Abstract
Purpose
Vegetable polyphenols (bioflavonoids) have been suggested to represent promising drugs for treating cancer and retinal diseases. We compared the effects of various bioflavonoids (epigallocatechin-3-gallate [EGCG], luteolin, apigenin, myricetin, quercetin, and cyanidin) on the physiological properties and viability of cultured human retinal pigment epithelial (RPE) cells.
Methods
Human RPE cells were obtained from several donors within 48 h of death. Secretion of vascular endothelial growth factor (VEGF) was determined with enzyme-linked immunosorbent assay. Messenger ribonucleic acid levels were determined with real-time reverse transcription polymerase chain reaction. Cellular proliferation was investigated with a bromodeoxyuridine immunoassay, and chemotaxis was examined with a Boyden chamber assay. The number of viable cells was determined by Trypan Blue exclusion. Apoptosis and necrosis rates were determined with a DNA fragmentation enzyme-linked immunosorbent assay. The phosphorylation level of signaling proteins was revealed by western blotting.
Results
With the exception of EGCG, all flavonoids tested decreased dose-dependently the RPE cell proliferation, migration, and secretion of VEGF. EGCG inhibited the secretion of VEGF evoked by CoCl2-induced hypoxia. The gene expression of VEGF was reduced by myricetin at low concentrations and elevated at higher concentrations. Luteolin, apigenin, myricetin, and quercetin induced significant decreases in the cell viability at higher concentration, by triggering cellular necrosis. Cyanidin reduced the rate of RPE cell necrosis. Myricetin caused caspase-3 independent RPE cell necrosis mediated by free radical generation and activation of calpain and phospholipase A2. The myricetin- and quercetin-induced RPE cell necrosis was partially inhibited by necrostatin-1, a blocker of programmed necrosis. Most flavonoids tested diminished the phosphorylation levels of extracellular signal-regulated kinases 1/2 and Akt proteins.
Conclusions
The intake of luteolin, apigenin, myricetin, and quercetin as supplemental cancer therapy or in treating retinal diseases should be accompanied by careful monitoring of the retinal function. The possible beneficial effects of EGCG and cyanidin, which had little effect on RPE cell viability, in treating retinal diseases should be examined in further investigations.
Introduction
Numerous studies performed in the last few years have shown that vegetable polyphenols (bioflavonoids) possess a wide range of activities in preventing common diseases including cancer, inflammation, infections, neovascularization, and neurodegenerative diseases [1-3]. Many dietary flavonoids have anti-inflammatory and antioxidant properties. For example, catechins of green tea, of which (-)-epigallocatechin-3-gallate (EGCG) is the most abundant, can inhibit tumorigenesis and angiogenesis in tumor tissues [4,5].
Enhanced production of free oxygen and nitrogen radicals contributes to the pathogenesis of important blinding diseases, including diabetic retinopathy, retinitis pigmentosa, and age-related macular degeneration [6-8]. Because bioflavonoids have anti-inflammatory and radical scavenging activities and suppress angiogenesis, they could have also potential benefits in inhibiting retinal diseases associated with oxidative stress, inflammation, and neovascularization. EGCG was shown to protect the retina from ischemic damage, mainly via its antioxidative activity [9,10]. Green tea, EGCG, and other flavonoids such as luteolin, myricetin, and quercetin, have also been shown to attenuate experimental retinal neovascularization, ischemic retinal injury, diabetic retinopathy, and light-induced photoreceptor apoptosis, respectively [11-16].
The mechanisms of the protective activities of flavonoids are not fully understood [5]. Many bioflavonoids including green tea catechins were shown to have antioxidant activity at low concentrations and prooxidant activity at high concentrations [1,5,17]. Antioxidant and prooxidant effects were suggested to be implicated in the anti-inflammatory and anticancer activities of dietary flavonoids [5]. The prooxidant effect appears to be responsible for inducing apoptosis in tumor cells and may also cause indirect antioxidant effects via induction of endogenous antioxidant systems in normal tissues that offer protection against oxidative stress [5]. In addition, excessive intake of vegetable polyphenols, as dietary supplements or natural food, may have adverse effects, for example, by inhibiting prosurvival pathways. The cytotoxicity of dietary flavonoids is helpful in treating cancer, but may also concern non-transformed cells [18]. We showed recently that curcumin (the yellow pigment of turmeric) at doses described to be effective in treating tumor cells has cytotoxic effects on human retinal pigment epithelial (RPE) cells and induces apoptosis and necrosis of the cells [19]. In another study, the flavonoids resveratrol (from red wine) and curcumin were shown to cause RPE cell death by inducing apoptosis and necrosis [20].
RPE cells play crucial roles in protecting the outer retina from photooxidative stress, in digesting shed photoreceptor outer segments which contain oxidized lipids, and in inhibiting retinal edema and neovascularization [21]. Dysfunction and degeneration of RPE cells are crucially involved in the pathogenesis of age-related macular degeneration [22,23]. The dry form of this blinding disease is characterized by the presence of lipofuscin within the RPE and drusen beneath the RPE, which contain photoreceptor-derived oxidized lipids, as well as by RPE cell death (geographic atrophy), while the hallmarks of the wet form are choroidal neovascularization and subretinal edema induced by outer retinal hypoxia [22,23]. Vascular endothelial growth factor (VEGF) is the main hypoxia-induced angiogenic factor that promotes retinal neovascularization and edema [24]. RPE cells are one source of VEGF in the retina [25].
Intake of bioflavonoids, as dietary supplements or natural food, is suggested to be helpful as supplemental therapy of cancer and chronic inflammation, and in preventing retinal disorders. However, it has been also suggested that excessive intake of vegetable polyphenols may have adverse effects that concern not only tumor cells but also non-transformed cells [18]. Therefore, further assessment for potential hazards of bioflavonoids should be considered before the compounds are used in the clinical setting [18]. In the present study, we compared the effects of various flavonoids on the physiologic properties of cultured human RPE cells involved in cellular responses to pathogenic conditions (secretion of VEGF, cellular proliferation and migration) and on the viability of the cells. The following compounds were tested: EGCG from green tea, luteolin from parsley, apigenin from celery and parsley, myricetin from black tea, grapes, walnuts, etc., quercetin from bulbs, and cyanidin from various plants such as red cabbage, blueberries, and strawberries. We found that, although most of the flavonoids investigated inhibited the release of VEGF and the proliferation and migration of RPE cells at higher concentrations, the flavonoids had also deleterious effects on cell viability and induced cellular necrosis. Two compounds, EGCG and cyanidin, were had little effect on cell viability and, thus, are suggested as candidates for further examination as possible therapeutic agents in retinal diseases.
Methods
Materials
All tissue culture components and solutions were purchased from Gibco BRL (Paisley, UK). Human recombinant platelet-derived growth factor (PDGF)-BB was purchased from R&D Systems (Minneapolis, MN). Necrostatin-1, inactive necrostatin-1, 3-(4-iodophenyl)-2-mercapto-(Z)-2-propenoic acid (PD150606), 2'-amino-3′-methoxyflavone (PD98059), and anthra(1–9-cd)pyrazol-6(2H)-one (SP600125) were from Calbiochem (Bad Soden, Germany). The peptides Ac-DEVD-CHO (asp-glu-val-asp) and Ac-IETD-CHO (ile-glu-thr-asp) were from Enzo Life Science (Lörrach, Germany). 3,4-Dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinoline (DPQ) and all other compounds used were from Sigma-Aldrich (Taufkirchen, Germany), unless stated otherwise. The following antibodies were used: rabbit antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH; New England Biolabs, Frankfurt/M., Germany; 1:2,000), rabbit antihuman extracellular signal-regulated kinases 1 and 2 (ERK1/2; New England Biolabs; 1:1,000), rabbit antiphosphorylated ERK1/2 (New England Biolabs; 1:1,000), rabbit antihuman p38 mitogen-activated protein kinase (MAPK; New England Biolabs; 1:1,000), rabbit antihuman phosphorylated p38 MAPK (New England Biolabs; 1:750), rabbit antihuman protein kinase B (Akt; New England Biolabs; 1:1,000), rabbit antihuman phosphorylated Akt (New England Biolabs; 1:1,000), and antirabbit immunoglobulin conjugated with alkaline phosphatase (Chemicon, Hofheim, Germany; 1:3,000).
