Highlights
-
•
Potassium (K+) channels are crucial determinants of neuronal excitability.
-
•
Nerve injury or inflammation alters K+ channel activity in neurons of the pain pathway.
-
•
These changes can render neurons hyperexcitable and cause chronic pain.
-
•
Therapies targeting K+ channels may provide improved pain relief in these states.
Keywords: potassium channel, pain, dorsal root ganglia, pharmacotherapy
Abstract
Chronic pain is associated with abnormal excitability of the somatosensory system and remains poorly treated in the clinic. Potassium (K+) channels are crucial determinants of neuronal activity throughout the nervous system. Opening of these channels facilitates a hyperpolarizing K+ efflux across the plasma membrane that counteracts inward ion conductance and therefore limits neuronal excitability. Accumulating research has highlighted a prominent involvement of K+ channels in nociceptive processing, particularly in determining peripheral hyperexcitability. We review salient findings from expression, pharmacological, and genetic studies that have untangled a hitherto undervalued contribution of K+ channels in maladaptive pain signaling. These emerging data provide a framework to explain enigmatic pain syndromes and to design novel pharmacological treatments for these debilitating states.
The problem of chronic pain
Chronic pain afflicts one in five adults in Europe and many diseases accompanied by pain are on the rise [1]. The diverse etiology of chronic pain encompasses trauma, metabolic or autoimmune disorders, infection, anti-retroviral treatment, and chemotherapy. Affected individuals typically report a combination of incapacitating sensory abnormalities, including spontaneous pain, hypersensitivity to stimulation, dysesthesias, and paresthesias. Despite significant progress, chronic pain remains refractory to treatment, with only one-third to two-thirds of patients reporting adequate (>50%) pain relief [1]. Moreover, our first-line drugs, non-steroidal anti-inflammatory agents (NSAIDS; e.g., aspirin) and opioids (e.g., morphine), are associated with adverse dose-limiting side-effects, dependence, and tolerance [2]. The lack of improved treatment reflects our incomplete understanding of the molecular pathophysiology underlying these pain states.
Nociceptive pathways
Pain is usually triggered by the activity of specialized damage-sensing neurons innervating the limbs and torso, whose cell somata cluster paraspinally in the dorsal root ganglion (DRG). These pseudo-unipolar cells project axons that bifurcate into peripheral fibers innervating the skin, muscle, or other organs, and central fibers that synapse with second-order spinal cord neurons. A similar architecture is encountered in trigeminal ganglion neurons located on each side of the cranium, which transduce sensory information from the face. Based on anatomical, neurochemical, and functional attributes, sensory neurons are distinguished into small-diameter with unmyelinated C-fibers, medium-diameter with thinly myelinated Aδ-fibers, and large-diameter that principally give rise to heavily myelinated Aβ-fibers. Because of their ability to encode noxious mechanical, thermal, or chemical stimuli, C- and Aδ-fibers are considered the main nociceptive afferents signaling pain. Aβ-fibers innervating the skin or muscles are predominantly low-threshold mechanoreceptive afferents responding to light touch or pressure, although a proportion are also activated by high-threshold stimuli. Signals initiated at sensory endings are relayed to the dorsal horn of the spinal cord and subsequently the brain via spinal projection systems including the spinothalamic tract, where the information is evaluated and an appropriate response generated. Spinal transmission is not a passive process but rather involves regulatory spinal processing, such as facilitatory or inhibitory modulation by interneurons, astroglia, and descending pathways, which can robustly increase or decrease the output [3].
Under normal conditions, generation of action potentials (APs) in sensory nerves typically originates at their peripheral nerve endings in the presence of a suprathreshold stimulus activating specialized receptors. However, following nerve trauma, electrogenesis can occur spontaneously at the site of injury (neuroma), DRG cell body, or even mid-nerve [4]. Furthermore, inflammation and neuropathic lesions are linked to enhanced responsiveness to supra- or even subthreshold stimulation 5, 6, 7. This hyperexcitability is thought to be a major driver of pain and is ascribed to injury-induced reorganization of membrane ion channels, which are the principal determinants of AP generation and propagation. These maladaptive changes also have downstream effects at the spinal level; C-fiber activity can induce central sensitization, a state of heightened responsiveness of spinal cord neurons, such that innocuous input can now result in abnormally painful responses (e.g., tactile allodynia after Aβ-fiber stimulation) [8]. In addition, lesioned Aβ-fibers can acquire de novo nociceptive qualities that may also contribute to central sensitization [9].
Until recently the search for ion channel correlates of pathological excitability primarily focused on sodium and calcium channels. Unfortunately, despite significant discoveries in acute and inflammatory pain, no decisive involvement has been definitely established yet, particularly in neuropathic pain [10]. New evidence however suggests a previously unappreciated contribution of K+ channels in chronic pain processing, which we review here.
K+ channels and pain signaling
K+ channels are the most populous, widely distributed, and diverse class of ion channels in neurons, governed by some 78 genes in humans [11]. Upon activation, K+ channels facilitate an extremely rapid transmembrane K+ efflux that can influence AP threshold, waveform and frequency. Because K+ channel opening repolarizes (or even hyperpolarizes) the neuronal membrane, this function can limit AP generation and firing rate.
Depending on the biophysical profile and precise subcellular localization in sensory neurons, K+ channel conduction is postulated to inhibit peripheral excitability by counteracting AP initiation at peripheral nerve terminals, reducing conduction fidelity across the axon, or limiting neurotransmitter release at central terminals (Figure 1). In addition, although normal sensory transduction does not rely on cell soma spiking, in chronic pain states K+ channels could provide a brake to the spontaneous activity developing in the DRG soma or other ectopic loci (e.g., the neuroma). Indeed, peripheral application of K+ channel openers on the cell body or terminals invariably decreases DRG excitability, whereas K+ channel blockers augment firing 5, 11, 12, 13. In the CNS, K+ channel opening could conceptually lead to enhanced nociception, for instance if the affected neuron participates in an inhibitory circuit. Nevertheless, the available data so far indicate that a variety of antinociceptive drugs mediate their action by directly opening spinal K+ channels [11].
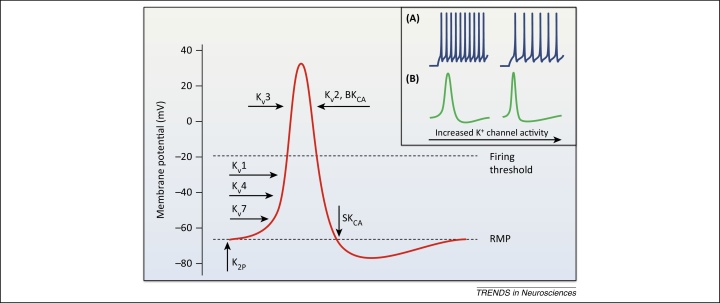
Figure 1.
Potassium channel activation during action potential (AP) firing in sensory neurons. A depiction of the sequential engagement of different K+ channels during neuronal activity, and typical effects of K+ channel opening on AP waveform and frequency (inset). The resting membrane potential (RMP) is primarily stabilized by two-pore K+ (K2P) channels and Kv7 background conductance, whereas KATP channels may also contribute in large neurons [95]. Basal excitability is also influenced by the opening of low-threshold Kv1 and Kv4 channels which filter out small depolarizations and therefore control the number of triggered APs. Kv4 channels are normally inactivated at RMP and require prior hyperpolarization (achieved during AP generation) to remove this steady-state inactivation. Once activated, however, Kv4 and other A-type channels may modulate firing threshold as well as repetitive spiking rate owing to their very fast kinetics [inset (A)] [47]. Following suprathreshold stimulation and initiation of an AP, high-threshold Kv3 channels open to limit AP duration and ensure quick recovery of voltage-gated Na+ channels from inactivation [inset (B)]. Kv2 channels are also high-threshold but with much slower activation and inactivation kinetics; they mainly contribute to the repolarizing/after-hyperpolarizing phases and are hence important for regulating interspike interval and conduction fidelity during sustained stimulation [inset (B)] [38]. Upon neuronal activity, Ca2+-activated K+ channels are engaged during repolarization (BKCA) and after-hyperpolarization (SKCA) to provide feedback inhibition at nerve terminals by restricting AP duration and thus neurotransmitter release [(inset (B)]. It is emphasized that this schematic is a simplified representation of most prominent K+ channel contributions to AP firing, based on in vitro assessment of recombinant counterparts. In vivo, however, the oligomeric composition, association with auxiliary proteins, post-translational modifications, and regulation by intracellular messengers can yield divergent biophysical properties. K+ channel opening will also have concurrent effects on the function of other ion channels, for instance by affecting their inactivation. For this reason the combined effect on firing behavior in a physiological context is often hard to predict. Abbreviations: BKCA, big conductance and SKCA, small conductance Ca2+-activated K+ channels.
Based on structural and physiological characteristics, K+ channels are organized into four distinct groups: voltage-gated, two-pore, calcium-activated, and inward rectifying, which we discuss in turn below.
