Abstract
The burn severity depends on the wound depth and area affected. Hitherto burn depth has been judged mainly by visual observation, although concerns have been raised about its validity. The regional tissue blood flow (rTBF) measured by laser Doppler imaging (LDI) in damaged tissue correlates with the depth. However, very few reports are available on the significance of the regional tissue oxygen saturation (rSO2) as an indicator of burn depth. We investigated whether rSO2 by Near-infrared spectroscopy (NIRS) in burn injuries correlates with rTBF by LDI, which would facilitate quantification of the severity of the tissue damage. Methods: We measured rTBF and rSO2 in 50 lesions from 14 patients of burn injury within 24 hours after injury. The correlation between rTBF and rSO2 was evaluated by Spearman rank correlation analysis. Results: The rSO2 (%; range, 52-82) by NIRS and the rTBF (perfusion unit; range, 61-704) by LDI in burn lesions were positively correlated (r=0.755, p<0.001). This statistically positive correlation still remained significant (r=0.678, p<0.001) after the rSO2 values were standardized. Conclusion: This study suggests that NIRS determination of rSO2 in burn injuries shows promise as a reliable and quick method to estimate the depth of burn lesion.
Keywords: NIRS, rSO2, laser Doppler imaging, burn depth
Introduction
An assessment of burn depth is the initial and critical step in guiding the treatment of patients with thermal injuries. Such assessment should be objective and non-biased, but in practice continues to rely on clinical observation and visual inspection as the most practical method. However, clinical estimation of burn depth by visual and tactile assessment of the wound is often inaccurate especially in determining whether a deep burn would heal without surgical intervention, even when performed by experienced clinicians [1]. This unsatisfactory current clinical status, namely that the surgical indication regarding whether or not a burn wound requires early excision and grafting is still decided based on imprecise methods cries out for improvement. In particular, there is an urgent need for a high-resolution imaging technique that would provide quantitative information on the depth of thermal damage.
Early studies demonstrated that estimates of perfusion in the burn wound by laser Doppler imaging (LDI) strongly correlated with the burn depth and healing time [2-7]. Laser Doppler perfusion imaging has shown very high sensitivity, specificity, and accuracy in determining the burn depth when compared to histological studies. However, LDI study is still not widely used clinically.
Near-infrared spectroscopy (NIRS) has been commonly used to determine the regional tissue oxygen saturation (rSO2) of brain tissue in patients with cerebral ischemia [8]. NIRS penetrates a broad range of tissues and utilizes reflection rather than direct transmission between an emitter and receiver pair. It is able to assess end-organ perfusion and uses a noninvasive, portable device that can be easily adapted to numerous settings including the emergency room, operating room for refinement of anesthesia, and hospital ward for bedside monitoring. However, very few reports are available on the utility and feasibility of NIRS to determine the rSO2 of burn wounds, and assess the burn depth. Since NIRS may represent a quick, easily accessible, and clinically practical method to assess burn depth, we investigated whether its usefulness is comparable to that of LDI in patients with severe burn injury.
Subjects and methods
This study was performed with permission obtained from the institutional ethics committee of Nara Medical University.
The patients recruited for the present study were admitted to Nara Medical University Advanced Emergency and Critical Care Medical Center during the period from March 2012 to February 2013 and treated within 24 hours of sustaining burn injuries. The total number of burn injury patients admitted to our center during this period was 25. Burn injuries in the body trunk were excluded from this study because the LDI and NIRS values obtained from them may be affected by the lung and/or the intestines. Since our intention was to measure LDI and NIRS focusing on burn sites in the 4 extremities we excluded those patients in whom these sites were not involved. Also excluded were patients administered drugs such as vasopressors and vasodilators during the measurement because of their effects on hemodynamics and skin blood flow. Further, patients with inhalation burn injury who showed hypoxia and/or carbon monoxide intoxication were excluded. Finally, 14 patients with extremity burns regardless of the presence/absence of inhalation injury or trunk burns were included for this study.
Fifty lesion sites in these 14 patients were analyzed by LDI and NIRS. There were 8 men and 6 women with a median age of 58 years (range 7-89 years) and a median Burn Index of 17 (6-55). At the time of the LDI and NIRS evaluation the median blood Hb concentration was 14.5 g/dl (10.8-16.2), and the median systemic circulation SpO2 was 98% (96-100) (Table 1).
Table 1.
