Abstract
Purpose
NADPH oxidase–generated reactive oxygen species (ROS) are implicated in angiogenesis. Isoforms of NADPH oxidase NOX1, NOX2, and NOX4 are reported to be expressed in endothelial cells (ECs). Of these, NOX1 and NOX2 have been reported to contribute to intravitreal neovascularization (IVNV) in oxygen-induced retinopathy (OIR) models. In this study, we tested the hypothesis that the isoform NOX4 in ECs contributed to vascular endothelial growth factor (VEGF)–induced angiogenesis and IVNV.
Methods
Isoforms of NADPH oxidase mRNA were measured in several types of cultured vascular ECs: human retinal microvascular ECs (hRMVECs), choroidal ECs (CECs), and human umbilical vascular ECs (HUVECs) using real-time PCR. Newborn rat pups and dams were placed into an OIR model that cycled oxygen concentration between 50% and 10% every 24 h for 14 days, and then were placed in room air (RA) for an additional 4 days (rat OIR model). NOX4 expression in retinal lysates from the RA–raised pups at postnatal day 0 (P0), P14, and P18 was determined with western blots. STAT3 activation was determined as the ratio of phosphorylated STAT3 to total STAT3 with western blot analysis of retinal lysates from pups raised in RA or from the rat OIR model at P18. Semiquantitative assessment of the density of NOX4 colabeling with lectin-stained retinal ECs was determined by immunolabeling of retinal cryosections from P18 pups in OIR or in RA. In hRMVECs transfected with NOX4 siRNA and treated with VEGF or control, 1) ROS generation was measured using the 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester fluorescence assay and 2) phosphorylated VEGF receptor 2 and STAT3, and total VEGFR2 and STAT3 were measured in western blot analyses. VEGF-stimulated hRMVEC proliferation was measured following transfection with NOX4 siRNA or STAT3 siRNA, or respective controls.
Results
NOX4 was the most prevalent isoform of NADPH oxidase in vascular ECs. NOX4 expression in retinal lysates was significantly decreased during development in RA. Compared to RA, the expression of retinal NOX4 increased at P18. At p18 OIR, semiquantitative assessment of the density of lectin and NOX4 colabeling in retinal vascular ECs was greater in retinal cryosections and activated STAT3 was greater in retinal lysates when compared to the RA-raised pups. In cultured hRMVECs, knockdown of NOX4 by siRNA transfection inhibited VEGF-induced ROS generation. VEGF induced a physical interaction of phosphorylated-VEGFR2 and NOX4. Knockdown of NOX4: 1) reduced VEGFR2 activation but did not abolish it and 2) abolished STAT3 activation in response to VEGF. Knockdown of either NOX4 or STAT3 inhibited VEGF-induced EC proliferation.
Conclusions
Our data suggest that in a model representative of human retinopathy of prematurity, NOX4 was increased at a time point when IVNV developed. VEGF-activated NOX4 led to an interaction between VEGF-activated VEGFR2 and NOX4 that mediated EC proliferation via activation of STAT3. Altogether, our results suggest that NOX4 may regulate VEGFR2-mediated IVNV through activated STAT3.
Introduction
Angiogenesis, a process of new blood vessel formation, plays important roles in development but also in pathologic conditions, including in cancer, diabetes mellitus, and ocular diseases. Endothelial cell (EC)–generated reactive oxygen species (ROS) can function as signaling molecules to promote EC proliferation, migration, and tube formation in angiogenesis [1]. NADPH oxidase, originally recognized for the superoxide burst generated by leukocytes to fight infection, is a source of ROS generation in ECs [1] that can affect angiogenesis [2,3]. Isoforms of NADPH oxidase, NOX 1–5 and Duox1 and -2, are differentially expressed in tissues and cells [4], and several papers support NADPH oxidase isoforms as having different roles in physiologic and pathological responses [2]. NOX1, NOX2, and NOX4 are expressed in ECs and have been associated with pathology in retinal diseases [5-10].
Models of oxygen-induced retinopathy (OIR) are useful for studying mechanisms of developmental [11] and pathologic angiogenesis caused by different oxygen stresses [12,13]. In these models [12,13], newborn animals that normally vascularize their retinas postnatally are exposed to oxygen stresses that cause intravitreal neovascularization (IVNV) in which blood vessels grow outside the plane of the retina into the vitreous. Several pathways involved in angiogenesis have been identified, including those activated by hypoxia and stabilization of hypoxia inducible factors [14], e.g., vascular endothelial growth factor (VEGF) and erythropoietin [15-18]; peroxisome proliferator activated receptor signaling [19,20]; and inflammatory pathways, such as cyclooxygenase signaling [21] and angiotensin II type I receptor signaling [22]. We and others found NADPH oxidase important in models of OIR [7,9,23,24]. In rat and mouse OIR models, NOX2 colocalized with retinal ECs and macrophages [8,25] and regulated IVNV [19,25] partly through apoptotic and inflammatory mechanisms [26]. Investigators also reported that activation of NOX1 in microglia or other cells contributed to ischemia-induced vascular pathologies and ganglion cell death [7,27]. The effects of NOX4 in vascular diseases are conflicting as having either protective [28] or pathologic [10] effects in ischemic vascular diseases. In one study involving inducible Nox4−/− mice, silencing of NOX4 led to aortic inflammation [28]. However, in a diabetic mouse model, NOX4 activation upregulated VEGF and reduced the integrity of the endothelial blood–retinal barrier [10].