Voltage-gated K+ channels (Kv)
The Kv superfamily is the most numerous among K+ channels, comprising of 40 genes in humans 14, 15, 16. They are further classified in 12 families of α subunits that can interact to form functional homo- or hetero-tetrameric channels. Members of Kv1-Kv4, Kv7 and Kv10-Kv12 are pore-forming subunits, whereas Kv5, Kv6, Kv8, and Kv9 members do not form conducting channels unless associated with pore-forming subunits (Box 1). Channel tetramerization leads to tremendous functional diversity, further elevated by association with auxiliary β subunits, splice variants, and post-translational modifications.
The largely overlapping pharmacology in neurons suggests a spectrum of Kv currents rather than fixed groups, reflecting the variant heterotetrameric composition, functional redundancy within families, and complex regulation. The majority of Kv channels are delayed rectifiers, because they are activated slowly to counteract (rectify) depolarization. On the basis of biophysical properties and sensitivity to tetraethylammonium (TEA), α-dendrotoxin, 4-aminopyridine, and muscarinic agonists, Kv currents are broadly distinguished into sustained delayed rectifying (IK), transient slowly inactivating (ID), transient fast-inactivating (IA) and non-inactivating (IM) that, as their names suggest, exhibit different kinetics. Although this classification is an oversimplification, it has value as a starting point to examine the different Kv components in physiological systems.
These typical currents are also present in dorsal root and trigeminal ganglia neurons, whereas Gold and colleagues described six distinct K+ currents, three of which in small nociceptors 17, 18, 19, 20. Although it has been known for some time that nerve injury results in a dramatic decrease in K+ conductance of peripheral nerves that correlates with the emergence of hyperexcitability and pain behaviors, it was not until recently that specific subunits were linked to these changes [21].
Kv1.1 and Kv1.2 are delayed rectifiers activated by modest membrane depolarizations, and mainly contribute to the ID current. In many CNS neurons, these channels are preferentially localized at the axon initial segment (AIS, the site of AP initiation in CNS neurons) where they regulate AP threshold and firing rates, as well as nerve terminals where they modulate neurotransmitter release by controlling AP invasion in axonal branches 22, 23. The dominant role of Kv1 becomes apparent in type 1 episodic ataxia, where Kv1.1 mutations drive excitability changes in the cerebellum that cause severe seizures and premature death [24].
In the peripheral nervous system (PNS), Kv1.1 and Kv1.2 are predominantly found in the soma and juxtaparanodes of medium-large DRG neurons, often in heterotetramers [25], and are largely decreased after axotomy 26, 27; this may contribute to the hyperexcitable phenotype. Indeed, Kv1.1 loss-of-function results in reduced firing thresholds, attenuated mechanical and heat pain, and increased sensitivity in both phases of the formalin test 28, 29. By contrast, diminished Kv1.2 activity contributes to mechanical and cold neuropathic pain by depolarizing the resting membrane potential (RMP), reducing threshold current, and augmenting firing rates in myelinated neurons [30]. Moreover, Hao et al. recently reported that Kv1.1 tetramers form a bona fide mechanosensor that acts as an excitability brake in Aβ-mechanoreceptors of mouse DRG, with a minor contribution of Kv1.2 [31]. Interestingly, this mechanosensitive current was also detected in some high-threshold C-mechano-nociceptors (C-HTMRs). Although the literature highlights predominant Kv1.1 expression in myelinated neurons, the authors confirmed the presence of Kv1.1 subunits in a subpopulation of capsaicin-insensitive small neurons and C-fiber terminals in the skin using a monoclonal antibody [30]. This pattern may correspond to the occasional expression Rasband et al. documented in small DRG neurons from rat [25]. Although species differences may account for the discrepancy (and multiple species variations are recognized), other studies implementing molecular, immunohistological, and electrophysiological techniques have also indicated presence of Kv1.1 subunits in rat small sensory neurons 26, 29, 32, 33. Intriguingly, an accumulating body of research indicates that some human neuropathic pain syndromes are caused by production of autoimmune antibodies against Kv1 subunits that disrupt normal A- or C-fiber function (Box 1).
Box 1. Pain syndromes associated with autoimmune Kv antibodies.
Compelling evidence suggests that several neurological disorders linked to peripheral hyperexcitability and pain of a neuropathic nature, such as neuromyotonia (NMT) or Morvan's and cramp fasciculation syndromes, may be caused by erroneous Kv function due to host production of autoantibodies. Kv antibodies are detected in approximately 40% of NMT patients [111], and when transferred to mouse cells they cause reduction of K+ currents, DRG hyperexcitability and other signs of the disease [112]. In agreement with an autoimmune etiology, immunomodulatory therapy can improve function and symptoms [113]. Interestingly, these conditions can also arise owing to autoantibodies against proteins of the functional Kv complexes, such as Caspr2 or LGI1 [114]. Thus Caspr2 dysfunction may affect Kv1 assembly at juxtaparanodes, whereas altered Kv1 association with LGI1 in presynaptic C-fiber complexes could explain symptoms such as heat hyperalgesia. Although still in its infancy, the concept of Kv complex autoimmunity is an exciting development that may explain idiosyncratic pain in the absence of injury (e.g., fibromyalgia) or other presently enigmatic congenital pain states.
Most of our knowledge on Kv2 comes from CNS studies, where Kv2.1 and Kv2.2 conduct the majority of delayed rectified IK current in several neuron subtypes 15, 34. Kv2 channels are activated slowly after significant depolarization, therefore their opening primarily influences membrane repolarization and inter-spike hyperpolarization during AP firing [15]. Importantly, because Kv2 feature characteristically slow activation and inactivation, the progressive channel recruitment during sustained activity can have a cumulative limiting effect on firing rates. The prominent CNS function of Kv2 is substantiated by specific localization in dendrites and AIS where the channel can exert intricate control over somal AP invasion and back-propagation [35]. Other interesting features of Kv2 are the phosphorylation-dependent regulation by neuronal activity, which can fine-tune excitability of CNS neurons by altering the channel membrane distribution and biophysical properties [36], as well as their modulation by several silent Kv subunits [37].
Despite the pivotal Kv2 role in shaping CNS signaling, an involvement in chronic pain was only recently uncovered. Kv2 subunits are present in small nociceptors (where Kv2.1 conducts the majority of IK [34]) but are also abundantly expressed in myelinated DRG neurons [38]. Transcript and protein Kv2 levels are downregulated by traumatic nerve injury, and this could augment firing by limiting the Kv2 inhibitory effect on spike frequency 26, 27, 38. Indeed, application of a Kv2 blocker on ex vivo DRG preparations promotes myelinated neuron hyperexcitability by increasing conduction fidelity to the cell soma during repetitive stimulation [38]. It is possible that particular subcellular Kv2 localization forms the basis of an important filtering capacity (for instance by controlling AP traffic through the T-junction [39]), similarly to somatodendritic Kv2 filtering of somatic input in the CNS. Finally, a role in supraspinal pain pathways has also been demonstrated; cortical expression of Kv2.2 is reduced in oxaliplatin-induced neuropathy, and reproducing this in vivo results in marked cold and mechanical hypersensitivity [40].
All Kv3 channels are high-threshold and are typically encountered in fast-spiking neurons where they facilitate AP repolarization and hence dictate AP duration, but without affecting AP threshold or interspike interval [41]. Kv3.1 and Kv3.2 are delayed rectifiers contributing a small fraction (20%) of IK in small nociceptors, with a possible participation of Kv3.3 heterotetramers [42]. The Kv3.4 member almost certainly underlies the TEA-sensitive high-threshold transient current detected in nociceptors by Gold et al. (1996). This rapid Kv3.4 current accelerates nociceptor repolarization, an effect that restricts Ca2+-dependent neurotransmitter release at central nerve endings, where Kv3.4 is localized 41, 43. Hence, the mechanical hypersensitivity reported after Kv3.4 antisense treatment can be explained by AP broadening and therefore increased neurotransmission [44], although a loss of protein kinase C (PKC)-dependent modulation has also been suggested [43]. It is noted that although APs only spend a brief time at voltages capable of activating Kv3 channels, this restriction may be overcome by enhanced Kv3 densities at sites of action [41]. In addition, in native channels the activation threshold of Kv3.4 could be hyperpolarized following association with other proteins. For instance, heterotetramers of Kv3.4 with the delayed rectifiers Kv3.1 or Kv3.2 are activated at –30 mV [45], whereas Kv3.4 association with the auxiliary MinK-related peptide 2 (MiRP2) yields subthreshold currents in skeletal muscle [46].