Cell culture
The use of human material was approved by the Ethics Committee of the University of Leipzig and was performed according to the Declaration of Helsinki. Human RPE cells were obtained from several donors within 48 h of death, and were prepared and cultured as follows. After the vitreous and the retina were removed, the RPE cells were mechanically harvested, separated by digestion with 0.05% trypsin and 0.02% EDTA, and washed two times with PBS pH 7.2 (1.54 mM KH2PO4; 155.17 mM NaCl; 2.71 mM Na2HPO4x7H2O, Invitrogen, Paisley, UK). The cells were suspended in complete Ham F-10 medium containing 10% fetal bovine serum, GlutaMAX II, and penicillin/streptomycin, and were cultured in tissue culture flasks (Greiner, Nürtingen, Germany) in 95% air/5% CO2 at 37 °C. Cells of passages 3 to 5 were used. The epithelial nature of the RPE cells was routinely identified with immunocytochemistry using the monoclonal antibodies AE1 (recognizing most of the acidic type I keratins) and AE3 (recognizing most of the basic type II keratins), both from Chemicon. To test the substances, cultures that reached approximately 90% confluency were growth arrested in medium without serum for 5 h. Subsequently, media containing 0.5% serum with and without test substances were added.
Cell proliferation
The proliferation rate of RPE cells was determined by measuring the incorporation of bromodeoxyuridine (BrdU) into the genomic DNA. The cells were seeded at 3×103 cells per well in 96-well microtiter plates (Greiner), and were allowed to attach for 48 h. Thereafter, the cells were growth arrested in medium without serum for 5 h, and subsequently, medium containing 0.5% serum with and without test substances was added for another 24 h. BrdU incorporation was determined by using the Cell Proliferation Enzyme-linked Immunosorbent Assay (ELISA) BrdU Kit (Roche, Mannheim, Germany). BrdU (10 μM) was added to the culture medium 5 h before fixation.
Chemotaxis
Chemotaxis was determined with a modified Boyden chamber assay. Suspensions of RPE cells (100 µl; 5×105 cells/ml serum-free medium) were seeded onto polyethylene terephthalate filters (diameter 6.4 mm, pore size 8 µm; Becton Dickinson, Heidelberg, Germany) coated with fibronectin (50 µg/ml) and gelatin (0.5 mg/ml). Within 16 h after seeding, the cells attached to the filter and formed a semiconfluent monolayer. The cells were pretreated with blocking substances for 30 min, and thereafter, the medium was changed into medium without additives in the upper well and medium containing test substances in the lower well. After incubation for 6 h, the inserts were washed with buffered saline, fixed with Karnofsky’s reagent, and stained with hematoxylin. Non-migrated cells were removed from the filters by gentle scrubbing with a cotton swab. The migrated cells were counted, and the results were expressed relative to the cell migration rate in the absence of the test substances.
Cell viability
Cell viability was determined by Trypan Blue exclusion. The cells were seeded at 5×104 cells per well in six-well plates. After the cells reached 90% confluency, they were cultured in serum-free medium 16 h, and then stimulated with test substances for 6 and 24 h, respectively. After trypsinization, the cells were stained with Trypan Blue (0.4%), and the number of viable (non-stained) and dead (stained) cells were determined using a hemocytometer.
Deoxyribonucleic fragmentation
The Cellular DNA Fragmentation ELISA (Roche) was used to determine whether the cells undergo apoptosis or necrosis in the absence and presence of test substances. The cells were seeded at 3×103 cells per well in 96-well plates, and were cultured until confluency was reached. After the culture media were changed, the cells were prelabeled with BrdU for 16 h and then incubated in the absence or presence of the test substances in F-10/0.5% fetal calf serum for 6 and 24 h, respectively. Necrosis was determined by analyzing the BrdU-labeled DNA fragments in the cell-free culture supernatants, and apoptosis was determined by using the cytoplasmic lysates of the cells.
Total ribonucleic acid isolation
Total RNA was extracted from cultured cells by using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The quality of the RNA was analyzed with agarose gel electrophoresis. The A260/A280 ratio of optical density was measured using the NanoDrop 1000 device (Peqlab, Erlangen, Germany), and was between 1.9 and 2.1 for all RNA samples, indicating sufficient quality.
Real-time reverse transcription polymerase chain reaction
After treatment with DNase I (Roche), cDNA was synthesized from 1 µg of total RNA using the RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Roth, Germany). For subsequent PCR amplification, the cDNA was diluted by addition of 20 µl RNase free water. Semiquantitative real-time reverse transcription (RT)–PCR was performed with the Single-Color Real-Time PCR Detection System (BioRad, Munich, Germany) using the following primer pairs: VEGFA (NM_001025370; 407, 347, and 275 bp), sense 5ʹ-CCT GGT GGA CAT CTT CCA GGA GTA-3ʹ, anti-sense 5ʹ-CTC ACC GCC TCG GCT TGT CAC A-3ʹ; ACTB (NM_001101; 237 bp), sense 5ʹ-ATG GCC ACG GCT GCT TCC AGC-3ʹ, anti-sense 5ʹ-CAT GGT GGT GCC GCC AGA CAG-3ʹ. The PCR solution contained 1 µl cDNA, a specific primer set (0.25 µM each), and 10 µl of iQ SYBR Green Supermix (BioRad) in a final volume of 20 µl. The following conditions were used: initial denaturation and enzyme activation (one cycle at 95 °C for 3 min); denaturation, amplification, and quantification, 45 cycles at 95 °C for 30 s, 58 °C for 20 s, and 72 °C for 45 s. This was followed by a melt curve analysis (81 cycles) to determine the product specificity where the temperature was gradually increased from 55 °C to 95 °C (0.5 °C/cycle). The amplified samples were analyzed with standard agarose gel electrophoresis. The mRNA expression was normalized to the level of ACTB expression. The changes in mRNA expression were calculated according to the 2-ΔΔCT method (CT, cycle threshold), with ΔCT=CTtarget gene - CTactb and ΔΔCT=ΔCTtreatment - ΔCTcontrol.
Enzyme-linked immunosorbent assay
The cells were cultured at 3×103 cells per well in 96-well plates (100 µl culture medium per well). At approximately 90% confluency, the cells were cultured in serum-free medium for 16 h. Subsequently, the culture medium was changed, and the cells were stimulated with test substances. The supernatants were collected after 6 h, and the level of VEGF-A165 in the cultured media (200 µl) was determined with ELISA (R&D Systems) according to the manufacturer's recommendations.
Western blotting
The cells were seeded at 1×105 cells per well in six well plates in 1.5 ml complete medium, and were allowed to grow up to approximately 90% confluency. After growth arrest for 16 h, the cells were treated with test substances for 15 min. Then, the medium was removed, the cells were washed twice with prechilled PBS (pH 7.2; Invitrogen), and the monolayer was scraped into 150 µl lysis buffer (Mammalian Cell Lysis-1 Kit; Sigma). The total cell lysates were centrifuged at 10,000 × g for 10 min, and the supernatants were analyzed with immunoblots. Equal amounts of protein (30 µg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Immunoblots were probed with primary and secondary antibodies, and immunoreactive bands were visualized using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium.
Statistics
For each test, at least three independent experiments were performed in triplicate using cells from different donors. Data are expressed as means±standard error of the mean (SEM). Statistical analysis was performed with the Prism program (GraphPad Software, San Diego, CA). Significance was determined with one-way ANOVA followed by Bonferroni's multiple comparison test, and was accepted at p<0.05.
Results
Production of vascular endothelial growth factor
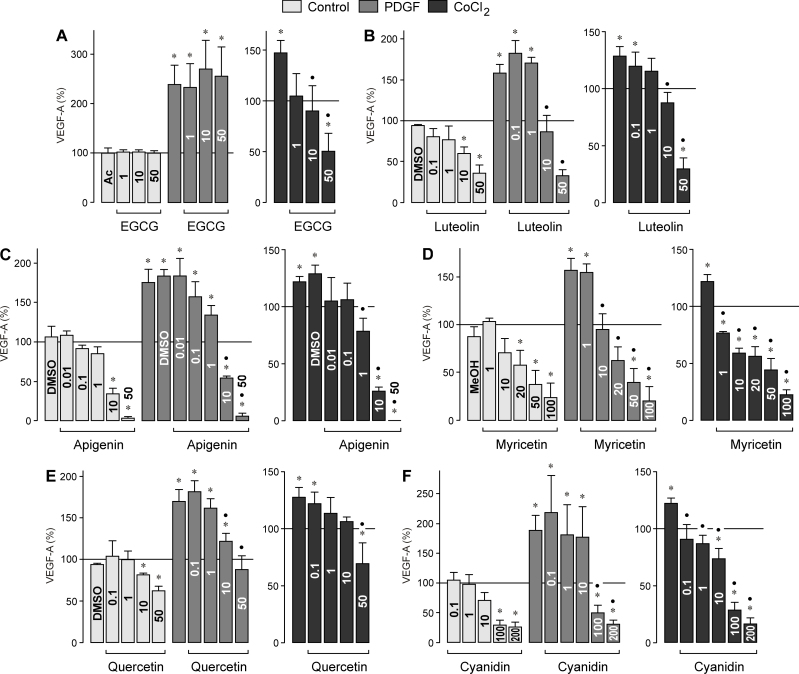
VEGF is a key player in choroidal neovascularization [24], and RPE cells are one source of VEGF in the retina [25]. To determine whether bioflavonoids modulate the secretion of VEGF protein from RPE cells, we determined the level of VEGF-A165 in the cultured media with ELISA. The release of VEGF was examined under control conditions and after the cells were stimulated with PDGF and CoCl2-induced chemical hypoxia, respectively. PDGF and hypoxia are known inducers of VEGF secretion from RPE cells [26]. As shown in Figure 1A, the major catechin of green tea, EGCG, did not alter the secretion of VEGF from RPE cells under control and PDGF-stimulated conditions when tested at concentrations between 1 and 50 µM. However, EGCG induced a dose-dependent decrease in the secretion of VEGF induced with chemical hypoxia. All other bioflavonoids tested, that is, luteolin (Figure 1B), apigenin (Figure 1C), myricetin (Figure 1D), quercetin (Figure 1E), and cyanidin (Figure 1F), induced dose-dependent decreases in the secretion of VEGF from RPE cells under all three conditions tested.
