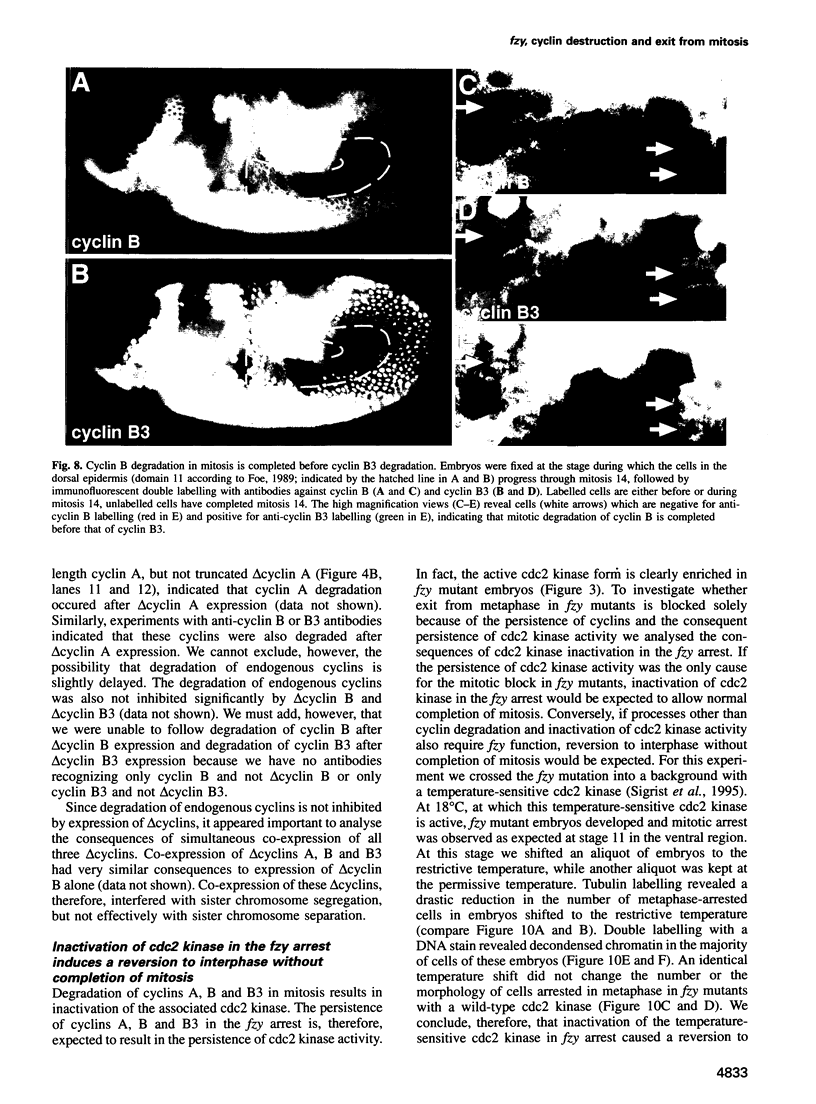
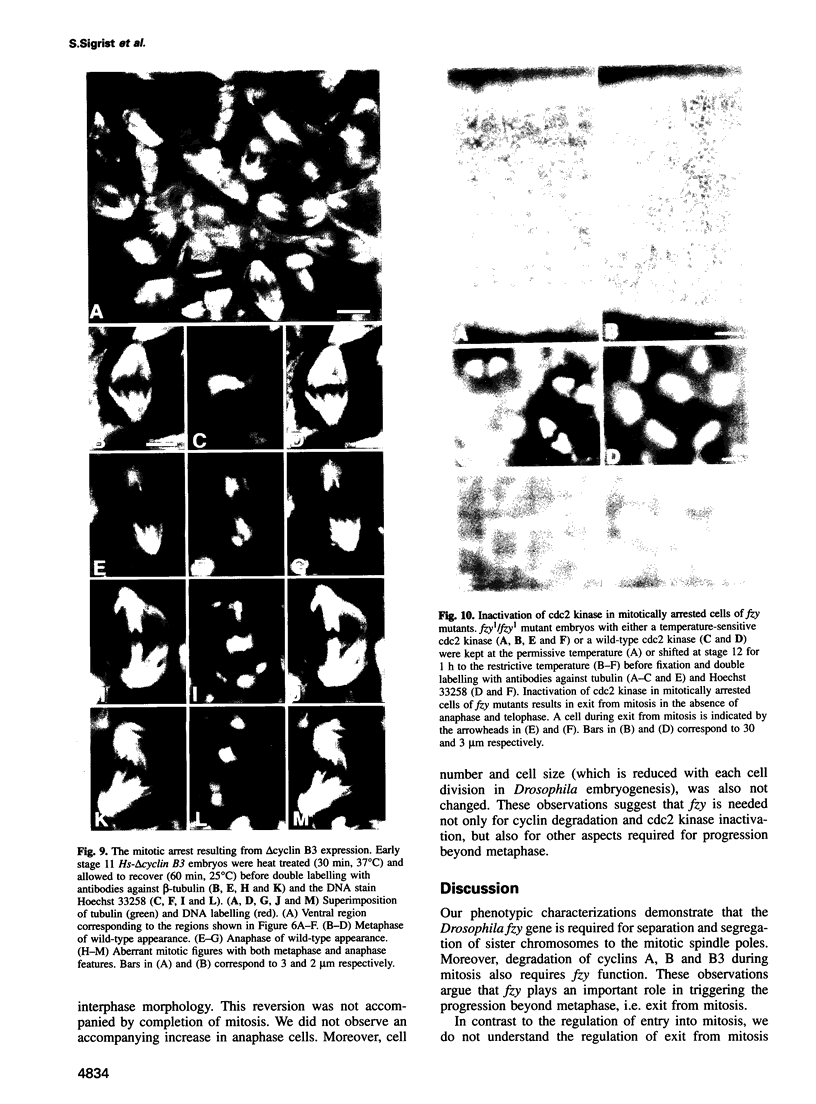
While entry into mitosis is triggered by activation of cdc2 kinase, exit from mitosis requires inactivation of this kinase. Inactivation results from proteolytic degradation of the regulatory cyclin subunits during mitosis. At least three different cyclin types, cyclins A, B and B3, associate with cdc2 kinase in higher eukaryotes and are sequentially degraded in mitosis. We show here that mutations in the Drosophila gene fizzy (fzy) block the mitotic degradation of these cyclins. Moreover, expression of mutant cyclins (delta cyclins) lacking the destruction box motif required for mitotic degradation affects mitotic progression at distinct stages. Deltacyclin A results in a delay in metaphase, deltacyclin B in an early anaphase arrest and deltacyclin B3 in a late anaphase arrest, suggesting that mitotic progression beyond metaphase is ordered by the sequential degradation of these different cyclins. Coexpression of deltacyclins A, B and B3 allows a delayed separation of sister chromosomes, but interferes wit chromosome segregation to the poles. Mutations in fzy block both sister chromosome separation and segregation, indicating that fzy plays a crucial role in the metaphase/anaphase transition.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad J. P., Carmena M., Baars S., Saunders R. D., Glover D. M., Ludeña P., Sentis C., Tyler-Smith C., Villasante A. Dodeca satellite: a conserved G+C-rich satellite from the centromeric heterochromatin of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4663–4667. doi: 10.1073/pnas.89.10.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M., Abad J. P., Villasante A., Gonzalez C. The Drosophila melanogaster dodecasatellite sequence is closely linked to the centromere and can form connections between sister chromatids during mitosis. J Cell Sci. 1993 May;105(Pt 1):41–50. doi: 10.1242/jcs.105.1.41. [DOI] [PubMed] [Google Scholar]
- Dawson I. A., Roth S., Akam M., Artavanis-Tsakonas S. Mutations of the fizzy locus cause metaphase arrest in Drosophila melanogaster embryos. Development. 1993 Jan;117(1):359–376. doi: 10.1242/dev.117.1.359. [DOI] [PubMed] [Google Scholar]
- Dawson I. A., Roth S., Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995 May;129(3):725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B. A., Sprenger F., Duronio R. J., Leopold P., O'Farrell P. H. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994 Feb 15;8(4):440–452. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V. E. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development. 1989 Sep;107(1):1–22. [PubMed] [Google Scholar]