In this study, we sought to study the role of endothelial NOX4 in VEGF-induced IVNV. We used the rat 50/10 OIR model, which recreates many of the features of human retinal diseases, including diabetic retinopathy [29] and retinopathy of prematurity (ROP) [30]. We addressed the hypothesis that VEGF-activated NOX4 in ECs contributes to pathologic angiogenesis through VEGF/VEGF receptor 2 (VEGFR2) signaling.
Methods
Rat model of oxygen-induced retinopathy
All animal studies were performed in compliance with the University of Utah (Guide for the Care and Use of Laboratory Animals) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The rat 50/10 OIR model has been previously described [31]. Entire litters of newborn Sprague-Dawley rat pups (Charles River, Wilmington, MA) and dams were placed in an oxygen environment that cycled oxygen concentration between 50% and 10% every 24 h for 14 days and then placed in room air. Pup number was maintained at 12 to 14 pups/litter. Pups were euthanized with intraperitoneal injections (IP) of ketamine (60 mg/kg) and xylazine (18 mg/kg) followed by IP pentobarbital (80 mg/kg).
Retinal section preparation and staining
Eyes were fixed in 4% paraformaldehyde (PFA) containing 10 mmol/l sodium orthovanadate for 10 min. Retinas were removed and placed into 4% PFA for 15 min followed by incubation in 30% sucrose/PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4, Ph 7.4) overnight. Each retina was immersed in optimal cutting temperature compound (OCT; Tissue-Tek; EMS, Hatfield, PA). Eyes were cut into 12 µm cryosections and were incubated overnight at 4 °C with primary antibody anti-NOX4 (Santa Cruz Biotechnology, Santa Cruz, CA). All sections were washed and then incubated for 1 h with a 1:500 dilution of Alexa 594-conjugated antibody for isolectin B4 and FITC-conjugated goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA) for NOX4. Sections stained with only secondary antibodies were controls. Sections were rinsed in PBS and mounted in Fluoromount-G (Southern Biotech, Birmingham, AL). Integrated density per image area of NOX4 fluorescence was quantified using ImageJ.
Cell culture
Human primary retinal microvascular endothelial cells (hRMVECs; Cell Systems, Kirkland, WA) and human umbilical vein endothelial cells (HUVECs; Lonza, Hopkinton, MA) were maintained in basal endothelial growth medium (EGM2-MV, Lonza), supplemented with 5% fetal bovine serum (FBS). Human choroidal endothelial cells (CECs) were isolated from donor eyes (Utah Eye Bank, Salt Lake City, UT) as described previously [32]. Briefly, the choroidal tissue was separated from the sclera, minced into small pieces. After three washes with cold Hank’s Balanced Salt Solution (HBSS) containing 0.5 mg ml−1 penicillin–streptomycin, the minced choroid was digested in a mixture containing 500 μg ml−1 collagenase 1A (Sigma-Aldrich, St. Louis, MO) and 1.2 U ml−1 Dispase II (Sigma) for 45 min at 37 °C in a rotating shaker. The choroidal digests were double filtered through 70 and 44 μm meshes. Enzymes were neutralized by adding two times the volume of DMEM with 10% FBS. The cell suspension was incubated with CD31-coated DynaBeads (Dynal Biotech, Inc., NY) beads for 15–30 min at room temperature (RT) with gentle rotation to isolate human choroidal ECs and minimize RPE and fibroblast contamination. The bead–EC complexes were then washed five times in HBSS with 5% FBS, mixed by gentle agitation for 1 min, and separated from the mixture with the Dynal Magnetic Particle Concentrator (MPC). The bead–choroidal EC complexes were resuspended in 150 μl EGM-2 with 10% FBS and 0.5 mg ml l−1 penicillin–streptomycin and plated onto a 1 cm2 area and incubated overnight at 37 °C and 5% CO2. Choroidal ECs were confirmed as ECs and not fibroblasts by 100% immunostaining with von Willebrand Factor (Sigma), CD31 (Serotec, NC), and VE-cadherin (R&D Systems, MN). Choroidal ECs were maintained in basal endothelial growth medium (EGM-2, Lonza) with 5% FBS at 37 °C in 5% CO2 and Passages 2–5 were used in the experiments. For treatment, cells were first starved in serum-free basal endothelial medium (EBM2; Lonza) overnight, pretreated with VEGFR2 tryosine-kinase inhibitor semaxanib (SU5416; 5 µM, Sigma-Aldrich) or dimethyl sulfoxide (DMSO) for 30 min, and then incubated with VEGF (20 ng/ml, R&D Systems, Minneapolis, MN) for 1 h. Cells were harvested for western blots and immunoprecipitation.
RNA isolation and real-time PCR analysis
Total RNA was extracted with the Tri Reagent (Sigma-Aldrich, St. Louis, MO). cDNA was generated with the use of a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative Real Time-PCR was performed on a Mastercycler ep Realplex (Eppendorf, Westbury, NY) with SYBR Green Master Mix (Roche Diagnostics, Indianapolis, IN). Quantitative real-time PCR conditions were Step 1 (50 °C for 2 min), Step 2 (95 °C for 10 min) and Step 3 (40 cycles of [95 °C for 15 s to 60 °C for 1 min]). Expression levels for NOX isoforms were normalized to the mean value of internal control GAPDH.
siRNA transfection in human retinal microvascular endothelial cells
For siRNA transfection, hRMVECs at 70%–80% confluence in six-well plates were transfected with siRNAs targeting human NOX4 or STAT3 (Applied Biosystems, Grand Island, NY) using Lipofectamine 2000 (Invitrogen). A silencer selective negative control siRNA was used as control.