Figure 1.
Dose-dependent effects of several vegetable polyphenols on the secretion of vascular endothelial growth factor from retinal pigment epithelial cells. (A) epigallocatechin-3-gallate, (B) luteolin, (C) apigenin, (D) myricetin, (E) quercetin, and (F) cyanidin. The effects were determined in the absence (control) and presence of platelet-derived growth factor (PDGF; 10 ng/ml) and CoCl2 (150 µM), respectively, for 6 h. The level of VEGF-A165 in the cultured media was determined with enzyme-linked immunosorbent assay. The concentrations of the test substances (in µM) are given in the bars. The vehicle controls were made with acetone (Ac; 0.1%), dimethyl sulfoxide (DMSO; 0.1%), and methanol (MeOH; 0.2%), respectively. Data are means±standard error of the mean (SEM) of three to six independent experiments performed in triplicate using cells from different donors, and are expressed in percent of untreated control (100%). Significant difference vs. untreated control: *p<0.05. Significant difference vs. PDGF and CoCl2 control, respectively: ●p<0.05.
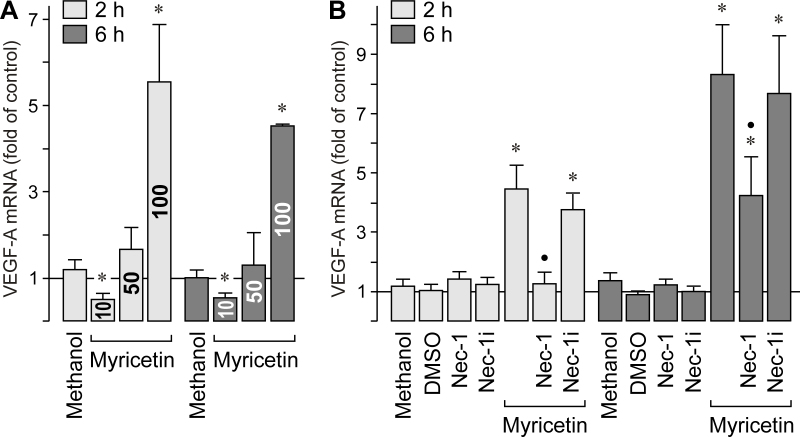
Myricetin induced a strong decrease in the secretion of VEGF protein from RPE cells (Figure 1D). To determine whether the compound also alters the expression of the VEGFA gene, we performed real-time RT–PCR. As shown in Figure 2A, myricetin induced a significant (p<0.05) decrease in the VEGFA expression at 10 µM, but a strong increase in VEGFA expression at 100 µM. Because the myricetin-induced increase in the gene expression of VEGF is reduced in the presence of an inhibitor of programmed necrosis, necrostatin-1 (Figure 2B), high concentrations of myricetin could induce cell stress, which is characterized by (among others) increased gene expression of VEGF.
Figure 2.
Effect of myricetin on the expression of the vascular endothelial growth factor gene. mRNA levels were determined by real-time RT–PCR after stimulation of the cells with myricetin for 2 and 6 h, respectively. A. Dose-dependency of the myricetin effect. The concentration of myricetin (in µM) is given in the bars. B. The stimulatory effect of myricetin (100 µM) on the gene expression of vascular endothelial growth factor (VEGF) is decreased in the presence of the inhibitor of programmed necrosis, necrostatin-1 (Nec-1; 30 µM), but not in the presence of inactive necrostatin-1 (Nec-1i; 30 µM). Vehicle controls were made with methanol (0.2%) and dimethyl sulfoxide (DMSO; 0.1%), respectively. Data are means±standard error of the mean (SEM) of three to six independent experiments performed in triplicate using cells from different donors. Significant difference vs. untreated control: *p<0.05. Significant difference vs. myricetin: ●p<0.05.
Proliferation and chemotaxis of retinal pigment epithelial cells
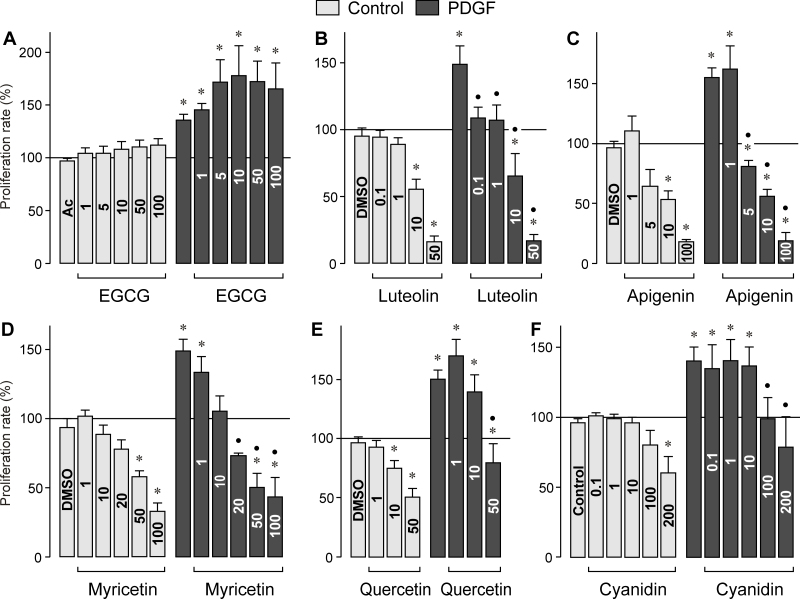
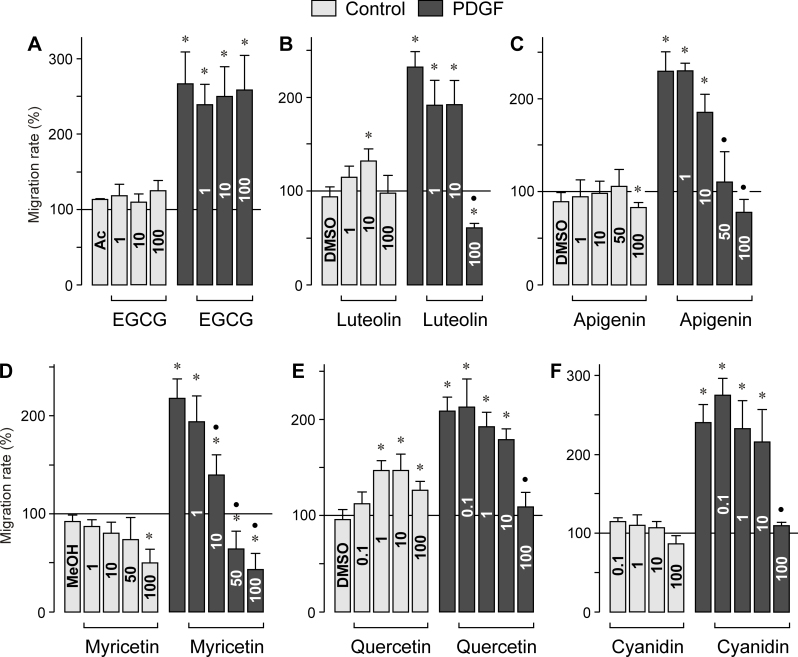
RPE cell proliferation and migration are characteristic features of proliferative retinal diseases. To determine whether bioflavonoids alter physiologic characteristics of RPE cells, we measured the proliferation and chemotaxis of cultured cells in the absence and presence of PDGF, a known mitogen and motogen of the cells [26]. As shown in Figure 3A, EGCG as the major green tea catechin did not significantly alter the proliferation rate of RPE cells under control and PDGF-stimulated conditions. Similarly, EGCG had no effect on the chemotaxis of RPE cells, under the control and PDGF-stimulated conditions (Figure 4A). All other bioflavonoids tested, that is, luteolin, apigenin, myricetin, quercetin, and cyanidin, induced dose-dependent decreases in the proliferation rate under control and PDGF-stimulated conditions (Figure 3B–F). Whereas the flavonoids (with the exception of EGCG) decreased dose-dependently the PDGF-induced chemotaxis of RPE cells (Figure 4B–F), the chemotaxis under non-stimulated control conditions was differently regulated by different flavonoids. Although apigenin and myricetin decreased the migration of the cells at the highest concentrations tested (Figure 4C, D), luteolin and quercetin induced increased chemotaxis of RPE cells under control conditions (Figure 4B, E), and cyanidin had no effect (Figure 4F).