In addition to Kv3.4, Kv4 members and Kv1.4 also give rise to transient A-currents (IA) that inactivate rapidly [15]. In contrast to Kv3.4, these A-channels are activated by small depolarizations, and their function in DRG can limit AP threshold, duration, and firing frequency [47]. Two low-threshold IA are detected in DRG neurons [17]; although Kv1.4 might contribute to the low-threshold IA in small DRG neurons, the fast voltage-dependent recovery from inactivation suggests the presence of Kv4 channels [25]. Therefore the low-threshold component may be predominantly mediated by Kv4.1, and the somatically confined Kv4.3 [48], because Kv4.2 is either absent or expressed at very low levels 27, 48. Consistent with a role in nociceptive pathways, A-type subunit expression and currents in the DRG are found to be reduced in a variety of pain models 21, 25, 44, 49. Mimicking Kv4.3 downregulation via intrathecal antisense is sufficient to induce mechanical hypersensitivity in naïve rats, presumably via reducing firing thresholds in a subset of Mrgprd (Mas-related G protein-coupled receptor D) neurons [44]. A-type blockers or short interfering RNA (siRNA) treatment can also diminish the analgesia by diclofenac in bone cancer [49], although K+ channel-related antinociception by this drug may be principally conferred via direct opening of other voltage-gated (e.g., Kv7 [50]), ATP-sensitive, or Ca2+-activated channels [51]. Finally, despite its negligible involvement in DRG excitability, Kv4.2 can strongly modulate pain plasticity in dorsal horn neurons; thus Kv4.2-null mice exhibit quicker mechanical pain resolution following nerve injury, as well as loss of extracellular signal-regulated kinase (ERK)-dependent sensitization in inflammatory models [52]. Although a few Kv4 activators are available (e.g., NS-5806 and KW-7158), the pacemaking activity of Kv4 channels in cardiac tissue is limiting for systemic applications.
Kv7 channels open near RMP and underlie the low-threshold, non-inactivating M-current (IM) [20]. IM serves as a native ‘voltage clamp’ that stabilizes RMP and regulates AP threshold and accommodation within AP trains, affording it a central role in modulation of neuronal excitability. Accordingly, mutations in the human Kv7.2/Kv7.3 encoding genes cause benign familial neonatal epilepsy due to excessive excitability in distal motor axons [20]. In the DRG, IM mediated by Kv7.2, Kv7.3 and Kv7.5 oligomers is the dominant subthreshold K+ current in small neurons and a significant component in larger neurons (together with Kv1.1/Kv1.2) [12]. Kv7.2 and Kv7.3 are enriched in nociceptor AIS and terminals (but see [53]) and in nodes of myelinated fibers, in contrast to the majority of Kv channels which occupy paranodes or juxtaparanodes 12, 53. Kv7.2 and associated currents are reduced in DRG following neuropathic lesions, although the delayed onset of downregulation suggests a link to the maintenance rather than initiation of pain [54]; nevertheless, enhancement of residual IM can reverse pain behaviors [55]. Reduced Kv7 function is also involved in inflammatory pain, where IM inhibition occurs via protease-activated receptor 2 (PAR2) activation and phospholipase C (PLC)-induced depletion of phosphatidylinositol-4,5-bisphosphate (PIP2), inositol trisphosphate (IP3)-mediated Ca2+ augmentation, or a combination of both [12]. Consistent with this, PLC activation by bradykinin results in Ca2+ release which inhibits IM, thus allowing Ca2+-activated Cl− channels to amplify depolarizing input and trigger spontaneous firing [56]. The general purpose anti-inflammatory diclofenac has also been shown to directly activate Kv7.2/Kv7.3 channels [50].
The anticonvulsant retigabine, the most advanced Kv modulator, reduces excitability of animal 12, 57, 58 and human [59] axotomized nociceptive fibers by enhancing IM via a hyperpolarizing shift in Kv7.2/Kv7.3 activation. Accordingly, both retigabine and its structural analogue flupirtine (used as an analgesic in Europe since 1984) are antinociceptive in a variety of inflammatory and neuropathic pain paradigms through both central and peripheral mechanisms 12, 60, 61, 62. Although retigabine failed to produce analgesia in a recent clinical trial of post-herpetic neuralgia, flupirtine is currently in Phase II trials for fibromyalgia pain. New activators such as the Kv7.2/Kv7.3-selective ICA-27243 are in the pharmaceutical pipeline because retigabine and flupirtine do not show strong selectivity among Kv7 subunits and can additionally cause side-effects by interacting with other targets such as GABA receptors [63].
Two-pore K+ (K2P) channels
K2P have emerged as promising candidates for pain modulation owing to their cell type-specific expression and lower inter-family sequence identity. They are unique among K+ channels in that they contain two pore domains and co-assemble as dimers rather than tetramers. Under physiological conditions K2P generate hyperpolarizing leak currents that stabilize cells below firing threshold, and disrupting this constitutive conductance results in depolarization and increased excitability [64]. Sensory neurons express many of the 15 members of the K2P superfamily, including TWIK1 (two-pore weak inwardly rectifying K+ channel), the TWIK-related (TR) channels TRESK, TREK1, and TRAAK, as well as TASK1 (acid-sensitive K+ channel), and marked reductions have been documented in pain states 65, 66. The importance of K2P in pain is highlighted by the discovery of a human K+ channelopathy; thus, familial migraine with aura is associated with a dominant-negative mutation in TRESK, a subunit strongly expressed in human trigeminal and dorsal root ganglia [67]. This fits well with the fact that migraine is associated with secretion of neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P by meningeal nociceptors of the trigeminal ganglia, which may lead to sensitization. In a traumatic injury context, TRESK expression is decreased by axotomy, whereas pharmacological or siRNA inhibition induces C-fiber hyperexcitability and pain behaviors [68]. Contrarily, adenovirus-mediated spinal delivery of TRESK can reverse nerve injury-induced mechanical allodynia [69]. Another interesting but relatively unexplored member, TWIK1, is selectively expressed in medium-large DRG neurons and undergoes robust and persistent reductions by neuropathic injury [66].
A particularly noteworthy feature of K2P channels is their activation by a wide range of physicochemical factors including volatile anesthetics [70]. For instance, TREK1 is coexpressed with TRPV1 in nociceptors and can be activated by heat, stretching or lipids; a corresponding current is recorded in small neurons from wild type, but not TREK1 knockout (KO) animals [71]. TREK1 KO animals also show increased sensitivity to heat and mechanical stimulation, suggesting that normal K2P function counterbalances inward currents generated by TRPV1 and mechanosensitive Na+-permeable channels, respectively. Interestingly, TREK1 activity is decreased by inflammatory mediators such as prostaglandin E2 (PGE2) and lysophosphatidic acid, and TREK1-null mice develop more modest mechanical and thermal hyperalgesia during inflammation, presumably due to loss of this inhibition 71, 72. These data suggest that TREK1-modulating drugs may be useful in acute and inflammatory pain. Although TREK1 KOs show reduced cold pain after SNL, the precise involvement of this channel in neuropathic pain has not been thoroughly examined [71]. The member TRAAK is also mechano- and heat-sensitive, and simultaneous deletion of TREK1 and TRAAK has additive effects that may explain some of the mechano-hypersensitivity in colitis 73, 74. Furthermore, double TREK1/TRAAK KOs exhibit defects in acute cold pain processing, traced back to menthol-insensitive nociceptors [74]. Interestingly, oxaliplatin reduces TREK1 and TRAAK expression, and double KOs have modified cold pain responses in this model [75].
Ca2+-activated K+ channels (KCA)
Opening of KCA during neuronal firing hyperpolarizes the membrane and provides feedback inhibition that limits Ca2+ influx and excitability, making them powerful regulators of synaptic transmission at nerve terminals [11]. Based on their conductance, they are further divided into BKCA (big conductance), IKCA (intermediate conductance), and SKCA (small conductance).
All KCA are found in DRG and respond to increases in intracellular calcium, whereas BKCA are also voltage-sensitive 76, 77. Big conductance KCA are thought to influence excitability more prominently; illustrative of their significance in pain transduction is the recent finding of a functional coupling with TRPV1 (transient receptor potential cation channel, subfamily V, member 1) in nociceptors [78]. Blocking these channels with iberiotoxin reduces outward currents, prolongs AP duration, and increases firing rates in small-medium sensory neurons, with no effect on RMP, AP threshold, or AP amplitude [77]. Accordingly, axotomy decreases BKCA expression and Ca2+-dependent post-spike after-hyperpolarization in small-medium DRG neurons [79]. Contrariwise, the specific BKCA opener NS-1619 suppresses DRG neuron firing and can even antagonize the hyperexcitability evoked by A-channel block [77]. Interestingly, PGE2 and other inflammatory mediators reduce BKCA channel activity in nociceptors 17, 76, 80 and BKCA deletion in these neurons enhances inflammatory pain without affecting acute or neuropathic behaviours [133]. The BKCA opener andolast is currently in Phase III trials as an anti-inflammatory for chronic obstructive pulmonary disease; it would be interesting to evaluate the antinociceptive properties of NS-1619 and andolast in chronic pain models.