Clinical characteristics of patients with burn injury
| Age | Sex | Burn Index | TBSA | Etiology | Location | Hb | SpO2 |
|---|---|---|---|---|---|---|---|
| 7 | M | 13 | 26 | Scald | Leg | 14.4 | 99% (room air) |
| 27 | M | 18 | 35 | Flame | Arm, Leg | 14.8 | 98% (room air) |
| 36 | F | 10 | 12 | Flame | Leg | 13.8 | 98% (room air) |
| 40 | F | 18 | 27 | Flame | Arm | 14.6 | 96% (room air) |
| 40 | M | 6 | 12 | Flame | Arm | 13.7 | 97% (room air) |
| 52 | F | 20 | 23 | Flame | Leg | 15.3 | 98% (room air) |
| 54 | M | 32 | 64 | Scald | Arm, Leg | 15.1 | 96% (room air) |
| 61 | M | 52 | 62 | Flame | Arm | 14.4 | 99% (FiO2 0.4) |
| 62 | M | 6 | 11 | Flame | Hand | 15.2 | 98% (room air) |
| 64 | M | 16 | 32 | Flame | Hand | 16.2 | 96% (room air) |
| 67 | F | 55 | 72 | Flame | Leg | 13.1 | 98% (FiO2 0.6) |
| 80 | F | 24 | 36 | Flame | Arm, Leg | 11.6 | 100% (FiO2 0.4) |
| 82 | M | 8.5 | 17 | Chemical | Arm | 10.8 | 97% (room air) |
| 89 | F | 7 | 11 | Flame | Leg | 15.2 | 96% (FiO2 0.4) |
TBSA, total burn surface area.
Regional tissue blood flow (rTBF) and rSO2 were measured by LDI and NIRS respectively within 48 hours of the burn injuries. The sites selected for the LDI and NIRS measurements were adequately rinsed beforehand, and any sites showing blisters or treated with ointment were excluded from the measurements. Then, to prevent any influence of this rinsing on the skin temperature and skin blood flow of the measured sites, for 1 hour afterwards the measured sites were protected with coating material. The patients were kept at rest, and with the systolic pressure maintained at ≥90 mmHg and the systemic circulation arterial blood SpO2 at ≥96% rTBF and rSO2 at the wound were measured.
Laser Doppler imaging (LDI)
LDI is already a well established non-contact blood flow imaging modality [2-7]. In LDI, a single wavelength laser beam is made to irradiate intravascular red blood cells in the burn wound, and the reflected light is analyzed. This light is reflected back within irradiating fiberglass. The intensity of this reflected light, and discrepancies in the wavelength phase of the reflected and irradiating light are determined, the red blood cell mass and velocity are calculated, and the state of blood flow is imaged from these data (blood flow 2-dimensional image) [7]. The area targeted for measurement is located at a depth about 1~2 mm below the skin surface, and usually the blood flow of only the skin is measured, with the measurements not thought to be influenced by the deep organs. It takes about 4~10 minutes for the measurements when images are prepared in a 256x256 pixel format. In this study, a Moor Co. (UK) LDI2-IR was used. From the “flux” value obtained from the measurement a Perfusion Unit (PU) in the range of 0-1000 was calculated, and was considered to reflect rTBF. PU has been documented to accurately evaluate burn depth [7].
Near-infrared spectroscopy (NIRS)
NIRS uses near-infrared rays of 3 wavelengths (735, 810, 850 nm). The oxyhemoglobin and deoxyhemoglobin concentration ratio is calculated by spatial resolution in the entire tissue including arterial blood, vein, capillary, and bone, and the oxygen saturation of the entire tissue (regional mixed blood oxygen saturation: rSO2) is measured. In the present study, a NIRO-200NX (Hamamatsu Photonics, Japan) was used. The surface of burn wound to be estimated by NIRS was covered with sterile sheet of transparent polyurethane film (3MTM Tegade-rmTM) for the indirect contact of the NIRS probes with the burn injury. The distance between the two NIRS probes (light emitting and detecting ones) were 3 cm, and thereby the current data for rSO2 were supposed to be obtained from the tissue with a depth up to 2.1 cm beneath the probes.
rSO2 measured by NIRS has been reported to be useful in diverse contexts such as for evaluation of cerebral blood flow in patients undergoing cardiopulmonary resuscitation [9,10], and assessment of intramuscular blood flow in sports medicine [11,12].
Statistical analysis
The correlation between rTBF (PU) measured by LDI and rSO2(%) measured by NIRS was determined statistically using the statistical software package SPSS® (Version 11.0 for Windows, SPSS Inc., Chicago, IL).