Figure 3.
Dose-dependent effects of vegetable polyphenols on the proliferation of retinal pigment epithelial cells. (A) epigallocatechin-3-gallate, (B) luteolin, (C) apigenin, (D) myricetin, (E) quercetin, and (F) cyanidin. The effects were determined in the absence (control) and presence of platelet-derived growth factor (PDGF; 10 ng/ml). The rate of bromodeoxyuridine (BrdU) incorporation was measured after 24-h incubation with the agents. The concentrations of the test substances (in µM) are given in the bars. The vehicle controls were made with acetone (Ac; 0.2%), dimethyl sulfoxide (DMSO; 0.1%), and methanol (MeOH; 0.2%), respectively. Data are means±standard error of the mean (SEM) of three to ten independent experiments using cells from different donors, and are expressed in percent of untreated control (100%). Significant difference vs. untreated control: *p<0.05. Significant difference vs. PDGF control: ●p<0.05.
Figure 4.
Dose-dependent effects of several vegetable polyphenols on the chemotaxis of retinal pigment epithelial cells. (A) epigallocatechin-3-gallate, (B) luteolin, (C) apigenin, (D) myricetin, (E) quercetin, and (F) cyanidin. The effects were determined in the absence (control) and presence of platelet-derived growth factor (PDGF) (10 ng/ml). The concentrations of the test substances (in µM) are given in the bars. The vehicle controls were made with acetone (Ac; 0.2%), dimethyl sulfoxide (DMSO; 0.1%), and methanol (MeOH; 0.2%), respectively. Data are means±standard error of the mean (SEM) of three to five independent experiments using cells from different donors, and are expressed in percent of untreated control (100%). Significant difference vs. untreated control: *p<0.05. Significant difference vs. PDGF control: ●p<0.05.
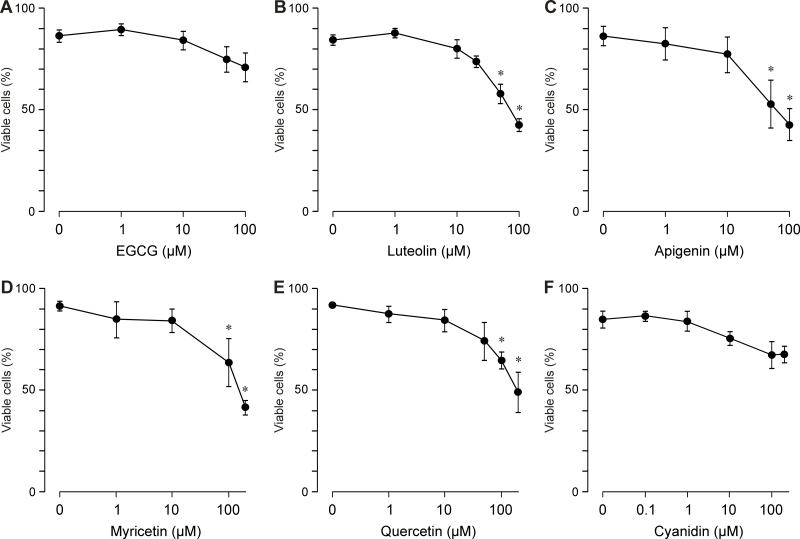
Cell viability
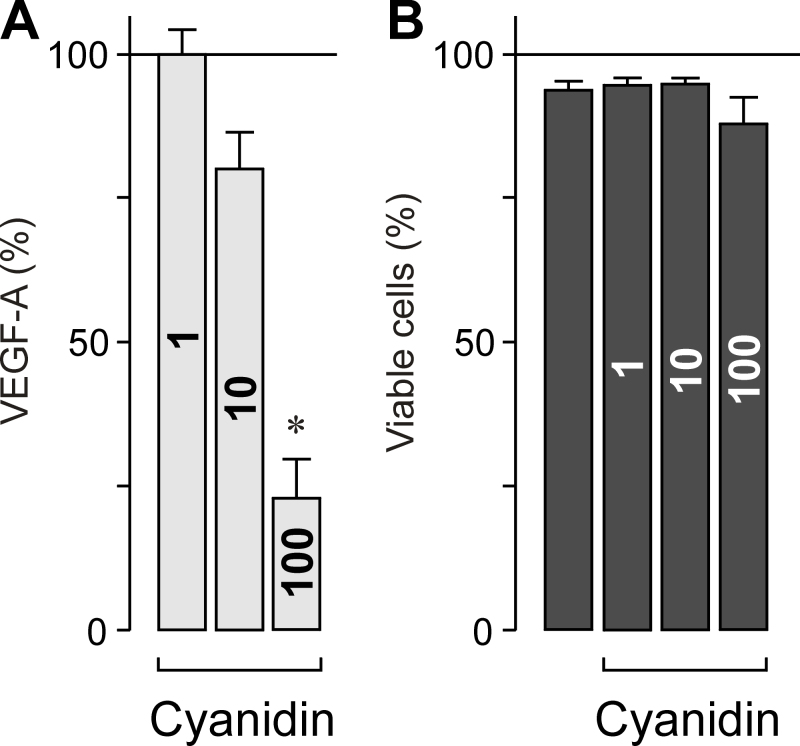
To determine whether the inhibitory effects of the flavonoids on the secretion of VEGF and the proliferation and chemotaxis of RPE cells involve a toxic effect on the cells, we determined cell viability by staining with Trypan Blue and counting the living and dead cells. Whereas EGCG (Figure 5A) and cyanidin (Figure 5F) up to a concentration of 100 µM did not significantly decrease the viability of the cells, luteolin (Figure 5B), apigenin (Figure 5C), myricetin (Figure 5D), and quercetin (Figure 5E) induced significant (p<0.05) decreases in the cell viability at the higher concentrations tested. The data suggest that the effects of different flavonoids on the secretion of VEGF and the proliferation and chemotaxis of RPE cells are, at least at higher concentrations, mediated by toxic effects of the compounds. However, cyanidin decreased the secretion of VEGF without a significant reduction in the cell viability (Figure 6).
Figure 5.
Dose-dependent effects of several vegetable polyphenols on the viability of retinal pigment epithelial cells. (A) epigallocatechin-3-gallate, (B) luteolin, (C) apigenin, (D) myricetin, (E) quercetin, and (F) cyanidin. The cells were stimulated with test substances for 24 h. Data are means±standard error of the mean (SEM) of three to seven independent experiments using cells from different donors. Significant difference vs. control: *p<0.05.
Figure 6.
Effects of Cyanidin on the secretion of vascular endothelial growth factor and cell viability of retinal pigment epithelial cells. Cyanidin decreases the secretion of vascular endothelial growth factor (A) without a significant reduction in the cell viability (B). The level of vascular endothelial growth factor (VEGF-A165) in the cultured media (measured with enzyme-linked immunosorbent assay) and the cell viability (measured with Trypan Blue exclusion) were determined by using the same cultures. The cells were stimulated with cyanidin for 6 h. The concentrations of cyanidin (in µM) are given in the bars. Data are means±standard error of the mean (SEM) of five to seven independent experiments using cells from different donors. Significant difference vs. control: *p<0.05.
