Summary
Pancreatic β cells adapt to compensate for increased metabolic demand during insulin resistance. Although the microRNA pathway has an essential role in β cell proliferation, the extent of its contribution is unclear. Here, we report that miR-184 is silenced in the pancreatic islets of insulin-resistant mouse models and type 2 diabetic human subjects. Reduction of miR-184 promotes the expression of its target Argonaute2 (Ago2), a component of the microRNA-induced silencing complex. Moreover, restoration of miR-184 in leptin-deficient ob/ob mice decreased Ago2 and prevented compensatory β cell expansion. Loss of Ago2 during insulin resistance blocked β cell growth and relieved the regulation of miR-375-targeted genes, including the growth suppressor Cadm1. Lastly, administration of a ketogenic diet to ob/ob mice rescued insulin sensitivity and miR-184 expression and restored Ago2 and β cell mass. This study identifies the targeting of Ago2 by miR-184 as an essential component of the compensatory response to regulate proliferation according to insulin sensitivity.
Graphical Abstract
Highlights
-
•
Silencing of miR-184 during insulin resistance promotes its target Ago2
-
•
Loss of Ago2 during insulin resistance blocks pancreatic β cell proliferation
-
•
Ago2 mediates the suppression of Cadm1 by miR-375 in the β cell
-
•
Administration of the ketogenic diet to ob/ob mice rescues miR-184 in islets
Tattikota et al. find that as β cells adapt to increased metabolic demand during insulin resistance in obesity, miR-184 is silenced to alleviate repression of its target Argonaute2, a component of the microRNA-induced silencing complex. Argonaute2 promotes compensatory β cell proliferation via miR-375 and its target genes, including the growth suppressor Cadm1.
Introduction
Adaptation to environmental stress is a fundamental cellular process that promotes the maintenance of the physiologic steady state (Spriggs et al., 2010). Stress responses have been shown to induce numerous changes, such as activation of gene expression programs, which have evolved to allow for the cell to promote its own survival (Kültz, 2005, Ebert and Sharp, 2012). For example, in response to insulin resistance, the pancreatic β cell undertakes measures to proliferate and increase its output of secreted insulin. A coordinated increase in both β cell mass and secretory function constitutes the compensatory response to maintain normoglycemia (Muoio and Newgard, 2008). Although the underlying mechanisms directing these processes are still not completely understood, several studies have illustrated a role for metabolic changes in catalyzing β cell expansion (Steil et al., 2001). Furthermore, cellular pathways enabling the β cells to proliferate and adapt to increases in metabolic load may act by ultimately promoting signaling cascades essential to increasing both secretion and islet mass (Rhodes, 2005).
Recent evidence has shown the microRNA (miRNA) pathway as an important regulator of gene expression in response to metabolic stress (Leung and Sharp, 2010). Central to this mechanism are the Argonaute (Ago) proteins, which mediate this pathway by facilitating the interaction between miRNAs and their target mRNAs (Höck and Meister, 2008, Bartel, 2009). In addition, Ago proteins have been shown to accumulate in stress granules upon exposure to oxidative stress; however, their role in this compartment is not understood (Leung et al., 2006). Although loss of Argonaute2 (Ago2) expression in the MIN6 β cell line model resulted in enhanced secretion, its role in the stress response of the β cell has not been described (Tattikota et al., 2013).
We have previously shown that loss of miR-375 expression, among the most abundant miRNA in the pancreatic islet, inhibited the compensatory β cell proliferation in leptin-deficient ob/ob mice and resulted in severe hyperglycemia and diabetes (Poy et al., 2009). The absence of any dramatic effect on the development or specification of the different cell populations in the miR-375 knockout mouse may indicate a larger role for this miRNA in stress responses (Mendell and Olson, 2012). Furthermore, these observations suggest that many of the targets of miR-375 are also relevant to the adaptive response of the β cell and likely play a role in proliferation during metabolic stress. Although extensive sequencing efforts have identified ∼2,000 mature miRNA sequences in human tissues, relatively little is understood regarding how small RNAs coordinately function in these cellular processes (Kozomara and Griffiths-Jones, 2011).
Here, we show that miR-184 is silenced during insulin resistance to promote the expression of Ago2 in the pancreatic β cell. Deletion of Ago2 in ob/ob mice reduced compensatory proliferation of this cell type, thereby underlining an integral role for the miRNA pathway in this process. Moreover, we observed that Ago2 mediates the function of miR-375 in regulating the growth suppressor Cadm1. Taken together, our results show that several components of the miRNA pathway contribute in a concerted effort to facilitate proliferation of the β cell to meet metabolic demand during insulin resistance.
Results
Silencing of miR-184 in the Pancreatic β-Cell Promotes Its Target Argonaute2
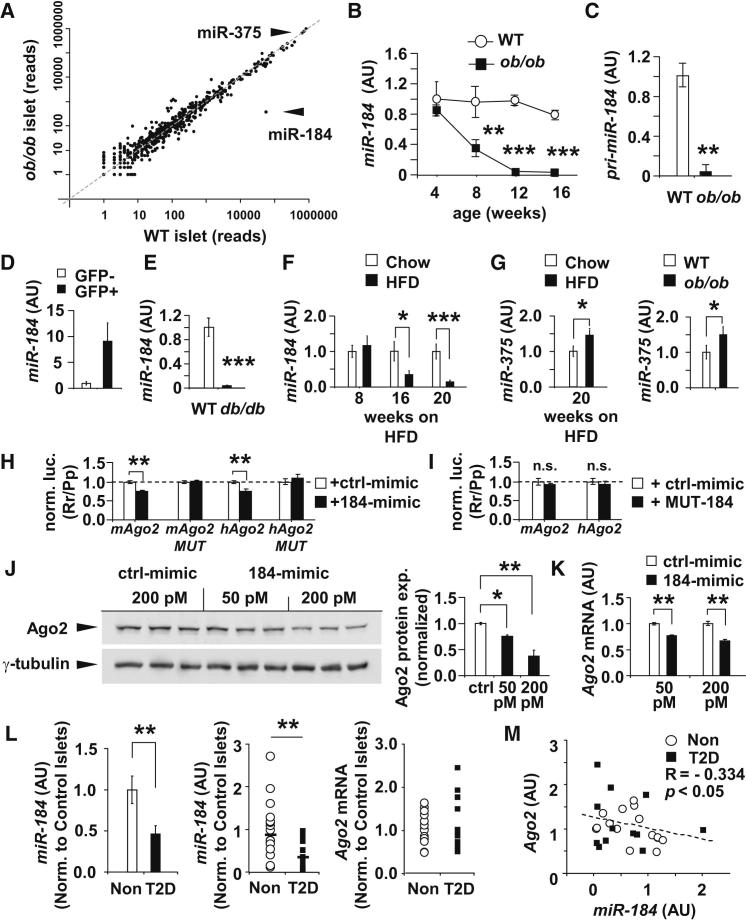
In light of the essential role of miR-375 in adaptive growth of the pancreatic β cell, we first sought to identify the additional components of the miRNA pathway that coordinately mediate this mechanism. We performed small RNA sequencing on total RNA from islets of 12-week-old ob/ob mice (Table S1 available online). Consistent with results by Zhao et al. (2009), expression of miR-184 was the most reduced miRNA identified (Figure 1A; Table S1). We then measured miR-184 in the islets of ob/ob mice from age 4–16 weeks and observed the decrease in expression starting at 8 weeks of age with the onset of resistance (Figures 1B, S1A, and S1B). Similarly, the pri-miR-184 transcript in the islets of ob/ob mice by quantitative real-time PCR was also silenced, indicating that this miRNA is regulated on a transcriptional level (Figure 1C). As recently described, miR-184 is enriched in pancreatic β cells as shown by quantitative real-time PCR from fluorescence-activated cell sorting (FACS)-sorted, GFP-positive β cells (MIP-GFP) (Figure 1D) (Hara et al., 2003, van de Bunt et al., 2013). We also observed a similar loss of expression of mature miR-184 in the islets of 12-week-old leptin receptor-deficient db/db mice and in mice on a high-fat diet (HFD; 60% calories from fat), all of which showed that this observation is not limited to one mouse model of obesity and insulin resistance (Figures 1E and 1F). In contrast to miR-184, miR-375 was modestly increased in HFD-fed animals as previously observed in ob/ob mice (Figure 1G) (Poy et al., 2009). In addition, the suppression of miR-184 was not observed in the eye of ob/ob mice, the highest site at which expression has been measured (Figures S1C and S1D).