Protein extraction, western blot, and coimmunoprecipitation
Retina and hRMVECs were lysed in modified radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors (Roche Diagnostics, Indianapolis, IN) and orthovanadate (2 mmol/l; Sigma Aldrich), and then homogenized and centrifuged at 15,700 ×g for 10 min at 4 °C. Protein concentration in the supernatant fluid was quantified with bicinchoninic acid (BCA) Protein Assay (Pierce, Rockford, IL); 30 µg total protein for each sample was used for western blots. Membranes were incubated overnight at 4 °C with the following primary antibodies: p-VEGFR2, total VEGFR2 (Santa Cruz Biotechnology), NOX4, p-STAT3 (Y705), and total STAT3 (Cell Signaling Technology, Danvers, MA). For the interaction of NOX4 and VEGFR2, coimmunoprecipitation was performed in 500 µl cell lysate containing 500 µg protein using anti-NOX4 antibody to pull down NOX4; the amount of VEGFR2 interacted with NOX4 was determined with western blots using anti-VEGFR2 antibody. Blots were visualized, and the relative densities of bands were calculated using UN-SCAN-IT Gel 6.1.
Intracellular reactive oxygen species generation and cell proliferation assay
hRMVECs were seeded into 96-well plates and transfected with siRNA. Forty-eight hours post-transfection, cells were loaded with 5 µm 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CMDCF-DA; Invitrogen) in serum- and growth factor-free medium for 30 min at 37 °C. After two washes with PBS, cells were incubated with VEGF (20 ng/ml) or control PBS for 30 min. ROS generation was measured in a fluorescent plate reader (excitation-488 nm and emission-520 nm). Cells incubated with 10 µM H2O2 were used as a positive control.
For the proliferation assay, 24 h post-transfection, cells were starved in serum- and growth factor-free medium for 24 h and then incubated with VEGF (20 ng/ml) for another 24 h. Cell number was measured with Vybrant 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay kit (Invitrogen).
Statistical analysis
Significant differences between treatment groups were determined with ANOVA with the Newman–Keuls multiple comparison post-hoc test. A minimum value of p<0.05 was considered statistically significant. For in vivo studies, three different litters were used for each experiment to account for potential effects within individual litters. Four to five retinas from different individual pups were analyzed for time points. For in vitro studies, at least three conditions were used in each experiment and experiments were repeated two additional times. For real-time PCR, six individual samples were used for NOX isoforms mRNA analysis. Results are means ± standard deviation (SD).
Results
NOX4 Is highly expressed in retinal endothelial cells and in rat oxygen-induced retinopathy model
To determine the roles of endothelial NADPH oxidase isoforms in angiogenesis, we first detected expression profiles of NOX isoforms in several types of cultured vascular ECs (hRMVECs, CECs, HUVECs) using real-time PCR. In support of our hypothesis and the literature [2], NOX4 was the most prevalent isoform in all ECs tested, and NOX2 was also expressed in CECs (Figure 1). We then measured NOX4 expression in retinal lysates from pups raised in RA at postnatal day 0 (P0), P14, and P18 and found that NOX4 expression was reduced during development (Figure 2A). In comparison to RA-raised rat pups, NOX4 protein was significantly increased in the OIR model at P18 (Figure 2C), but not at P14 (Figure 2B). We then determined NOX4 localization in retinal cryosections from rat pups in OIR or RA at P18 (Figure 2D). Compared to the RA-raised rat pups, semiquantitative assessment of NOX4 density was greater in lectin-stained ECs in the P18 OIR model than the P18 RA (Figure 2E). Thus, NOX4 expression in retinal ECs was increased in the rat OIR model at the time point when IVNV occurred, whereas NOX4 was less prevalent during retinal vascular development.
Figure 1.
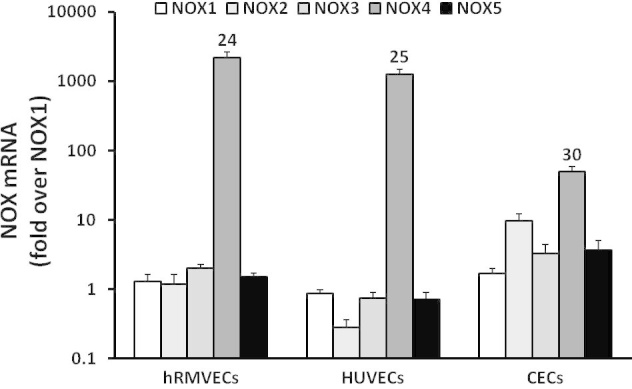
Expression of NOX4 in cultured vascular endothelial cells. Quantitative Real-time PCR of NOX isoforms was measured in human retinal microvascular endothelial cells (hRMVECs), human umbilical vein cells (HUVECs), and human choroidal endothelial cells (CECs). Numbers represent cycle of threshold (Ct) values.
Figure 2.
