Abstract
Purpose
To investigate whether variants in a set of eight candidate genes are associated with diabetic retinopathy (DR) in a cohort of Chinese patients with type 2 diabetes mellitus (T2DM).
Methods
Case-control study. Patients with T2DM were recruited from the Desheng community in urban Beijing and assigned into a DR group or diabetic without retinopathy (DWR) group, based on the duration of diabetes and grading of fundus images. Twenty-six single-nucleotide polymorphisms (SNPs) within eight candidate genes, including PPARγ, vascular endothelial growth factor (VEGF) and its receptor kinase insert domain receptor (KDR), erythropoietin, aldose reductase, protein kinase C-β, angiotensin-converting enzyme, and intercellular adhesion molecule 1, were analyzed using the MassARRAY genotyping system.
Results
A total of 500 patients with T2DM (216 with DR and 284 with DWR) were enrolled in the study. Significant associations of DR were noted with genotypes of four SNPs—rs699947 (p<0.001), rs833061 (p=0.001), rs13207351 (p<0.001), and rs2146323 (p=0.006)—in the VEGF gene and one variant, rs2071559, in the KDR gene (p=0.034). After adjustment for covariates, significant association of DR remained with the homozygous genotype of the minor allele for the SNPs rs699947 (odds ratio [OR] = 3.54, 95% confidence interval [CI]: 1.12–11.19), rs833061 (OR = 3.72, 95% CI: 1.17–11.85), rs13207351 (OR = 3.76, 95% CI: 1.21–11.71), and rs2146323 (OR = 2.8, 95% CI: 1.46–5.37) in the VEGF gene as well as the SNP rs2071559 (OR = 1.62, 95% CI: 1.08–2.41) in the KDR gene. However, only rs699947 and rs13207351 in the VEGF gene remained statistically significant after Bonferroni correction. No associations were found in other genes tested.
Conclusions
These data expanded previous observations on the association of DR with variants in the VEGF gene in Chinese patients with T2DM. Moreover, a possible association between DR and KDR polymorphisms is suggested.
Introduction
Diabetic retinopathy (DR) is one of the most common complications of diabetes and the leading cause of irreversible visual loss in people of working age in the United States [1]. The etiology of DR is complex and largely unknown. Hyperglycemia and long duration of diabetes along with hypertension are widely recognized as major risk factors for its development [2,3]; however, these factors seem to explain only a part of the risk of DR [4,5]. In clinical practice, for example, some patients with poorly controlled diabetes or a long duration of diabetes do not develop retinopathy, whereas others with relatively good glycemic control have advanced retinopathy [6]. Accumulated evidence has suggested that genetic factors may play a role in the development and progression of DR, with heritability estimated to be as high as 27% [7] for DR and 50% for proliferative diabetic retinopathy (PDR) [8].
Several studies have used a genome-wide association approach [9-12] and multipoint sib-pair analysis [13,14] to detect regions of the genome that potentially contain genes involved in the etiology of DR. While different regions of the genome have been implicated, the reported regions were not consistently identified among the studies. In addition, many studies have taken a candidate gene approach to investigate the genetic etiology of DR, implicating the potential candidate genes in DR etiology, which include genes for PPARγ [15], vascular endothelial growth factor (VEGF) [16-19], erythropoietin (EPO) [20,21], aldose reductase (AKR1B1) [22-25], protein kinase C (PKC)-β [26,27], angiotensin-converting enzyme (ACE) [28], and intercellular adhesion molecule 1 (ICAM-1) [29-31]. The validity of these results, however, remains unconfirmed, with few of the reported susceptibility variants having been consistently replicated [25].
Kinase insert domain receptor (KDR), also called VEGF receptor (VEGFR) 2, is believed to be responsible for the majority of the angiogenic and permeability-enhancing effects of VEGF [32,33]. An increase expression of KDR has been reported in the retinas from diabetic rats [34,35]. One study investigated the fibrovascular tissues obtained from 22 PDR patients and suggested the co-expression of KDR and neuropilin-1 facilitated fibrovascular proliferation in DR [36]. Moreover, microvascular expression of KDR was found to be associated with leaky vessels in diabetic retina from eyes of diabetic donors as compared to the retina from eyes of nondiabetic control donors [37]. Taken together, these findings imply that KDR might be a major contributor in the pathogenesis of DR. Furthermore, the KDR gene has been found to be associated with other pathogenic conditions featured for vascular permeability and angiogenesis, such as age-related macular degeneration (AMD) [38], cancer [39], and coronary heart/artery disease [40,41]. However, genetic association studies with DR have not been performed for the KDR gene. In this study, we tested whether the candidate genes, including PPARγ, VEGF, EPO, AKR1B1, PKC-β, ACE, and ICAM-1 genes as well as the KDR gene, were associated with DR in our independent cohort of Chinese patients with T2DM.
Methods
Subjects and clinical evaluation
The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital (TRECKY200907) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants before their enrollment. Patients with T2DM were recruited between November 2009 and September 2011 from the Desheng Community of urban Beijing. Diabetes was defined as a self-reported history of physician-diagnosed T2DM treated with insulin, oral hypoglycemic agents, or diet only; or by a fasting plasma glucose (FPG) concentration of 7.0 mmol/l (126 mg/dl) or more in at least two previous examinations; or a random plasma glucose concentration of ≥11.1 mmol/l (200 mg/dl). All subjects underwent a standardized evaluation consisting of a questionnaire, ocular and anthropometric examinations, and a laboratory test. The questionnaire elicited basic information (age, sex, ethnicity, income, education), lifestyle information (such as smoking and alcohol intake), health status information (such as the use of insulin therapy and any history of systemic diseases), and family history of diseases. Anthropometric parameters included body weight and height, waist and hip circumference, and three measurements, 5 min apart, of blood pressure in a resting state. Body mass index (BMI, kg/m2) was calculated according to the height and weight of the participant, and waist-to-hip ratio was calculated. A comprehensive ophthalmological examination included corrected visual acuity, slit-lamp biomicroscopy, and dilated fundus photography. Seven fields of 30°color fundus photographs with stereoscopic images of the optic disc and macula were taken through the dilated pupils of each patient, using a digital fundus camera (Zeiss Visucam Pro; Oberkochen, Germany).
Based on the duration of diabetes and grading of fundus photographs, patients were assigned to the diabetic-without retinopathy (DWR) group if they had more than 10 years of T2DM with no signs of DR (microaneurysms, hemorrhages, exudates) or if they had more than 15 years of T2DM with fewer than five microaneurysms. Patients with five or more microaneurysms in at least one eye were assigned to the DR group. Patients who did not meet the DWR or DR criteria were excluded from this study. The duration of diabetes was defined as the interval between the first diagnosis of diabetes and the time of enrollment in the present study.