Figure 1.
miR-184 Is Silenced during Insulin Resistance and Directly Targets Argonaute2
(A) Comparison of small RNA sequencing analysis from total RNA from islets of 12-week-old ob/ob and wild-type (WT) littermates.
(B) Quantitative real-time PCR analysis of miR-184 in islets of ob/ob and WT mice from 4–16 weeks of age (n = 3–5).
(C) Quantitative real-time PCR analysis of pri-miR-184 in islets of ob/ob mice and WT littermates at 16 weeks of age (n = 3–5).
(D) Quantitative real-time PCR analysis of miR-184 in FACS-sorted β cells from 16-week-old mouse insulin promoter-GFP mice (n = 6).
(E) Quantitative real-time PCR analysis of miR-184 in islets of db/db mice and WT littermates at age 12 weeks (n = 4).
(F) Quantitative real-time PCR analysis of miR-184 in islets of C57BL/6 mice on high-fat diet (HFD) or chow diet.
(G) Quantitative real-time PCR analysis of miR-375 in islets of C57BL/6 mice on HFD or chow diet and in islets of ob/ob mice and littermates at age 12 weeks (n = 6).
(H and I) Luciferase assays in MIN6 cells testing direct targeting of mouse and human Ago2 genes by miR-184 (184-mimic) or mutant (MUT-184).
(J and K) Western blot and quantitative real-time PCR analysis of Ago2 after transfection of miR-184-mimic and scrambled control.
(L) Quantitative real-time PCR analysis of miR-184 and Ago2 in islets from nondiabetic (Non) and type-2 diabetic human subjects (T2D) after normalization to RNU6b and TBP, respectively, expressed as fraction of control islets. p values represent a Mann-Whitney significance test (p = 0.009 for miR-184).
(M) Correlation of quantitative real-time PCR analysis from (L) of Ago2 and miR-184 in individual T2D (n = 12) and nondiabetic (n = 15) human subjects. Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
In order to identify direct targets of miR-184, we induced its expression in the MIN6 line with doxycycline after transfection with plasmids expressing the rtTA transactivator and miR-184 under control of the operator sequence of the Escherichia coli tetracycline-resistance operon (184-tetO). Illumina WG6v2 arrays measured changes in gene expression after 16 hr of treatment with doxycycline in triplicate (Figure S1E). Several computationally predicted genes for miR-184 were downregulated in both the array and luciferase-based experiments, including Ago2 (Figure 1H; Figures S1F–S1H) (Friedman et al., 2009). We cloned a portion of the mouse 3′ UTR of Ago2 (912 nt) and the complete human UTR of Ago2 (899 nt) into a luciferase reporter construct and observed decreased activity in the presence of the miR-184 mimic. In addition, mutating six nucleotides in the binding site within the mouse or human UTR (at position 152) from UCCGUCC to CGGGCGG abolished the inhibitory effect of miR-184 (Figure 1H). Conversely, transfecting a mutant miR-184 mimic had no effect on reporter activity from constructs bearing either the mouse or human Ago2 3′ UTR sequences (Figure 1I). Western blotting after overexpression of miR-184 resulted in an approximately 60% decrease in Ago2 protein levels, whereas quantitative real-time PCR revealed a 40% decrease in Ago2 mRNA in MIN6 cells (Figures 1J and 1K).
To address the relevance of this microRNA:target interaction in the islets of human subjects, we measured the expression of miR-184 and Ago2 in the pancreatic islets from 15 nondiabetic and 12 type 2 diabetic (T2D) donors. miR-184 was significantly decreased in T2D islets, indicating that the expression of this specific miRNA is linked to changes in metabolic status in both mice and humans (Figure 1L; Table S2). Whereas levels of Ago2 were not statistically significantly increased in T2D donor islets compared to the nondiabetic cohort, the inverse correlation between Ago2 and miR-184 expression was significant across the entire cohort (Figure 1M).
Argonaute2 Regulates β-Cell Proliferation
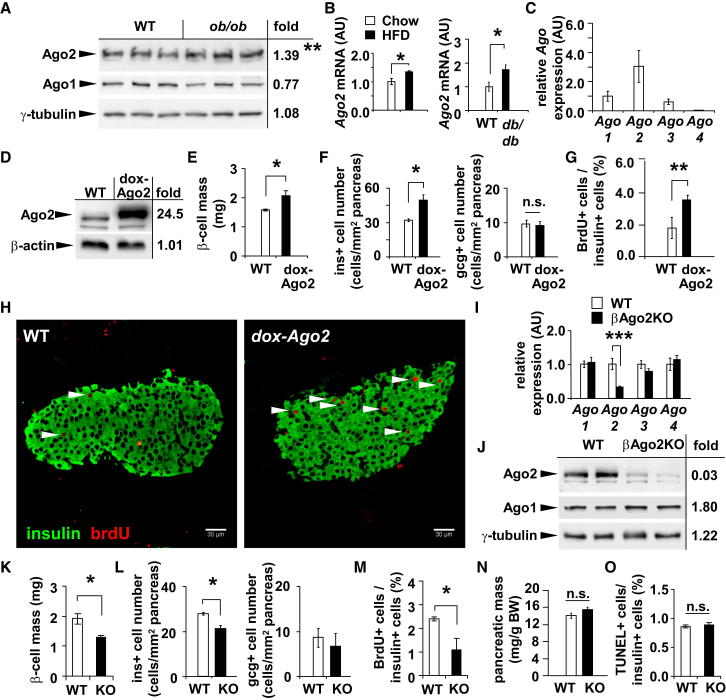
We next measured Ago2 expression in the islets of insulin-resistant ob/ob mice and observed increased levels at 8 weeks of age, whereas Ago1 was not altered (Figure 2A). Similarly, Ago2 levels were increased in islets isolated from 12-week-old db/db mice and C57BL/6 mice on a HFD for 16 weeks (Figures 2B). As shown in other tissues, Ago2 is the most abundant of the Ago family in GFP-positive β cells as measured by quantitative real-time PCR (Figures 2C and S2A) (Wang et al., 2012). To understand the contribution of Ago2 to growth and function of the β cell, we generated transgenic mice bearing the mouse Ago2 cDNA under the regulatory control of a tetracycline-responsive promoter element and crossed them with mice expressing the reverse tetracycline-controlled transactivator (rtTA) protein under the control of the rat insulin2 (Ins-rtTA) promoter as described (dox-Ago2) (Nir et al., 2007). Administration of doxycycline via drinking water for 30 days to four different lines (14, 30, 61, and 69) resulted in ∼1.5- to 25-fold increase of Ago2 protein levels in isolated islets (Figures 2D and S2B). Characterization of all lines showed no changes in body weight, random glucose, or insulin levels. After a glucose challenge, dox-Ago2 mice (line 30) exhibited transiently elevated glucose levels compared to littermates; however, no difference was observed in insulin sensitivity (Figures S2C and S2D). Moreover, acute-phase plasma insulin levels were diminished after glucose challenge, indicating reduced insulin release (Figure S2E). Morphometric analysis in dox-Ago2 (line 30) animals showed increased β cell mass in addition to increased insulin-positive cell number and BrdU incorporation compared to littermates (Figures 2E–2H).
Figure 2.
Argonaute2 Is Increased during Insulin Resistance and Promotes β-Cell Proliferation
(A) Western blot analysis of Ago1 and Ago2 from islets of 8-week-old ob/ob mice and wild-type (WT) littermates.
(B) Quantitative real-time PCR analysis of Ago2 in islets after 16 weeks HFD and from 12-week-old db/db mice (n = 3–6).
(C) Quantitative real-time PCR analysis of Argonaute genes in FACS-sorted β cells from 12-week-old MIP-GFP mice (n = 3).
(D) Western blot analysis of Ago2 from islets of 10-week-old dox-Ago2 mice (line 30) and WT littermates.
(E) β cell mass analysis of 10-week-old dox-Ago2 mice (line 30) and WT (n = 3).
(F) Morphometric analysis of insulin+ and glucagon+ cells in dox-Ago2 and WT at age 10 weeks (n = 5–6).
(G and H) Ratio of BrdU (red) and insulin+ cells (green) in 10-week-old dox-Ago2 mice and WT littermates (n = 4) after immunostaining. Scale bars = 30 μm.