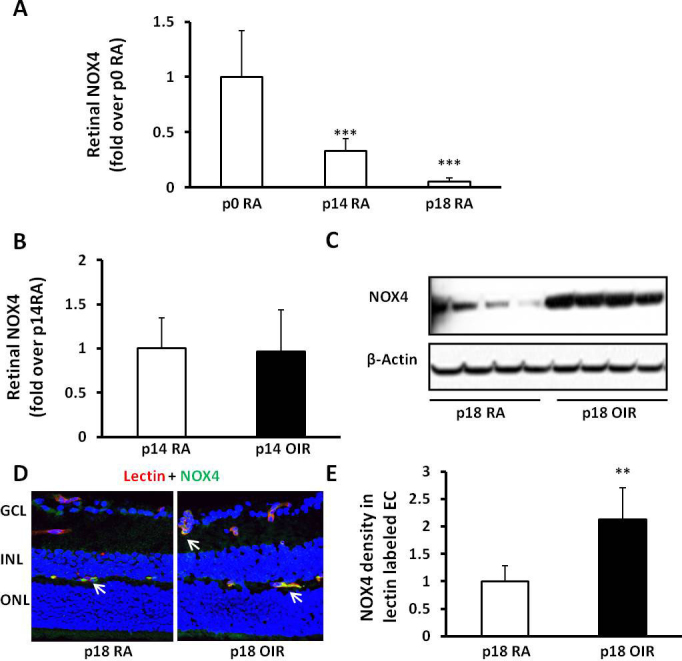
NOX4 expression in retina and retinal endothelial cells is increased at postnatal day18 (P18) in the rat 50/10 oxygen-induced retinopathy model (ROP model). Western blots of NOX4 expression in retinal lysates from room air (RA)-raised pups at P0, P14, and P18 (A; ANOVA ***p<0.001; ***p<0.001 versus P0 by one-way ANOVA) and in the retina from RA- and ROP model raised pups at P18 (B, representative gels) and P14 (C, densitometry); immunohistochemistry (IHC) of NOX4 and lectin colabeling in retinal sections at P18 (D) and greater in the ROP model (E; **p<0.01 versus standard deviation [SD], RA; n = 4–5, two-way ANOVA).
Upregulation of NOX4 is associated with activation of STAT3 in the rat oxygen-induced retinopathy model
We previously found that VEGF-activated STAT3 caused retinal endothelial cell proliferation in vitro [33] and that NADPH oxidase mediated IVNV through activated STAT3 in a rat OIR+supplemental oxygen model in which pups were placed in 28% supplemental oxygen from P14 to P18, rather than 21% oxygen as in the rat OIR model used in the study [24]. We also previously reported that, compared to RA-raised pups, retinal VEGF expression increased at several time points including P18 in the rat OIR model [34]. Therefore, to determine if increased EC NOX4 was associated with activation of retinal STAT3 at P18 when VEGF is also increased in the rat OIR model, we measured phosphorylated STAT3 and total STAT3 in retinal lysates from the P18 OIR model and RA pups (Figure 3A). STAT3 activation, determined as a ratio of phosphorylated STAT3 to total STAT3, was significantly increased at P18 in the OIR model compared to the RA pups (Figure 3B). This suggested that increased NOX4 was associated with activation of STAT3 and VEGF signaling at the time point when IVNV occurred.
Figure 3.
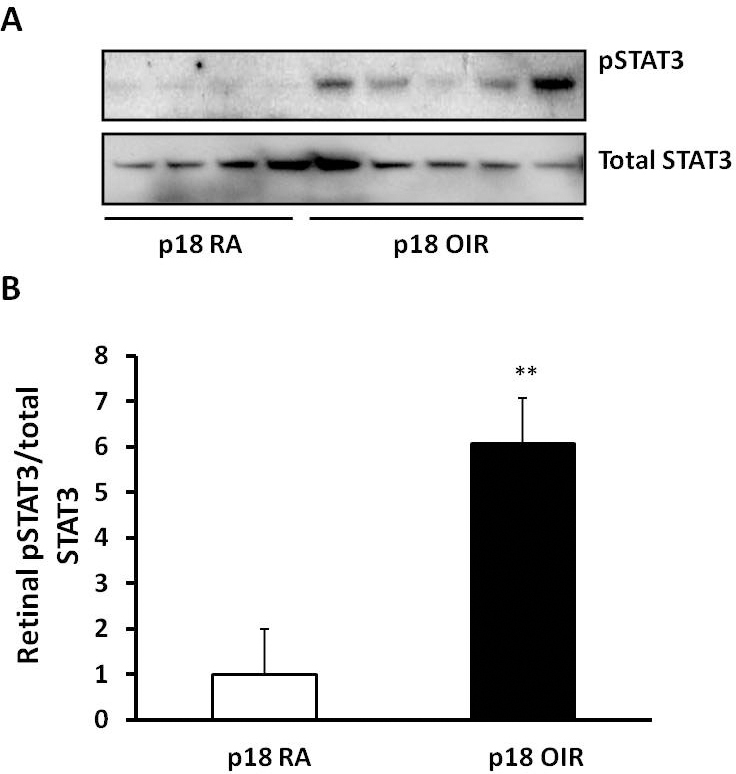
STAT3 is activated at P18 in the rat 50/10 oxygen-induced retinopathy model (ROP model). (A) p-STAT3 and total STAT3 in the retinas from postnatal day 18 (P18) pups raised in room air (RA) and ROP model were measured by western blots; (B) STAT3 activity determined by the ratio of pSTAT3 to total STAT3 is increased in the ROP model (**p<0.01 versus standard deviation [SD], RA, two-way ANOVA).
NOX4 regulates vascular endothelial growth factor-induced reactive oxygen species generation in retinal endothelial cells
Increasing evidence indicates that NADPH oxidase participates in retinal angiogenesis through ROS generation [2]. We previously reported that VEGF activated NADPH oxidase in CECs and contributed to choroidal neovascularization in a laser-induced choroidal neovascularization (CNV) model [35]. To understand the relationship between NOX4 and VEGF, we determined whether NOX4 was involved in VEGF-induced ROS generation in hRMVECs. In hRMVECs, knockdown of NOX4 by siRNA transfection (Figure 4A) inhibited basal and VEGF-induced ROS generation (Figure 4B). This suggests that VEGF activates NOX4 and leads to ROS generation.