- Félix M. A., Labbé J. C., Dorée M., Hunt T., Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990 Jul 26;346(6282):379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Gallant P., Nigg E. A. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J Cell Biol. 1992 Apr;117(1):213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P., Nigg E. A. Identification of a novel vertebrate cyclin: cyclin B3 shares properties with both A- and B-type cyclins. EMBO J. 1994 Feb 1;13(3):595–605. doi: 10.1002/j.1460-2075.1994.tb06297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara J. B., Richardson H. E., Sugimoto K., Henze M., Lew D. J., Wittenberg C., Reed S. I. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991 Apr 5;65(1):163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Casal Jimenez J., Ripoll P., Sunkel C. E. The spindle is required for the process of sister chromatid separation in Drosophila neuroblasts. Exp Cell Res. 1991 Jan;192(1):10–15. doi: 10.1016/0014-4827(91)90150-s. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J. Kinetochores, microtubules and the metaphase checkpoint. Trends Cell Biol. 1995 Apr;5(4):143–148. doi: 10.1016/s0962-8924(00)88968-0. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J., Ricketts W. A. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993 Sep;122(6):1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y., Dernburg A. F., Parmelee S. J., Rykowski M. C., Agard D. A., Sedat J. W. The onset of homologous chromosome pairing during Drosophila melanogaster embryogenesis. J Cell Biol. 1993 Feb;120(3):591–600. doi: 10.1083/jcb.120.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway S. L., Glotzer M., King R. W., Murray A. W. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993 Jul 2;73(7):1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Hunt T., Luca F. C., Ruderman J. V. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992 Feb;116(3):707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J. A., Lehner C. F. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J. 1993 Jan;12(1):65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer M. A., Richards J. P., De Silva-Udawatta M. N., Temenak J. J., Knoblich J. A., Lehner C. F., Bennett K. L. Caenorhabditis elegans cyclin A- and B-type genes: a cyclin A multigene family, an ancestral cyclin B3 and differential germline expression. J Cell Sci. 1995 Jun;108(Pt 6):2415–2424. doi: 10.1242/jcs.108.6.2415. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989 Mar 24;56(6):957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F. The pebble gene is required for cytokinesis in Drosophila. J Cell Sci. 1992 Dec;103(Pt 4):1021–1030. doi: 10.1242/jcs.103.4.1021. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Shibuya E. K., Dohrmann C. E., Ruderman J. V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO J. 1991 Dec;10(13):4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Codina G., Llamazares S., Glover D. M. Heat shock results in cell cycle delay and synchronisation of mitotic domains in cellularised Drosophila melanogaster embryos. J Cell Sci. 1993 Jul;105(Pt 3):711–720. doi: 10.1242/jcs.105.3.711. [DOI] [PubMed] [Google Scholar]
- Minshull J., Pines J., Golsteyn R., Standart N., Mackie S., Colman A., Blow J., Ruderman J. V., Wu M., Hunt T. The role of cyclin synthesis, modification and destruction in the control of cell division. J Cell Sci Suppl. 1989;12:77–97. doi: 10.1242/jcs.1989.supplement_12.8. [DOI] [PubMed] [Google Scholar]
- Minshull J., Sun H., Tonks N. K., Murray A. W. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 1994 Nov 4;79(3):475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- Morin N., Abrieu A., Lorca T., Martin F., Dorée M. The proteolysis-dependent metaphase to anaphase transition: calcium/calmodulin-dependent protein kinase II mediates onset of anaphase in extracts prepared from unfertilized Xenopus eggs. EMBO J. 1994 Sep 15;13(18):4343–4352. doi: 10.1002/j.1460-2075.1994.tb06754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Rimmington G., Dalby B., Glover D. M. Expression of N-terminally truncated cyclin B in the Drosophila larval brain leads to mitotic delay at late anaphase. J Cell Sci. 1994 Oct;107(Pt 10):2729–2738. doi: 10.1242/jcs.107.10.2729. [DOI] [PubMed] [Google Scholar]
- Sethi N., Monteagudo M. C., Koshland D., Hogan E., Burke D. J. The CDC20 gene product of Saccharomyces cerevisiae, a beta-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol Cell Biol. 1991 Nov;11(11):5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., Futcher B., Jentsch S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature. 1995 Jan 5;373(6509):78–81. doi: 10.1038/373078a0. [DOI] [PubMed] [Google Scholar]
- Stern B., Ried G., Clegg N. J., Grigliatti T. A., Lehner C. F. Genetic analysis of the Drosophila cdc2 homolog. Development. 1993 Jan;117(1):219–232. doi: 10.1242/dev.117.1.219. [DOI] [PubMed] [Google Scholar]
- Sudakin V., Ganoth D., Dahan A., Heller H., Hershko J., Luca F. C., Ruderman J. V., Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995 Feb;6(2):185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U., Amon A., Dowzer C., McGrew J., Byers B., Nasmyth K. Destruction of the CDC28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993 May;12(5):1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]