Deoxyribonucleic acid fragmentation
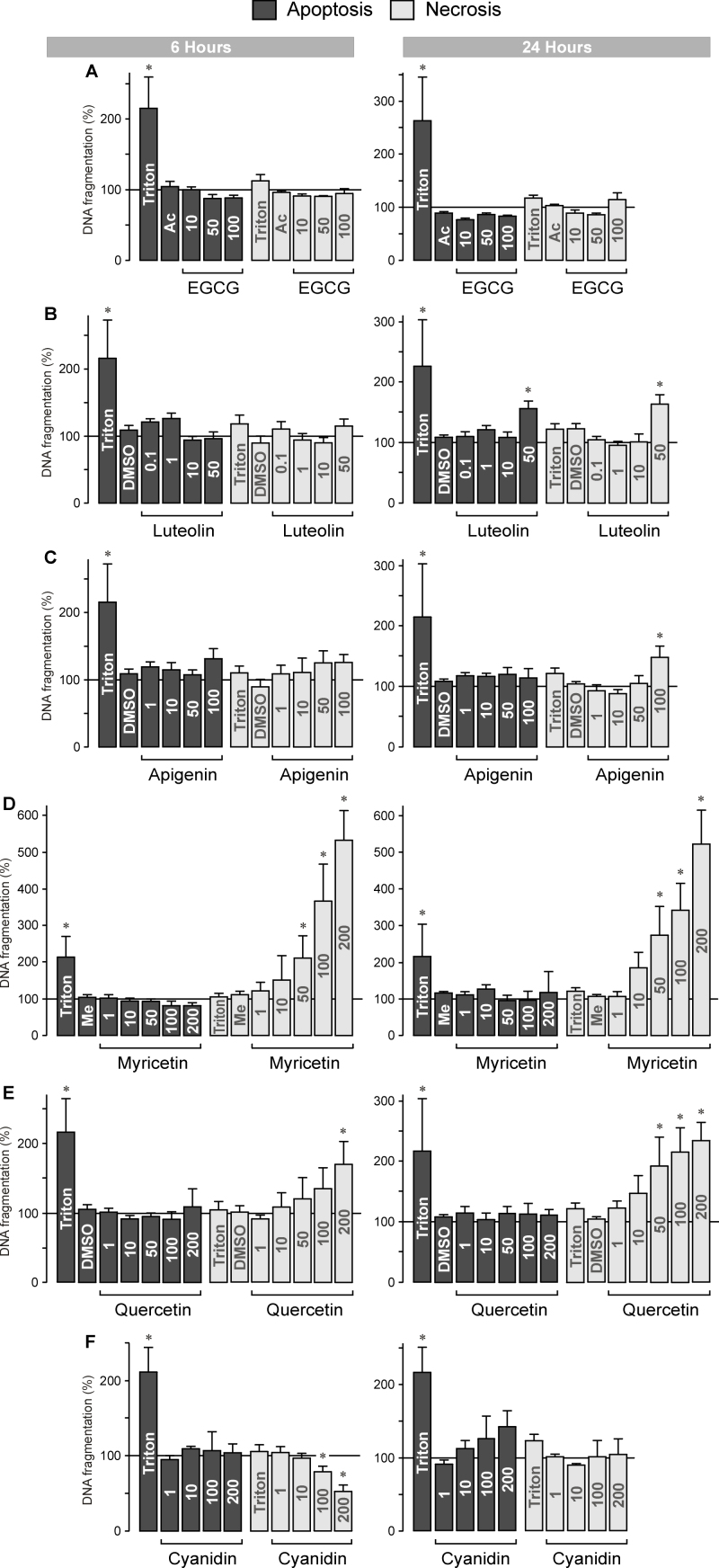
By measuring the internucleosomal DNA fragmentation rate in the cultured media and cell lysates, we determined whether the decrease in RPE cell viability induced by various flavonoids was mediated by induction of apoptosis and/or necrosis. An increased level of BrdU-labeled DNA fragments in the cell-free culture supernatants reflects cellular necrosis, while an increased level of BrdU-labeled DNA fragments in the cell lysates reflects apoptosis of the cells. Triton X-100 (1%) was used as the positive control. As previously described [27], Triton induced an increase in the DNA fragmentation rate in the RPE cell lysate after 6 and 24 h of stimulation, while the DNA fragmentation rate in the cultured media remained unchanged (Figure 7). The data suggest that Triton evoked apoptosis but not necrosis of RPE cells. The green tea catechin EGCG did not induce apoptosis or necrosis of RPE cells at both time periods investigated when tested at concentrations between 10 and 100 µM (Figure 7A). Luteolin (at 50 µM) induced significant (p<0.05) increases in the DNA fragmentation rates in the cell lysates and in the cultured media after 24 h of exposure (Figure 7B), suggesting that it induced apoptosis and necrosis of RPE cells. Apigenin (at 100 µM) induced necrosis but not apoptosis of RPE cells (Figure 7C). Myricetin did not induce apoptosis of RPE cells; however, it dose-dependently induced strong necrosis of the cells (Figure 7D). Similarly, quercetin induced dose-dependently necrosis of RPE cells, whereas it did not induce apoptosis (Figure 7E). Cyanidin did not induce apoptosis but decreased the rate of cellular necrosis after 6 h of exposure (Figure 7F).
Figure 7.
Dose-dependent effects of several vegetable polyphenols on the rate of DNA fragmentation of retinal pigment epithelial (RPE) cells. (A) epigallocatechin-3-gallate, (B) luteolin, (C) apigenin, (D) myricetin, (E) quercetin, and (F) cyanidin. The rate of DNA fragmentation was determined in the cell lysates (to determine the level of cellular apoptosis) and culture supernatants (to determine the level of cellular necrosis) after 6 h (left side) and 24 h of cell culturing (right side). Triton X-100 (1%) was used as positive control. The vehicle controls were made with acetone (Ac; 0.2%), dimethyl sulfoxide (DMSO; 0.2%), and methanol (Me; 0.4%), respectively. The concentrations of the test substances (in µM) are given in the bars. Data are means±standard error of the mean (SEM) of three to six independent experiments using cells from different donors, and are expressed in percent of untreated control (100%). Significant difference vs. untreated control: *p<0.05.
Mechanisms of myricetin-induced retinal pigment epithelial cell death
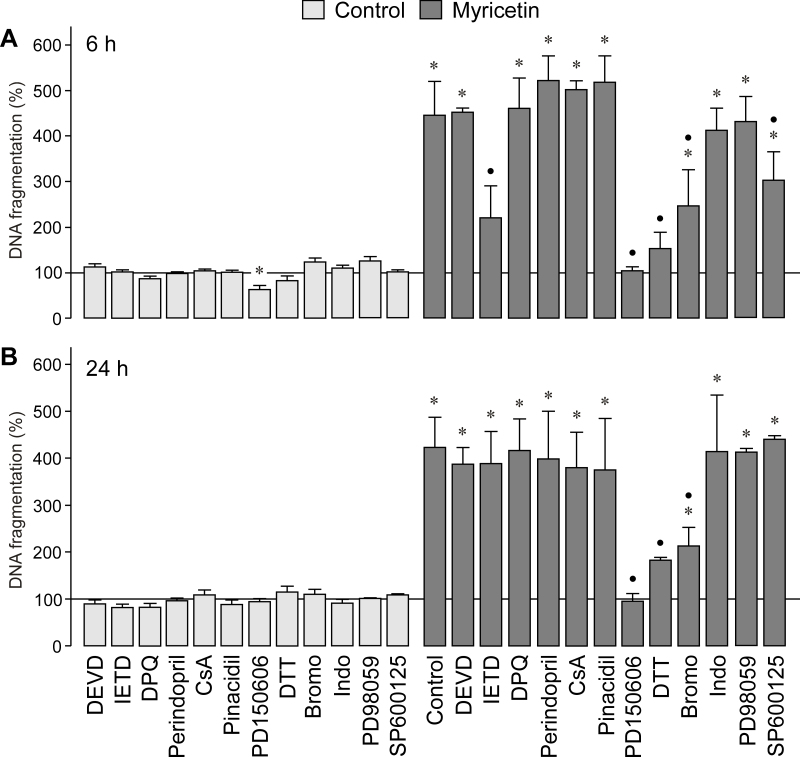
Myricetin induced severe necrosis of RPE cells (Figure 7D). To determine the molecular mechanisms of myricetin-induced cytotoxicity, we evaluated the rate of DNA fragmentation in the cultured media in the presence of different caspase inhibitors. As shown in Figure 8A and B, the selective inhibitor of the effector caspase-3, DEVD, did not alter the rate of RPE cell necrosis induced by myricetin. The inhibitor of caspase-8, IETD, inhibited in part myricetin-induced RPE cell necrosis after 6 h (Figure 8A), but not after 24 h of stimulation (Figure 8B).
Figure 8.
Mechanisms of myricetin-induced retinal pigment epithelial cell necrosis. The rate of DNA fragmentation in the cultured media was determined after stimulation of the cells with myricetin for 6 h (A) and 24 h (B), respectively. The following agents were tested in the absence (control) and presence of myricetin (200 µM): the caspase-3 and -8 specific inhibitors DEVD (100 μM) and IETD (100 µM), the poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinoline (DPQ; 30 µM), the inhibitor of the nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase pathway and the uncoupling protein-2/mitochondrial pathway, perindopril (4 µM), the inhibitor of the mitochondrial permeability transition cyclosporin A (CsA; 1 µM), the mitochondrial KATP channel opener pinacidil (10 µM), the calpain inhibitor PD150606 (100 µM), the reducing agent dithiothreitol (DTT; 3 mM), the inhibitor of phospholipase A2 4-bromophenacyl bromide (Bromo; 300 µM), the cyclooxygenase inhibitor indomethacin (Indo; 10 µM), the mitogen-activated protein kinase (MEK) inhibitor, PD98059 (20 µM), and the c-Jun NH2-terminal kinase (JNK) inhibitor, SP600125 (10 µM). Data are means±standard error of the mean (SEM) of three to six independent experiments using cells from different donors, and are expressed in percent of untreated control (100%). Significant difference vs. untreated control: *p<0.05. Significant difference vs. myricetin control: ●p<0.05.