Smaller conductance KCA are detected in a mixture of human and rodent DRGs, and may also contribute to pain phenotypes 76, 81. In small neurons, SKCA are downstream targets of NMDA receptor (NMDAR)-mediated Ca2+ influx because deleting the NR1 subunit in DRG induces hyperexcitability and pain hypersensitivity that can be reproduced by NMDAR antagonism or pharmacological SKCA inhibition [82]. Although IKCA expression in large neurons is decreased by nerve injury, SKCA and IKCA subunits in small neurons appear unaltered, suggesting that opening these channels may be a viable approach for chronic pain relief [76]. In line with this, the channel opener 1-ethyl-2-benzimidazolone (1-EBIO) reduces excitability in response to mechanical stimulation; however, the analgesic properties of such compounds remain to be robustly tested 11, 83.
KCA also participate in central pain processing. Nerve injury leads to enhanced BKCA expression in second-order neurons near the dorsal root entry zone, and activating these channels by intrathecal NS-1619 reverses pain hypersensitivity [79]. Conversely, KCA blockers can antagonize the antinociceptive effects of muscarinic receptor agonists, gabapentin, and perhaps some NSAIDS [11].
Inward rectifiers (Kir)
These channels are expressed mainly (but not exclusively) in supporting cells (Box 2) and can conduct atypical inward (rather than outward) K+ currents at depolarized membrane potentials. This buffering activity adds to glial K+ uptake through electrogenic Na+/K+ pumps to offset extracellular K+ accumulation during neuronal firing [84], thus preventing AP ‘short-circuiting’ and uncontrolled excitability changes [85]. They belong to one of seven families (Kir1-Kir7) and have a relatively simple structure with two transmembrane domains flanking the pore region [11]. Three families implicated in nociception are Kir3 (also known as G protein-regulated inward rectifiers K+ channels, GIRK), Kir2, and the ATP-sensitive channels (KATP).
Box 2. The involvement of glial K+ channels.
Accumulating evidence points towards a pain-modulating role of K+ channels in satellite glial cells (SGC). For example, an inward current is detected in SGC of the trigeminal ganglion. The member responsible appears to be Kir4.1 because no inward currents are detected in Kir4.1-null animals, alongside depolarized RMP and inhibition of K+ uptake 115, 116. Attenuation of Kir4.1 expression in SGC of the trigeminal ganglion by neuropathic lesions or antisense treatment results in spontaneous and evoked facial pain-like behaviors [117]. Similarly, CFA inflammation can suppress Kir4.1 expression and associated currents, leading to depolarized RMP [118]. In the spinal cord, nerve injury reduces expression of Kir6.1/SUR, whereas the KATP opener cromakalim relieves pain via regulation of astroglial gap junctions [119]. Put together, these studies suggest that heightened extracellular K+ due to impaired glial K+ homeostasis can cause downstream hyperexcitability of adjacent neurons, although a glutamate involvement is also plausible. Interestingly, Kir2.1, Kir2.3, and Kir6.2 are also present in Schwann cells at the nodes of peripheral nerves, and putative regulation by injury might regulate excitability of myelinated neurons in a similar fashion 94, 120. Another class of K+ channels involved in spinal SGC-dependent hyperexcitability are the microglia-expressed KCA, which participate in microglia activation and migration following injury [121]. Hence, intrathecal treatment with a BKCA blocker inhibits P2X4 expression and BDNF synthesis in spinal microglia, and precludes injury-induced tactile allodynia. The analgesic effects of ketamine in neuropathic pain and inflammation may also be partly mediated via inhibition of microglia activation following KCA current attenuation [121].
Neuronal GIRK channels are important determinants of spinal analgesia. As their name suggests they can interact with G proteins, an association thought to underlie the analgesic effects of opioids, endocannabinoids, and endogenous pain modulators [11]. Interestingly, enhanced GIRK1 phosphorylation in the dorsal horn following neuropathy or inflammation suggests reduced channel activity [86], whereas ‘pain risk’ GIRK2 alleles are associated with intensity of chronic back pain in humans [87]. Although no GIRK openers are currently available, their development could provide a viable alternative to opiates because this interaction may set in motion the same analgesic pathway without the unwanted side-effects of direct opioid activation 88, 89, 90. Furthermore, a recent study suggests that GIRK2 expressed in sensory neurons also contribute to peripheral opioid-mediated antinociception [134]. Finally, although normally expressed in low levels in the periphery, Kir2.1 channels could also be useful for therapeutic interventions; virus-mediated expression of Kir2.1 in DRG neurons can restore excitability following compression injury, and even preclude pain symptoms when applied pre-emptively [91].
KATP members are tetramers of Kir6.1 or Kir6.2 surrounded by four sulfonylurea receptor subunits (SUR1 or SUR2) [92]. These channels are inhibited by ATP but also modulated by ligands such as ADP, adenosine, NO, vasoactive intestinal polypeptide (VIP) and CGRP. KATP currents are generally thought to play a minor role in setting basal excitability of DRG neurons, where Kv7 and K2P conductances dominate [93]. However, a therapeutic potential in pathological conditions has been proposed. Thus, although Kir6.2 activity is reduced in large DRG neurons post-injury, the ability of KATP openers to hyperpolarize RMP is retained, which could be exploited for neuropathic pain treatments 94, 95. Similarly, the inhibition of Kv7 activity in nociceptors during inflammation may also reveal analgesic roles for KATP channels. Indeed, the activators pinacidil and diazoxide reduce the hyperexcitability and pain induced by a range of peripheral inflammatory stimuli 51, 93, 96.
Finally, KATP opening in the CNS is linked to the antinociception produced by systemic treatment with morphine, NSAIDs, or even gabapentin 11, 97. Unfortunately, the involvement of KATP in modulation of cardiac rhythmicity, pancreatic insulin secretion, and intestinal function necessitates therapeutic strategies that selectively target the tissues of interest [92].
How does nerve injury trigger K+ channel dysfunction?
In the preceding paragraphs we reviewed studies describing distinct expression patterns of K+ channels involved in the peripheral and central processing of painful stimuli (Figure 2) as well as their extensive downregulation after nerve lesions (Table 1). The latter finding has implications for treatment because the analgesia produced by pharmacologically enhancing the remaining K+ activity may be of limited scope. In these cases, targeting upstream cascades that orchestrate K+ channel dysfunction could yield more efficacious treatments. For instance, it was recently reported that an injury-induced endogenous non-coding RNA attenuates Kv1.2 expression, and blocking this pathway diminishes neuropathic pain [30]. Whether similar non-coding RNAs modulate the activity of other K+ channels is a question that warrants further investigation. Similarly, expression of Kv7.2, Kv4.3, and other ion channels in DRG is inhibited by the transcription factor REST (RE1-silencing transcription factor), which is induced by injury or inflammation 54, 98. Accordingly, blocking REST with antisense restores transcript levels and reverses some neuropathic pain symptoms [99].
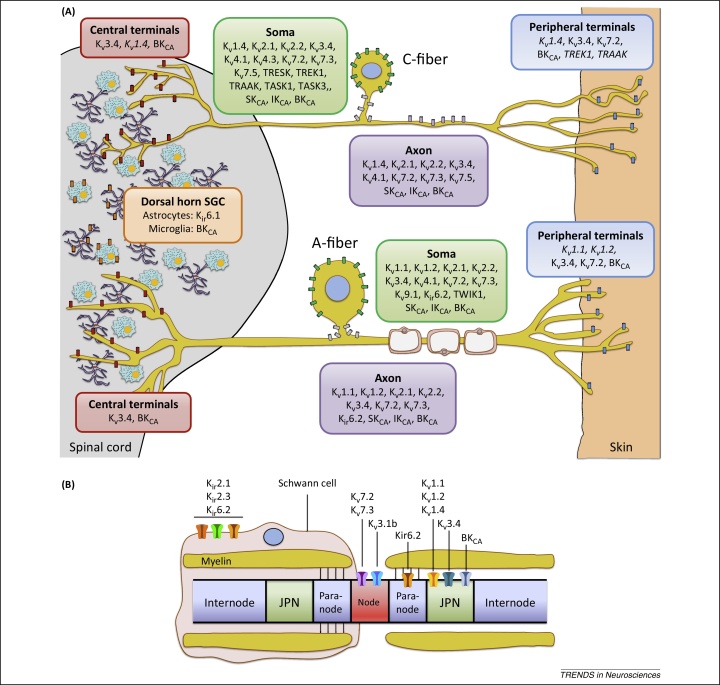
Figure 2.