(I) Quantitative real-time PCR analysis of Argonaute family members in islets of 10-week-old βAgo2KO mice and littermates (n = 4).
(J) Western blot analysis of Ago2 and Ago1 from islets of 10-week-old βAgo2KO mice and WT.
(K) β cell mass analysis of 10-week-old βAgo2KO and WT mice (n = 3).
(L) Morphometric analysis of insulin+ and glucagon+ cells in βAgo2KO and littermates at 10 weeks of age (n = 5–6).
(M) Ratio of BrdU+ and insulin+ cells in βAgo2KO and littermates at 10 weeks of age (n = 5–6).
(N) Pancreatic mass to total body mass ratio of βAgo2KO and littermates at 10 weeks of age (n = 5).
(O) Ratio of TUNEL+ and insulin+ cells in βAgo2KO and littermates (n = 3). Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01.
See also Figure S2.
We then established the conditional deletion in β cells by crossing mice bearing the floxed allele of the Ago2 gene with animals expressing Cre-recombinase under control of the rat insulin promoter (βAgo2KO) (O’Carroll et al., 2007, Herrera, 2000). Quantitative RT-PCR and western blotting analysis confirmed an efficient reduction in Ago2 expression in islets from βAgo2KO mice compared to littermate controls (Figures 2I and 2J). In contrast, Ago1 was upregulated in islets isolated from βAgo2KO mice, suggesting a degree of compensation by this family member on the protein level (Figure 2J). Both random-fed and fasted glucose and insulin levels were unchanged; however, βAgo2KO mice exhibited improved glucose tolerance without any alteration in insulin sensitivity at 10 weeks of age compared to littermates (Figures S2F and S2G). As previously observed in the MIN6 model, loss of Ago2 expression in vivo resulted in enhanced secretion of insulin after glucose challenge (Figure S2H) (Tattikota et al., 2013). In contrast to dox-Ago2 mice, we observed decreased β cell mass and number, BrdU incorporation, and pancreatic insulin content in βAgo2KO animals without any change in islet architecture, α cell number, or total pancreatic mass (Figures 2K–2N, S2I, and S2J). Moreover, no change was detected in TUNEL-positive β cells, indicating cell death was not a primary mechanism for the effect on β cell mass (Figure 2O). Lastly, we measured an increase in both β cell size and the total number of granules (Nv, measured as LDCV volume density), whereas the number of docked granules remained unchanged (Ns, LDCV surface density) in βAgo2KO mice compared to their littermates using electron micrographs (Figure S2K).
Loss of miR-184 Expression Promotes β-Cell Proliferation
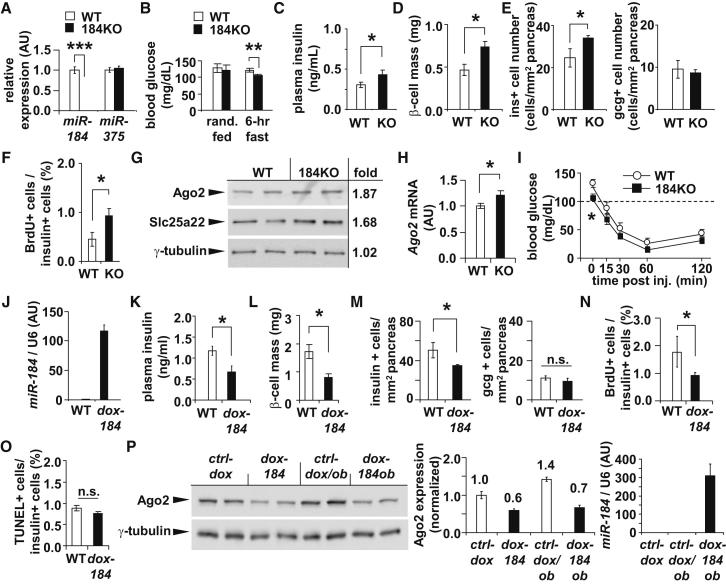
To determine the functional role of miR-184 in the β cell, we characterized the constitutive miR-184-knockout mouse (184KO) for changes in β cell proliferation, insulin release, and glucose homeostasis. The loss of miR-184 expression resulted in decreased fasted glucose levels and increased fasted plasma insulin levels (Figures 3A–3C). Consistent with loss of miR-184 expression during insulin resistance, morphometric analysis of pancreatic sections from 184KO mice showed an increase in β cell mass and number and BrdU incorporation rate (Figures 3D–3F). Moreover, in line with islet expression analysis in insulin-resistant models, Ago2 expression was increased in miR-184-deficient islets (Figures 3G and 3H). Knockout mice exhibited transiently elevated insulin release and improved tolerance after glucose challenge, indicating additional targets, such as Slc25a22, an established regulator of insulin release, may mediate the effect of miR-184 on secretion (Figures S1H, S3A, and S3B) (Casimir et al., 2009). Lastly, insulin sensitivity was unchanged in 184KO mice, indicating that loss of this miRNA in the β cell does not contribute to insulin resistance (Figure 3I).
Figure 3.
miR-184 Regulates Ago2 and Pancreatic β-Cell Proliferation In Vivo
(A) Quantitative real-time PCR analysis of miR-184 and miR-375 in islets of 10-week-old 184KO mice and wild-type (WT) littermates (n = 4).
(B) Blood glucose of 10-week-old 184KO mice and littermates (n = 4).
(C) Fasted plasma insulin levels of 10-week-old 184KO mice and littermates (n = 4).
(D) β cell mass analysis of 10-week-old 184KO and WT mice (n = 4).
(E) Morphometric analysis of insulin+ and glucagon+ cells in 184KO and WT at 10 weeks of age (n = 3).
(F) Ratio of BrdU (red) and insulin+ cells (green) in 12-week-old 184KO mice and WT (n = 3).
(G) Western blot analysis of Ago2, Slc25a22, and γ-tubulin from islets of 184KO mice and WT.
(H) Quantitative real-time PCR analysis of Ago2 in islets of 10-week-old 184KO mice and littermates (n = 4–5).
(I) Blood glucose levels during an ITT on 10-week-old 184KO mice and WT (n = 4–5).
(J) Quantitative real-time PCR analysis of miR-184 in islets of 12-week-old dox-184 mice and WT mice after 15 days on doxycycline (n = 4).
(K) Plasma insulin levels of 10-week-old dox-184 mice and WT after 15 days on doxycycline (n = 4).
(L) β cell mass analysis of 10-week-old dox-184 mice and WT after 15 days on doxycycline (n = 3).
(M) Morphometric analysis of insulin+ and glucagon+ cells in 10-week-old dox-184 mice and WT (n = 4).
(N) Ratio of BrdU and insulin+ cells in dox-184 mice and WT (n = 4).
(O) Ratio of TUNEL+ and insulin+ cells in dox-184 mice and WT (n = 3).
(P) Western blot analysis of Ago2 and γ-tubulin after ex vivo treatment of doxycycline on the islets of dox-184 and dox-184ob mice compared to islets from respective control lean or ob/ob littermates. Densitometry is normalized to γ-tubulin expression of ctrl-dox islets. Quantitative real-time PCR analysis of miR-184 in dox-184ob mice and ob/ob littermates. Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01.
See also Figure S3.