Because of skewed data distributions and small sample sizes, nonparametric statistical procedures were used. The Spearman rank correlation coefficient was used to assess the association between rTBF (PU) and rSO2(%). Also, since the oxygen saturation of the arterial blood in the systemic circulation differs between individual patients, we standardized burn wound rSO2 values so as to exclude any influence of this systemic circulation SO2 on wound SO2. These values were obtained by dividing the burn wound rSO2 by rSO2 of uninvolved skin (rSO2 ratio: burn wound rSO2/normal site rSO2). The correlation between this standardized rSO2 and rTBF measured by LDI was also statistically. P value less than 0.05 was considered statistically significant.
Results
rSO2 determined by NIRS showed a range of values of 50~83%, while PU obtained by LDI showed a range of values of 80~700.
The rSO2 (perfusion unit; range, 61-704 and median, 406) by NIRS and the rTBF (%; 52-82, 72) by LDI in the severe burn lesions were positively correlated (r=0.755, p<0.001) (Figure 1). This statistically positive correlation remained significant (r=0.678, p<0.001) after the values of rSO2 standardized by dividing them with ones from the contralateral or adjacent intact skin regions (Figure 2).
Figure 1.
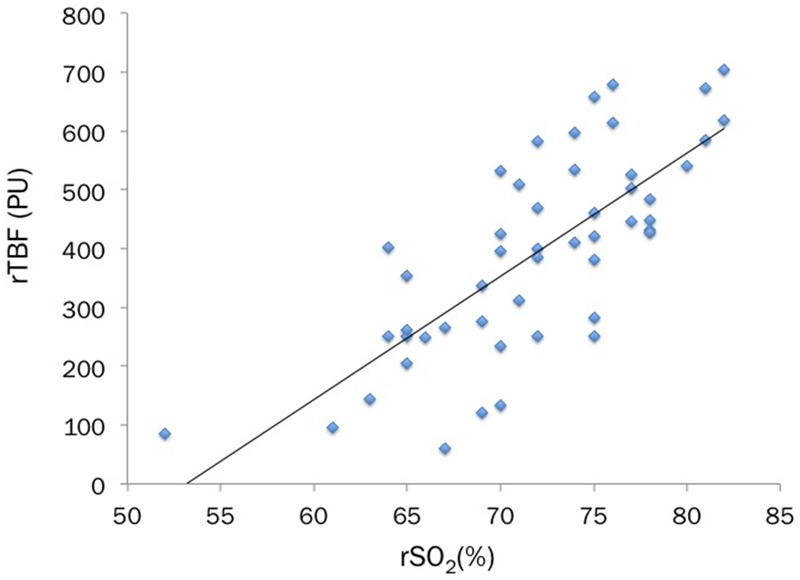
The rSO2(%) measured by NIRS is compared to the rTBF (perfusion unit) by LDI in the 50 burn sites in 14 patients. The statistical analysis shows a significant positive correlation between these two values of rSO2 and rTBF (Spearman rank correlation coefficient, r=0.755, p<0.001).
Figure 2.
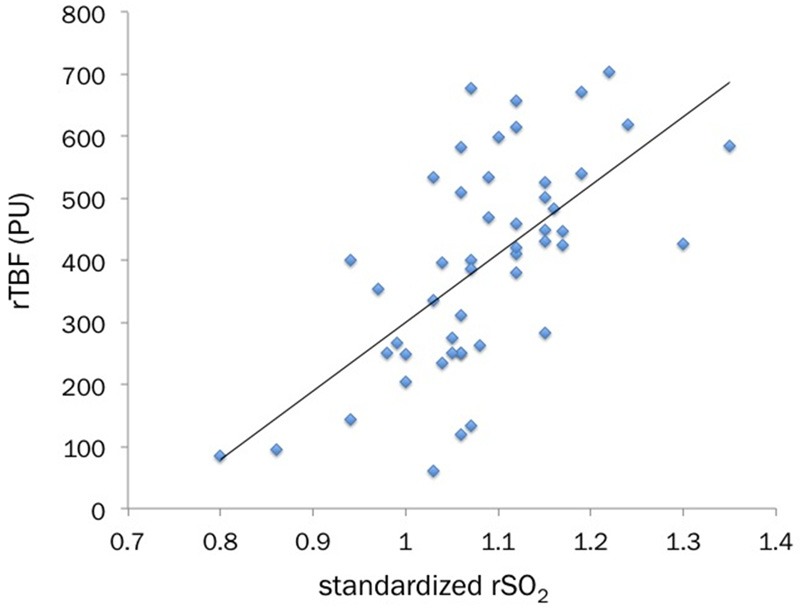
The standardized rSO2 values (rSO2 ratio; burn wound rSO2/normal site rSO2) also shows a statistically-significant positive correlation with rTBF (Spearman rank correlation coefficient, r=0.678, p<0.001).