Figure 4.
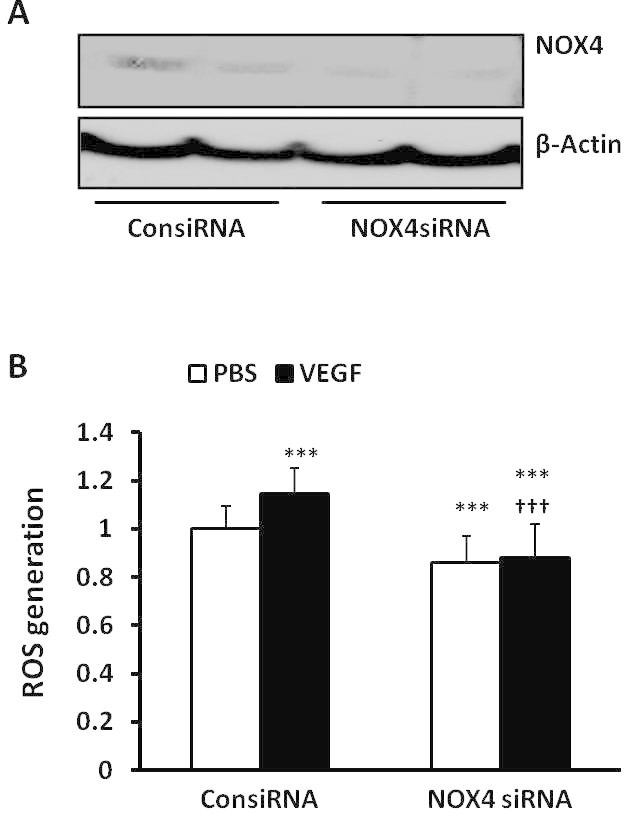
Knockdown of NOX4 by siRNA transfection inhibits vascular endothelial growth factor–induced reactive oxygen species generation in human retinal microvascular endothelial cells. (A) western blots of NOX4 expression and (B) reactive oxygen species (ROS) generation were determined with 434 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CMDCF-DA) fluorescence in human retinal microvascular endothelial cells (hRMVECs) transfected with control siRNA (ConsiRNA) or NOX4 siRNA (NOX4siRNA) and treated with PBS or vascular endothelial growth factor (VEGF; 20 ng/ml; ***p<0.001 versus PBS of ConsiRNA; †††p<0.001 versus standard deviation [SD], VEGF of ConsiRNA; one-way ANOVA with post-hoc Newman–Keuls multiple comparison testing).
NOX4 interacts with phosphorylated vascular endothelial growth factor receptor 2 to regulate vascular endothelial growth factor-mediated STAT3 activation and endothelial cell proliferation
Activated VEGF/ VEGFR2 signaling can cause IVNV in rat [13,36,37] and mouse models of OIR [12,17]. We determined if endothelial NOX4 was involved in VEGF/VEGFR2 signaling. We first measured VEGF-induced VEGFR2 and STAT3 phosphorylation in cultured hRMVECs that were transfected with NOX4 siRNA compared to control (Figure 5A). NOX4 silencing had no effect on basal levels of phosphorylated VEGFR2 (p-VEGFR2; Figure 5A) or STAT3 (p-STAT3; Figure 5B), but partly reduced p-VEGFR2 (Figure 5A) and completely inhibited VEGF-induced p-STAT3 (Figure 5B), providing support that STAT3 was a downstream effector of activated NOX4 and that NOX4 involved VEGFR2 signaling. To determine the mechanism in which VEGF/VEGFR2 and NOX4 were involved in downstream STAT3 activation, hRMVECs were pretreated with the VEGFR2 tyrosine kinase inhibitor, SU5416, or vehicle, and then stimulated with VEGF. In response to VEGF stimulation, coimmunoprecipitation of NOX4 with VEGFR2 was increased (Figure 5C). However, inhibition of VEGFR2 activation by SU5416 inhibited coimmunoprecipitation of NOX4 and VEGFR2 and SU5416 treatment effectively blocked VEGF-induced p-VEGFR2 in the same cell lysates (Figure 5C). Altogether, these results suggest that a structural relationship between NOX4 and p-VEGFR2 existed following VEGF-induced activation of VEGFR2 and that together the interaction over-activated STAT3.
Figure 5.