Laboratory assays
Fasting venous blood samples were collected for measurement of FPG, glycosylated hemoglobin A1c (HbA1c), creatinine, uric acid, and lipid profile (levels of total cholesterol, triglycerides, and high-density and low-density lipoprotein cholesterol), which were measured in an automated system with reagents for routine biomarkers. HbA1c was assessed by the enzymatic method using a Hitachi analyzer 7080 (Hitachi, Ibaraki, Japan). A first-void, midstream, morning, spot urine sample was collected, and albuminuria was measured by immunonephelometry with a Roche/Cobas C501 analyzer (Ibaraki). High albuminuria was defined as ≥20 mg/l.
Candidate genes and single-nucleotide polymorphisms selection
Eight candidate genes that are involved in the pathogenesis of DR or previously identified as being associated with DR were tested, including the PPARγ, VEGF and its receptor (KDR), EPO, AKR1B1, PKC-β, ACE and ICAM-1 genes. Twenty-six single-nucleotide polymorphisms (SNPs) in these genes were chosen initially for availability of assays, informativeness, and spacing across the gene. More information about the candidate genes is given in Table 1. It should be noted that the VEGF gene has been reported in previous literature [16], and the present study repeated the association between the VEGF gene and DR in an expanded larger sample size.
Table 1. Description of candidate genes investigated.
| Gene | Gene function | Locus | References |
|---|---|---|---|
|
PPARγ |
responsible for differentiation of fibroblasts to adipocytes and regulation of their function |
3p25 |
Maciej et al. 2008 [13] |
|
KDR |
functions as the main mediator of VEGF-induced endothelial permeability, proliferation, survival, migration, tubular morphogenesis and sprouting |
4q11-q12 |
Galan et al. 2010 [46] |
|
VEGF |
a critical angiogenic and vasopermeability factor that is upregulated in various conditions involving retinal ischemia |
6p12 |
Churchill et al. 2008 [15]
Al-Kateb et al. 2007 [16] |
|
EPO |
been linked to angiogenesis, vasculogenesis, and neuroprotection |
7q21–7q22 |
Tong et al. 2008 [18]
Abhary et al. 2010 [19] |
|
AKR1B1 |
the first and rate-limiting enzyme in the polyol pathway |
7q35 |
Richeti, F et al. 2007 [22]
Abhary et al. 2009 [23] |
|
PKC-β |
PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways |
16p11.2 |
Uthra et al. 2010 [24]
Ikeda et al. 2004 [25] |
|
ACE |
involved in the conversion of angiotensin I to angiotensin II (ATII). |
17q23 |
Kondo et al. 2009 [26] |
| ICAM-1 | a member of the immunoglobulin superfamily of adhesion molecules, involved in blood-retina barrier breakdown, capillary nonperfusion and endothelial cell damage and death | 19p13 | Kamiuchi, et al. 2002 [21] L. Liu, et al. 2006 [22] Petrovic, et al. 2008 [23] |
Locus: the chromosomal region of gene; p: long arm of chromosome; q: short arm of chromosome
Genotyping
Blood samples were collected from all participants and stored at −80 °C before DNA extraction. Genomic DNA was extracted from venous blood leukocytes using a genomic DNA extraction and purification kit (TIANamp Swab DNA Kit; Tiangen Biotech, Beijing, China). Study participants were genotyped for the SNPs using Sequenom MassARRAY technology (Bioyong Technologies, Beijing, China). All DNA samples passing initial quality checks were plated at a concentration of ≥5 ng/μl for processing on the platform. Quality measures taken into account for genotyped SNPs to be excluded from the subsequent analysis were minor allele frequency (MAF) <0.05, genotyping success <80%, and failed Hardy–Weinberg equilibrium (HWE) test in control samples (p<0.001). Quality control criteria for each SNP were implemented in our data set.
Statistical analysis
Statistical analysis was performed using the R statistical analysis package. Baseline characteristics of diabetic patients in the DR and DWR groups were compared using a t test for continuous variables or a chi-square test for categorical variables. The genotype frequencies of genes were checked for HWE in all groups using a chi-square test. The chi-square test was also used to analyze the distribution of genotypes and alleles. When the expected frequency was less than 5, Fisher’s exact test was used. The pairwise linkage disequilibrium was calculated using Haploview, version 4.2 (Broad Institute, Cambridge, MA). Univariate and multivariate logistic regression analyses were performed to identify the association between SNPs/haplotypes and the presence of DR. Statistical results were expressed as p values, odds ratios (OR), and 95% confidence intervals (CI). The Bonferroni correction (p<0.05 divided by the number of SNPs analyzed) was used to account for multiple testing.
Results
In total, 1,433 subjects with T2DM were enrolled from the community, of which, 500 subjects (284 in the DWR group and 216 in the DR group) participated in the genetic study based on the inclusion criteria. The distribution of demographic, behavioral, and clinical characteristics of participants are listed in Table 2. In the DR group, 59 patients were further classified as PDR. Compared with the DWR group, individuals in the DR group had a younger age of diabetic onset (p=0.008); higher percentage of high albuminuria (p<0.001); higher levels of creatinine (p=0.027), FPG (p<0.001), and HbA1c (p<0.001); and were more likely to use insulin (p<0.001). Duration of diabetes in the DWR group was longer than the DR group (p=0.013), which could be due to the definition of DWR in this study (more than 10 years of T2DM without retinopathy). No statistically significant differences were found between the DR and DWR groups in terms of gender, BMI, waist-to-hip ratio, blood pressure, serum levels of uric acid, or lipid profile.
Table 2. Clinical and biochemical markers for the studied groups.