Expression and function of K+ channels in sensory neurons. (A) Subcellular localization of K+ channel subunits in unmyelinated (top) and myelinated (bottom) murine dorsal root ganglia (DRG) neurons. The panoply of K+ channels endows sensory neurons with a sophisticated machinery for the regulation of neuronal excitability. The depiction illustrated here is not absolute but rather reflects most prominent expression patterns in pain-relevant subpopulations, as reported in the literature. In addition, it is noted that K+ channel distribution patterns can vary tremendously between species, and validation against human data is currently very limited 59, 81. In the pain pathway, the TWIK-related (TR) channels TREK1 and TRAAK (and possibly TRESK) located at C-fiber terminals can counteract the activation of inward-conducting ion channels by pressure, heat or cold, whereas steady Kv7 currents also stabilize RMP and regulate action potential (AP) threshold. In myelinated neurons, low-threshold Kv1.1/Kv1.2 heterotetramers appear to modulate acute and neuropathic pain modalities 28, 30, 31, whereas Kv1.4 and Kv4 members may exert similar roles in small nociceptors 25, 44. In addition, recent evidence suggest that Kv1.1/Kv1.2 may also function as mechanoreceptors in some C-fibers (not shown) [31]. Transmission of signals generated at the periphery is reliant on numerous axonal K+ channels, which influence the fidelity of AP conduction and therefore the fiber following frequency. Although normal sensory transduction is independent of spiking in the DRG soma, this can become a site of spontaneous firing in neuropathic conditions. In these scenarios, the activity of somal K+ channels may become an important regulator of excitability by influencing somal AP generation as well as propagation past the DRG T-junction. Potential candidates here are channels that preferentially localize at the soma or axon initial segments (in grey), such as Kv4.3 in mechanosensitive C-fibers [44] or Kv2/Kv9.1 in A-fibers 38, 122. At the central terminals, Ca2+-activated channels BKCA fine-tune activity and regulate neurotransmitter release in the spinal cord in response to calcium influx during AP firing. The high-threshold Kv3.4 limits AP duration and thus may play a key role in synaptic transmission, whereas Kv1.2 may also regulate presynaptic terminal excitability [22]. Finally, pain processing can be influenced by K+ channels expressed by glial satellite cells (GSC) in the dorsal horn. Astrocyte-expressed Kir6.1 (and perhaps Kir3.1 [86]) buffers the extracellular K+ to maintain equilibrium potential during neuronal firing [119], and BKCA conduction is involved in microgliosis following injury [121]. In addition, satellite cell-expressed Kir4.1 is involved in facial pain processing in the trigeminal ganglion (not shown). Subunits denoted in italics represent localizations that are indirectly implied by pharmacological profiling in DRG neurons, or by extrapolating on known localization in other neuronal types. For example, the Kv1.1 and Kv1.2 subunits are typically detected in dendrites and terminals of CNS neurons 22, 23, whereas TREK1 and TRAAK are axonally trafficked in sciatic nerves and are present at synaptic sites in cerebellar cultures [123]. (B) K+ channel composition of a myelinated DRG axon, illustrating nodes, paranodes, juxtaparanodes (JPN), internode segments, and a myelinating Schwann cell. The Kv7.2 and Kv7.3 subunits (together with a splice variant of Kv3.1) are found in the nodes, and may therefore more prominently affect saltatory conduction under physiological conditions. Following axonal injury and demyelination, however, other channels such as the juxtaparanodal Kv1 subunits may become exposed, leading to reduced conduction velocity and negative symptoms including sensory loss. In other cases, reduced axonal K+ channel function due to disrupted node organization (e.g., autoantibodies against Kv complex proteins) may induce peripheral hyperexcitability. Schwann cells also express inward rectifiers that regulate the node microenvironment during neuronal activity.
Table 1.
Summary of studies investigating the effect of altered K+ channel expression and function on acute, neuropathic, and inflammatory pain phenotypes
| Superfamily | Subunit | Manipulation | Expression/excitability changes | Pain phenotype | Comments | Refs |
|---|---|---|---|---|---|---|
| Voltage-gated | Kv1.1 | KO transgenic | Loss of IKmech currents ↓Mechanical threshold (HTM-C fibers) ↓Firing adaptation (Aβ-fibers) |
↑Mechanical ↑Heat ↑Formalin |
Reduced morphine antinociception | 28, 31 |
| Morvan's syndrome Neuromyotonia | ↑Peripheral excitability | ↑Mechanical allodynia ↑Heat hyperalgesia |
Autoantibodies to Kv1 complexes; cause disease when transferred to cells; immunomodulatory therapy useful | 111, 112, 113 | ||
| Kv1.2 | Nerve injury (SNL) Kv1.2 knockdown |
↓K+ current, ↓firing threshold ↑RMP, ↑firing rate |
↑Mechanical ↑Cold |
SNL induces Kv1.2 antisense RNA; pre-emptive sense RNA alleviates pain | [30] | |
| Kv1.4 | Nerve injury (SNT, SNL) Diabetic neuropathy Inflammation |
↓Kv1.4 expression | ND | – | 25, 101, 124 | |
| Kv2.1 | Nerve injury (SNL, SNT) | ↓Kv2.1 expression | ND | – | 26, 38 | |
| Kv2.2 | Nerve injury (SNL, CCI) | ↓Kv2.2 expression | ND | – | 27, 38 | |
| Oxaliplatin Kv2.2 knockdown (cortex) |
↓Kv2.2 expression (cortex) | ↑Mechanical ↑Cold |
– | [40] | ||
| Kv3.4 | Nerve injury (SNL) Kv3.4 knockdown Diabetic neuropathy |
↓Kv3.4 expression ↑AP duration |
↑Mechanical | PKC phosphorylation slows Kv3.4 inactivation and decreases AP duration | 43, 44, 101 | |
| Kv4.2 | KO transgenic | DH neurons: ↑RMP, ↓firing threshold ↑Repetitive firing ↓IA current,↑excitability |
↑Mechanical, ↑heat ↓Formalin, ↓Carrageenan |
Quicker resolution of mechanical allodynia after CCI; defects in central sensitization | [52] | |
| Kv4.3 | Nerve injury (SNL) Diabetic neuropathy Kv4.3 knockdown |
↓Kv4.3 expression | ↑Mechanical | REST antisense blocks Kv4.3 downregulation | 44, 99, 101 | |
| Kv7.2 Kv7.3 |
Nerve injury (PSNL) | ↓Kv7.2 expression ↓IM current |
↑Mechanical ↑Heat |
Perisciatic flupirtine reverses pain; antagonized by XE-991 blocker | [54] | |
| Bone cancer | ↓IM current, ↑excitability | ↑Mechanical ↑Heat |
– | [55] | ||
| Retigabine (opener) after nerve injury or inflammation | ↓Excitability (DRG, neuroma, DH neurons) |
↓Mechanical (CCI, SNI, bone cancer) ↓Heat (bone cancer) ↓Cold (CCI) ↓Formalin, ↓Carrageenan ↓CFA ↓Visceral pain |
Antinociception reversed by XE-991; no effect on intact fibers or acute pain | 12, 55, 58, 60, 61, 62 | ||
| Kv9.1 | Kv9.1 knockdown Nerve injury (SNL) |
↓Kv9.1 expression ↑SA, ↑firing, ↑after-discharge |
↑Mechanical | Mediated through Kv2 | [122] | |
| Human SNPs | ND | ↑Risk of phantom limb pain ↑Risk of chronic back pain ↑HIV neuropathy pain intensity |
– | 125, 126 | ||
| Two-pore | TRESK | Human DN mutation | ↓TRESK currents | ↑Migraine pain | – | [67] |
| Nerve injury (SNT) TRESK knockdown |
↓TRESK expression | ↑Mechanical | Trend for thermal pain | [68] | ||
| TRESK overexpression after nerve injury (SNI) |
↑TRESK expression | ↓Mechanical allodynia | – | [69] | ||
| TREK1 | KO transgenic | ↓TREK1 expression ↑AP firing |
↑Mechanical, ↑heat ↓Inflammatory |
Reduced cold hypersensitivity after SNL; TREK1 inhibited by PGE2 and cAMP | [71] | |
| TRAAK | KO transgenic | ↓TRAAK expression | ↑Mechanical, ↑heat | TREK1/TRAAK KO: ↑noxious cold pain but reduced cold sensitivity after oxaliplatin | 74, 75 | |
| TASK | Inflammation (CFA) | ↓TASK1, TASK2, TASK3 expression | ND | – | [65] | |
| TWIK1 | Nerve injury (SNI) | ↓TWIK1 expression | ND | – | [66] | |
| Inward rectifiers | Kir2.1 | Nerve injury (CCD) plus Kir2.1 overexpression | ↑Kir2.1 expression ↓RMP, ↑firing threshold, ↓SA |
↓Mechanical hyperalgesia | Late treatment had no affect | [91] |
| Kir3.1 | Nerve injury (PSNL) Inflammation (formalin) |
↑Phospho-Kir3.1 (SC) | ND | Predicted to increase excitability by decreasing Kir3.1 activity | [86] | |
| Kir3.2 | Eight human SNPs | ND | ↓Acute pain tolerance ↑Chronic back pain intensity |
– | [87] | |
| Kir4.1 | Nerve injury (CCI) Kir4.1 knockdown |
↓Kir4.1 expression (SGC of TG) | ↑Facial pain | – | [117] | |
| Inflammation (CFA) | ↓Kir4.1 expression (SGC of TG) ↓Kir4.1 currents, ↑RMP (SGC of TG) |
↑Facial pain | – | [118] | ||