The lack of an effect of the caspase-3 inhibitor suggests that the myricetin-induced cytotoxicity is mediated by caspase 3-independent necrotic death pathways. An important activator of the caspase-independent cell death is the mitochondrial flavoprotein apoptosis-induced factor, which mediates chromatin condensation and DNA fragmentation when translocated to the nucleus [28]. The release of the apoptosis-induced factor from the mitochondria can be triggered by different mechanisms, including activation of poly(ADP-ribose) polymerase-1 (PARP-1). To determine whether activation of this nuclear enzyme participates in myricetin-induced RPE cell death, we used the selective PARP-1 inhibitor DPQ. DPQ did not prevent RPE cell necrosis induced by myricetin (Figure 8A,B). Concomitant inhibition of PARP-1 and caspase-3 using DPQ plus DEVD did also not block myricetin-induced RPE cell death (not shown). Similarly, the inhibitor of the NADPH oxidase pathway and the uncoupling protein-2/mitochondrial pathway, perindopril, and the inhibitor of mitochondrial permeability transition, cyclosporin A, displayed no effects (Figure 8A,B). In addition, the mitochondrial KATP channel opener pinacidil had no effect (Figure 8A,B). The data suggest that activation of the mitochondrial apoptotic pathway is not involved in mediating the cytotoxic effect of myricetin.
Another mechanism that could contribute to the myricetin-induced cytotoxicity is activation of the cysteine protease calpain. Calpain activation has been recently implicated in the toxic effect of curcumin in RPE cells [19]. Pretreatment of RPE cells with the calpain inhibitor PD150606 (which prevents the binding of calcium to calpain and does not significantly inhibit cathepsins and caspases [29]) fully abrogated the RPE cell necrosis induced by myricetin (Figure 8A,B). The data suggest that calpain activation is involved in mediating the toxic effect of myricetin. Activation of apoptotic pathways [30] and the excitotoxic death of oligodendrocytes [31] were shown to depend on the generation of reactive oxygen species. Preincubation of the cells with the cell-permeable dithiol-reducing agent dithiothreitol significantly (p<0.05) reduced the rate of RPE cell necrosis induced by myricetin (Figure 8A,B). The data suggest that generation of free oxygen radicals contributes to the myricetin-induced cytotoxicity. We found also that the inhibitor of phospholipase A2, 4-bromophenacyl bromide, reduced the cytotoxic effect of myricetin (Figure 8A,B). However, the cyclooxygenase inhibitor indomethacin had no effect (Figure 8A,B). Furthermore, inhibition of ERK1/2 activation with the specific MAPK kinase (MEK) antagonist PD98059 did not suppress the myricetin-induced DNA fragmentation (Figure 8A,B). Inhibition of c-Jun NH2-terminal kinase (JNK) activation by SP600125 reduced slightly the DNA fragmentation rate after 6 h (Figure 8A), but not after 24 h of myricetin stimulation (Figure 8B).
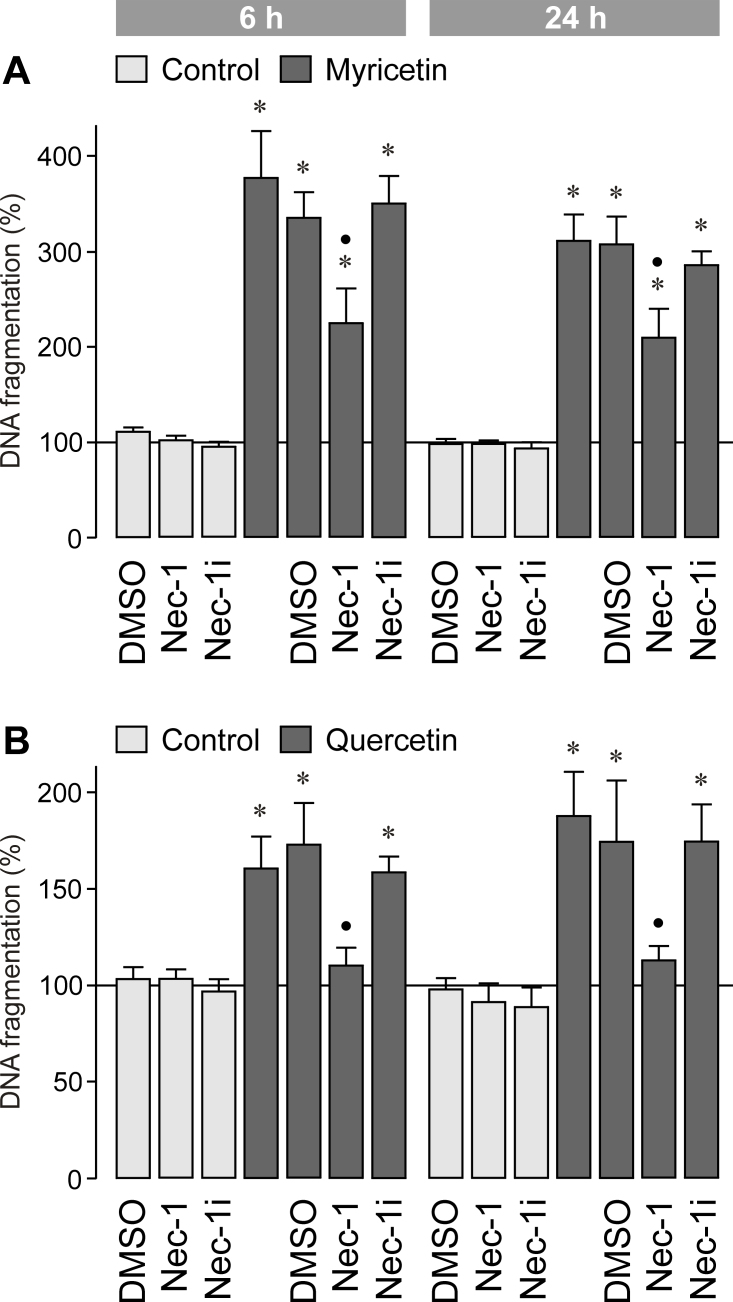
To determine whether the myricetin-induced cytotoxicity is mediated by programmed necrosis, we tested the inhibitor of necroptosis, necrostatin-1 [32]. As shown in Figure 9A, necrostatin-1 decreased significantly (p<0.05) the myricetin-induced increase in the DNA fragmentation rate of the cultured media. However, the inactive derivative of necrostatin-1 [32] had no effect (Figure 9A). We also found that the quercetin-induced increase in the DNA fragmentation rate of the cultured media is largely inhibited by necrostatin-1 (Figure 9B). The data suggest that the myricetin-induced RPE cell necrosis is mainly mediated by activation of death pathways that involve generating free oxygen radicals and activating calpain and phospholipase A2, as well as activating caspase-8 and JNK in the early phase of cell death. The myricetin-induced cytotoxicity is in part mediated by the induction of programmed necrosis.
Figure 9.
Effect of the inhibitor of programmed necrosis, necrostatin-1 (Nec-1; 30 µM), on the DNA fragmentation rate in the cultured media of retinal pigment epithelial (RPE) cells. The cells were cultured for 6 and 24 h in the presence of myricetin (200 µM; A) and quercetin (200 µM; B), respectively. As negative control, inactive necrostatin-1 (Nec-1i; 30 µM) was tested. The vehicle control was made with dimethyl sulfoxide (DMSO; 0.2%). Data are means±standard error of the mean (SEM) of three to seven independent experiments using cells from different donors, and are expressed in percent of untreated control (100%). Significant difference vs. untreated control: *p<0.05. Significant difference vs. quercetin and myricetin control, respectively: ●p<0.05.
Activation of intracellular signal transduction proteins
We found that various bioflavonoids decrease the proliferation (Figure 3B–F), migration (Figure 4B–F), and viability of RPE cells (Figure 5B–E). Therefore, we determined with western blotting analysis whether the flavonoids alter the phosphorylation levels of three major ligand-induced signal transduction pathways. Activation of the Ras-Raf-ERK MAPK pathway is an important step in intracellular signaling that stimulates the proliferation of RPE cells [26,33]. Activation of p38 MAPK is implicated in stimulation of cellular migration [26], while activation of the phosphatidylinositol-3 kinase-Akt signaling pathway stimulates the protein synthesis at the translational level required for cell growth and survival [34]. As shown in Figure 10A, the green tea catechin EGCG did not induce significant alterations in the phosphorylation levels of ERK1/2, p38 MAPK, and Akt in RPE cells when the cells were stimulated for 15 min and the compound was tested at concentrations between 1 and 50 µM. However, PDGF induced increases in the phosphorylation levels of the proteins (Figure 10A), as previously described [26]. Luteolin (Figure 10B), apigenin (Figure 10C), myricetin (Figure 10D), quercetin (Figure 10E), and cyanidin (Figure 10F) induced decreases in the phosphorylation level of ERK1/2 at higher concentrations.