| Clinical characteristics | DWR (n=284) | DR (n=216) | p value |
|---|---|---|---|
| Age of diabetic onset (years) |
52.7±7.61 |
50.67±9.41 |
0.008 |
| Sex (Male/Female) |
111/173 |
103/113 |
0.054 |
| Duration of diabetes (years) |
14.79±4.97 |
13.44±7.22 |
0.013 |
| BMI (kg/m2) |
25.26±3.95 |
25.8±4.13 |
0.14 |
| WHR |
0.92±0.06 |
0.93±0.06 |
0.5 |
| High albuminuria(-/+) |
240/38 |
143/68 |
<0.001 |
| Systolic blood pressure (mmHg) |
136.7±16.65 |
138.5±17.18 |
0.22 |
| Diastolic blood pressure (mmHg) |
77.65±9.36 |
79.23±9.69 |
0.066 |
| Insulin therapy (yes/no) |
92/191 |
118/97 |
<0.001 |
| HbA1c (%) |
6.97±1.40 |
7.84±1.70 |
<0.001 |
| FPG (mmol/l) |
8.09±2.43 |
9.22±3.21 |
<0.001 |
| Creatinine (µmol/l) |
67.43±17.69 |
73.66±49.84 |
0.027 |
| Uric acid (µmol/l) |
281.9±82.42 |
281.1±76.58 |
0.98 |
| Cholesterol (mmol/l) |
5.05±0.98 |
5.18±1.17 |
0.19 |
| Triglycerides (mmol/l) |
1.55±0.93 |
1.73±1.52 |
0.11 |
| HDL cholesterol (mmol/l) |
1.25±0.30 |
1.23±0.30 |
0.38 |
| LDL cholesterol (mmol/l) | 3.07±0.83 | 3.08±0.90 | 0.83 |
The data are expressed as mean ± standard deviation (SD) or number of subjects. Differences between diabetic retinopathy (DR) and diabetic without retinopathy (DWR) groups were compared using t test or Chi-square test. BMI, body mass index; WHR, waist and hip ratio; HbA1c, glycosylated hemoglobin A1c; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Basic information of SNPs analyzed with allelic distributions is shown in Table 3. Out of the 26 SNPs selected, rs11645239 in the PKC-β gene had a low genotyping call rate (<52%); rs1805192 in the PPARγ gene and rs1799969 in the ICAM-1 gene had a minor allele frequency of less than 5% in the studied population. These three SNPS were not studied further. The remaining 23 SNPs were tested in the DR and DWR groups for any departure from HWE. All were in HWE except rs3900008 (p<0.001), which failed to be genotyped in 53 individuals, indicating a possible genotyping error. The SNP rs3900008 was therefore excluded, and a total of 22 SNPs were included for subsequent analysis. For each of the SNPs evaluated, the number of participants varied slightly depending on the genotype call rate (≥94%).
Table 3. Basic information of Alleles and allele association results Between DR and DWR groups.
| Gene | locus | SNP | position |
Minor |
MAF |
HWpval |
OR (CI) |
P |
|
|---|---|---|---|---|---|---|---|---|---|
| Allele | DR DWR | value | |||||||
|
PPARγ |
3 |
rs1805192 |
12,396,238 |
G |
0 |
||||
|
KDR |
4 |
rs2071559 |
55,687,123 |
C |
0.34 |
0.39 |
0.022 |
1.41(1.07,1.86) |
0.011 |
| rs13109660 |
55,665,437 |
A |
0.31 |
0.63 |
0.49 |
1.06(0.8,1.41) |
0.66 |
||
| rs1870378 |
55,661,210 |
A |
0.48 |
0.67 |
0.33 |
0.92(0.71,1.21) |
0.55 |
||
| rs1870377 |
55,667,731 |
A |
0.48 |
0.4 |
0.73 |
0.95(0.73,1.23) |
0.67 |
||
|
VEGF |
6 |
rs699947 |
43,844,367 |
A |
0.28 |
0.009 |
0.51 |
1.63(1.22,2.18) |
0.001 |
| rs833061 |
43,845,464 |
C |
0.28 |
0.021 |
0.33 |
1.53(1.14,2.04) |
0.003 |
||
| rs13207351 |
43,845,772 |
A |
0.28 |
0.01 |
0.41 |
1.58(1.18,2.12) |
0.001 |
||
| rs2010963 |
43,846,328 |
C |
0.44 |
0.41 |
0.9 |
0.87(0.67,1.13) |
0.29 |
||
| rs833069 |
43,850,557 |
G |
0.44 |
1 |
0.9 |
0.92(0.71,1.2) |
0.53 |
||
| rs2146323 |
43,853,073 |
A |
0.26 |
0.003 |
0.49 |
1.52(1.13,2.05) |
0.004 |
||
| rs3025021 |
43,857,141 |
T |
0.16 |
0.58 |
1 |
1.36(0.95,1.97) |
0.083 |
||
| rs3025039 |
43,860,514 |
T |
0.09 |
1 |
0.38 |
1.34(0.96,1.88) |
0.077 |
||
|
EPO |
7 |
rs507392 |
100,157,872 |
C |
0.2 |
0.4 |
0.03 |
0.93(0.67,1.28) |
0.64 |
| rs551238 |
100,159,464 |
C |
0.2 |
0.39 |
0.045 |
1.04(0.75,1.45) |
0.8 |
||
| rs1617640 |
100,155,234 |
G |
0.19 |
0.1 |
0.14 |
1.18(0.85,1.66) |
0.31 |
||
|
AKR1B1 |
7 |
rs759853 |
133,794,498 |
A |
0.07 |
0.79 |
0.34 |
1.31(0.92,1.87) |
0.11 |
|
PKC-β |
16 |
rs3900007 |
23,754,453 |
G |
0.42 |
0.49 |
0.22 |
1.01(0.78,1.31) |
0.95 |
| rs3900008 |
23,754,514 |
C |
0.3 |
<0.001 |
<0.001 |
1.05(0.78,1.41) |
0.75 |
||
| rs11645239 |
23,754,563 |
G |
0.36 |
||||||
| rs2575390 |
23,754,255 |
G |
0.06 |
1 |
1 |
0.95(0.53,1.69) |
0.85 |
||
| rs3760106 |
23,753,297 |
A |
0.06 |
1 |
1 |
1.02(0.57,1.84) |
0.95 |
||
|
ACE |
17 |
rs1800764 |
58,904,261 |
C |
0.39 |
1 |
1 |
1.23(0.94,1.6) |
0.12 |
| rs9896208 |
58,929,841 |
T |
0.42 |
0.26 |
0.021 |
0.95(0.73,1,24) |
0.71 |
||
| ICAM-1 | 19 | rs5498 |
10,256,683 |
G |
0.31 |
0.64 |
0.1 |
1.32(1,1.75) |
0.044 |
| rs1799969 | 10,255,792 | A | 0.003 | ||||||
MAF: minor allele frequency; HWpval: p value for Hardy–Weinberg (H-W) equilibrium; OR: odds ratio; CI:95% confidence interval; Genotype distributions for SNPs were in Hardy–Weinberg (H-W) equilibrium, except rs3900008 in both groups.
Table 4 shows the genotypic distribution in the DR and DWR groups for the 22 informative SNPs. A strong association with DR was detected at three promoter variants (rs833061, rs13207351, rs699947) and one intronic SNP (rs2146323) in the VEGF gene, with the minor alleles of SNPs rs833061, rs13207351, rs699947, and rs2146323 showing a similar magnitude of risk (OR≥1.52; p<0.003). The ORs for the homozygote minor alleles were 3.80 (95% CI: 1.91-7.57) for rs699947, 3.6 (95% CI: 1.79- 7.22) for rs833061, 3.7 (95% CI: 1.86-7.39) for rs13207351, and 2.8 (95% CI: 1.46-5.37) for the intronic SNP rs2146323. The three promoter variants rs833061, rs13207351, rs699947 in the VEGF gene showed strong linkage disequilibrium (r2≥0.98). SNP rs2071559 in the KDR gene was also significantly associated with DR with an OR of 1.41 (95% CI: 1.07-1.86) for the minor allele and 1.57 (95% CI: 1.05-2.28) for the minor homozygote.
