Abstract
Background: The majority of patients with periampullary cancer develop local or metastatic recurrence despite successful negative margin resection. Unfortunately, there are no established therapeutic strategies for managing these patients. The literature on the surgical resection of recurrent disease is limited.
Methods: This is a retrospective study evaluating patients who underwent reoperative resection of recurrent periampullary cancer at a single institution between 1990 and 2011. Perioperative outcomes were compared with those of the original primary resections for patients with local recurrence. Kaplan–Meier curves were used to evaluate survival.
Results: Twenty-two patients underwent reoperative resection following the successful primary resection of periampullary cancers. Median survival from the time of reoperation was 28.1 months. A greater survival benefit was seen in patients undergoing reoperative resection with >15 months between the primary resection and recurrence (40.6 months versus 8.2 months; P < 0.05). Complication rates were lower after reoperative resection compared with the primary resection (20% versus 70%). Perioperative characteristics including operative time, estimated blood loss and hospital stay were similar in both the primary and reoperation procedures.
Conclusions: Surgical resection of periampullary cancer recurrence is feasible, safe and may offer survival benefits in comparison with alternative treatment modalities. Reoperative resection should be considered, especially in patients in whom the time to recurrence is lengthy.
Introduction
Despite being only the 10th most common cancer, periampullary cancer is the fourth most common cause of cancer-related death in the USA and just over 40 000 new cases are estimated to have occurred in 2012.1 Surgical resection offers the only hope for longterm survival, albeit that overall 5-year survival in resected pancreatic cancer patients is poor and amounts roughly 10–20%.2–4 The poor survival in periampullary cancer patients following resection reflects in part the substantial number of patients who develop local recurrence despite surgical resection. Up to 86% of patients have evidence of locoregional recurrence following successful primary resection and an even larger number develop hepatic disease.5,6 Distant metastases without evidence of local recurrence or liver metastasis(es) are present in only 3% of patients.5
Despite the high recurrence rates in periampullary cancer following primary resection, there are no established therapeutic strategies for managing these patients.7,8 Improved survival has been demonstrated following either surgery or chemotherapy; therefore, treatment in some form may be warranted.9 Reoperation for local recurrence is challenging and complex; however, repeat pancreatic procedures have been demonstrated to be safe in the hands of experienced surgeons.10 The surgical resection of locally recurrent or isolated metastatic disease represents the best hope for longterm survival. However, few patients are offered reoperation and only a small number of studies have evaluated the efficacy of reoperative surgery in these patients. This report represents a single-institution experience of reoperation for locally recurrent or isolated metastatic periampullary adenocarcinoma following successful primary resection.
Materials and methods
Patient identification and data collection
The Institutional Review Board at the University of Pittsburgh Medical Center (UPMC) approved this study prior to its initiation (approval no. PRO11070165).
A retrospective search of the UPMC electronic health records databases was performed to identify patients who had undergone primary resection for periampullary cancer over the previous 12 years (1990–2011). Patients who underwent subsequent reoperative resection with curative intent for locally recurrent or metastatic disease were then identified. All pertinent medical records were reviewed; these included operative reports, anaesthesia records, daily progress notes, consultation notes, nursing notes, and laboratory and radiology records and images. Perioperative mortality and morbidity were evaluated over a 90-day postoperative period. Complications were graded according to the Clavien–Dindo system of classifying surgical complications.11
Patient follow-up after primary resection
There is no standardized postoperative surveillance protocol for patients who undergo resection of primary periampullary cancer at this institution. Most surgeons rely on carbohydrate antigen 19-9 (CA 19-9) levels and routine standard computed tomography (CT) to evaluate for recurrence, typically every 3 months during the first 2 years postoperatively and then yearly until 5 years. Positron emission tomography (PET) CT scanning is then utilized to confirm recurrent disease and monitor response to treatment. All challenging cases are presented at a multidisciplinary hepatopancreatobiliary conference at which input from various specialty teams is obtained before the surgeon makes any decision on how best to manage recurrent disease.
Statistical analysis
IBM spss Version 20.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Continuous data are presented as the mean, median and/or range. Categorical variables are expressed as numbers and percentages of the group from which they were derived. Independent-sample t-tests were used to evaluate continuous data. Survival was estimated using the Kaplan–Meier method. Survival was calculated from the time of resection. Patients alive at the last follow-up were censored. Results were considered statistically significant if they achieved P-values of <0.05.