| KATP | Nerve injury (CCI) | ↓Kir6.1 expression (SGC of spinal cord) | ↑Mechanical, ↑heat | Cromakalim (opener) reversed pain; antagonized by carbenoxolone | [119] | |
| Nerve injury (SNL) | ↓Kir6.2 expression (DRG, Schwann cells) ↓KATP activity |
↑Mechanical | Diazoxide (opener) or CAMKII activate residual KATP channels | 94, 95 | ||
| Pinacidil and diazoxide (openers) | ↓DRG excitability | ↓Inflammatory ↓Mechanical, ↓thermal |
Antagonized by KATP blocker glyburide | [93] | ||
| Calcium-activated | SKCA/IKCA | Apamin (blocker) | ↑AP frequency, ↑excitatory transmission | ↑Mechanical, ↑heat | NR1 KO in DRG has same effects | [82] |
| Nerve injury (CCI) Inflammation (CFA) |
No change in SK1–3 expression | ND | Targeting residual SK may be viable for pain treatment |
[76] | ||
| Nerve injury (avulsion) | ↓IK1 expression | ND | IK1 was upregulated in vitro by NT-3 | [81] | ||
| 1-EBIO (opener) | ↓Excitability after mechanical stimulation | ND | Effects reversed by UCL-1848 (blocker) | [83] | ||
| BKCA | Iberiotoxin (blocker) | ↓BKCA current ↑AP duration, ↑firing frequency |
ND | NS-1619 (opener) decreases excitability | [77] | |
| Nerve injury (SNL) | DRG: ↓BKCA expression SC: ↑BKCA expression (DREZ) |
↑Mechanical, ↑heat | Iberiotoxin reduces mechanical thresholds; BKCA opener (NS-1619) reverses SNL pain | [79] | ||
| Nerve injury (SNL) | ↓BKCA current (DRG) ↓ BKα1 mRNA (DRG) |
ND | BDNF reduced BKCA currents. Anti-BDNF reversed BKCA reduction |
[102] | ||
| Inflammation (CFA) | ↓BKCA current (DRG) | ND | No change in BKCA expression | [80] | ||
| Charybdotoxin (blocker) | ↓P2X4 and BDNF in microglia | ↓Tactile allodynia | Mediated via inhibition of microglial activation |
[121] | ||
| KO transgenic | ↓BKCA expression (nociceptors) | ↑Inflammatory | Acute and neuropathic pain unaffected | [133] |
Unless stated otherwise, entries in expression and excitability refer to sensory neurons. Abbreviations: AGJ, astroglial gap junction; AP, action potential; CCD, chronic compression of the dorsal root ganglion; CFA, complete Freund's adjuvant; CCI, chronic constriction injury; DH, dorsal horn; DN, dominant-negative; DREZ, dorsal root entry zone; DRG, dorsal root ganglion; HTM, high-threshold mechanoreceptor; KO, knockout; ND, not determined; PSNL, partial sciatic nerve ligation; RMP, resting membrane potential; SA, spontaneous activity; SC, spinal cord; SGC, satellite glial cells; SNI, spared nerve injury; SNL, spinal nerve ligation; SNP, single-nucleotide polymorphism; SNT, sciatic nerve transection; TG, trigeminal ganglion.
Ion channel expression is typically controlled by carefully balanced neurotrophic support, which may become disrupted in pain pathology [100]. One of the most interesting messengers downstream of REST is brain-derived neurotrophic factor (BDNF), which has an established sensitizing role; in a diabetic neuropathy model, pre-emptive anti-BDNF treatment can reverse the IA reduction in myelinated neurons [101]. The regulatory role of BDNF may be more general among K+ channels, because the injury-induced BKCA downregulation in DRG can also be reversed by anti-BDNF [102]. There is also evidence that KCA activity can be regulated by nerve growth factor (NGF), glial-derived neurotrophic factor (GDNF), and neurotrophin 3 (NT3) 81, 103, 104. Early research showed that NGF treatment can normalize axotomy-induced IA and IK reductions in DRG; however, an inhibitory effect on IA and IM may occur during inflammation 105, 106, 107. The exact influence of these growth factors and the responsive K+ channel subunits remains to be systematically tested and clarified.
Concluding remarks
The exceptional abundance and breadth of function encountered in K+ channels has complicated efforts to untangle explicit roles in pain syndromes. Owing to advances in molecular, biochemical, electrophysiological, and genetic methods, however, we can now appreciate the involvement of specific subunits in maladaptive pain signaling after injury or inflammation. Nevertheless, there are many potential avenues of K+ involvement that have hardly been explored. It seems likely that unknown mutations in K+ channel genes might contribute to inherited pain syndromes. There are many ‘silent’ K+ channel subunits for which we have little idea of whether and how they might affect pain processing (Box 3). Auxiliary subunits can provide alternative substrates for pharmacological modulation; however, our understanding of these interactions in the PNS is also limited. In many chronic pain models an extensive dysregulation of several K+ channels is seen, and it is unknown whether a common epigenetic control exists.
Box 3. Why so many silent and auxiliary Kv subunits?
Emerging evidence suggests that the rich complement of modulatory Kv partners may have previously overlooked significance in fine-tuning neuronal activity. The silent Kv9.1 subunit is selectively localized in myelinated DRG neurons, the principal source of spontaneous activity after nerve injury [5]. Injury-induced Kv9.1 downregulation triggers spontaneous and evoked hyperexcitability as well as mechanical allodynia [122]. Intriguingly, a human Kv9.1 polymorphism is associated with high risk of developing chronic back pain or persistent pain after amputation [125], whereas another study found a link with pain intensity in HIV neuropathy [126]. These effects are most likely mediated via a regulation of Kv2 currents, which are modulated by Kv9.1 in vitro [37]. Similar Kv2 modification by the non-conducting Kv5.1, Kv6.1, Kv6.3, Kv8.1, and Kv9.3 has been reported in heterologous systems and DRG neurons; it is therefore appealing to suggest that silent subunits might be instrumental in pain pathophysiology 34, 37.
Auxiliary proteins can modulate Kv function by affecting gating, expression levels and trafficking. For instance, Kvβ2 subunits enhance Kv1 currents by promoting trafficking and membrane incorporation as well as inhibiting inactivation, whereas Kvβ1 subunits confer the opposite effects [127]. Genetic Kvβ2 deletion leads to defects in axonal targeting of Kv1.1 and Kv1.2, reduced after-hyperpolarization, and increased amygdala hyperexcitability associated with memory impairments [128]. In myelinated DRG neurons, Kvβ2.1 colocalizes with Kv1.1 and Kv1.2; however, the modest Kvβ2.1 reduction by injury (25%) argues against a significant role in Kv1 dysfunction in chronic pain 25, 26. Nevertheless, targeting auxiliary subunits may be of therapeutic value; for example Kv1.1 ‘disinactivators’ reduce excitability by preventing Kv1.1 inactivation by Kvβ1 [129].
Similarly, the localization of AMIGO (amphoterin-induced gene and ORF) dynamically follows that of Kv2.1, and AMIGO increases Kv2.1 conductance [130]. Kv4 surface expression and gating can be enhanced by Kv channel interacting proteins (KChIPs), which respond to intracellular calcium fluxes during AP firing, and by dipeptidyl-peptidase-like proteins (DPPs) [131]. Interestingly, these auxiliary proteins often have distinctive distributions that may be relevant to pain processing. For instance, KChIP3 is abundant in medium-large DRG neurons, whereas DPP10 is restricted to small neurons [132]. Finally, Kv7.2/Kv7.3 can be modulated by MinK-related peptides (MiRPs, pluripotent proteins that also interact with Kv2, Kv3, and Kv4), calmodulin, and A-kinase anchor proteins (AKAPs) [131]. The above description is by no means exhaustive; interested readers are referred towards excellent topical reviews [131].
Manipulation of K+ channel subunits with dominant contributions in neuron excitability is likely to play a key role in shaping future pain treatments. The development of novel technologies and increasing availability of structural information creates an optimistic outlook for pharmacological design of K+ channel modulators 108, 109, 110. In the next few years these advancements may be complemented by gene therapy strategies to introduce K+ channel copies at lesioned sites of the nervous system. Given the considerable convergence of pain mechanisms, it is plausible that synergistic treatments with K+ channel openers and other drugs (e.g., sodium or calcium channel blockers) can improve analgesic outcomes and/or circumvent side-effects by expanding the therapeutic window of present drugs to lower, more tolerable doses.
Acknowledgments
This work was supported by the Wellcome Trust-funded London Pain Consortium (grants 080504 and 083259).