Figure 10.
Dose-dependent effects of several vegetable polyphenols on the phosphorylation levels of extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, and Akt protein in retinal pigment epithelial (RPE) cells. (A) epigallocatechin-3-gallate, (B) luteolin, (C) apigenin, (D) myricetin, (E) quercetin, and (F) cyanidin. Platelet-derived growth factor (PDGF; 10 ng/ml) was used as the positive control. The vehicle controls were made with acetone (Ac; 0.1%) and dimethyl sulfoxide (DMSO; 0.1%), respectively. The cultures were stimulated for 15 min. Amounts of phosphorylated proteins are shown above; amounts of total proteins are shown below. Similar results were obtained in three independent experiments.
With the exception of cyanidin, the bioflavonoids tested did not alter the phosphorylation level of p38 MAPK in RPE cells (Figure 10A–E). Cyanidin decreased the phosphorylation level of p38 MAPK at higher doses (Figure 10F). With the exception of EGCG (Figure 10A), all flavonoids tested decreased dose-dependently the phosphorylation level of Akt protein (Figure 10B–F).
Discussion
Vegetable polyphenols are suggested to represent promising drugs for the supplemental therapy of cancer in particular because of their capability to induce apoptosis in tumor cells [4,5]. Bioflavonoids may have also benefits in treating retinal diseases [12-16]. However, an excessive intake of bioflavonoids may have adverse effects that may concern non-transformed cells [18]. In RPE cells, for example, curcumin and other flavonoids were shown to have cytotoxic effects and may induce apoptosis and necrosis [19,20]. Therefore, excess intake of bioflavonoids, either as supplemental cancer therapy or in the treatment of retinal diseases, may have adverse effects on the retina. In the present study, we compared the effects of various vegetable polyphenols in cultured human RPE cells. We found that the various flavonoids differentially affect the physiologic properties of RPE cells including the secretion of VEGF and cellular proliferation and migration, and that some of the compounds tested (luteolin, apigenin, myricetin, quercetin) decreased dose-dependently the viability of the cells (Figure 5) and induced apoptosis and/or necrosis (Figure 7). Overall, the effects of the compounds on the physiologic parameters were observed at concentrations lower than doses that induced a decrease in cell viability. However, the concentration windows between the beneficial and detrimental effects depended on the compound investigated and were larger in the case of apigenin and smaller in the case of myricetin, for example. We assume that the polyphenols tested at low concentrations have beneficial effects, as suggested by the VEGF-decreasing effect and the inhibition of cellular migration and proliferation, and at higher concentrations, the polyphenols (with the exception of EGCG and cyanidin) have toxic effects on the cells. However, further experiments are required to support this assumption.
EGCG and cyanidin did not significantly affect the viability of RPE cells (Figures 5A,F and Figure 6B) and did not induce apoptosis or necrosis (Figure 7A,F) at the concentrations tested. EGCG decreased the secretion of VEGF under conditions of chemical hypoxia, but not under control and PDGF-stimulated conditions (Figure 1A). EGCG did not prevent the proliferation and migration induced by PDGF (Figure 3A and Figure 4A). PDGF-induced cellular signaling is a major causative factor of proliferative retinopathies [35,36]. However, cyanidine inhibited the release of VEGF (Figure 1F and Figure 6A), the proliferation and migration of RPE cells (Figure 3F and Figure 4F), and the cellular necrosis (Figure 7F) without a significant reduction in the cell viability (Figure 5F and Figure 6B). The data suggest that EGCG and in particular cyanidin may have certain benefits to prevent RPE cell responses characteristically for neovascular and proliferative retinopathies.
Among the compounds tested, myricetin and quercetin induced strong increases in the rate of DNA fragmentation in the culture supernatants at relatively low concentrations (Figure 7D,E). Data obtained with pharmacological inhibitors (Figure 8A,B) suggest that the toxic effect of myricetin in RPE cells (Figure 5D) was mainly mediated by non-apoptotic death modes such as classical and/or programmed necrosis [37]. Various indications suggest that activating apoptotic pathways did not significantly contribute to the toxic effect of myricetin in RPE cells: (1) We did not find any myricetin-induced increase in the DNA fragmentation rate in the cell lysates (Figure 7D). (2) The caspase-3 inhibitor DEVD did not prevent the myricetin-induced increase in the DNA fragmentation rate in the cultured media (Figure 8A,B). (3) The inhibitor of the mitochondrial permeability transition, cyclosporin A, had no effect on the myricetin-induced increase in the DNA fragmentation rate (Figure 8A,B). The independence of the myricetin-induced cell death from the mitochondrial apoptotic pathway is further suggested by the facts that the PARP-1 inhibitor DPQ, the inhibitor of the NADPH oxidase pathway and the uncoupling protein-2/mitochondrial pathway, perindopril, and the mitochondrial KATP channel opener pinacidil had no effects (Figure 8A,B). However, the contribution of distinct apoptotic pathways, at least of signaling steps upstream from the effector caspase-3, cannot be fully ruled out because the caspase-8 inhibitor IETD and the JNK inhibitor SP600125 decreased the DNA fragmentation rate in the culture supernatants after 6 h of stimulation with myricetin (Figure 8A). Prolonged activation of the stress-activated JNK was shown to be an important factor in apoptotic and necrotic cell death [37].
We found that the toxic effect of myricetin was mediated by induction of oxidative stress and activation of calpains and phospholipase A2 (Figure 8A,B). Reactive oxygen species are known to induce apoptosis and necrosis [37]. They may contribute to JNK activation [37] which stimulates the mitochondrial production of superoxide [38], the main source of cellular oxidative stress involved in inducing necrosis [37]. Increased levels of reactive oxygen species are known to activate calpains likely by increasing the intracellular free calcium level [39-41]. Sustained activation of calpain is known to trigger various intracellular signaling processes that lead to progressive plasma membrane damage, a hallmark of necrosis [42,43]. Activated calpains may also induce lysosome destabilization [43], at least in part by inducing mitochondrial permeability transition [44]. However, because the inhibitor of mitochondrial permeability transition, cyclosporin A, did not prevent myricetin-induced RPE cell necrosis (Figure 8A,B), it seems to be unlikely that myricetin induces a rupture of lysosomes in RPE cells via this pathway. There are various necrotic pathways, including programmed necrosis, in which caspase-8 and calpains play a role [45]. Caspase-8 is a subunit of the ripoptosome involved in inducing programmed necrosis [46,47]. We found that the inhibitor of programmed necrosis, necrostatin-1, decreased the myricetin- (Figure 9A) and quercetin- (Figure 9B) induced increase in the DNA fragmentation rate of the cultured media. Because necrostatin-1 does not block “classic” oxidative stress-induced necrosis [32], the data may suggest that the cytotoxicity induced by both compounds is in part mediated by inducing programmed necrosis. However, the contribution of various apoptotic and/or necrotic pathways to myricetin-induced RPE cell death remains to be better clarified in future experiments.
Further mechanisms may contribute to the induction of RPE cell necrosis by myricetin. By degrading the anchorage to the membrane cytoskeleton, activated calpains may impair the activity of the Na,K-ATPase [48]. The activity of phospholipase A2 is increased in response to oxidative stress, resulting in lipid peroxidation and release of arachidonic acid [49]. Arachidonic acid is a potent inhibitor of the Na,K-ATPase that leads to intracellular sodium overload, influx of water with consecutive cellular swelling, and possibly membrane rupture [50,51]. The inhibition of ERK1/2 and Akt activation (Figure 10B-F) may contribute to the decreases in RPE cell proliferation (Figure 3B-F) and survival (Figure 5B-E) induced by various flavonoids.
Because low concentrations of dietary flavonoids (up to 10 µM) did not decrease the viability of the cells (Figure 5) and did not induce significant apoptosis or necrosis (Figure 7), we assume that normal and moderate intake of the compounds as natural food will have no deleterious consequences in the RPE, and may have even beneficial effects, for example, in the cases of EGCG and cyanidin. Further experiments are required to compare the in vivo bioavailability and retinal effects of different vegetable polyphenols. However, it cannot be ruled out that increased intake of distinct flavonoids as dietary supplement in the (self-) therapy of cancer, for example, may have adverse effects on the retina resulting in dysregulation and degeneration of the RPE, in particular in subjects with decreased levels of antioxidant enzymes. To avoid accelerated development of age-related macular degeneration, which is characterized by dysfunction and degeneration of RPE cells, the intake of flavonoids at higher doses as supplemental cancer therapy or in the treatment of retinal diseases should be accompanied by careful monitoring of the retinal function. Possible beneficial effects of EGCG and cyanidin, which had little effects on the RPE cell viability in the concentration range investigated (Figure 5), in treating retinal diseases should be further examined.