Table 4. Genotype frequencies of polymorphisms in the studied groups.
| Gene | SNP | Sample size | genotype | DR | DWR | OR (CI) | p-value |
|---|---|---|---|---|---|---|---|
| KDR |
rs2071559 |
492 |
TT |
84(39.3) |
141(50.7) |
0.034 |
|
| CT |
95(44.4) |
103(37.1) |
1.73 (1,2.98) |
||||
| CC |
35(16.4) |
34(12.2) |
1.57 (1.05,2.28) |
||||
| rs1870377 |
490 |
TT |
53(24.8) |
78(28.3) |
0.6 |
||
| TA |
113(52.8) |
134(48.6) |
1.24 (0.81,1.91) |
||||
| AA |
48(22.4) |
64(23.2) |
1.1 (0.66,1.84) |
||||
| rs1870378 |
471 |
GG |
54(26.9) |
80(29.6) |
0.8 |
||
| GA |
97(48.3) |
126(46.7) |
1.14 (0.74,1.76) |
||||
| AA |
50(24.9) |
64(23.7) |
1.16 (0.7,1.92) |
||||
| rs13109660 |
490 |
GG |
102(47.9) |
127(45.8) |
0.9 |
||
| GA |
93(43.7) |
125(45.1) |
0.93 (0.64,1.35) |
||||
| AA |
18(8.5) |
25(9) |
0.9 (0.46,1.73) |
||||
| VEGF |
rs699947 |
495 |
CC |
105(48.6) |
162(58.1) |
<0.001 |
|
| CA |
79(36.6) |
104(37.3) |
1.17 (0.8,1.72) |
||||
| AA |
32(18.4) |
13(4.7) |
3.80 (1.91,7.57) |
||||
| rs833061 |
491 |
TT |
104(49.1) |
158(56.6) |
0.001 |
||
| CT |
78(36.8) |
108(38.7) |
1.1 (0.75,1.61) |
||||
| CC |
30(14.2) |
13(4.7) |
3.6 (1.79,7.22) |
||||
| rs13207351 |
491 |
GG |
105(48.8) |
158(57.2) |
<0.001 |
||
| GA |
78(36.3) |
105(38) |
1.12 (0.76,1.64) |
||||
| AA |
32(14.9) |
13(4.7) |
3.7 (1.86,7.39) |
||||
| rs2146323 |
490 |
CC |
112(52.3) |
167(60.5) |
0.006 |
||
| CA |
72(33.6) |
93(33.7) |
1.15 (0.78,1.7) |
||||
| AA |
30(14) |
16(5.8) |
2.8 (1.46,5.37) |
||||
| rs3025021 |
491 |
TT |
5(2.4) |
9(3.2) |
0.21 |
||
| CT |
48(22.6) |
81(29) |
1.07 (0.34,3.37) |
||||
| CC |
159(75) |
189(67.7) |
1.51 (0.5,4.61) |
||||
| rs833069 |
497 |
GG |
40(18.6) |
58(20.6) |
0.82 |
||
| GA |
105(48.8) |
138(51.8) |
1.1 (0.69,1.78) |
||||
| AA |
70(18.6) |
86(30.5) |
1.18 (0.71,1.97) |
||||
| rs2010963 |
495 |
GG |
70(32.6) |
84(30) |
0.47 |
||
| GC |
111(51.6) |
140(50) |
0.95 (0.64,1.42) |
||||
| CC |
34(15.8) |
56(20) |
0.73 (0.43,1.24) |
||||
| rs3025039 |
498 |
CC |
137(63.7) |
198(70.0) |
0.15 |
||
| CT |
69(32.1) |
47(28.3) |
2.6 (0.85,7.93) |
||||
| TT |
9(4.2) |
5(1.8) |
1.25 (0.85,1.84) |
||||
| ICAM-1 |
rs5498 |
492 |
AA |
92(42.8) |
138(49.8) |
0.078 |
|
| GA |
100(46.5) |
123(44.4) |
1.22 (0.84,1.77) |
||||
| GG |
23(10.7) |
16(5.8) |
2.16 (1.08,4.30) |
||||
| EPO |
rs507392 |
496 |
TT |
141(65.3) |
181(64.6) |
0.68 |
|
| CT |
65(30.1) |
81(28.9) |
1.03 (0.69,1.53) |
||||
| CC |
10(4.6) |
18(6.4) |
0.71 (0.32,1.59) |
||||
| rs551238 |
494 |
AA |
141(65.3) |
182(65.5) |
|||
| CA |
65(30.1) |
79(28.4) |
1.06 (0.72,1.58) |
0.74 |
|||
| CC |
10(4.6) |
17(6.1) |
0.76 (0.34,1.71) |
||||
| rs1617640 |
491 |
TT |
146(69.2) |
182(65) |
|||
| GT |
55(26.1) |
82(29.3) |
0.84 (0.56,1.25) |
0.61 |
|||
| GG |
10(4.7) |
16(5.7) |
0.78 (0.34,1.77) |
||||
| AKR1B1 |
759853 |
471 |
GG |
145(70.7) |
167(62.8) |
||
| GA |
54(26.3) |
91(34.2) |
0.68 (0.46,1.02) |
0.18 |
|||
| AA |
6(2.9) |
8(3.0) |
0.86 (0.29,2.55) |
||||
| ACE |
1800764 |
494 |
TT |
88(40.9) |
98(35.1) |
0.31 |
|
| CT |
99(46.0) |
134(48) |
0.83 (0.56,1.23) |
||||
| CC |
28(13) |
47(16.8) |
0.65 (0.38,1.14) |
||||
| rs9896208 |
496 |
CC |
79(36.6) |
84(30) |
0.056 |
||
| TC |
97(44.9) |
156(55.7) |
0.66 (0.44,0.98) |
||||
| TT |
40(18.5) |
40(14.3) |
1.06 (0.62,1.82) |
||||
| PKC-β | 3,900,007 |
496 |
AA |
69(31.9) |
99(35.4) |
0.36 |
|
| GA |
111(51.4) |
126(45) |
1.26 (0.85,1.88) |
||||
| GG |
36(16.7) |
55(19.6) |
0.94 (0.56,1.58) |
||||
| 2575390 |
499 |
CC |
193(89.4) |
251(88.7) |
0.95 |
||
| CG |
22(10.2) |
31(11.0) |
0.92 (0.52,1.64) |
||||
| GG |
1(0.5) |
1(0.4) |
1.3 (0.08,20.92) |
||||
| 3760106 |
492 |
GG |
190(89.2) |
248(88.9) |
0.97 |
||
| GA |
22(10.3) |
30(10.8) |
1.31 (0.08,21) |
||||
| AA | 1(0.5) | 1(0.4) | 0.96 (0.54,1.71) | ||||
Data are expressed as number (%).The p value represents comparison between DR and diabetic without retinopathy (DWR) groups, using χ2 or Fisher’s exact test.