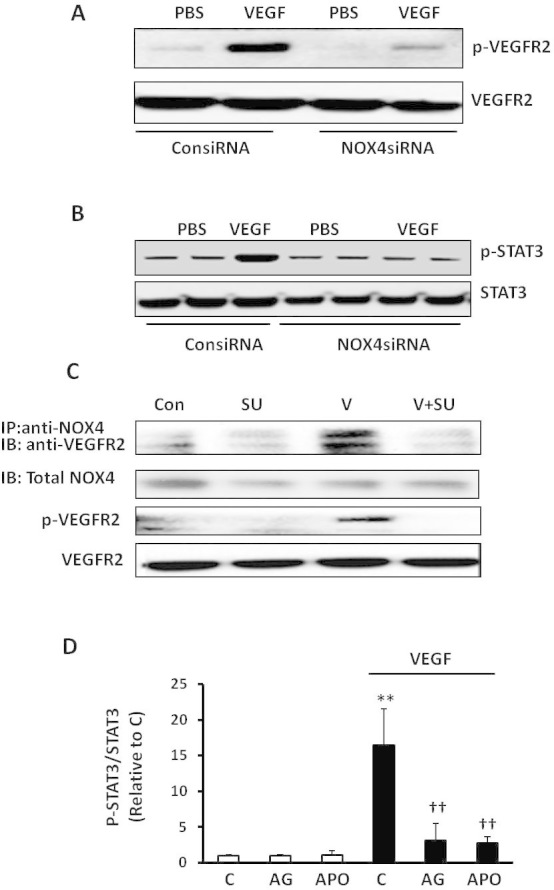
NOX4 regulates vascular endothelial growth factor–induced STAT3 activation through interacting with phosphorylated vascular endothelial growth factor receptor 2 in human retinal microvascular endothelial cells. Western blots of phosphorylated vascular endothelial growth factor receptor 2 (p-VEGFR2) and total VEGFR2 (A) and phosphorylated STAT3 (p-STAT3) and total STAT3 (B) in human retinal microvascular endothelial cells (hRMVECs) transfected with ConsiRNA or NOX4siRNA and treated with PBS or VEGF (20 ng/ml); (C) coimmunoprecipitation of NOX4 with VEGFR2 and western blots of p-VEGFR2 and total VEGFR2 in hRMVECs treated with VEGF with or without pretreatment of SU5416 (Con, control; SU, SU5416; V, VEGF). D: Western blots of p-STAT3 and total STAT3 were performed in hRMVECs treated with VEGF and pretreated with AG490, apocynin or respective controls (C, control; AG, AG490; APO, apocynin; **p<0.01 versus control and ††p<0.01 versus VEGF, one-way ANOVA with post-hoc Newman–Keuls multiple comparison testing; n=3 and results are means±SD).
To assess the effect of NOX4 and STAT3 on EC proliferation, hRMVECs transfected with siRNAs to NOX4 or STAT3 or control siRNAs were treated with VEGF or PBS for 24 h. Compared to control, NOX4 siRNA (Figure 6A) and STAT3 siRNA (Figure 6B) significantly inhibited VEGF-induced hRMVEC proliferation. These and the previous data provide evidence that VEGF-VEGFR2 signaling mediated NOX4 enhanced EC proliferation through activation of STAT3.
Figure 6.
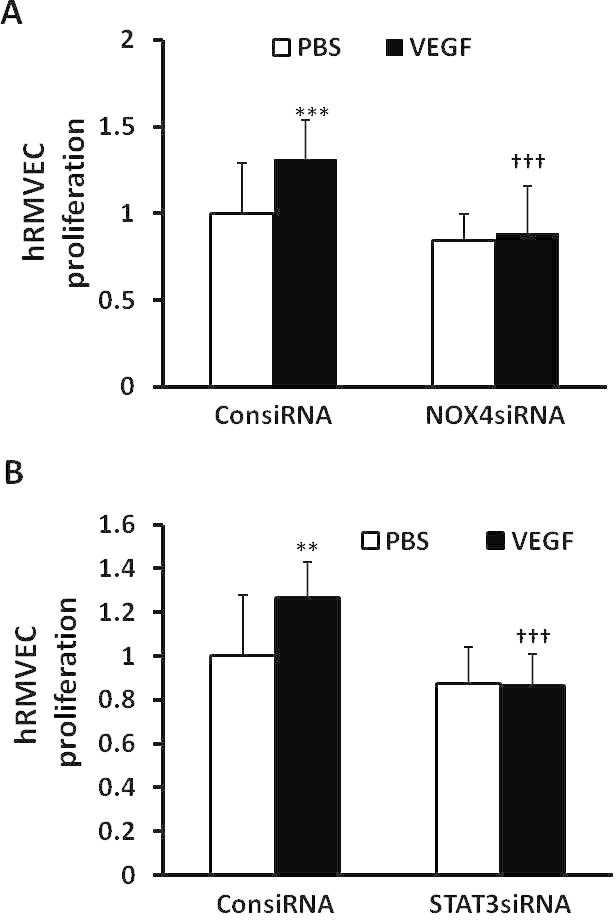
NOX4 regulates vascular endothelial growth factor–induced endothelial cell proliferation through activation of STAT3. Cell proliferation was measured in human retinal microvascular endothelial cells (hRMVECs) transfected with control siRNA (ConsiRNA) or siRNA to either NOX4 (NOX4siRNA; A) or STAT3 (STAT3siRNA; B) and treated with PBS or vascular endothelial growth factor (VEGF; 20 ng/ml; **p<0.01 and ***p<0.001 versus PBS of ConsiRNA; †††p<0.001 versus standard deviation [SD], VEGF of ConsiRNA; one-way ANOVA with post-hoc Newman–Keuls multiple comparison testing).
Discussion
VEGF plays important roles in physiologic angiogenesis in development and in the adult [38,39] as well as in pathologic angiogenesis in cancer [40,41] and eye diseases [30,42-44]. Inhibition of the bioactivity of VEGF has been used in several retinal and choroidal diseases to reduce pathologic angiogenesis [30,45], including several leading causes of blindness worldwide, neovascular age-related macular degeneration (AMD) [46], diabetic retinopathy [47], and retinopathy of prematurity [48]. However, in AMD, repeated anti-VEGF injections are usually required, and reports of retinal and choroidal atrophy raise concerns regarding long-term vision loss [49,50]. In addition, a single dose of intravitreal anti-VEGF can reduce circulating VEGF levels [30,51], raising questions about reducing beneficial effects of VEGF as a neuroprotective agent in development or after brain ischemia or stroke [52,53]. We previously found excessive signaling through VEGFR2-triggered signaling pathways that caused IVNV [54]. To preserve beneficial aspects of VEGF signaling and inhibit IVNV, we sought to identify effectors that regulate VEGFR2 activation and downstream angiogenic signaling pathways as a means of supporting physiologic angiogenesis while inhibiting IVNV.