Results
Reoperations and indications
A total of 1707 patients who underwent primary resection for periampullary cancer during the study period were identified (1099 pancreaticoduodenectomies, 495 distal pancreatectomies, 113 total pancreatectomies). Of these, 22 patients (1.3%) subsequently underwent reoperative resection with curative intent for local or metastatic periampullary cancer recurrence following primary resection (Table 1). The median age of these patients was 67 years and 68% of them were female. Of the patients submitted to resection, 10 (45%) underwent resection of local recurrence and 12 (55%) underwent resection of metastatic disease. Of the latter 12 patients, five (42%) were resected for lung metastases, one (8%) for ovarian metastases and six (50%) for liver metastases.
Table 1.
Surgical and survival data for patients undergoing reoperative resection for locally recurrent or metastatic periampullary cancer (n = 22)
| Patient | TNM | Primary cancer | Initial operation | Margin | Recurrence | Site | Reoperation | Margin | Time to reoperation, months | Survival post-reoperation, months |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T3N0M0 | Ampullary | Whipple with en bloc extended right hemicolectomy, SMVR | R0 | Metastatic | Liver | Liver resection × 4, RFA × 2 | R0 | 36.6 | 28.6 |
| 2 | T2N0M0 | Pancreatic | DPS | R0 | Metastatic | Lung | LUL wedge × 2 | R0 | 52.8 | 73.0 |
| 3 | T3N1M0 | Pancreatic | ppPD | R1 | Metastatic | Ovary | TAH/BSO | R0 | 7.6 | 12.7 |
| 4 | T2N0M0 | Ampullary | ppPD | R0 | Local | Pancreas, small bowel | DPS, SBR | R0 | 33.4 | 40.6 |
| 5 | T2NxM0 | Pancreatic | ppPD, PVR | R0 | Metastatic | Liver | RFA | R0 | 8.2 | 2.9 |
| 6 | T2N1M0 | Ampullary | ppPD | R0 | Local | Resection bed | Resection pancreatic bed mass | R0 | 24.7 | 33.0a |
| 7 | T3N1M0 | Ampullary | Whipple | R0 | Metastatic | Lung | LLL wedge | R0 | 19.4 | 12.6a |
| 8 | T3N1M0 | Pancreatic | ppPD | R1 | Local | Resection bed | Resection pancreatic bed mass | R0 | 14.9 | 4.2a |
| 9 | T3N1M0 | Pancreatic | ppPD, PVR | R0 | Metastatic | Liver | Wedge resection | R0 | 17.6 | 4.9 |
| 10 | T2N0M0 | Pancreatic | ppPD | R0 | Metastatic | Liver | Partial R lobectomy | R0 | 32.7 | 34.7a |
| 11 | T2N0M0 | Pancreatic | DP, partial colectomy, adrenalectomy | R0 | Local | Stomach | Partial gastrectomy | R0 | 20.1 | 80.8 |
| 12 | T3N0M0 | Pancreatic | ppPD | R0 | Local | Pancreas, small bowel, stomach | Completion pancreatectomy, splenectomy, SBR, gastrectomy | R1 | 14.5 | 8.2 |
| 13 | T3N1M0 | Pancreatic | ppPD with SMVR | R0 | Metastatic | Lung | VATS, RML/RUL wedge | R0 | 43.2 | 27.6 |
| 14 | T3N0M0 | Pancreatic | DPS | R0 | Metastatic | Lung | RLL segmentectomy | R0 | 63.6 | 3.6 |
| 15 | T2N0M0 | Pancreatic | ppPD | R0 | Local | Pancreas, small bowel | Completion pancreatectomy, SBR | R0 | 60.8 | 67.1 |
| 16 | T3N0M0 | Pancreatic | ppPD, PVR | R1 | Local | Pancreas, colon | DPS, partial colectomy | R0 | 31.6 | 29.0 |
| 17 | T3N0M0 | Pancreatic | Whipple | R0 | Metastatic | Lung | VATS, wedge | R0 | 25.5 | 46.7a |
| 18 | T3N1M0 | Ampullary | Whipple | R0 | Metastatic | Liver | Wedge resection liver, RFA × 2 | R0 | 16.4 | 20.7 |
| 19 | T4N0M0 | Ampullary | Whipple | R0 | Local | Resection bed | Resection pancreatic bed mass | R1 | 8.8 | 46.3 |
| 20 | T2N1M0 | Pancreatic | Robotic Whipple | R0 | Metastatic | Liver | Laparoscopic RFA × 2 | R0 | 9.5 | 7.1 |
| 21 | T3N0M0 | Pancreatic | ppPD | R0 | Local | Stomach | LOA, small bowel resection | R0 | 36.4 | 31.8a |
| 22 | T3N0M0 | Pancreatic | DPS | R0 | Local | Stomach | Partial gastrectomy | R0 | 26.0 | 11.9 |
Patient alive at last follow-up.