Restored Expression of miR-184 in ob/ob Mice Inhibits Ago2 and β-Cell Proliferation
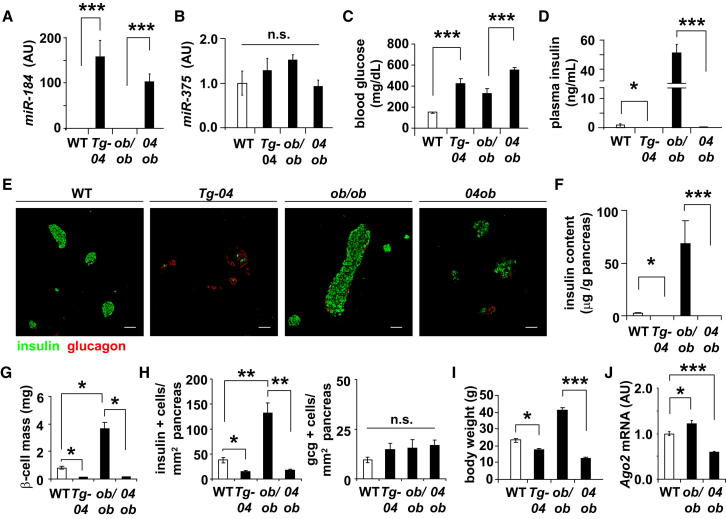
Two gain-of-function models for miR-184 were then generated in order to restore its expression in the β cell and validate the regulation of Ago2 by this miRNA during insulin resistance. Similar to dox-Ago2 mice, Ins2-rtTA mice were crossed to newly generated mice bearing the mouse miR-184 precursor under regulatory control of a tetracycline-responsive promoter element (dox-184). Administration of doxycycline for 15 days via drinking water (1 mg/ml) resulted in ∼100-fold increase in expression (Figure 3J). No change was observed in body weight; however, dox-184 animals exhibited decreased plasma insulin levels, β cell mass and number, and BrdU incorporation (Figures 3K–3N and S3C). The number of apoptotic cells was unchanged, indicating the hyperglycemia (>250 mg/dl) and glucose intolerance in dox-184 mice were due to a decrease in β cell mass and rate of proliferation (Figures 3O and S3D–S3F). Isolated islets from dox-184 mice treated with doxycycline for 48 hr ex vivo exhibited decreased Ago2 expression (Figure 3P). Moreover, we crossed dox-184 mice onto the ob/ob background (dox-184ob) and observed a 50% reduction in Ago2 expression after doxycycline treatment and restoring expression of miR-184 (Figure 3P). Furthermore, whereas islet levels of miR-375 and Ago1 were unchanged, random blood glucose levels increased and plasma insulin levels were significantly reduced in dox-184ob mice (Figures S3G–S3J). An additional transgenic model of constitutive overexpression was developed to further confirm the role of miR-184 in β cell growth and function. We focused on three independent mouse lines (Tg-96, Tg-04, and Tg-32), which showed by Southern analysis varying numbers of the transgene containing the miR-184 precursor sequence under control of the rat insulin promoter (Figure S3K). All lines exhibited a mild decrease in body weight, but the highest overexpression of miR-184 (∼100-fold in Tg-04 and 32) resulted in severe hyperglycemia and reduced systemic insulin levels, whereas a 5-fold increase (Tg-96) induced glucose intolerance and impaired insulin release (Figures S3L–S3Q). Similar to dox-184 animals, Tg-04 and Tg-32 animals exhibited hyperglycemia and reduced circulating insulin due to loss of β cell mass and pancreatic insulin content (Figures 4A–4G and S4A–S4G). Crossing Tg-04 animals onto the ob/ob background (04ob) resulted in sustained expression of miR-184 and did not compromise the most abundant miRNAs, such as miR-375 (Figures 4A and 4B). As in dox-184ob mice, 04ob mice exhibited severe hyperglycemia as a result of diminished insulin levels and β cell mass and ultimately contributed to weight loss (Figures 4C–4I). Lastly, restoring expression of miR-184 in β cells of 04ob mice suppressed Ago2 expression compared to ob/ob littermates (Figure 4J).
Figure 4.
Restoration of miR-184 during Insulin Resistance Inhibits Compensatory β-Cell Proliferation
(A) Quantitative real-time PCR analysis of miR-184 in islets of 8-week-old wild-type (WT), Tg-04, ob/ob, and 04ob mice (n = 3).
(B) Quantitative real-time PCR analysis of miR-375 in islets of 8-week-old WT, Tg-04, ob/ob, and 04ob mice (n = 4).
(C) Random blood glucose of 8-week-old WT, Tg-04, ob/ob, and 04ob mice (n = 4–12).
(D) Plasma insulin concentrations of 8-week-old WT, Tg-04, ob/ob, and 04ob mice (n = 3–6).
(E) Immunostaining analysis of pancreatic sections in 8-week-old WT, Tg-04, ob/ob, and 04ob mice for insulin (green) and glucagon (red). Scale bars = 50 μm.
(F) Pancreatic insulin content in 8-week-old WT, Tg-04, ob/ob, and 04ob mice (n = 4–6).
(G) β cell mass analysis of 10-week-old WT, Tg-04, ob/ob, and 04ob mice (n = 3).
(H) Quantification of insulin+ and glucagon+ cells per area of pancreas in WT, Tg-04, ob/ob, and 04ob mice (n = 4).
(I) Body weight analysis of WT, Tg-04, ob/ob, and 04ob mice (n = 4–12).
(J) Quantitative real-time PCR analysis of Ago2 in islets of 10-week-old WT, ob/ob, and 04ob mice (n = 4–5). Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
See also Figure S4.
Argonaute2 Mediates miR-375 Function
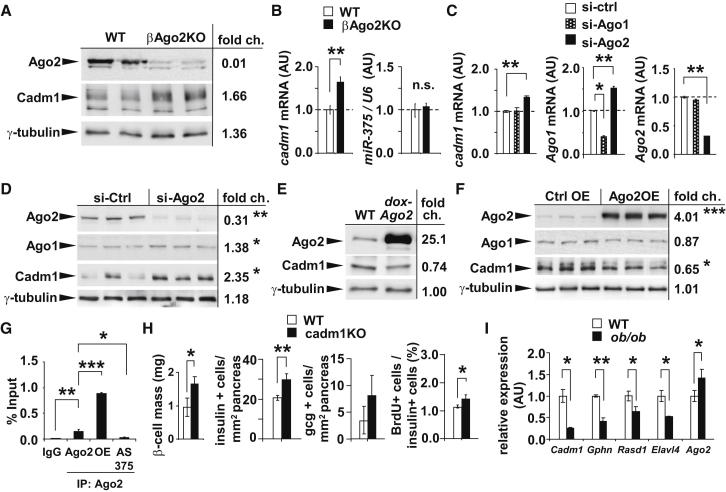
We next measured the expression of miR-375-targeted genes in islets from βAgo2KO mice and confirmed increased protein expression in vivo (Figure S5A) (Tattikota et al., 2013). Conversely, expression analysis of these miR-375 targets in islets from dox-Ago2 mice showed decreased levels by quantitative real-time PCR (Figure S5B). While immunoprecipitation of Ago2 from untransfected MIN6 cells efficiently recovered miR-375, we observed an enrichment of Gephyrin, Elavl4/HuD, and Rasd1 in the Ago2-containing complexes compared to control pull-downs (Figures S5C and S5D). Moreover, overexpression (OE) of Ago2 recovered higher levels of these specific mRNAs, whereas transfection of inhibitory antisense oligonucleotides against miR-375 (AS 375) depleted their presence in the protein complex (Figure S5D). We also observed that expression of the growth suppressor Cadm1 was increased in islets of βAgo2KO mice, further indicating that the regulation of several targets of miR-375 was dependent on Ago2 (Figures 5A and 5B) (Poy et al., 2009). The higher levels of miR-375 target genes were accompanied by no change in miR-375 expression, indicating that the loss of Ago2 can nullify the impact of abundant miRNAs on their respective targets (Figure 5B). We next measured Cadm1 expression after siRNA-mediated knockdown of Ago2 in MIN6 cells and observed a similar increase, but not after knockdown of Ago1 (Figures 5C and 5D). In addition, increasing Ago2 levels in both islets of dox-Ago2 mice and in MIN6 cells (Ago2OE) decreased Cadm1 expression (Figures 5E and 5F). As observed with the other miR-375 targets, we detected enrichment of Cadm1 in Ago2-containing complexes, indicating its regulation was also dependent upon this member of the Ago family (Figure 5G). In line with its established role as a growth suppressor, morphometric analysis in cadm1-deficient mice (cadm1KO) showed increased β cell mass and number and BrdU incorporation without any change in total pancreatic mass (van der Weyden et al., 2006, van der Weyden et al., 2012) (Figures 5H and S5E). Lastly, consistent with observations in dox-Ago2 animals, quantitative real-time PCR analysis in the islets of ob/ob mice and littermate controls showed the miR-375 targets Cadm1, Gephyrin, Rasd1, and Elavl4/HuD to be decreased, whereas Ago2 expression was significantly upregulated (Figure 5I).
Figure 5.
Argonaute2 Mediates miR-375 Function in the Pancreatic β-Cell
(A) Western blot analysis of Ago2 and Cadm1 from islets of 10-week-old βAgo2KO mice and WT littermates.