References
- 1.van Hecke O. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013;111:13–18. doi: 10.1093/bja/aet123. [DOI] [PubMed] [Google Scholar]
- 2.Labianca R. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin. Drug Investig. 2012;32(Suppl. 1):53–63. doi: 10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.D’Mello R., Dickenson A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 4.Kajander K.C. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci. Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- 5.Kajander K.C., Bennett G.J. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in Abeta and Adelta primary afferent neurons. J. Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- 6.Serra J. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Djouhri L. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J. Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costigan M. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp. Brain Res. 2009;196:115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu M., Wood J.N. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;12(Suppl. 3):S93–S99. doi: 10.1111/j.1526-4637.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 11.Ocana M. Potassium channels and pain: present realities and future opportunities. Eur. J. Pharmacol. 2004;500:203–219. doi: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Passmore G.M. KCNQ/M currents in sensory neurons: significance for pain therapy. J. Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhoff C. Excitation of cutaneous sensory nerve endings in the rat by 4-aminopyridine and tetraethylammonium. J. Neurophysiol. 1992;67:125–131. doi: 10.1152/jn.1992.67.1.125. [DOI] [PubMed] [Google Scholar]
- 14.MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- 15.Johnston J. Going native: voltage-gated potassium channels controlling neuronal excitability. J. Physiol. 2010;588:3187–3200. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutman G.A. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 17.Gold M.S. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J. Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- 18.Everill B. Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J. Neurophysiol. 1998;79:1814–1824. doi: 10.1152/jn.1998.79.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto S. The roles of I(D), I(A) and I(K) in the electrophysiological functions of small diameter rat trigeminal ganglion neurons. Curr. Mol. Pharmacol. 2010;3:30–36. doi: 10.2174/1874467211003010030. [DOI] [PubMed] [Google Scholar]
- 20.Brown D.A., Passmore G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everill B., Kocsis J.D. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J. Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- 22.Dodson P.D. Presynaptic rat Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. J. Physiol. 2003;550:27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J. Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Browne D.L. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat. Genet. 1994;8:136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 25.Rasband M.N. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa K. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve. 1999;22:502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.S. Downregulation of voltage-gated potassium channel alpha gene expression in dorsal root ganglia following chronic constriction injury of the rat sciatic nerve. Brain Res. Mol. Brain Res. 2002;105:146–152. doi: 10.1016/s0169-328x(02)00388-1. [DOI] [PubMed] [Google Scholar]
- 28.Clark J.D., Tempel B.L. Hyperalgesia in mice lacking the Kv1.1 potassium channel gene. Neurosci. Lett. 1998;251:121–124. doi: 10.1016/s0304-3940(98)00516-3. [DOI] [PubMed] [Google Scholar]
- 29.Chi X.X., Nicol G.D. Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. J. Neurophysiol. 2007;98:2683–2692. doi: 10.1152/jn.00437.2007. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 2013;16:1024–1031. doi: 10.1038/nn.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao J. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Glazebrook P.A. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J. Physiol. 2002;541:467–482. doi: 10.1113/jphysiol.2001.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beekwilder J.P. Kv1.1 channels of dorsal root ganglion neurons are inhibited by n-butyl-p-aminobenzoate, a promising anesthetic for the treatment of chronic pain. J. Pharmacol. Exp. Ther. 2003;304:531–538. doi: 10.1124/jpet.102.042135. [DOI] [PubMed] [Google Scholar]
- 34.Bocksteins E. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am. J. Physiol. Cell Physiol. 2009;296:C1271–C1278. doi: 10.1152/ajpcell.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarmiere P.D. The Kv2.1K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connell K.M. Localization-dependent activity of the Kv2.1 delayed-rectifier K+ channel. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12351–12356. doi: 10.1073/pnas.1003028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocksteins E., Snyders D.J. Electrically silent Kv subunits: their molecular and functional characteristics. Physiology (Bethesda) 2012;27:73–84. doi: 10.1152/physiol.00023.2011. [DOI] [PubMed] [Google Scholar]
- 38.Tsantoulas C. Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input. Exp. Neurol. 2013;251C:115–116. doi: 10.1016/j.expneurol.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amir R., Devor M. Electrical excitability of the soma of sensory neurons is required for spike invasion of the soma, but not for through-conduction. Biophys. J. 2003;84:2181–2191. doi: 10.1016/S0006-3495(03)75024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thibault K. Cortical effect of oxaliplatin associated with sustained neuropathic pain: exacerbation of cortical activity and down-regulation of potassium channel expression in somatosensory cortex. Pain. 2012;153:1636–1647. doi: 10.1016/j.pain.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Rudy B., McBain C.J. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 42.Bocksteins E. Kv3 channels contribute to the delayed rectifier current in small cultured mouse dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 2012;303:C406–C415. doi: 10.1152/ajpcell.00343.2011. [DOI] [PubMed] [Google Scholar]
- 43.Ritter D.M. Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J. Physiol. 2012;590:145–161. doi: 10.1113/jphysiol.2011.218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chien L.Y. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J. Neurosci. 2007;27:9855–9865. doi: 10.1523/JNEUROSCI.0604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baranauskas G. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat. Neurosci. 2003;6:258–266. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- 46.Abbott G.W. MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis. Cell. 2001;104:217–231. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- 47.Vydyanathan A. A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons. J. Neurophysiol. 2005;93:3401–3409. doi: 10.1152/jn.01267.2004. [DOI] [PubMed] [Google Scholar]
- 48.Phuket T.R., Covarrubias M. Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front. Mol. Neurosci. 2009;2:3. doi: 10.3389/neuro.02.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan K.Z. Targeting A-type K+ channels in primary sensory neurons for bone cancer pain in a rat model. Pain. 2012;153:562–574. doi: 10.1016/j.pain.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Peretz A. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol. Pharmacol. 2005;67:1053–1066. doi: 10.1124/mol.104.007112. [DOI] [PubMed] [Google Scholar]
- 51.Ortiz M.I. Pharmacological evidence for the activation of K+ channels by diclofenac. Eur. J. Pharmacol. 2002;438:85–91. doi: 10.1016/s0014-2999(02)01288-8. [DOI] [PubMed] [Google Scholar]
- 52.Hu H.J. The Kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 53.King C.H., Scherer S.S. Kv7.5 is the primary Kv7 subunit expressed in C-fibers. J. Comp. Neurol. 2012;520:1940–1950. doi: 10.1002/cne.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rose K. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 2011;152:742–754. doi: 10.1016/j.pain.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Q. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Liu B. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J. Clin. Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera-Arconada I. Enhancing M currents: a way out for neuropathic pain? Front. Mol. Neurosci. 2009;2:10. doi: 10.3389/neuro.02.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roza C., Lopez-Garcia J.A. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axotomized sensory fibres. Pain. 2008;138:537–545. doi: 10.1016/j.pain.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 59.Lang P.M. Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology. 2008;54:1271–1278. doi: 10.1016/j.neuropharm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Blackburn-Munro G., Jensen B.S. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur. J. Pharmacol. 2003;460:109–116. doi: 10.1016/s0014-2999(02)02924-2. [DOI] [PubMed] [Google Scholar]
- 61.Xu W. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol. Pain. 2010;6:49. doi: 10.1186/1744-8069-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirano K. Kv7.2-7.5 voltage-gated potassium channel (KCNQ2-5) opener, retigabine, reduces capsaicin-induced visceral pain in mice. Neurosci. Lett. 2007;413:159–162. doi: 10.1016/j.neulet.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 63.Wickenden A.D. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): a novel, selective KCNQ2/Q3 potassium channel activator. Mol. Pharmacol. 2008;73:977–986. doi: 10.1124/mol.107.043216. [DOI] [PubMed] [Google Scholar]
- 64.Plant L.D. A eole for K2P channels in the operation of somatosensory nociceptors. Front. Mol. Neurosci. 2012;5:21. doi: 10.3389/fnmol.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marsh B. Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol. Cell. Neurosci. 2012;49:375–386. doi: 10.1016/j.mcn.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollema-Mays S.L. Expression of background potassium channels in rat DRG is cell-specific and down-regulated in a neuropathic pain model. Mol. Cell. Neurosci. 2013;57:1–9. doi: 10.1016/j.mcn.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lafreniere R.G. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 2010;16:1157–1160. doi: 10.1038/nm.2216. [DOI] [PubMed] [Google Scholar]
- 68.Tulleuda A. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol. Pain. 2011;7:30. doi: 10.1186/1744-8069-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J. Intrathecal TRESK gene recombinant adenovirus attenuates spared nerve injury-induced neuropathic pain in rats. Neuroreport. 2013;24:131–136. doi: 10.1097/WNR.0b013e32835d8431. [DOI] [PubMed] [Google Scholar]