BSO, bilateral salpingo-oophorectomy; DPS, distal pancreactectomy and splenectomy; LOA, lysis of adhesions; LLL, left lower lobe; LUL, left upper lobe; ppPD, pylorus-preserving pancreaticoduodenectomy; PVR, portal vein resection; RFA, radiofrequency ablation; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SBR, small bowel resection; SMVR, superior mesenteric vein resection; TAH, total abdominal hysterectomy; TNM, tumour–node–metastasis; VATS, video-assisted thoracic surgery.
Perioperative course
Perioperative outcomes in all patients submitted to resection of local and metastatic recurrence are reported in Table 2. In Table 3, perioperative outcomes following primary resection are compared with outcomes of reoperation in patients with locally recurrent disease. Reoperation was associated with a lower median operative time than primary resection (364 min versus 616 min; P < 0.05). Patients were discharged sooner (12 days versus 9 days; P < 0.05) and were more likely to be discharged to home (100% versus 90%) following reoperation than after primary resection.
Table 2.
Perioperative outcomes in patients undergoing reoperative resection of recurrent periampullary cancer (n = 22)
| Operative time, min, median (range) | 232 (77–435) |
| Blood loss, ml, median (range) | 300 (25–6000) |
| Required PRBC Transfusion, n (%) | 6 (27%) |
| Length of stay, days, mean (median; range) | 8 (7; 2–28) |
| Disposition, n (%) | |
| Home | 22 (100%) |
| SNF/rehabilitation | 0 |
| Morbidity, n (%) | |
| All grades | 7 (32%) |
| Grade 1 | 2 (29%) |
| Grade II | 2 (29%) |
| Grade III | 3 (42%) |
| Grade IV | 0 |
| Perioperative mortality, n (%) | 0 |
PRBC, packed red blood cells; SNF, skilled nursing facility.
Table 3.
Perioperative outcomes in patients undergoing reoperative resection for periampullary cancer local recurrence at the primary resection and at reoperation (n = 22)
| At primary resection | At reoperation | P-value | |
|---|---|---|---|
| Operative time, min, median (range) | 616 (223–771) | 364 (109–558) | <0.05 |
| Blood loss, ml, median (range) | 450 (75–700) | 300 (50–6000) | 0.38 |
| Required PRBC Transfusion, n (%) | 3 (30%) | 2 (20%) | |
| Length of stay, days, mean (median; range) | 12 (12; 6–25) | 9 (10; 3–15) | <0.05 |
| Disposition, n (%) | |||
| Home | 9 (90%) | 10 (100%) | |
| SNF/rehabilitation | 1 (10%) | 0 | |
| Morbidity, n (%) | |||
| All grades | 7 (70%) | 2 (20%) | |
| Grade I | 2 (29%) | 0 | |
| Grade II | 3 (43%) | 1 (50%) | |
| Grade III | 2 (29%) | 1 (50%) | |
| Grade IV | 0 | 0 | |
| Perioperative mortality, n (%) | 0 | 0 |
PRBC, packed red blood cells; SNF, skilled nursing facility.
Morbidity and mortality
There was no perioperative mortality. Perioperative complications affected 32% of patients. All complications were classed as ≤Grade III.