Table 5 presents the multivariate analyses data adjusted for variables including the age of diabetic onset, duration of diabetes, high albuminuria, insulin use, HbA1c, and creatinine levels. The association with DR remained statistically significant with the homozygous genotype of the minor allele for SNPs rs699947 (OR=3.74, 95% CI: 1.75-8.00), rs833061 (OR=3.50, 95% CI: 1.62-7.54), rs13207351 (OR=3.64, 95% CI: 1.71-7.77), and rs2146323 (OR=2.88, 95% CI: 1.4-5.93) in the VEGF gene as well as SNP rs2071559 (OR=1.88, 95% CI: 1.01-3.48) in the KDR gene. However, only rs699947 and rs13207351 in the VEGF gene remained statistically significant after Bonferroni correction for the 22 SNPs analyzed. No statistically significant association with DR was found for all other SNPs in genes for EPO, AKR1B1, PKC-β, ACE, and ICAM-1.
Table 5. Association analysis of polymorphisms in multivariate models.
| Gene | SNP | genotype | Ajusted OR (CI) | P value |
|---|---|---|---|---|
| KDR |
rs2071559 |
TT |
0.05 |
|
| CT |
1.57 (1.01,2.44) |
|||
| CC |
1.87 (1.01,3.48) |
|||
| rs1870377 |
TT |
0.64 |
||
| TA |
1.13 (0.69,1.85) |
|||
| AA |
0.88 (0.48,1.6) |
|||
| rs1870378 |
GG |
0.79 |
||
| GA |
1.1 (0.66,1.81) |
|||
| AA |
0.91 (0.5,1.66) |
|||
| rs13109660 |
GG |
0.93 |
||
| GA |
1.01 (0.48,2.13) |
|||
| AA |
1.09 (0.7,1.68) |
|||
| VEGF |
rs699947 |
CC |
0.002 |
|
| CA |
1.26 (0.82,1.95) |
|||
| AA |
3.74 (1.75,8.00) |
|||
| rs833061 |
TT |
0.004 |
||
| CT |
1.19 (0.77,1.85) |
|||
| CC |
3.50 (1.62,7.54) |
|||
| rs13207351 |
GG |
0.002 |
||
| GA |
1.22 (0.79,1.89) |
|||
| AA |
3.64 (1.71,7.77) |
|||
| rs2146323 |
CC |
0.014 |
||
| CA |
1.14 (0.73,1.77) |
|||
| AA |
2.88 (1.4,5.93) |
|||
| rs3025021 |
TT |
|||
| CT |
0.75 (0.2,2.78) |
0.22 |
||
| CC |
1.14 (0.32,4.06 |
|||
| rs833069 |
GG |
|||
| GA |
1.09 (0.63,1.89) |
0.63 |
||
| AA |
1.3 (0.73,2.34) |
|||
| rs2010963 |
GG |
|||
| GC |
0.89 (0.56,1.41) |
0.51 |
||
| CC |
0.7 (0.38,1.28) |
|||
| rs3025039 |
CC |
0.23 |
||
| CT |
1.23 (0.79,1.92) |
|||
| TT |
2.8 (0.76,10.27) |
|||
| ICAM-1 |
rs5498 |
AA |
0.091 |
|
| GA |
1.44 (0.94,2.22) |
|||
| GG |
2.04 (0.94,4.44) |
|||
| EPO |
rs507392 |
TT |
0.88 |
|
| CT |
1.02 (0.65,1.61) |
|||
| CC |
0.8 (0.32,2.02) |
|||
| rs551238 |
AA |
0.94 |
||
| CA |
1.07 (0.67,1.68) |
|||
| CC |
0.92 (0.36,2.36) |
|||
| rs1617640 |
TT |
|||
| GT |
0.85 (0.53,1.36) |
0.79 |
||
| GG |
0.94 (0.36,2.41) |
|||
| AKR1B1 |
759853 |
GG |
0.11 |
|
| GA |
0.62 (0.39,0.99) |
|||
| AA |
0.59 (0.17,2.05) |
|||
| ACE |
1800764 |
TT |
0.33 |
|
| CT |
0.87 (0.56,1.37) |
|||
| CC |
0.62 (0.33,1.16) |
|||
| rs9896208 |
CC |
|||
| TC |
0.62 (0.39,0.97) |
0.097 |
||
| TT |
0.88 (0.47,1.63) |
|||
| PRK-β | 3900007 |
AA |
0.82 |
|
| GA |
1.14 (0.72,1.8) |
|||
| GG |
1.17 (0.64,2.14) |
|||
| 2575390 |
CC |
0.78 |
||
| CG |
0.89 (0.46,1.71) |
|||
| GG |
2.73 (0.1,73.85) |
|||
| 3760106 |
GG |
0.81 |
||
| GA |
2.67 (0.1,71.35) |
|||
| AA | 0.92 (0.48,1.77) |
Multiple logistic regression analysis was used to calculate odds ratio (OR) and 95% confidence interval (CI) by comparing diabetic retinopathy (DR) group with diabetic without retinopathy (DWR) group, which include the most significant SNP at kinase insert domain receptor gene (KDR) gene (rs2071559), vascular endothelial growth factor (VEGF) gene (rs833061, rs13207351, rs699947, rs2146323). Adjusted OR represents data after adjustment for covariates including age of diabetic onset, duration, high albuminuria, insulin use, glycosylated hemoglobin A1c (HbA1c), fasting plasma glucose and creatinine levels.
Haplotype analysis revealed five SNP blocks (Figure 1). Only two haplotypes in the VEGF gene showed strong evidence of an association with DR, and the others did not show any statistically significant association (Table 6). The frequency of haplotype ACGA in the VEGF gene, defined by the minor alleles of the three SNPs rs699947, rs833061, rs13207351 at the promoter region and rs2010963 at the 5′ untranslated region, was 32.4% in the DR group and 23.2% in the DWR group (p=0.002, OR=1.58, 95% CI: 1.19–2.10). The haplotype AA defined by the minor alleles of the SNPs rs833069 and rs2146323 at intron 2 of the VEGF gene was 30.7% in the DR group and 22.5% in the DWR group (p=0.004), with an OR of 1.53 (95% CI: 1.15–2.04) for the increased risk of DR.