(B) Quantitative real-time PCR analysis of cadm1 and miR-375 in islets of 10-week-old βAgo2KO mice and WT littermates (n = 4).
(C) Quantitative real-time PCR analysis of Cadm1, Ago1, and Ago2 after siRNA-mediated knockdown of Ago1 and Ago2 in MIN6 cells (n = 4).
(D) Western blot analysis of Ago2, Ago1, and Cadm1 after siRNA-mediated knockdown of Ago2 compared to scrambled control.
(E) Western blot analysis of Ago2 and Cadm1 in islets from 10-week-old dox-Ago2 mice and WT.
(F) Western blot analysis of Ago2, Ago1, and Cadm1 after overexpression of Ago2 compared to transfection control.
(G) Quantitative real-time PCR analysis of Cadm1 after immunopreciptation of Ago2 from MIN6 cells untransfected (Ago2), after overexpression of Ago2 (OE), and after inhibition of miR-375 with antisense oligonucleotides (AS375) (n = 4).
(H) β cell mass, morphometric analysis of insulin+ and glucagon+ cells, and ratio of BrdU to insulin+ cells in 18-week-old cadm1-knockout mice and littermates (n = 4).
(I) Quantitative real-time PCR analysis of Cadm1, Gephyrin, Rasd1, Elavl4, and Ago2 in islets of ob/ob and WT mice at 16 weeks of age (n = 5–6). Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01.
See also Figure S5.
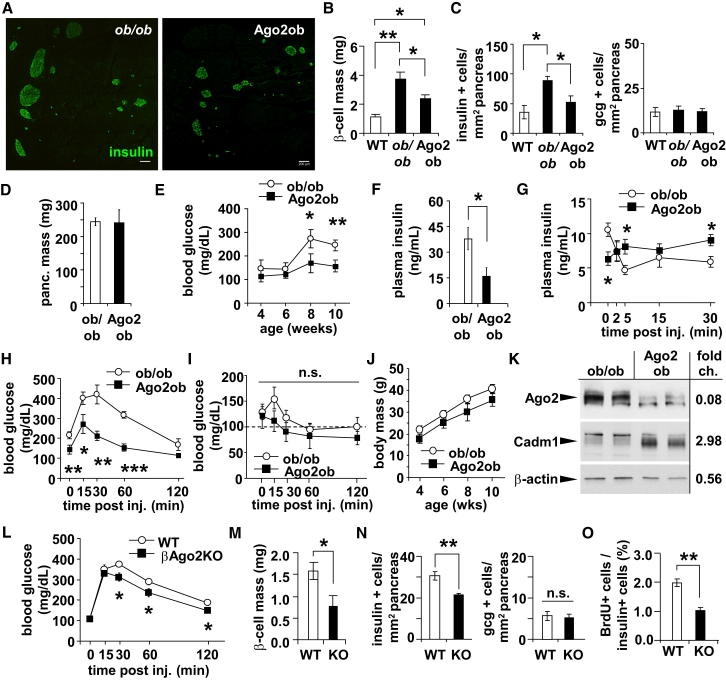
Loss of Argonaute2 Blocks Proliferation during Insulin Resistance
To address whether Ago2 mediates the compensatory expansion of β cells during insulin resistance, we next crossed mice carrying the Ago2-floxed allele onto the ob/ob background to generate mice deficient in both Ago2 and leptin (Ago2ob). In line with βAgo2KO mice, β cell mass and the number of insulin-positive cells were significantly reduced in Ago2ob animals compared to ob/ob littermates without any alterations in the number of glucagon-positive cells or total pancreatic mass (Figures 6A–6D). Furthermore, random glucose levels and the corresponding insulin levels were decreased, thereby underlining an important functional role for Ago2 in the growth and function of the β cell (Figures 6E and 6F). In spite of the decrease in β cell mass, Ago2ob mice exhibited higher circulating insulin levels after challenge and improved glucose tolerance, confirming again that loss of Ago2 increases secretion in vivo (Figures 6G and 6H). Insulin sensitivity, body weight, or food intake (5.2 ± 0.4 in ob/ob and 5.0 ± 0.3 g/day in Ago2ob mice) were not significantly altered, but the decrease in both circulating glucose and insulin suggests insulin resistance has been partially attenuated (Figures 6I and 6J). In addition, western blotting analysis of isolated islets showed that loss of Ago2 expression resulted in increased levels of the miR-375 target Cadm1 (Figure 6K). Similar results in islet morphometry and glucose tolerance were observed in βAgo2KO mice placed on a HFD, indicating that the effect on β cell mass was consistent in multiple forms of insulin resistance and, although extrapancreatic recombination has been shown with this Cre line, including in the hypothalamus, energy expenditure or food intake was not affected (Figures 6L–6O and S6A–S6D).
Figure 6.
Loss of Argonaute2 during Insulin Resistance Inhibits Compensatory Proliferation
(A) Immunostaining of pancreatic sections from Ago2ob mice and ob/ob littermates with antibodies to insulin (green) and glucagon (red). Scale bars = 200 μm.
(B) β cell mass analysis of 10-week-old wild-type (WT), ob/ob, and Ago2ob mice (n = 3).
(C) Morphometric analysis of insulin+ and glucagon+ cells in 10-week-old WT, ob/ob, and Ago2ob (n = 4–5).
(D) Pancreatic weight in 10-week-old Ago2ob mice and ob/ob littermates (n = 4–5).
(E and F) Random blood glucose and plasma insulin levels of 10-week-old Ago2ob and littermate ob/ob mice (n = 4–5).
(G) Plasma insulin levels after glucose bolus on 10-week-old Ago2ob mice and ob/ob littermates (n = 4–5).
(H) Blood glucose levels during a GTT on 10-week-old Ago2ob mice and ob/ob littermates (n = 4–5).
(I) Blood glucose levels during an ITT on 10-week-old Ago2ob mice and ob/ob littermates (n = 4–5).
(J) Body weight of Ago2ob mice and ob/ob mice from 4–10 weeks of age (n = 4–5).
(K) Western blot analysis of Ago2 and Cadm1 in islets from 10-week-old Ago2ob mice and ob/ob littermates.
(L) Blood glucose levels during a GTT on 16-week-old βAgo2KO mice and littermates after 12 weeks on a HFD (n = 6).
(M) β cell mass analysis in 16-week-old βAgo2KO mice and littermates after 12 weeks on a HFD (n = 6).
(N and O) Morphometric analysis of insulin+, glucagon+, and BrdU+ cells in 16-week-old βAgo2KO mice and littermates after 12 weeks on a HFD (n = 6). Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
See also Figure S6.