Survival
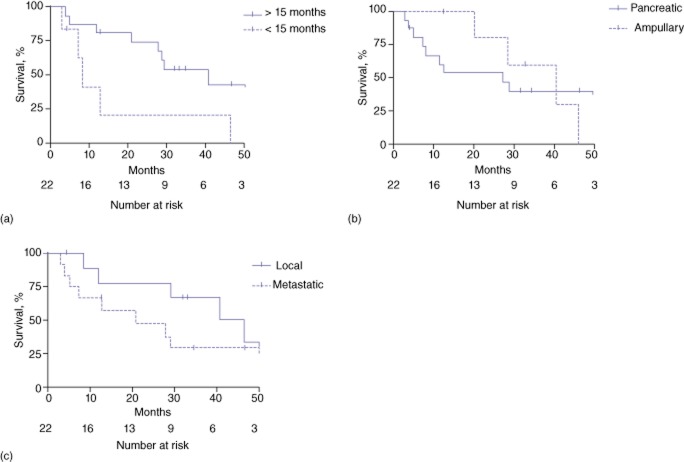
Reoperative resection with curative intent resulted in median postoperative survival of 28.1 months; six patients (27%) remained alive at the last follow-up. Three patients (14%) survived for >5 years. Median survival in these patients from the date of primary resection was 60.6 months. Survival in patients undergoing curative resection was greater in those who experienced >15 months of recurrence-free survival after the primary resection (40.6 months versus 8.2 months; P < 0.05) (Fig. 1a). There was no significant difference in median survival between patients with ampullary versus patients with pancreatic cancers (30.8 months versus 20.2 months; P = 0.9) (Fig. 1b). Median survival in patients with local recurrence was 31.8 months, whereas median survival in patients with lung recurrence was 27.6 months and patients with liver recurrence was 13.9 months; however, there was no statistical difference in outcomes according to whether resection was carried out for local or metastatic disease (P = 0.14) (Fig. 1c). Additionally, there was no statistically significant benefit to resection of isolated lung metastases compared to local recurrence or liver metastases. Univariate analyses were performed to identify which patients would benefit most from reoperative resection; however, no variables generated differences of statistical significance.
Figure 1.
Kaplan–Meier curves showing survival following reoperative resection of periampullary cancer recurrence by (a) time to recurrence, (b) type of cancer and (c) site of recurrent disease
Discussion
Despite the unfortunate frequency with which surgeons are confronted with recurring periampullary cancer following curative resection, there are few reports of the surgical resection of recurrent disease. The present data represent a series of 22 patients submitted to reoperative resection for locally recurrent or isolated metastatic periampullary adenocarcinoma. These results suggest that the resection of recurrent cancer may provide a possible survival benefit without incurring significant morbidity.
Surgical resection of locally recurrent or isolated metastatic disease represents the best hope for longterm survival; however, few patients are offered reoperation. The potential resection of local or metastatic recurrence is an uncommon treatment scenario. This series of 22 patients undergoing reoperative resection represents 1.3% of all patients submitted to primary resection of periampullary cancer during the study period at this centre. This is likely to reflect the fact that few patients present with isolated, stable local or metastatic disease that is suitable for resection. Additionally, this uncommon group of patients is unlikely to be offered resection, largely because data evaluating surgery as a treatment modality for recurrent disease are lacking. A number of single case reports of completion pancreatectomy for disease in the remnant pancreas are available and many of these report significant longterm survival (up to 48 months without considerable morbidity).12–22 Only three case series have evaluated reoperation for recurrent periampullary adenocarcinoma. Most recently, Thomas et al. reported a series of 21 patients undergoing reoperative resection of local or metastatic recurrence of pancreatic ductal adenocarcinoma following primary resection.23 The authors demonstrated a survival benefit in patients who had experienced >20 months of disease-free survival prior to recurrence; however, the greatest benefit was seen in patients with isolated pulmonary metastases. The authors concluded that patients with isolated pulmonary metastases with a long disease-free interval should be considered for reoperation.23 Zacharias et al. published the results of 15 re-laparotomies for recurrent periampullary malignancies and found slightly improved survival in patients who presented for elective resection.24 A study by Kleef et al. evaluated 30 patients with recurrent pancreatic cancer, of whom 15 underwent either palliative bypass or abdominal exploration alone and 15 underwent resection of local recurrence or metastatic disease.25 Although the resection group showed a tendency towards improved survival, the difference did not reach statistical significance.25
Although a small number of patients present with isolated, stable metastatic periampullary cancer (most commonly to the lungs and liver), the literature on the optimal management of such disease is lacking. A small number of single case reports have described the resection of stable liver metastases and reported survival of up to 29 months.18,19,26,27 Arnaouakis et al. reported a series of nine patients submitted to pulmonary metastasectomy with curative intent for pancreatic cancer and demonstrated an impressive longterm survival of 51 months with no morbidity or perioperative mortality.28
These data demonstrate that reoperative resection of periampullary cancer recurrence is feasible and safe. No operative complications or perioperative mortality occurred in the present series of 22 patients. Compared with the primary resection, reoperation for local recurrence was associated with a shorter operative time and length of stay. Despite the greater technical demands associated with reoperation, there was no difference between reoperation and primary resection procedures with a similar operative risk in estimated blood loss, transfusion rate or morbidity. This lower morbidity in part reflects the lack of risk for pancreatic fistula following reoperation, which contributes significantly to morbidity following to primary resection.