Figure 1.
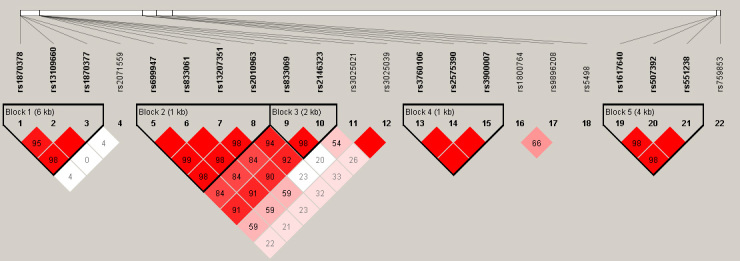
Linkage disequilibrium plot generated by Haploview 4.2 software. Five haplotype blocks (bold) were identified for single-nucleotide polymorphisms (SNPs) in the kinase insert domain receptor, vascular endothelial growth factor gene, protein kinase C-β gene, and in erythropoietin gene. Linkage disequilibrium (LD) is displayed as the pairwise D’ value multiplied by 100 and given for each SNP combination. Shading represents the magnitude and significance of the pairwise LD, with a red-to-white gradient reflecting higher-to-lower LD values. Red diamond without a number corresponds to a D’ value of 1.0.
Table 6. Haplotype association analysis between polymorphisms and risk of diabetic retinopathy in patients with type 2 diabetes mellitus.
| Haplotype blocks | DR group (%) | DWR group (%) | OR (95%CI) | Chi Square | p value |
|---|---|---|---|---|---|
| Block 1 |
|||||
| AAG |
48.7 |
47.2 |
1.07 (0.82, 1.39) |
0.26 |
61 |
| TGA |
29.5 |
31.2 |
0.93 (0.69, 1.23) |
0.28 |
0.59 |
| TGG |
20.7 |
20.8 |
0.99(0.72–1.37) |
0.001 |
0.98 |
| Block 2 |
|||||
| ACGA |
32.4 |
23.2 |
1.58 (1.19, 2.10) |
9.87 |
0.002 |
| CTCG |
42.1 |
44.6 |
0.90 (0.69, 1.16) |
0.68 |
0.41 |
| CTGG |
25.2 |
31.6 |
0.73(0.55, 0.97) |
4.8 |
0.03 |
| Block 3 |
|||||
| AA |
30.7 |
22.5 |
1.53 (1.15, 2.04) |
8.39 |
0.04 |
| AC |
25.8 |
32.4 |
0.72 (0.55, 0.96) |
5.16 |
0.02 |
| GC |
43.4 |
44.8 |
0.94 (0.73, 1.21) |
0.23 |
0.63 |
| Block 4 |
|||||
| CGA |
57.3 |
57.9 |
0.97(0.75, 1.26) |
0.044 |
0.83 |
| CGG |
37.1 |
36.3 |
1.04(0.80, 1.35) |
0.068 |
0.79 |
| GAG |
5.6 |
5.8 |
0.97(0.57, 1.68) |
0.009 |
0.92 |
| Block 5 |
|||||
| CCG |
17.5 |
20 |
0.86(0.62, 1.20) |
0.79 |
0.37 |
| TAT | 80.6 | 79.4 | 1.16(0.84, 1.61) | 0.79 | 0.37 |
Data were given as frequency of each haplotype within diabetic retinopathy (DR) group or diabetic without retinopathy (DWR) group. Block 1 included rs1870378, rs13109660, and rs1870377 in kinase insert domain receptor gene. block 2 included promoter SNPs rs699947, rs833061, rs13207351 and rs2010963 at 5′ UTR, and Block3 included SNPs two intronic SNPs of rs833069 rs2146323 in vascular endothelial growth factor gene, Block4 included SNPs rs3760106 rs2575390 rs3900007 in protein kinase C-β gene, block 5, rs1617640 rs507392 rs551238 in erythropoietin gene. Only haplotypes with frequency over 3% were included in this table. OR indicates odds ratio and CI refers to confidence interval.
Discussion
We analyzed the possible association between polymorphisms in eight candidate genes and DR in a well-defined cohort of Chinese patients with T2DM. Our data showed significant association between SNPs in the VEGF gene and the risk of DR after adjustment for possible confounding risk factors. However, no statistically significant association was found for other genes, including KDR, PPARγ, EPO, AKR1B1, PKC-β, ACE, and ICAM-1.
The pathogenesis of DR is complex with multifactorial biochemical causes influenced by genetic and environmental factors. Many vasoactive factors stimulated by hyperglycemia or oxidative stress have been identified [1]. Among them, VEGF has been characterized as a critical angiogenic and vasopermeability factor that is upregulated in various retinal ischemic disorders, such as retinopathy of prematurity, AMD, and DR [32], whereas anti-VEGF therapy has been shown to be effective for patients with AMD [42] or diabetic macular edema [43]. The human VEGF gene is highly polymorphic and has a large number of individual SNPs examined in relationship with DR [16-19,44]. In our previous study, three SNPs (rs833061, rs13207351, rs699947) at the promoter region of the VEGF gene showed a strong association with the risk of DR [16]. This current study with an expanded sample size confirmed our previous report. Discussions of the three promoter SNPs have been described in detail in our previous literature [16]. Moreover, the association of DR with an intronic SNP rs2146323 in the VEGF gene has been suggested in this current study. Although the intronic region of VEGF is believed to contain binding sites for transcription factors that regulate VEGF production [45], there is no functional data to date on the intronic SNPs.
There are three high-affinity VEGF tyrosine kinase receptors: VEGFR-1, VEGFR-2 (KDR), and VEGFR-3 that are expressed mainly in the vascular endothelial cells [32,33]. KDR is the primary responder to the VEGF signal regulating endothelial cell migration and proliferation [32,33]. Several lines of evidence have implicated that KDR plays a role in the etiology of DR [34-37]. Although it became insignificant after Bonferroni correction, the present study suggested a possible association between the SNP rs2071559 in the KDR gene and DR. Further studies with a larger sample size to confirm this observation would be warranted. The present study failed to find an association of DR with other previously reported genes, including ACE, AKR1B1, PKC-β, PPARγ, ICAM-1, and EPO. Discrepancies between our study and previous reports could be due to different ethnic origin, diagnostic criteria, or sample size.
Strengths of the current study include the enrollment of participants from a relatively homogeneous population of a single community, collection of detailed information on a variety of risk factors, and the standardized fundus photograph and assessment of DR severity. Additionally, previous genetic association studies for DR have been limited to studies of one or a modest number of candidate genes [25,46]. To our knowledge, this is the first and largest study in the Chinese population to address the question of whether multiple candidate genetic polymorphisms are associated with DR.