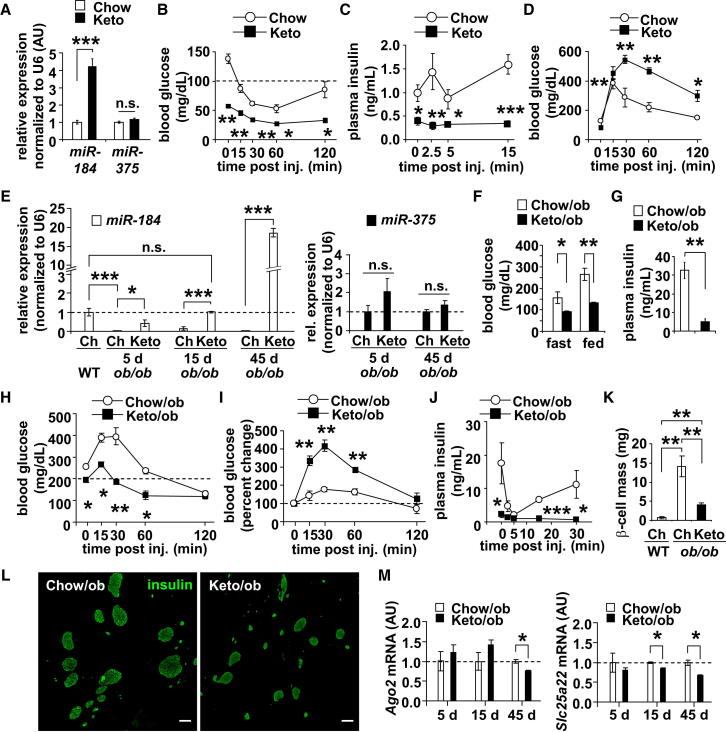
Administration of the Ketogenic Diet to ob/ob Mice Restores miR-184 Expression
Published studies have shown that the administration of the ketogenic diet improves insulin sensitivity and glycemic status (Badman et al., 2009). To further support the functional relevance of miR-184 and Ago2 in the β cell, we next tested whether miR-184 is regulated after administering the ketogenic diet to C57BL/6 mice (Keto mice). After 24 days on the diet, we observed ∼4-fold increase of miR-184 in islets from Keto mice (Figure 7A). As previously shown, Keto mice exhibited decreased body weight and reduced glucose levels at steady state and during an ITT in line with improved insulin sensitivity (Figures 7B, S7A, and S7B). Consistent with mild ∼4-fold, overexpression of miR-184, Keto mice exhibited reduced insulin levels and impaired tolerance compared to control mice after a glucose challenge; however, no change was observed in β cell mass (Figures 7C, 7D, and S7C). Moreover, administration of the ketogenic diet to ob/ob mice (Keto/ob) for 15 days was sufficient to restore normal expression of miR-184; however, after 45 days, we observed a ∼18-fold increase in the expression of this miRNA in islets from Keto/ob mice compared to chow-fed, nonobese littermate controls (Ch-WT) (Figure 7E). Keto/ob mice also exhibited lower glucose levels during an ITT, reduced random glucose and plasma insulin levels, and no change in body weight (Figures 7F–7H, S7D, and S7E). Similar to Keto mice, Keto/ob mice were relatively more glucose intolerant as a result of decreased insulin release compared to Chow/ob littermates (Figures 7I and 7J). These results may reflect a significant reduction in the demand for insulin because Keto/ob mice are normoglycemic. Moreover, after 45 days on the ketogenic diet, we observed a decrease in β cell mass and Ki-67+ β cells in Keto/ob animals, and quantitative real-time PCR analysis at this time point showed Ago2 and Slc25a22 expression to be significantly reduced, whereas measurements produced by western blotting showed Cadm1 to be increased (Figures 7K–7M, S7F, and S7G). Taken together, these data further show that both miR-184 and Ago2 expression are inversely regulated in accordance with changes in the requirement for insulin.
Figure 7.
Administration of the Ketogenic Diet during Insulin Resistance Rescues miR-184 Expression
(A) Quantitative real-time PCR analysis of miR-184 and miR-375 in islets of 10-week-old C57BL/6 mice on chow (Chow) or ketogenic diet (Keto) for 24 days (n = 4).
(B) Blood glucose levels during an ITT on 10-week-old Chow and Keto mice for 24 days (n = 4–5).
(C and D) Plasma insulin and blood glucose levels after glucose challenge on 10-week-old Chow and Keto mice for 24 days (n = 4–5).
(E) Quantitative real-time PCR analysis of miR-184 and miR-375 in islets of 16-week-old ob/ob mice on chow or ketogenic diet and WT littermates (n = 4).
(F and G) Blood glucose and random plasma insulin levels in 16-week-old ob/ob mice on chow (Chow/ob) or ketogenic diet (Keto/ob) for 15 days (n = 4).
(H) Blood glucose levels during an ITT on 16-week-old ob/ob mice on chow or ketogenic diet for 15 days (n = 4–5).
(I and J) Plasma insulin and blood glucose levels after glucose challenge on 16-week-old ob/ob mice on chow or ketogenic diet for 15 days (n = 4-5).
(K and L) Quantification of β cell mass after immunostaining pancreatic sections for insulin (green) of 16-week-old ob/ob mice on chow or ketogenic diet for 45 days (n = 3). Scale bars = 200 μm.
(M) Quantitative real-time PCR analysis of Ago2 and Slc25a22 in islets of 16-week-old ob/ob mice on chow or ketogenic diet (n = 4–5). Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
See also Figure S7.
Discussion
Here, we show that the onset of insulin resistance induces the silencing of miR-184 in order to promote the function of Ago2 in the pancreatic β cell. Our data suggest that miR-184 acts as a “brake” on Ago2 that becomes released to increase proliferation and accommodate the elevated demand for insulin. Moreover, we show that the increased expression of Ago2 will facilitate the function of miR-375 in suppressing genes, including Cadm1 in vivo. Our observations functionally associating miR-184 together with Ago2 and miR-375 now identify a network within the miRNA pathway that coordinately regulates the compensatory proliferation of the β cell.
Interestingly, miR-184 is unique as the most downregulated islet miRNA during insulin resistance, and additionally, administration of the ketogenic diet in leptin-deficient ob/ob mice rescued its expression. Furthermore, the increase of miR-184 expression in the islets of ob/ob mice after restoring insulin sensitivity further underlines a dynamic role for the miRNA pathway in regulating β cell growth and function according to the demand for insulin. Published work identified the regulation of miRNAs in the retina according to light levels, thereby linking turnover of small RNAs with physiologic stimuli in neuronal cells; together, our results may indicate another common property between these cell types (Krol et al., 2010). Likewise, after treatment with serotonin in Aplysia sea snails, miR-184 was significantly downregulated in the nervous system, the site of orthologous insulin expression (Rajasethupathy et al., 2009, Floyd et al., 1999). Moreover, serotonin has been previously shown to mediate compensatory expansion of the β cell during pregnancy (Kim et al., 2010). In line with these studies, we observed that miR-184 was transiently decreased at day 18.5 of gestation in C57BL/6 mice and that the expression of this miRNA had been restored by day 5 in the postpartum phase. Neither tryptophan hydroxlase 1 nor 2 (Tph1/2) were regulated in the pancreatic islets of ob/ob mice, further suggesting that multiple independent pathways promote proliferation in these models (Rieck et al., 2009). However, the robust consistency of the silencing of miR-184 in both models of pregnancy and insulin resistance suggests that additional genes in the miRNA pathway will be identified as mediators of β cell proliferation (Jacovetti et al., 2012).
Importantly, our results continue to build on the numerous studies now supporting a role for miRNAs in mediating cellular stress responses (Leung and Sharp, 2010, Mendell and Olson, 2012). The observations made in Ago2ob animals would continue to indicate that several abundant miRNAs in the β cell coordinately contribute to this process. In addition, our current investigation continues to reinforce the role of miRNAs as inhibitors of insulin release (Poy et al., 2004, Tattikota et al., 2013, Bolmeson et al., 2011). Although the precise role of miRNAs remains unclear, the accumulating evidence may suggest that “brakes” on gene expression are essential in managing the dynamic nature of both first- and second-phase insulin release (Wang and Thurmond, 2009). Of note, the increased release of insulin after loss of Ago2 expression in the β cells of ob/ob mice improved many aspects of the diabetic phenotype, including circulating glucose and insulin levels, and glucose tolerance; however, this came at the expense of β cell mass and may suggest that the miRNA pathway mediates a delicate balance between both growth and secretion. Many of the genes being targeted by small RNAs in the β cell may regulate this balance via independent mechanisms. Several studies indicate F-actin and the microtubule networks are actively engaged in managing insulin release and many of the targets of miR-375, including Cadm1 and Gephyrin, may contribute to the remodeling of the cytoskeleton (Seino et al., 2011, Charrier et al., 2010, Masuda et al., 2010). In addition, published studies have already shown that during pregnancy, Rasd1 acts as a growth suppressor and is inhibited during islet expansion and then is increased in the postpartum phase when islet mass is restored (Lellis-Santos et al., 2012). Also, inhibition of the RNA-binding protein HuD/Elavl4 was shown to promote expression of insulin (Lee et al., 2012). Lastly, as evidenced in the differences in the phenotypes of dox-184 and βAgo2KO mice, our observations supporting Slc25a22, a regulator of glutamate transport and secretion in the β cell, as an additional target of miR-184 indicates that this miRNA may also regulate both proliferation and exocytosis through the targeting of different genes (Casimir et al., 2009). Future studies will presumably continue to establish that numerous targets of all miRNAs conspire to orchestrate the demands placed on the cell during insulin resistance, including growth and the continual recruitment of insulin-containing granules. It will also remain to be seen whether specific miRNA-mediated gene regulations are unique to their metabolic context, including insulin resistance, fasting, hypoxia, and cold exposure (Dumortier et al., 2013). Published studies have illustrated reduced expression of miR-375 in the pancreatic islets of db/db mice and Goto-Kakizaki rats, suggesting that differential roles may exist for miRNAs and their targets according to alterations in the metabolic environment (Zhu et al., 2013, El Ouaamari et al., 2008).