This series revealed a median survival following reoperative resection of 28.1 months and six patients remained alive at the last follow-up timepoint. As previous authors reporting on reoperative resection have concluded,25 survival was dramatically improved in patients who underwent resection of recurrent disease at >15 months after primary resection. There are two hypotheses that may explain why a longer latency period results in improved survival. The two-hit hypothesis centres on the idea that ‘recurrent’ cancer actually represents a second primary cancer, which would support the resection of ‘recurrent’ tumours. An alternative explanation is that, as a result of their biology, slow-growing tumours that have a longer latency between presentations portend a better overall prognosis, which again supports the consideration of surgical resection in these patients. The present analysis found a trend towards higher survival in local recurrence (31.8 months) and lung metastases (27.6 months) compared with liver metastases (13.9 months); however, this trend did not reach statistical significance. It is possible that a larger study population might indicate a survival advantage associated with resection of local recurrence or lung metastases compared with liver metastases.
Periampullary cancers are notoriously insensitive to chemotherapy, especially in the setting of recurrence; response rates are reported to be 10–30%.29,30 As a result, chemotherapy is combined with radiation to improve response rates and is the most commonly utilized treatment for local recurrence. Wilkowski et al. described a series of 18 patients with isolated local recurrence who underwent radiation plus combination chemotherapy of 5-fluorouracil (5-FU) with gemcitabine and/or cisplatin.31 Median overall survival from the start of chemoradiotherapy was 17.5 months and only 37% of patients achieved complete remission. These data put the 28.1-month postoperative survival demonstrated in the present study into perspective. Because this is a retrospective study with significant selection bias, it does not support conclusions on survival outcomes in patients treated with surgery compared with those treated with chemoradiation. More recently, FOLFIRINOX [folinic acid (leucovorin calcium), fluorouracil (5-FU), irinotecan hydrochloride, oxaliplatin] has shown promising results in treating pancreatic cancer.32 However, data on the treatment of recurrent disease are limited and further study is warranted.33,34 To date, studies evaluating FOLFIRINOX have assessed the regimen in the metastatic setting, demonstrating survival of 8–11 months.32,35,36
The present data should be interpreted with caution as this study has several limitations. This is a retrospective series and definitive conclusions cannot be made without a prospective, randomized controlled trial, which is, admittedly, a difficult proposal that is unlikely to be enacted. This series of patients is clearly a heterogeneous population in that it includes patients with local recurrence as well as those with metastatic disease, and patients with both ampullary and pancreatic adenocarcinoma, and thus the data are more difficult to extrapolate. Unfortunately, this population heterogeneity is necessary because the number of patients undergoing reoperation for pancreatic cancer recurrence is limited. This series represents a limited number of patients over a 12-year period, which suggests that only a select number of patients have isolated recurrent disease and are thus suitable for surgical resection. In addition, the study is limited by its methodology, which allowed for the identification of patients who underwent a primary resection at this institution only; it is likely that the present data exclude a small number of patients who underwent primary resection at an outside institution and resection of recurrent disease at the study institution. Unfortunately, there is no way of identifying these patients by searching operative records. Despite these limitations, these data suggest that the resection of recurrent periampullary cancer is feasible, can be safely accomplished in a wide variety of patients, and preliminarily appears to offer a significant survival advantage over alternative treatments. Not all patients are suitable for surgical re-resection and therefore further research is warranted to identify factors that predict which patients may benefit most from the resection of recurrent disease.