Acknowledgments
The authors thank Ute Weinbrecht for excellent technical support. This work was supported by grants from the Deutsche Forschungsgemeinschaft (KO 1547/7–1 to L.K; GRK 1097/1, RE 849/16–1, to A.R.) and the Geschwister Freter Stiftung (Hannover, Germany).
References
- 1.Hadi SM, Asad SF, Singh S, Ahmad A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life. 2000;50:167–71. doi: 10.1080/152165400300001471. [DOI] [PubMed] [Google Scholar]
- 2.Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76:1044–63. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- 3.González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104:S15–27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 5.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 7.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Rev Endocr Metab Disord. 2008;9:315–27. doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 8.Usui S, Oveson BC, Lee SY, Jo YJ, Yoshida T, Miki A, Miki K, Iwase T, Lu L, Campochiaro PA. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009;110:1028–37. doi: 10.1111/j.1471-4159.2009.06195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda T, Ueda T, Armstrong D. Preventive effect of natural and synthetic antioxidants on lipid peroxidation in the mammalian eye. Ophthalmic Res. 1996;28:184–92. doi: 10.1159/000267901. [DOI] [PubMed] [Google Scholar]
- 10.Chida M, Suzuki K, Nakanishi-Ueda T, Ueda T, Yasuhara H, Koide R, Armstrong D. In vitro testing of antioxidants and biochemical end-points in bovine retinal tissue. Ophthalmic Res. 1999;31:407–15. doi: 10.1159/000055565. [DOI] [PubMed] [Google Scholar]
- 11.Laabich A, Manmoto CC, Kuksa V, Leung DW, Vissvesvaran GP, Karliga I, Kamat M, Scott IL, Fawzi A, Kubota R. Protective effects of myricetin and related flavonols against A2E and light mediated-cell death in bovine retinal primary cell culture. Exp Eye Res. 2007;85:154–65. doi: 10.1016/j.exer.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Rusciano D, Osborne NN. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 2008;1198:141–52. doi: 10.1016/j.brainres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Costa BL, Fawcett R, Li GY, Safa R, Osborne NN. Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull. 2008;76:412–23. doi: 10.1016/j.brainresbull.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Peng PH, Ko ML, Chen CF. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp Eye Res. 2008;86:637–46. doi: 10.1016/j.exer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Park SW, Cho CS, Jun HO, Ryu NH, Kim JH, Yu YS, Kim JS, Kim JH. Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Invest Ophthalmol Vis Sci. 2012;53:7718–26. doi: 10.1167/iovs.11-8790. [DOI] [PubMed] [Google Scholar]
- 16.Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, Lopes de Faria JB, Lopes de Faria JM. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:1325–36. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 17.Galati G, Sabzevari O, Wilson JX, O'Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 18.Burgos-Morón E, Calderón-Montaño JM, Salvador J, Robles A, López-Lázaro M. The dark side of curcumin. Int J Cancer. 2010;126:1771–5. doi: 10.1002/ijc.24967. [DOI] [PubMed] [Google Scholar]
- 19.Hollborn M, Chen R, Wiedemann P, Reichenbach A, Bringmann A, Kohen L. Cytotoxic effects of curcumin in human retinal pigment epithelial cells. PLoS ONE. 2013;8:e59603. doi: 10.1371/journal.pone.0059603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alex AF, Spitznas M, Tittel AP, Kurts C, Eter N. Inhibitory effect of epigallocatechin gallate (EGCG), resveratrol, and curcumin on proliferation of human retinal pigment epithelial cells in vitro. Curr Eye Res. 2010;35:1021–33. doi: 10.3109/02713683.2010.506970. [DOI] [PubMed] [Google Scholar]
- 21.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 22.Roth F, Bindewald A, Holz FG. Keypathophysiologic pathways in age-related macular disease. Graefes Arch Clin Exp Ophthalmol. 2004;242:710–6. doi: 10.1007/s00417-004-0976-x. [DOI] [PubMed] [Google Scholar]
- 23.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–63. [PubMed] [Google Scholar]
- 24.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 25.Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–8. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollborn M, Bringmann A, Faude F, Wiedemann P, Kohen L. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem Biophys Res Commun. 2006;344:912–9. doi: 10.1016/j.bbrc.2006.03.185. [DOI] [PubMed] [Google Scholar]
- 27.Hollborn M, Wiedemann P, Bringmann A, Kohen L. Chemotactic and cytotoxic effects of minocycline on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:2721–9. doi: 10.1167/iovs.09-4661. [DOI] [PubMed] [Google Scholar]
- 28.Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci. 2004;25:259–64. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Wang KK, Nath R, Posner A, Raser KJ, Buroker-Kilgore M, Hajimohammadrezal I, Probert AWJ, Marcoux FW, Ye Q, Takano E, Hatanaka M, Maki M, Caner H, Collins JL, Fergus A, Lee KS, Lunney EA, Hays SJ, Yuen P. An α-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci USA. 1996;93:6687–92. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci. 2009;10:481–94. doi: 10.1038/nrn2665. [DOI] [PubMed] [Google Scholar]
- 31.Liu HN, Giasson BI, Mushynski WE, Almazan G. AMPA receptor-mediated toxicity in oligodendrocyte progenitors involves free radical generation and activation of JNK, calpain and caspase 3. J Neurochem. 2002;82:398–409. doi: 10.1046/j.1471-4159.2002.00981.x. [DOI] [PubMed] [Google Scholar]
- 32.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 33.Hecquet C, Lefevre G, Valtink M, Engelmann K, Mascarelli F. Activation and role of MAP kinase-dependent pathways in retinal pigment epithelial cells: ERK and RPE cell proliferation. Invest Ophthalmol Vis Sci. 2002;43:3091–8. [PubMed] [Google Scholar]
- 34.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–50. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 35.Andrews A, Balciunaite E, Leong FL, Tallquist M, Soriano P, Refojo M, Kazlauskas A. Platelet-derived growth factor plays a key role in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1999;40:2683–9. [PubMed] [Google Scholar]
- 36.Ikuno Y, Leong F-L, Kazlauskas A. Attenuation of experimental proliferative vitreoretinopathy by inhibiting the platelet-derived growth factor receptor. Invest Ophthalmol Vis Sci. 2000;41:3107–16. [PubMed] [Google Scholar]
- 37.Morgan MJ, Liu Z-G. Programmed cell death with a necrotic-like phenotype. BioMol Concepts. 2013;4:259–75. doi: 10.1515/bmc-2012-0056. [DOI] [PubMed] [Google Scholar]
- 38.Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem. 2011;286:16052–62. doi: 10.1074/jbc.M111.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanvicens N, Gómez-Vicente V, Masip I, Messeguer A, Cotter TG. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J Biol Chem. 2004;279:39268–78. doi: 10.1074/jbc.M402202200. [DOI] [PubMed] [Google Scholar]
- 40.Weber H, Huhns S, Luthen F, Jonas L, Schuff-Werner P. Calpain activation contributes to oxidative stress-induced pancreatic acinar cell injury. Biochem Pharmacol. 2005;70:1241–52. doi: 10.1016/j.bcp.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med. 2009;46:51–61. doi: 10.1016/j.freeradbiomed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Schnellmann RG. Calpain mediates progressive plasma membrane permeability and proteolysis of cytoskeleton-associated paxillin, talin, and vinculin during renal cell death. J Pharmacol Exp Ther. 2003;304:63–70. doi: 10.1124/jpet.102.043406. [DOI] [PubMed] [Google Scholar]
- 43.Harwood SM, Yaqoob MM, Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem. 2005;42:415–31. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- 44.Yang YT, Whiteman M, Gieseg SP. HOCl causes necrotic cell death in human monocyte derived macrophages through calcium dependent calpain activation. Biochim Biophys Acta. 2012;xxx:420–9. doi: 10.1016/j.bbamcr.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012;27:3412–9. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 46.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Häcker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J. Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ Res. 2005;97:465–73. doi: 10.1161/01.RES.0000181170.87738.f3. [DOI] [PubMed] [Google Scholar]
- 49.Balboa MA, Balsinde J. Oxidative stress and arachidonic acid mobilization. Biochim Biophys Acta. 2006;1761:385–91. doi: 10.1016/j.bbalip.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Lees GJ. Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Brain Res Rev. 1991;16:283–300. doi: 10.1016/0165-0173(91)90011-v. [DOI] [PubMed] [Google Scholar]
- 51.Staub F, Winkler A, Peters J, Kempski O, Kachel V, Baethmann A. Swelling, acidosis, and irreversible damage of glial cells from exposure to arachidonic acid in vitro. J Cereb Blood Flow Metab. 1994;14:1030–9. doi: 10.1038/jcbfm.1994.135. [DOI] [PubMed] [Google Scholar]