Therefore, it will be important to understand the factors that dictate the kinetics of miRNA regulation in the β cell. It also remains to be determined whether the same genes that expedite the decay of miR-184 during insulin resistance are the same factors that promote its expression as sensitivity improves after administration of the ketogenic diet. Moreover, the increase in miR-184 that results from this diet and the resulting suppression of insulin release indicate that miRNAs play a significant role in the maintenance of steady-state circulating glucose and insulin levels. Furthermore, the identification of increased miR-184 expression can explain the simultaneous occurrence of both improved insulin sensitivity and glucose intolerance observed in mice on ketogenic diet. Although the results appear paradoxical at first, this may be explained by the drastic reduction in both circulating glucose and the demand for insulin. Another apparent conflict appears in the mouse models exhibiting hypersecretion and decreased β cell mass. However, in spite of the reduction in β cell mass in Ago2ob mice (35% lower compared to ob/ob littermates), the decrease is significantly below the threshold that would induce hyperglycemia (Orland et al., 1985). Ago2-deficient models still maintain the capacity to transiently secrete higher amounts of insulin because β cells release a relatively small percentage of its stored granule pool in response to glucose (Seino et al., 2011). Conversely, although increased expression of Ago2 causes transient glucose intolerance in dox-Ago2 mice, these mice have normal circulating glucose and insulin levels and insulin sensitivity, and therefore it is unlikely that the increase in Ago2 levels in the islets of ob/ob mice plays a causal role in the hyperglycemia or hyperinsulinemia. As shown in dox-184ob animals, restoring miR-184 in the β cell and reducing Ago2 levels in an insulin-resistant model exacerbated the diabetic phenotype. In contrast, reducing glucose levels and the demand for insulin in ob/ob mice by administration of ketogenic diet to ultimately suppress Ago2 and restore β cell mass underlines an important function this gene plays in the adaptation of the β cell according to metabolic need.
Many aspects of β cell physiology remain largely unexplored in terms of nutrient sensing, cellular metabolism, and intercellular signaling pathways. Our observations on the effects of the ketogenic diet on miRNA function in the β cell unify several poorly understood mechanisms and reinforce the potential in studying the role of small RNAs in physiologic stresses. Continued emphasis on characterizing the functional role of miRNA targets in this cell type should bring clarity in understanding how the network of small RNAs contribute to maintaining essential metabolic processes and how their failure ultimately leads to disease.
Experimental Procedures
Generation and Maintenance of Animals
Mice were maintained on a 12 hr light/dark cycle with ad libitum access to regular chow food, a HFD (containing 60% kcal fat, cat. no. E15741-347, ssniff Spezialdiäten GmbH), or a ketogenic diet (cat. No. E15149-30, ssniff Spezialdiäten GmbH) in accordance with requirements established by Landesamt für Gesundheit und Soziales (Lageso). All experimental procedures were approved under protocols G 0357/10, O 0405/09, and T 0436/08. Results were consistent in both genders; however, data from female mice are not shown. Total Cadm1 knockouts and floxed Ago2 mice were generated as described (van der Weyden et al., 2006, O’Carroll et al., 2007). The total miR-184 knockout was generated by D. Aberdam and R. Shalom-Feuerstein (Institut Clinique de la Souris) and genotyped with forward and reverse primers 5′ ACTGAACATTATTTCATGGGCCGGG and 5′ AACTACAACTGTTTGGCTAGCAGGGTG (knockout allele) and the substitute reverse primer 5′ CGCTGAGACCTTGTGATAAACCGTT (wild-type allele). To generate dox-184 mice, a 292 bp genome fragment encompassing miR-184 precursor was amplified using the primers 5′ TGCTGAAGAGTGGCCTGCTAGG and 5′ CTCCTCCTCACGTCCTGTGGTA, cloned into a pTRE-2 vector (Clontech), and the resulting construct was used for microinjections. The Ago2-tetO mice were generated from a mouse Ago2 cDNA construct (a kind gift of G. Meister, University of Regensburg) (Nir et al., 2007).
Gene Expression Analysis in Mouse and Human Islets
Small RNA sequencing and expression analysis from cell lines and mouse islets was performed as described; primers used with FastStart SYBR Green PCR Master Mix (Roche) are available upon request (Tattikota et al., 2013). Relative quantification of Ago genes was determined after normalization with standard curves established with mouse Ago1-4 cDNA constructs (G. Meister). RIP experiments using Ago1 and Ago2 antibodies were performed as described (Keene et al., 2006). Human islets were obtained with research consent and selected based on islet cell purity and isolated at the University of Virginia, Oxford DRWF Human Islet Isolation Facility and Prodo Laboratories (USA) (Morán et al., 2012). Information for nondiabetic and diabetic donors is provided in Table S2.
Analytic Procedures
Insulin measurements from plasma and pancreatic extracts were measured by radioimmunoassay (RIA) (Millipore), and blood glucose and luciferase assays were measured as described previously (Poy et al., 2009). Islet morphometric analysis after intraperitoneal injections of BrdU on four consecutive days (50 μg/g BW, Sigma B5002) was performed on 8 μm sections of paraffin-embedded pancreas approximately 150–200 μm apart. Sections were dewaxed, washed, and stained for insulin (Dako A0564), glucagon (Millipore AB932), BrdU (Abcam ab6326), Ki-67 (Dako), or TUNEL (Roche cat. no.12156792910). Cell numbers from all islets in 3–7 sections were counted with ImageJ software from 20X images obtained using a Zeiss LSM700 (Schneider et al., 2012). β cell mass was measured as the ratio of insulin-positive cell area to the total tissue area, multiplied by the weight of the pancreas using Imaris software (Bitplane). In vivo insulin release and glucose (GTT) or insulin (ITT) tolerance tests were performed following a 6 hr fast and injected intraperitoneally with either glucose (2 g/kg BW) or insulin (0.75 U/kg BW).
Cell Culture, Immunoprecipitation, and Antibodies
MIN6 cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) containing 4.5 g/l glucose supplemented with 15% v/v heat-inactivated FCS, 50 μM β-mercaptoethanol, and 50 mg/ml penicillin, and 100 mg/ml streptomycin and insulin release was performed as described (Poy et al., 2004). Antibodies were as described: Gephyrin (BD 610584), Rasd1 (Millipore AB15794), Ago2 (for immunoprecipitation, Wako 018-22021), Ago2 (for western blotting, Cell Signaling C34C6), Ago1 (MBL RN028PW), Slc25a22 (Sigma AV44041), HuD/Elavl4 (Santa Cruz sc-48421), γ-tubulin (Sigma T6557), Cadm1 (Sigma S4945), and β-actin (Sigma). Guinea pig anti-insulin and rabbit anti-glucagon antibodies (Millipore) were used on paraffin-embedded pancreata fixed in 4% paraformaldehyde for 3 hr.
Statistical Analysis
Pearson’s R coefficient and independent two-group Mann-Whitney tests were implemented using the R statistical package (http://www.r-project.org). Comparisons between data sets with two groups were evaluated using an unpaired Student’s t test. ANOVA analysis was performed for comparisons of three or more groups. A p value of less than or equal to 0.05 was considered statistically significant. Results presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Acknowledgments
This work was funded by the Helmholtz Gemeinschaft, an ERC Starting Grant (IsletVasc 260744), the Fritz Thyssen Stiftung, the Deutsches Zentrum fuer Herz-Kreislauf Forschung, E.V. (DZHK), ANR11-03 erare2-SkinDev (to D.A.), Swedish Diabetes Association, Swedish Research Council, and the Wellcome Trust (095101/Z/10/Z [to A.L.G.] and 101033/Z/13/Z [to J.F.]). The authors thank L.v.d. Weyden for providing the Cadm1 knockout mouse and T. Horvath, M. Landthaler, T. Willnow, M. Gotthardt, F. Spagnoli, R. Schweiker, K.L. Brayman, H.P. Rahn, M. Richter, A. Sporbert, and the MDC Microscopy Core Facility for assistance in conducting this work.
Published: December 19, 2013
Footnotes
Supplemental Information includes seven figures, Supplemental Experimental Procedures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.cmet.2013.11.015.
Accession Numbers
The NCBI Gene Expression Omnibus (GEO) accession number for referenced array data is GSE46623.