Conclusions
Surgical resection of periampullary cancer recurrence should at least be considered by specialized, experienced oncology surgeons, especially in patients in whom the time to recurrence is relatively long. Despite its significant technical challenges, reoperative resection is feasible and safe, and offers the best hope for longterm survival in patients with isolated metastatic or locally recurrent periampullary cancer.
Acknowledgements*
This work was supported by the National Institutes of Health under the Ruth L. Kirschstein National Research Service Award, grant number T32CA113263.
Footnotes
This information was added on 7 August 2013, after first online publication.
Conflicts of interest
None declared.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon KC, Klimstra DS, Brennan MF. Longterm survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecki SS, Sarr MG, Colby TV, Heerden van JA. Longterm survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- Westerdahl J, Andren-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer – local or hepatic? Hepatogastroenterology. 1993;40:384–387. [PubMed] [Google Scholar]
- Kyriazanos ID, Tsoukalos GG, Papageorgiou G, Verigos KE, Miliadis L, Stoidis CN. Local recurrence of pancreatic cancer after primary surgical intervention: how to deal with this devastating scenario? Surg Oncol. 2011;20:e133–e142. doi: 10.1016/j.suronc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Sunamura M, Egawa S, Shibuya K, Shimamura H, Takeda K, Kobari M, et al. [Therapeutic strategy for the recurrence of pancreatic cancer following pancreatectomy.] Nihon Geka Gakkai Zasshi. 1999;100:200–205. [PubMed] [Google Scholar]
- Menke-Pluymers MB, Klinkenbijl JH, Tjioe M, Jeekel J. Treatment of locoregional recurrence after intentional curative resection of pancreatic cancer. Hepatogastroenterology. 1992;39:429–432. [PubMed] [Google Scholar]
- Kersting S, Janot MS, Chromik AM, Suelberg D, Uhl W, Seelig MH. Contemporary single-centre surgical experiences in redo procedures of the pancreas: improved outcome and reduction of operative risk. J Gastrointest Surg. 2011;15:191–198. doi: 10.1007/s11605-010-1384-7. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Barkun J, Oliveira de ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- Eriguchi N, Aoyagi S, Imayama H, Okuda K, Hara M, Fukuda S, et al. Resectable carcinoma of the pancreatic head developing 7 years and 4 months after distal pancreatectomy for carcinoma of the pancreatic tail. J Hepatobiliary Pancreat Surg. 2000;7:316–320. doi: 10.1007/s005340070055. [DOI] [PubMed] [Google Scholar]
- Niiyama H, Yamaguchi K, Shimizu S, Yokohata K, Chijiiwa K, Yonemasu H, et al. Pancreatic carcinoma in remnant pancreas after pancreatectomy for mucinous cystadenoma. Eur J Gastroenterol Hepatol. 1998;10:703–707. [PubMed] [Google Scholar]
- Wada K, Takada T, Yasuda H, Amano H, Yoshida M. A repeated pancreatectomy in the remnant pancreas 22 months after pylorus-preserving pancreatoduodenectomy for pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2001;8:174–178. doi: 10.1007/s005340170043. [DOI] [PubMed] [Google Scholar]
- Dalla Valle R, Mancini C, Crafa P, Passalacqua R. Pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. JOP. 2006;7:473–477. [PubMed] [Google Scholar]
- D'Amato A, Gentili V, Santella S, Boschetto A, Pronio A, Montesani C. [Carcinoma of the pancreatic remnant developing after pancreaticoduodenectomy for adenocarcinoma of the head of pancreas.] Chir Ital. 2002;54:539–544. [PubMed] [Google Scholar]
- Takamatsu S, Ban D, Irie T, Noguchi N, Kudoh A, Nakamura N, et al. Resection of a cancer developing in the remnant pancreas after a pancreaticoduodenectomy for pancreas head cancer. J Gastrointest Surg. 2005;9:263–269. doi: 10.1016/j.gassur.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Soma I, Nakano K, Higaki N, Murakami M, Hayashida H, et al. [Repeated resections of asynchronous liver metastases after pancreatomy for pancreatic cancer – a case report.] Gan To Kagaku Ryoho. 2009;36:2410–2412. [PubMed] [Google Scholar]