- 70.Heurteaux C. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alloui A. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen A. Pain-associated signals, acidosis and lysophosphatidic acid, modulate the neuronal K(2P)2.1 channel. Mol. Cell. Neurosci. 2009;40:382–389. doi: 10.1016/j.mcn.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 73.La J.H., Gebhart G.F. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G165–G174. doi: 10.1152/ajpgi.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noel J. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Descoeur J. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mongan L.C. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience. 2005;131:161–175. doi: 10.1016/j.neuroscience.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X.F. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 78.Wu Y. TRPV1 channels are functionally coupled with BK(mSlo1) channels in rat dorsal root ganglion (DRG) neurons. PLoS ONE. 2013;8:e78203. doi: 10.1371/journal.pone.0078203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen S.R. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J. Neurochem. 2009;110:352–362. doi: 10.1111/j.1471-4159.2009.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X.L. Inflammation-induced changes in BK(Ca) currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol. Pain. 2012;8:37. doi: 10.1186/1744-8069-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boettger M.K. Calcium-activated potassium channel SK1- and IK1-like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain. 2002;125:252–263. doi: 10.1093/brain/awf026. [DOI] [PubMed] [Google Scholar]
- 82.Pagadala P. Loss of NR1 aubunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: involvement of Ca2+-activated small conductance potassium channels. J. Neurosci. 2013;33:13425–13430. doi: 10.1523/JNEUROSCI.0454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahia P.K. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J. Neurosci. 2005;25:3489–3498. doi: 10.1523/JNEUROSCI.0597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D’Ambrosio R. Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J. Neurophysiol. 2002;87:87–102. doi: 10.1152/jn.00240.2001. [DOI] [PubMed] [Google Scholar]
- 85.Janigro D. Reduction of K+ uptake in glia prevents long-term depression maintenance and causes epileptiform activity. J. Neurosci. 1997;17:2813–2824. doi: 10.1523/JNEUROSCI.17-08-02813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ippolito D.L. Tyrosine phosphorylation of K(ir)3.1 in spinal cord is induced by acute inflammation, chronic neuropathic pain, and behavioral stress. J. Biol. Chem. 2005;280:41683–41693. doi: 10.1074/jbc.M507069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruehl S. Associations between KCNJ6 (GIRK2) gene polymorphisms and pain-related phenotypes. Pain. 2013;154:2853–2859. doi: 10.1016/j.pain.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marker C.L. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J. Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marker C.L. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J. Neurosci. 2004;24:2806–2812. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cruz H.G. Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J. Neurosci. 2008;28:4069–4077. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma C. Expression of inwardly rectifying potassium channels by an inducible adenoviral vector reduced the neuronal hyperexcitability and hyperalgesia produced by chronic compression of the spinal ganglion. Mol. Pain. 2010;6:65. doi: 10.1186/1744-8069-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark R., Proks P. ATP-sensitive potassium channels in health and disease. Adv. Exp. Med. Biol. 2010;654:165–192. doi: 10.1007/978-90-481-3271-3_8. [DOI] [PubMed] [Google Scholar]
- 93.Du X. Activation of ATP-sensitive potassium channels antagonize nociceptive behavior and hyperexcitability of DRG neurons from rats. Mol. Pain. 2011;7:35. doi: 10.1186/1744-8069-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zoga V. KATP channel subunits in rat dorsal root ganglia: alterations by painful axotomy. Mol. Pain. 2010;6:6. doi: 10.1186/1744-8069-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawano T. Suppressed Ca2+/CaM/CaMKII-dependent K(ATP) channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8725–8730. doi: 10.1073/pnas.0901815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alves D.P. Additive antinociceptive effect of the combination of diazoxide, an activator of ATP-sensitive K+ channels, and sodium nitroprusside and dibutyryl-cGMP. Eur. J. Pharmacol. 2004;489:59–65. doi: 10.1016/j.ejphar.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 97.Ortiz M.I. Role of ATP-sensitive K+ channels in the antinociception induced by non-steroidal anti-inflammatory drugs in streptozotocin-diabetic and non-diabetic rats. Pharmacol. Biochem. Behav. 2012;102:163–169. doi: 10.1016/j.pbb.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 98.Mucha M. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J. Neurosci. 2010;30:13235–13245. doi: 10.1523/JNEUROSCI.1981-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uchida H. Epigenetic gene silencing underlies C-fiber dysfunctions in neuropathic pain. J. Neurosci. 2010;30:4806–4814. doi: 10.1523/JNEUROSCI.5541-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pezet S., McMahon S.B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 101.Cao X.H. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J. Neurochem. 2010;114:1460–1475. doi: 10.1111/j.1471-4159.2010.06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cao X.H. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J. Neurochem. 2012;121:944–953. doi: 10.1111/j.1471-4159.2012.07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holm N.R. Activation of calcium-dependent potassium channels in mouse brain neurons by neurotrophin-3 and nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1002–1006. doi: 10.1073/pnas.94.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hendrich J. GDNF induces mechanical hyperalgesia in muscle by reducing I(BK) in isolectin B4-positive nociceptors. Neuroscience. 2012;219:204–213. doi: 10.1016/j.neuroscience.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Everill B., Kocsis J.D. Nerve growth factor maintains potassium conductance after nerve injury in adult cutaneous afferent dorsal root ganglion neurons. Neuroscience. 2000;100:417–422. doi: 10.1016/s0306-4522(00)00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu Y. Systemic administration of anti-NGF increases A-type potassium currents and decreases pancreatic nociceptor excitability in a rat model of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G176–G181. doi: 10.1152/ajpgi.00053.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia Z. NGF inhibits M/KCNQ currents and selectively alters neuronal excitability in subsets of sympathetic neurons depending on their M/KCNQ current background. J. Gen. Physiol. 2008;131:575–587. doi: 10.1085/jgp.200709924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sandoz G., Levitz J. Optogenetic techniques for the study of native potassium channels. Front. Mol. Neurosci. 2013;6:6. doi: 10.3389/fnmol.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brohawn S.G. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L., Sigworth F.J. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hart I.K. Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain. 2002;125:1887–1895. doi: 10.1093/brain/awf178. [DOI] [PubMed] [Google Scholar]
- 112.Shillito P. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann. Neurol. 1995;38:714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- 113.Sinha S. Autoimmune aetiology for acquired neuromyotonia (Isaacs syndrome) Lancet. 1991;338:75–77. doi: 10.1016/0140-6736(91)90073-x. [DOI] [PubMed] [Google Scholar]
- 114.Irani S.R. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang X. Inwardly rectifying potassium channel Kir4.1 is responsible for the native inward potassium conductance of satellite glial cells in sensory ganglia. Neuroscience. 2010;166:397–407. doi: 10.1016/j.neuroscience.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Djukic B. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vit J.P. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J. Neurosci. 2008;28:4161–4171. doi: 10.1523/JNEUROSCI.5053-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takeda M. Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain. 2011;152:2147–2156. doi: 10.1016/j.pain.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 119.Wu X.F. Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain. 2011;152:2605–2615. doi: 10.1016/j.pain.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 120.Mi H. Inwardly rectifying K+ channels that may participate in K+ buffering are localized in microvilli of Schwann cells. J. Neurosci. 1996;16:2421–2429. doi: 10.1523/JNEUROSCI.16-08-02421.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hayashi Y. Microglial Ca2+-activated K+ channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J. Neurosci. 2011;31:17370–17382. doi: 10.1523/JNEUROSCI.4152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsantoulas C. Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury. J. Neurosci. 2012;32:17502–17513. doi: 10.1523/JNEUROSCI.3561-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bearzatto B. Axonal transport of TREK and TRAAK potassium channels in rat sciatic nerves. Neuroreport. 2000;11:927–930. doi: 10.1097/00001756-200004070-00006. [DOI] [PubMed] [Google Scholar]
- 124.Hayashi Y. Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1661–R1670. doi: 10.1152/ajpregu.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Costigan M. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hendry L. KCNS1, but not GCH1, is associated with pain intensity in a black southern African population with HIV-associated sensory neuropathy: a genetic association study. J Acquir. Immune Defic. Syndr. 2013;63:27–30. doi: 10.1097/QAI.0b013e318285cf36. [DOI] [PubMed] [Google Scholar]
- 127.Gu Y., Gu C. Dynamics of Kv1 channel transport in axons. PLoS ONE. 2010;5:e11931. doi: 10.1371/journal.pone.0011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perkowski J.J., Murphy G.G. Deletion of the mouse homolog of KCNAB2, a gene linked to monosomy 1p36, results in associative memory impairments and amygdala hyperexcitability. J. Neurosci. 2011;31:46–54. doi: 10.1523/JNEUROSCI.2634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu Q. Disruption of Kv1.1 N-type inactivation by novel small molecule inhibitors (disinactivators) Bioorg. Med. Chem. 2008;16:3067–3075. doi: 10.1016/j.bmc.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 130.Peltola M.A. AMIGO is an auxiliary subunit of the Kv2.1 potassium channel. EMBO Rep. 2011;12:1293–1299. doi: 10.1038/embor.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pongs O., Schwarz J.R. Ancillary subunits associated with voltage-dependent K+ channels. Physiol. Rev. 2010;90:755–796. doi: 10.1152/physrev.00020.2009. [DOI] [PubMed] [Google Scholar]
- 132.Matsuyoshi H. Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons. Life Sci. 2012;91:258–263. doi: 10.1016/j.lfs.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu R. BKCa channels expressed in sensory neurons modulate inflammatory pain in mice. Pain. 2013 doi: 10.1016/j.pain.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Nockemann D. The K(+) channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol Med. 2013;5:1263–1277. doi: 10.1002/emmm.201201980. [DOI] [PMC free article] [PubMed] [Google Scholar]