Supplemental Information
References
- Badman M.K., Kennedy A.R., Adams A.C., Pissios P., Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am. J. Physiol. Endocrinol. Metab. 2009;297:E1197–E1204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmeson C., Esguerra J.L.S., Salehi A., Speidel D., Eliasson L., Cilio C.M. Differences in islet-enriched miRNAs in healthy and glucose intolerant human subjects. Biochem. Biophys. Res. Commun. 2011;404:16–22. doi: 10.1016/j.bbrc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Casimir M., Lasorsa F.M., Rubi B., Caille D., Palmieri F., Meda P., Maechler P. Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J. Biol. Chem. 2009;284:25004–25014. doi: 10.1074/jbc.M109.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier C., Machado P., Tweedie-Cullen R.Y., Rutishauser D., Mansuy I.M., Triller A. A crosstalk between β1 and β3 integrins controls glycine receptor and gephyrin trafficking at synapses. Nat. Neurosci. 2010;13:1388–1395. doi: 10.1038/nn.2645. [DOI] [PubMed] [Google Scholar]
- Dumortier O., Hinault C., Van Obberghen E. MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metab. 2013;18:312–324. doi: 10.1016/j.cmet.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Ebert M.S., Sharp P.A. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ouaamari A., Baroukh N., Martens G.A., Lebrun P., Pipeleers D., van Obberghen E. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes. 2008;57:2708–2717. doi: 10.2337/db07-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd P.D., Li L., Rubakhin S.S., Sweedler J.V., Horn C.C., Kupfermann I., Alexeeva V.Y., Ellis T.A., Dembrow N.C., Weiss K.R., Vilim F.S. Insulin prohormone processing, distribution, and relation to metabolism in Aplysia californica. J. Neurosci. 1999;19:7732–7741. doi: 10.1523/JNEUROSCI.19-18-07732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R.C., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Wang X., Kawamura T., Bindokas V.P., Dizon R.F., Alcoser S.Y., Magnuson M.A., Bell G.I. Transgenic mice with green fluorescent protein-labeled pancreatic β -cells. Am. J. Physiol. Endocrinol. Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- Herrera P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Höck J., Meister G. The Argonaute protein family. Genome Biol. 2008;9:210. doi: 10.1186/gb-2008-9-2-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacovetti C., Abderrahmani A., Parnaud G., Jonas J.-C., Peyot M.-L., Cornu M., Laybutt R., Meugnier E., Rome S., Thorens B. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. J. Clin. Invest. 2012;122:3541–3551. doi: 10.1172/JCI64151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J.D., Komisarow J.M., Friedersdorf M.B. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 2006;1:302–307. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
- Kim H., Toyofuku Y., Lynn F.C., Chak E., Uchida T., Mizukami H., Fujitani Y., Kawamori R., Miyatsuka T., Kosaka Y. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A., Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J., Busskamp V., Markiewicz I., Stadler M.B., Ribi S., Richter J., Duebel J., Bicker S., Fehling H.J., Schübeler D. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Lee E.K., Kim W., Tominaga K., Martindale J.L., Yang X., Subaran S.S., Carlson O.D., Mercken E.M., Kulkarni R.N., Akamatsu W. RNA-binding protein HuD controls insulin translation. Mol. Cell. 2012;45:826–835. doi: 10.1016/j.molcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellis-Santos C., Sakamoto L.H., Bromati C.R., Nogueira T.C.A., Leite A.R., Yamanaka T.S., Kinote A., Anhê G.F., Bordin S. The regulation of Rasd1 expression by glucocorticoids and prolactin controls peripartum maternal insulin secretion. Endocrinology. 2012;153:3668–3678. doi: 10.1210/en.2012-1135. [DOI] [PubMed] [Google Scholar]
- Leung A.K.L., Sharp P.A. MicroRNA functions in stress responses. Mol. Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K.L., Calabrese J.M., Sharp P.A. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. USA. 2006;103:18125–18130. doi: 10.1073/pnas.0608845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M., Maruyama T., Ohta T., Ito A., Hayashi T., Tsukasaki K., Kamihira S., Yamaoka S., Hoshino H., Yoshida T. CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T-cell leukemia cells. J. Biol. Chem. 2010;285:15511–15522. doi: 10.1074/jbc.M109.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán I., Akerman I., van de Bunt M., Xie R., Benazra M., Nammo T., Arnes L., Nakić N., García-Hurtado J., Rodríguez-Seguí S. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012;16:435–448. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio D.M., Newgard C.B. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- Nir T., Melton D.A., Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll D., Mecklenbrauker I., Das P.P., Santana A., Koenig U., Enright A.J., Miska E.A., Tarakhovsky A. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orland M.J., Chyn R., Permutt M.A. Modulation of proinsulin messenger RNA after partial pancreatectomy in rats. Relationships to glucose homeostasis. J. Clin. Invest. 1985;75:2047–2055. doi: 10.1172/JCI111924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy M.N., Eliasson L., Krutzfeldt J., Kuwajima S., Ma X., Macdonald P.E., Pfeffer S., Tuschl T., Rajewsky N., Rorsman P., Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Poy M.N., Hausser J., Trajkovski M., Braun M., Collins S., Rorsman P., Zavolan M., Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc. Natl. Acad. Sci. USA. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasethupathy P., Fiumara F., Sheridan R., Betel D., Puthanveettil S.V., Russo J.J., Sander C., Tuschl T., Kandel E. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C.J. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Rieck S., White P., Schug J., Fox A.J., Smirnova O., Gao N., Gupta R.K., Wang Z.V., Scherer P.E., Keller M.P. The transcriptional response of the islet to pregnancy in mice. Mol. Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S., Shibasaki T., Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J. Clin. Invest. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs K.A., Bushell M., Willis A.E. Translational regulation of gene expression during conditions of cell stress. Mol. Cell. 2010;40:228–237. doi: 10.1016/j.molcel.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Steil G.M., Trivedi N., Jonas J.C., Hasenkamp W.M., Sharma A., Bonner-Weir S., Weir G.C. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am. J. Physiol. Endocrinol. Metab. 2001;280:E788–E796. doi: 10.1152/ajpendo.2001.280.5.E788. [DOI] [PubMed] [Google Scholar]
- Tattikota S.G., Sury M.D., Rathjen T., Wessels H.-H., Pandey A.K., You X., Becker C., Chen W., Selbach M., Poy M.N. Argonaute2 regulates the pancreatic β-cell secretome. Mol. Cell. Proteomics. 2013;12:1214–1225. doi: 10.1074/mcp.M112.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Thurmond D.C. Mechanisms of biphasic insulin-granule exocytosis - roles of the cytoskeleton, small GTPases and SNARE proteins. J. Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhang Z., O’Loughlin E., Lee T., Houel S., O’Carroll D., Tarakhovsky A., Ahn N.G., Yi R. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 2012;26:693–704. doi: 10.1101/gad.182758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Bunt M., Gaulton K.J., Parts L., Moran I., Johnson P.R., Lindgren C.M., Ferrer J., Gloyn A.L., McCarthy M.I. The miRNA profile of human pancreatic islets and beta-cells and relationship to type 2 diabetes pathogenesis. PLoS ONE. 2013;8:e55272. doi: 10.1371/journal.pone.0055272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L., Arends M.J., Chausiaux O.E., Ellis P.J., Lange U.C., Surani M.A., Affara N., Murakami Y., Adams D.J., Bradley A. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Mol. Cell. Biol. 2006;26:3595–3609. doi: 10.1128/MCB.26.9.3595-3609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L., Arends M.J., Rust A.G., Poulogiannis G., McIntyre R.E., Adams D.J. Increased tumorigenesis associated with loss of the tumor suppressor gene Cadm1. Mol. Cancer. 2012;11:29. doi: 10.1186/1476-4598-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao E., Keller M.P., Rabaglia M.E., Oler A.T., Stapleton D.S., Schueler K.L., Neto E.C., Moon J.Y., Wang P., Wang I.-M. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm. Genome. 2009;20:476–485. doi: 10.1007/s00335-009-9217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., You W., Wang H., Li Y., Qiao N., Shi Y., Zhang C., Bleich D., Han X. MicroRNA-24/MODY gene regulatory pathway mediates pancreatic beta-cell dysfunction. Diabetes. 2013;62:3194–3206. doi: 10.2337/db13-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.