Discussion
In previously conducted studies [2-7] in which regional tissue blood perfusion was evaluated in acute phase burn sites by LDI to determine burn depth/severity, the diagnostic accuracy and reliability of this method were deemed to be high. Riordan et al. [3] compared burn depth judged by pathological examination of burn wound tissues under a microscope with blood flow determined by LDI in acute phase burn wounds, and found an extremely close correlation between the two. In this investigation Riordan et al. reported a sensitivity of 95% and specificity of 94% for LDI in the evaluation of burn depth. Moreover, Pape et al. [7] found a close correlation between rTBF (PU) of acute phase burn wounds as measured by LDI and the time needed for burn wounds to heal with conservative therapy alone. They also noted that acute phase LDI values shown by burn wounds that do not heal with conservative therapy alone exist. Such values obtained by LDI in the acute phase may be useful in judging the surgical indications for burn wounds. In this way, it has been considered that rTBF (PU) measured by LDI is useful in determining burn depth and in estimating the healing time. However, whether LDI will find a role as a widely applicable and versatile modality in the actual clinical setting remains to be clarified. The hurdle for its widespread use at the bedside in the intensive care unit includes the size and cost of the LDI machine, and the fact that the time for LDI measurement is reratively long to apply for the restless patients such as babies.
On the other hand, NIRS to evaluate rSO2 is already widely used in diverse clinical settings [8]. In recent years rSO2 has also been used as an index of cerebral circulatory metabolism during resuscitation of cardiopulmonary arrest patients in the emergency outpatient clinic [9,10]. Determining rSO2 at the brain surface by attaching an NIRS measurement probe to the cardiopulmonary arrest patient’s forehead makes it possible to estimate in real time the extent to which the cerebral circulation is being maintained. Similarly in the field of motor rehabilitation, by attaching a probe to muscles in the femoral or other sites and using measurements of rSO2 as an index of changes in blood flow and oxygen metabolism associated with muscle contraction during exercise, NIRS has been found useful in assessing patients’ state of recovery in a number of studies [11,12]. The application of NIRS has been proposed in the field of burn injuries too [13-15]. In these reports, rSO2 was suggested to closely correlate with macroscopic determinations of burn depth. However, no studies thus far have analyzed the correlation between rTBF (PU) measured by LDI and rSO2 measured by NIRS in burn wounds. Already in the clinical setting there is anticipation that using highly versatile NIRS to evaluate rSO2, the obtained value may facilitate judgment regarding the severity of burn wounds, thereby facilitating more appropriate therapeutic decision-making from the early phase of burn injury, more accurate estimates of prognosis, and better outcomes.
In the present study undertaken to explore a novel index to evaluate the severity of burn wounds, the rSO2(%) value in burn wounds measured by NIRS was suggested to correlate extremely well with rTBF (PU) measured by LDI, which has previously been demonstrated to accurately evaluate burn depth. Moreover, since the oxygen saturation of the arterial blood in the systemic circulation differs between individual patients we standardized burn wound rSO2 values so as to exclude any influence of the systemic circulation SO2 on wound SO2 by dividing the burn wound rSO2 by the rSO2 of unaffected skin of each patient (burn wound rSO2/normal site rSO2). Subsequent analysis showed a close correlation between this rSO2 ratio and rTBF (PU), with these results thus suggesting that NIRS, which is highly versatile in the clinical setting, may also be useful in assessing the severity of acute phase burns.
This study has several limitations. The number of studied patients was small, the correlation between acute phase burn wound rSO2 and histological burn depth was not determined, the correlation between acute phase burn wound rSO2 and the subsequent burn wound healing period was not analyzed, and the study focused solely on burn wounds of the extremities while not considering those of the trunk. In future, we plan to analyze greater numbers of cases and burn sites to address these limitations, and in this way better define the role of acute phase burn wound rSO2 in the assessment of burn depth.
Conclusion
This study suggested the possibility that burn wound rSO2 measured by clinically highly versatile NIRS may be as practical and useful as evaluation of burn tissue blood flow by LDI in the determination of burn depth. If these results using a practical modality such as NIRS to objectively evaluate the severity of acute phase burn wounds are reproduced, NIRS may facilitate decision-making in the early phase on the most appropriate interventions thereby helping to improve the therapeutic strategies and outcome of severe burn patients.