In summary, the present study confirmed our previous observation on the association between DR and VEGF polymorphisms independent of other clinical factors in the Chinese population. Moreover, a possible association between DR and KDR polymorphisms is suggested and further studies are warranted.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) Grant 2007CB512201, the Beijing Municipal Health Bureau Grant 2,009,208, and the Beijing Natural Science Foundation Grant 7,131,007.
References
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM, Study PD. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus - UKPDS 69. Arch Ophthalmol-Chic. 2004;122:1631–40. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 3.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR, Grp U. UKPDS 50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–63. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy - A systematic review. JAMA. 2007;298:902–16. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 5.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Sobrin L, Green T, Sim X, Jensen RA, Tai ES, Tay WT, Wang JJ, Mitchell P, Sandholm N, Liu Y, Hietala K, Iyengar SK, Brooks M, Buraczynska M, Van Zuydam N, Smith AV, Gudnason V, Doney AS, Morris AD, Leese GP, Palmer CN, Swaroop A, Taylor HA, Jr, Wilson JG, Penman A, Chen CJ, Groop PH, Saw SM, Aung T, Klein BE, Rotter JI, Siscovick DS, Cotch MF, Klein R, Daly MJ, Wong TY. Candidate gene association study for diabetic retinopathy in persons with type 2 diabetes: the Candidate gene Association Resource (CARe). Invest Ophthalmol Vis Sci. 2011;52:7593–602. doi: 10.1167/iovs.11-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arar NH, Freedman BI, Adler SG, Iyengar SK, Chew EY, Davis MD, Satko SG, Bowden DW, Duggirala R, Elston RC, Guo X, Hanson RL, Igo RP, Ipp E, Kimmel PL, Knowler WC, Molineros J, Nelson RG, Pahl MV, Quade SRE, Rasooly RS, Rotter JI, Saad MF, Scavini M, Schelling JR, Sedor JR, Shah VO, Zager PG, Abboud HE, Diabet FIN. Heritability of the severity of diabetic retinopathy: The FIND-Eye study. Invest Ophthalmol Vis Sci. 2008;49:3839–45. doi: 10.1167/iovs.07-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hietala K, Forsblom C, Summanen P, Groop PH, Grp FS. Heritability of proliferative diabetic retinopathy. Diabetes. 2008;57:2176–80. doi: 10.2337/db07-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YC, Tsai FJ, Lin JM, Lin HJ, Chen CC, Chen SY, Tsai CH. Genome-wide Association Study of Diabetic Retinopathy in a Taiwanese Population. Ophthalmology. 2011;118:642–8. doi: 10.1016/j.ophtha.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Paterson AD, Waggott D, Shen EQ, Boright AP, Hosseini M, Cleary PA, Lachin JM, Sun L, Bull S, Grp DER. Genome-Wide Association Study of Risk for Diabetic Retinopathy in Type 1 Diabetes. Diabetes. 2010;2010 [Google Scholar]
- 11.Fu YP, Hallman DM, Gonzalez VH, Klein BE, Klein R, Hayes MG, Cox NJ, Bell GI, Hanis CL. Identification of Diabetic Retinopathy Genes through a Genome-Wide Association Study among Mexican-Americans from Starr County, Texas. J Ophthalmol. 2010;2010 doi: 10.1155/2010/861291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallman DM, Boerwinkle E, Gonzalez VH, Klein BE, Klein R, Hanis CL. A genome-wide linkage scan for diabetic retinopathy susceptibility genes in Mexican Americans with type 2 diabetes from Starr County, Texas. Diabetes. 2007;56:1167–73. doi: 10.2337/db06-1373. [DOI] [PubMed] [Google Scholar]
- 13.Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes. 1998;47:821–30. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- 14.Looker HC, Nelson RG, Chew E, Klein R, Klein BE, Knowler WC, Hanson RL. Genome-wide linkage analyses to identify Loci for diabetic retinopathy. Diabetes. 2007;56:1160–6. doi: 10.2337/db06-1299. [DOI] [PubMed] [Google Scholar]
- 15.Malecki MT, Cyganek K, Mirkiewicz-Sieradzka B, Wolkow PP, Wanic K, Skupien J, Solnica B, Sieradzki J. Alanine variant of the Pro12Ala polymorphism of the PPARgamma gene might be associated with decreased risk of diabetic retinopathy in type 2 diabetes. Diabetes Res Clin Pract. 2008;80:139–45. doi: 10.1016/j.diabres.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Deng Y, Gu H, Lim A, Altankhuyag A, Jia W, Ma K, Xu J, Zou Y, Snellingen T, Liu X, Wang N, Liu N. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol Vis. 2011;17:3088–96. [PMC free article] [PubMed] [Google Scholar]
- 17.Churchill AJ, Carter JG, Ramsden C, Turner SJ, Yeung A, Brenchley PEC, Ray DW. VEGF polymorphisms are associated with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3611–6. doi: 10.1167/iovs.07-1383. [DOI] [PubMed] [Google Scholar]
- 18.Al-Kateb H, Mirea L, Xie XL, Sun L, Liu M, Chen HT, Bull SB, Boright AP, Paterson AD, Group DER. Multiple variants in vascular endothelial growth factor (VEGFA) are risk factors for time to severe retinopathy in type 1 diabetes - The DCCT/EDIC genetics study. Diabetes. 2007;56:2161–8. doi: 10.2337/db07-0376. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi K, Watanabe C. Single nucleotide polymorphisms of vascular endothelial growth factor gene intron 2 are markers for early progression of diabetic retinopathy in Japanese with type 1 diabetes. Clin Chim Acta. 2009;402:171–5. doi: 10.1016/j.cca.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Tong Z, Yang ZL, Patel S, Chen HY, Gibbs D, Yang X, Hau VS, Kaminoh Y, Harmon J, Pearson E, Buehler J, Chen YH, Yu BF, Tinkham NH, Zabriskie NA, Zeng JX, Luo L, Sun JK, Prakash M, Hamam RN, Tonna S, Constantine R, Ronquillo CC, Sadda S, Avery RL, Brand JM, London N, Anduze AL, King GL, Bernstein PS, Watkins S, Jorde LB, Li DY, Aiello LP, Pollak MR, Zhang K, Genet D, Diabe Complicat S. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci USA. 2008;105:6998–7003. doi: 10.1073/pnas.0800454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abhary. Association Between Erythropoietin Gene Polymorphisms and Diabetic Retinopathy (vol 128, pg 102, 2010). Arch Ophthalmol. 2010;128:311. doi: 10.1001/archophthalmol.2009.355. [DOI] [PubMed] [Google Scholar]