NADPH oxidase isoforms NOX1, NOX2, and NOX4 have been implicated in pathologic angiogenesis of the retina and choroid in different models [5-10]. We previously found that activation of NADPH oxidase delayed physiologic retinal vascular development in the rat OIR model [8] but caused IVNV mediated through STAT3 in the rat OIR model rescued in 28% supplemental oxygen [24]. We and others also found that VEGF expression increased at several time points in the rat OIR model, including at P18 in association with IVNV [36]. In this study, we focused on the potential role of NOX4 in retinal ECs in VEGF-induced IVNV. At P18 in the OIR model compared to RA pups, STAT3 activation was also increased in retinal lysates, in association with greater expression of retinal NOX4 and the development of IVNV.
To study the role of NOX4 and VEGFR2 signaling in retinal ECs, a series of experiments were performed in cultured hRMVECs. Knockdown of NOX4 by siRNA transfection inhibited basal and VEGF-induced ROS generation. In addition, knockdown of NOX4 partially reduced VEGF-induced VEGFR2 activation and abolished VEGF-induced STAT3 activation, suggesting the expression level of NOX4 and its activation to generate ROS were important for the interaction of NOX4/p-VEGFR2 and in maintaining activation of downstream signaling effectors of VEGFR2. The results from in vitro studies support a structural interaction between NOX4 and VEGFR2, perhaps through an adaptor protein, that relies on activation of VEGFR2, and that triggered STAT3 activation to cause hRMVEC proliferation.
In summary, our study provides new evidence that VEGF-induced VEGFR2 can exacerbate downstream STAT3 activation through an interaction with NOX4 and may contribute to IVNV in the rat OIR model. Future studies to understand the interaction between NOX4 and p-VEGFR2 are important, because inhibiting NOX4 or its generated ROS may be a method to regulate VEGFR2 singaling and safely reduce VEGFR2-mediated IVNV.
Acknowledgments
This work was supported by the National Institutes of Health R01EY015130 and R01EY017011 to M.E.H. and in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah.
References
- 1.Ushio-Fukai M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc Res. 2006;71:226–35. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson-Berka JL, Rana I, Armani R, Agrotis A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clin Sci (Lond) 2013;124:597–615. doi: 10.1042/CS20120212. [DOI] [PubMed] [Google Scholar]
- 3.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:S28–9. [PubMed] [Google Scholar]
- 5.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11:747–64. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Rüegg C, Krause KH, Imhof BA. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS ONE. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson-Berka JL, Deliyanti D, Rana I, Miller AG, Agrotis A, Armani R, Szyndralewiez C, Wingler K, Touyz RM, Cooper ME, Jandeleit-Dahm KA, Schmidt HH. NADPH oxidase, NOX1, mediates vascular injury in ischemic retinopathy. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2013.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–53. [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Shabrawey M, Bartoli M, El Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH Oxidase and Stat3 in Statin-Mediated Protection against Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–8. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–38. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushner EJ, Bautch VL. Building blood vessels in development and disease. Curr Opin Hematol. 2013;20:231–6. doi: 10.1097/MOH.0b013e328360614b. [DOI] [PubMed] [Google Scholar]
- 12.Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 13.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularisation in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34:576–85. [PubMed] [Google Scholar]
- 14.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 15.Caprara C, Grimm C. From oxygen to erythropoietin: Relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012;31:89–119. doi: 10.1016/j.preteyeres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LEH. Suppression of Retinal Neovascularization by Erythropoietin siRNA in a Mouse Model of Proliferative Retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–35. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996;98:2507–11. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tawfik A, Sanders T, Kahook K, Akeel S, Elmarakby A, Al Shabrawey M. Suppression of Retinal Peroxisome Proliferator-Activated Receptor {gamma} in Experimental Diabetes and Oxygen-Induced Retinopathy: Role of NADPH Oxidase. Invest Ophthalmol Vis Sci. 2009;50:878–84. doi: 10.1167/iovs.08-2005. [DOI] [PubMed] [Google Scholar]
- 20.Capozzi ME, McCollum GW, Savage SR, Penn JS. Peroxisome Proliferator-Activated Receptor-beta/delta Regulates Angiogenic Cell Behaviors and Oxygen-Induced Retinopathy. Invest Ophthalmol Vis Sci. 2013;54:4197–207. doi: 10.1167/iovs.13-11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 Inhibition and Retinal Angiogenesis in a Mouse Model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2003;44:974–9. doi: 10.1167/iovs.02-0392. [DOI] [PubMed] [Google Scholar]