Disclosure of conflict of interest
None.
References
- 1.Heimbach D, Engrav L, Grube B, Marvin J. Burn depth: A review. World J Surg. 1992;16:10–5. doi: 10.1007/BF02067108. [DOI] [PubMed] [Google Scholar]
- 2.Kloppenberg FW, Beerthuizen GI, ten Duis HJ. Perfusion of burn wounds assessed by Laser Doppler Imaging is related to burn depth and healing time. Burns. 2001;27:359–63. doi: 10.1016/s0305-4179(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 3.Riordan CL, McDonough M, Davidson JM, Corley R, Perlov C, Barton R, Guy J, Nanney LB. Noncontact Laser Doppler Imaging in burn depth analysis of the extremities. J Burn Care Rehabil. 2003;24:177–86. doi: 10.1097/01.BCR.0000075966.50533.B0. [DOI] [PubMed] [Google Scholar]
- 4.Pape SA, Skouras CA, Byrne PO. An audit of the use of laser Doppler imaging (LDI) in the assessment of burns of intermediate depth. Burns. 2001;27:233–9. doi: 10.1016/s0305-4179(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 5.Atiles L, Mileski W, Spann K, Purdue G, Hunt J, Baxter C. Early Assessment of Pediatric Burn Wounds By Laser Doppler Flowmetry. J Burn Care Rehabil. 1995;16:596–601. doi: 10.1097/00004630-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Atiles L, Mileski W, Purdue G, Hunt J, Baxter C. Laser Doppler Flowmetry in Burn Wounds. J Burn Care Rehabil. 1995;16:388–93. doi: 10.1097/00004630-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Pape SA, Wilson D, Hoeksema H, Jeng JC, Spence RJ, Monstrey S. Burn wound healing time assessed by laser Doppler imaging (LDI). Part 1: Derivation of a dedicated colour code for image interpretation. Burns. 2012;38:187–94. doi: 10.1016/j.burns.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Hampton DA, Schreider MA. Near infrared spectroscopy: clinical and research uses. Transfusion. 2013;53:52S–58S. doi: 10.1111/trf.12036. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs H, Linder W, Buschko A, Almazam M, Hummler HD, Scmid MB. Brain oxygenation monitoring during neonatal resuscitation of very low birth weight infants. J Perinatol. 2012;32:356–62. doi: 10.1038/jp.2011.110. [DOI] [PubMed] [Google Scholar]
- 10.Frisch A, Suffoletto BP, Frank R, Martin-Gill C, Menegazzi JJ. Potential Utility of Near-Infrared Spectroscopy in Out-of-Hospital Cardiac Arrest: An Illustrative Case Series. Prehosp Emerg Care. 2012;16:564–70. doi: 10.3109/10903127.2012.702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCully KK. The influence of passive stretch on muscle oxygen saturation. Adv Exp Med Biol. 2010;662:317–22. doi: 10.1007/978-1-4419-1241-1_45. [DOI] [PubMed] [Google Scholar]
- 12.Otsuki A, Fujita E, Ikegawa S, Kuno-Mizumura M. Muscle oxygen and fascicle length during passive muscle stretching in ballet-trained subjects. Int J Sports Med. 2011;32:496–502. doi: 10.1055/s-0031-1275297. [DOI] [PubMed] [Google Scholar]
- 13.Sowa MG, Leonardi L, Payette JR, Cross KM, Gomez M, Fish JS. Classification of burn injuries using near-infrared spectroscopy. J Biomed Opt. 2006;11:054002. doi: 10.1117/1.2362722. [DOI] [PubMed] [Google Scholar]
- 14.Sowa MG, Leonardi L, Payette JR, Fish JS, Mantsch HH. Near infrared spectroscopic assessment of hemodynamic changes in the early post-burn period. Burns. 2001;27:241–9. doi: 10.1016/s0305-4179(00)00111-x. [DOI] [PubMed] [Google Scholar]
- 15.Cross KM, Leonardi L, Payette JR, Gomez M, Levasseur MA, Schattka BJ, Sowa MG, Fish JS. Clinical utilization of near-infrared spectroscopy devices for burn depth assessment. Wound Repair Regen. 2007;15:332–40. doi: 10.1111/j.1524-475X.2007.00235.x. [DOI] [PubMed] [Google Scholar]