- 22.Kao YL, Donaghue K, Chan A, Knight J, Silink M. A novel polymorphism in the aldose reductase gene promoter region is strongly associated with diabetic retinopathy in adolescents with type 1 diabetes. Diabetes. 1999;48:1338–40. doi: 10.2337/diabetes.48.6.1338. [DOI] [PubMed] [Google Scholar]
- 23.Olmos P, Futers S, Acosta AM, Siegel S, Maiz A, Schiaffino R, Morales P, Diaz R, Arriagada P, Claro JC, Vega R, Vollrath V, Velasco S, Emmerich M. (AC)(23) Z-2 polymorphism of the aldose reductase gene and fast progression of retinopathy in Chilean type 2 diabetics. Diabetes Res Clin Pract. 2000;47:169–76. doi: 10.1016/s0168-8227(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Richeti F, Noronha RM, Waetge RTL, de Vasconcellos JPC, de Souza OF, Kneipp B, Assis N, Rocha MN, Calliari LEP, Longui CA, Monte O, de Melo MB. Evaluation of AC(n) and C(−106)T polymorphisms of the aldose reductase gene in Brazilian patients with DM1 and susceptibility to diabetic retinopathy. Mol Vis. 2007;13:740–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Abhary S, Hewitt AW, Burdon KP, Craig JE. A Systematic Meta-Analysis of Genetic Association Studies for Diabetic Retinopathy. Diabetes. 2009;58:2137–47. doi: 10.2337/db09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uthra S, Raman R, Mukesh BN, Rajkumar SA, Kumari PR, Lakshmipathy P, Gnanamoorthy P, Sharma T, McCarty CA, Kumaramanickavel G. Protein kinase C beta (PRKCB1) and pigment epithelium derived factor (PEDF) gene polymorphisms and diabetic retinopathy in a south Indian cohort. Ophthalmic Genet. 2010;31:18–23. doi: 10.3109/13816810903426231. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda Y, Suehiro T, Osaki F, Tsuzura S, Kumon Y, Hashimoto K. Polymorphisms in the 5 '-upstream region of the PKC beta gene in Japanese patients with Type 2 diabetes. Diabet Med. 2004;21:1113–20. doi: 10.1111/j.1464-5491.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto A, Iwashima Y, Abiko A, Morikawa A, Sekiguchi M, Eto M, Makino I. Detection of the association between a deletion polymorphism in the gene encoding angiotensin I-converting enzyme and advanced diabetic retinopathy. Diabetes Res Clin Pract. 2000;50:195–202. doi: 10.1016/s0168-8227(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 29.Kamiuchi K, Hasegawa G, Obayashi H, Kitamura A, Ishii M, Yano M, Kanatsuna T, Yoshikawa T, Nakamura N. Intercellular adhesion molecule-1 (ICAM-1) polymorphism is associated with diabetic retinopathy in Type 2 diabetes mellitus. Diabet Med. 2002;19:371–6. doi: 10.1046/j.1464-5491.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Yu Q, Wang H, Zhang SX, Huang C, Chen X. Association of intercellular adhesion molecule 1 polymorphisms with retinopathy in Chinese patients with Type 2 diabetes. Diabet Med. 2006;23:643–8. doi: 10.1111/j.1464-5491.2006.01884.x. [DOI] [PubMed] [Google Scholar]
- 31.Petrovic MG, Osredkar J, Saraga-Babic M, Petrovic D. K469E polymorphism of the intracellular adhesion molecule 1 gene is associated with proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin Experiment Ophthalmol. 2008;36:468–72. doi: 10.1111/j.1442-9071.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 32.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–71. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res. 2006;83:1005–16. doi: 10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert RE, Vranes D, Berka JL, Kelly DJ, Cox A, Wu LL, Stacker SA, Cooper ME. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab Invest. 1998;78:1017–27. [PubMed] [Google Scholar]
- 35.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–9. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 36.Ishida S, Shinoda K, Kawashima S, Oguchi Y, Okada Y, Ikeda E. Coexpression of VEGF receptors VEGF-R2 and neuropilin-1 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1649–56. [PubMed] [Google Scholar]
- 37.Witmer AN, Blaauwgeers HG, Weich HA, Alitalo K, Vrensen GF, Schlingemann RO. Altered expression patterns of VEGF receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Invest Ophthalmol Vis Sci. 2002;43:849–57. [PubMed] [Google Scholar]
- 38.Huang H, Shen J, Vinores SA. Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS ONE. 2011;6:e21411. doi: 10.1371/journal.pone.0021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Försti A, Jin Q, Altieri A, Johansson R, Wagner K, Enquist K, Grzybowska E, Pamula J, Pekala W, Hallmans G, Lenner P, Hemminki K. Polymorphisms in the KDR and POSTN genes: association with breast cancer susceptibility and prognosis. Breast Cancer Res Treat. 2007;101:83–93. doi: 10.1007/s10549-006-9265-1. [DOI] [PubMed] [Google Scholar]
- 40.Kariyazono H, Ohno T, Khajoee V, Ihara K, Kusuhara K, Kinukawa N, Mizuno Y, Hara T. Association of vascular endothelial growth factor (VEGF) and VEGF receptor gene polymorphisms with coronary artery lesions of Kawasaki disease. Pediatr Res. 2004;56:953–9. doi: 10.1203/01.PDR.0000145280.26284.B9. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Zheng Y, Zhang W, Yu H, Lou K, Zhang Y, Qin Q, Zhao B, Yang Y, Hui R. Polymorphisms of KDR gene are associated with coronary heart disease. J Am Coll Cardiol. 2007;50:760–7. doi: 10.1016/j.jacc.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 42.Abouammoh M, Sharma S. Ranibizumab versus bevacizumab for the treatment of neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2011;22:152–8. doi: 10.1097/ICU.0b013e32834595d0. [DOI] [PubMed] [Google Scholar]
- 43.Ozturk BT, Kerimoglu H, Bozkurt B, Okudan S. Comparison of intravitreal bevacizumab and ranibizumab treatment for diabetic macular edema. J Ocul Pharmacol Ther. 2011;27:373–7. doi: 10.1089/jop.2010.0195. [DOI] [PubMed] [Google Scholar]
- 44.Abhary S, Burdon KP, Gupta A, Lake S, Selva D, Petrovsky N, Craig JE. Common Sequence Variation in the VEGFA Gene Predicts Risk of Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2009;50:5552–8. doi: 10.1167/iovs.09-3694. [DOI] [PubMed] [Google Scholar]
- 45.Churchill AJ, Carter JG, Lovell HC, Ramsden C, Turner SJ, Yeung A, Escardo J, Atan D. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15:2955–61. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- 46.Liew G, Klein R, Wong TY. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin. 2009;49:35–52. doi: 10.1097/IIO.0b013e31819fd5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]