- Seelig MH, Janot M, Chromik AM, Herzog T, Belyaev O, Weyhe D, et al. Redo-surgery following curative resection of pancreatic carcinoma: the difference between true and suspected recurrence. Dig Surg. 2009;26:222–228. doi: 10.1159/000219332. [DOI] [PubMed] [Google Scholar]
- Kim C, Tono T, Kimura Y, Watanabe A, Nakamura H, Inadome J, et al. [Re-resection for local recurrence in the remnant pancreas after pancreaticoduodenectomy for pancreatic cancer – a case report.] Gan To Kagaku Ryoho. 2011;38:2448–2450. [PubMed] [Google Scholar]
- Kinoshita H, Yamade N, Nakai H, Sasaya T, Matsumura S, Kimura A, et al. Successful resection of pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. Hepatogastroenterology. 2011;58:1406–1408. doi: 10.5754/hge09366. [DOI] [PubMed] [Google Scholar]
- Ogino T, Ueda J, Sato N, Takahata S, Mizumoto K, Nakamura M, et al. Repeated pancreatectomy for recurrent pancreatic carcinoma after pylorus-preserving pancreatoduodenectomy: report of two patients. Case Rep Gastroenterol. 2010;4:429–434. doi: 10.1159/000321513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Truty MJ, Nogueras-Gonzalez GM, Fleming JB, Vauthey JN, Pisters PW, et al. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg. 2012;16:1696–1704. doi: 10.1007/s11605-012-1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias T, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Surgery for recurrence of periampullary malignancies. J Gastrointest Surg. 13:760–767. doi: 10.1007/s11605-008-0769-3. [DOI] [PubMed] [Google Scholar]
- Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2009;245:566–572. doi: 10.1097/01.sla.0000245845.06772.7d. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Ohashi M, Tenma N, Matsuura H. [A case with a single liver metastasis from pancreatic cancer surviving for 29 months.] Gan To Kagaku Ryoho. 2007;34:1671–1674. [PubMed] [Google Scholar]
- Ota K, Yamamoto T, Matsumura T, Fukunaga M, Ohzato H, Miwa H, et al. [A case of surgical treatment of solitary liver metastasis from pancreatic cancer.] Gan To Kagaku Ryoho. 2009;36:2407–2409. [PubMed] [Google Scholar]
- Arnaoutakis GJ, Rangachari D, Laheru DA, Iacobuzio-Donahue CA, Hruban RH, Herman JM, et al. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg. 2011;15:1611–1617. doi: 10.1007/s11605-011-1605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts SR, Schroeder M, Erlichman C, Steen PD, Foster NR, Moore DF, Jr, et al. Gemcitabine and ISIS-2503 for patients with locally advanced or metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group Phase II trial. J Clin Oncol. 2004;22:4944–4950. doi: 10.1200/JCO.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Heinemann V, Labianca R, Hinke A, Louvet C. Increased survival using platinum analogue combined with gemcitabine as compared to single-agent gemcitabine in advanced pancreatic cancer: pooled analysis of two randomized trials, the GERCOR/GISCAD intergroup study and a German multicentre study. Ann Oncol. 2007;18:1652–1659. doi: 10.1093/annonc/mdm283. [DOI] [PubMed] [Google Scholar]
- Wilkowski R, Thoma M, Bruns C, Duhmke E, Heinemann V. Combined chemoradiotherapy for isolated local recurrence after primary resection of pancreatic cancer. JOP. 2006;7:34–40. [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- dos Santos LV, Andrade de DP, Lima JP. FOLFIRINOX: a great leap forward, but for whom? J Clin Oncol. 2012;30:114–115. doi: 10.1200/JCO.2011.39.4056. [Authors' reply.] [DOI] [PubMed] [Google Scholar]
- Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12:8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- Assaf E, Verlinde-Carvalho M, Delbaldo C, Grenier J, Sellam Z, Pouessel D, et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301–306. doi: 10.1159/000329803. [DOI] [PubMed] [Google Scholar]
- Conroy T, Mitry E. [Chemotherapy of metastatic pancreatic adenocarcinoma: challenges and encouraging results.] Bull Cancer. 2011;98:1439–1446. doi: 10.1684/bdc.2011.1494. [DOI] [PubMed] [Google Scholar]