- 22.Nagai N, Noda K, Urano T, Kubota Y, Shinoda H, Koto T, Shinoda K, Inoue M, Shiomi T, Ikeda E, Tsubota K, Suda T, Oike Y, Ishida S. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2005;46:1078–84. doi: 10.1167/iovs.04-1101. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H Oxidase from Supplemental Oxygen Induces Neovascularization Independent of VEGF in Retinopathy of Prematurity Model. Invest Ophthalmol Vis Sci. 2008;49:1591–8. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byfield G, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009;50:3360–5. doi: 10.1167/iovs.08-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Shabrawey M, Bartoli M, El Remessy AB, Platt DH, Matragoon S, Behzadian MA, Caldwell RW, Caldwell RB. Inhibition of NAD(P)H Oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci. 2008;49:3239–44. doi: 10.1167/iovs.08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvoriantchikova G, Grant J, Santos AR, Hernandez E, Ivanov D. Neuronal NAD(P)H oxidases contribute to ROS production and mediate RGC death after ischemia. Invest Ophthalmol Vis Sci. 2012;53:2823–30. doi: 10.1167/iovs.12-9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase / Novelty and Significance. Circ Res. 2012;110:1217–25. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 29.Maile LA, Gollahon K, Wai C, Byfield G, Hartnett ME, Clemmons D. Disruption of the association of integrin-associated protein (IAP) with tyrosine phosphatase non-receptor type substrate-1 (SHPS)-1 inhibits pathophysiological changes in retinal endothelial function in a rat model of diabetes. Diabetologia. 2012;55:835–44. doi: 10.1007/s00125-011-2416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartnett ME, Penn JS. Mechanisms and Management of Retinopathy of Prematurity. N Engl J Med. 2012;367:2515–26. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–31. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Geisen P, Wittchen ES, King B, Burridge K, D'Amore PA, Hartnett ME. The Role of RPE Cell-Associated VEGF189 in Choroidal Endothelial Cell Transmigration across the RPE. Invest Ophthalmol Vis Sci. 2011;52:570–8. doi: 10.1167/iovs.10-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. Anti-VEGF Antibody Leads to Later Atypical Intravitreous Neovascularization and Activation of Angiogenic Pathways in a Rat Model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2013;54:2020–6. doi: 10.1167/iovs.13-11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budd SJ, Thompson H, Hartnett ME. Association of Retinal Vascular Endothelial Growth Factor With Avascular Retina in a Rat Model of Retinopathy of Prematurity. Arch Ophthalmol. 2010;128:1014–21. doi: 10.1001/archophthalmol.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaghan-Benson E, Hartmann J, Vendrov AE, Budd S, Byfield G, Parker A, Ahmad F, Huang W, Runge M, Burridge K, Madamanchi N, Hartnett ME. The Role of Vascular Endothelial Growth Factor-Induced Activation of NADPH Oxidase in Choroidal Endothelial Cells and Choroidal Neovascularization. Am J Pathol. 2010;177:2091–102. doi: 10.2353/ajpath.2010.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisen P, Peterson L, Martiniuk D, Uppal A, Saito Y, Hartnett M. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Mol Vis. 2008;14:345–57. [PMC free article] [PubMed] [Google Scholar]
- 37.Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 2009;89:718–27. doi: 10.1016/j.exer.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF Expression and Receptor Activation in the Choroid during Development and in the Adult. Invest Ophthalmol Vis Sci. 2006;47:3135–42. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- 39.Bautch VL. VEGF-directed blood vessel patterning: from cells to organism. Cold Spring Harbor 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dvorak HF. Vascular Permeability Factor/Vascular Endothelial Growth Factor: A Critical Cytokine in Tumor Angiogenesis and a Potential Target for Diagnosis and Therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, New T, O'Toole K, Zabski S, Rudge JS, Holash J, Yancopoulos GD, Yamashiro DJ, Kandel JJ. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proceedings of the National Academy of Sciences 2003:1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, Nguyen HV, Aiello LM, Ferrara N, King GL. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 43.Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LEH. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–61. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng EWM, Adamis AP. Anti-VEGF Aptamer (Pegaptanib) Therapy for Ocular Vascular Diseases. Ann N Y Acad Sci. 2006;1082:151–71. doi: 10.1196/annals.1348.062. [DOI] [PubMed] [Google Scholar]
- 45.Kami J, Muranaka K, Yanagi Y, Obata R, Tamaki Y, Shibuya M. Inhibition of choroidal neovascularization by blocking vascular endothelial growth factor receptor tyrosine kinase. Jpn J Ophthalmol. 2008;52:91–8. doi: 10.1007/s10384-007-0506-6. [DOI] [PubMed] [Google Scholar]
- 46.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010;120:3033–41. doi: 10.1172/JCI42437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholson BP, Schachat A. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:915–30. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 48.Raizada S, Al Kandari J, Al Sabti K. Will the BEAT-ROP Study Results Really Beat ROP? Invest Ophthalmol Vis Sci. 2011;52:9288–9. doi: 10.1167/iovs.11-8479. [DOI] [PubMed] [Google Scholar]
- 49.Mititelu M, Chaudhary KM, Lieberman RM. An Evidence-Based Meta-analysis of Vascular Endothelial Growth Factor Inhibition in Pediatric Retinal Diseases: Part 1. Retinopathy of Prematurity. J Pediatr Ophthalmol Strabismus. 2012;49:332–40. doi: 10.3928/01913913-20120821-03. [DOI] [PubMed] [Google Scholar]
- 50.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: A Multicenter Cohort Study (SEVEN-UP). Ophthalmology. 2013;120:2292–9. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 51.Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, Kusaka S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu H, Tang Y, Dong Q. Protection of Vascular Endothelial Growth Factor to Brain Edema Following Intracerebral Hemorrhage and Its Involved Mechanisms: Effect of Aquaporin-4. PLoS ONE. 2013;8:e66051. doi: 10.1371/journal.pone.0066051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49:3107–14. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]


