Abstract
Objectives: An aberrant right hepatic artery (aRHA) may pose technical and oncologic challenges during pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma (PA) as a result of its proximity to the head of the pancreas. The aim of this study was to assess the impact of an aRHA on resectability, and perioperative and oncologic outcomes after PD for PA.
Methods: An 11-year retrospective cohort study was conducted. A total of 289 patients with PA scheduled for PD with intent for resection were included in the study.
Results: Of 289 patients, 249 underwent PD and 40 were found to have unresectable tumours. Incidences of aRHA in the resectable (14.9%) and unresectable (7.5%) groups were similar (P = 0.2); the main reasons for aborting PD were not directly related to the presence of an aRHA. In patients who underwent resection, complications occurred more frequently in the standard PD group (41.5% versus 24.3%; P = 0.04), but there was no difference in rates of positive margin (R1) resection (10.8% versus 16.0%; P = 0.4) or median overall survival (17 months versus 23 months; P = 0.1) between patients with and without an aRHA.
Conclusions: The presence of an aRHA in patients with PA does not affect resectability. In patients with resectable tumours, the presence of an aRHA does not increase morbidity or R1 resection rates and does not impact on overall survival.
Introduction
An aberrant right hepatic artery (aRHA) occurs in 15–20% of the population.1,2 It arises from the superior mesenteric artery (SMA), courses behind or through the head of the pancreas, and runs along the posterior aspect of the common bile duct (Fig. 1). The relevance of the aRHA in pancreaticoduodenectomy (PD) for cancer is two-fold. Firstly, the artery creates a technical challenge through its proximity to the structures involved in the PD dissection and the necessity of preserving it to maintain the blood supply to the right liver and the bile duct. Secondly, it must be dissected and preserved during PD in an oncologically sound manner that achieves negative margin (R0) resection.
Figure 1.
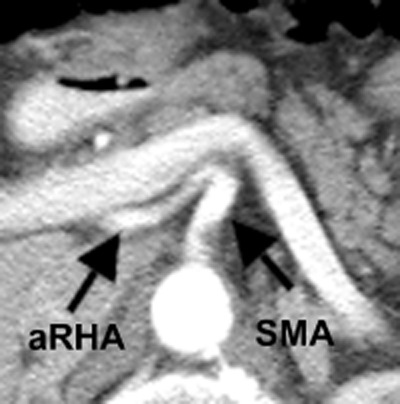
Axial computed tomography of an aberrant right hepatic artery (aRHA) arising from the superior mesenteric artery (SMA)
Previous studies have investigated outcomes in patients with an aRHA after PD for various cancers in the head of the pancreas and found that postoperative outcomes were not compromised by the presence of an aRHA.3–6 Turrini et al. reported a series of 41 patients with pancreatic adenocarcinoma (PA) and aRHA who underwent PD, in whom postoperative morbidity and positive margin rates were not increased.6 However, these studies have focused on patients submitted to PD and thus the question of whether the presence of an aRHA affects resectability remains unanswered. The present study was undertaken to analyse outcomes in 289 patients with PA who were initially deemed to be resectable and were scheduled for PD in order to determine: (i) the impact of the presence of an aRHA on eventual resectability, and (ii) the impact of an aRHA on oncologic and postoperative outcomes after PD at a high-volume hepatopancreatobiliary (HPB) surgical oncology centre.
Materials and methods
Study design and patient selection
This study was approved by the research ethics board of the University Health Network, University of Toronto, Toronto, Ontario, Canada (REB no. 11–0073-CE).
The study population included 289 patients with PA who were initially deemed resectable and were scheduled for PD at the study institution (University Health Network) from 1 January 2000 to 30 December 2010. The University Health Network is an academic, university-affiliated, high-volume Canadian HPB centre with 11 subspecialty trained HPB surgeons. A total of 635 PDs were performed at this institution during the study time period. Using retrospective chart review methodology, demographic, pathologic, postoperative and survival data were analysed.
Of the 289 patients, 249 underwent PD and 40 were found to have unresectable tumours at the time of laparotomy as a result of the intraoperative detection of metastatic or locally advanced disease. To address whether the presence of an aRHA affected resectability, demographic data, tumour size, National Comprehensive Cancer Network (NCCN) stage (resectable versus borderline versus locally advanced pancreas cancer)7 and the proportions of patients in the two groups were compared.
To establish whether the presence of an aRHA affects oncologic and postoperative outcomes in resected patients, the 249 patients who underwent PD were further divided into those with (aRHA group) and without (standard PD group) an aRHA. Patient demographics, tumour characteristics and postoperative outcomes were compared between these two groups.
The patients with variant upper abdominal arterial anatomy were identified using the Michels classification system.1 The different types of aRHA are as follows: (i) a replaced RHA that arises from the SMA, accompanied by a left hepatic artery (LHA) that arises from the common hepatic artery (CHA); (ii) a replaced RHA that arises from the SMA, accompanied by a replaced LHA that arises from the left gastric artery, and (iii) an accessory RHA that arises from the SMA and accompanies a main RHA that arises from the CHA. The total replacement of the hepatic artery from the SMA (coeliomesenteric trunk) and an early take-off of the RHA from the coeliac artery were also considered to be types of aRHA (not described by Michels1).
Preoperative management
Preoperative assessment included clinical assessment by an HPB surgeon, laboratory investigations and imaging by computed tomography (CT) of the chest and abdomen and/or magnetic resonance imaging (MRI) of the abdomen.
Surgical technique
A classic PD was performed in all cases. The pancreaticojejunostomy was performed with a retrocolic jejunal limb in end-to-side fashion with the duct-to-mucosa technique or the invagination technique according to the surgeon's preference. This was followed by an end-to-side choledochojejunostomy and a standard gastrojejunostomy. Intraoperative drains were used in 141 (56.6%) patients.
Pathologic analysis
All specimens were processed and interpreted by an experienced pancreas pathologist. The retroperitoneal margin was defined as the posterior section of the specimen where the uncinate process was dissected from the SMA and retroperitoneum (also known as the SMA margin). The pancreas margin was defined as the cut surface of the pancreas where the neck of the pancreas was divided. Pathologic staging was reported according to the seventh edition of the tumour–node–metastasis (TNM) staging system supported by the International Union against Cancer (UICC) and the American Joint Committee on Cancer (AJCC).8
Postoperative complications
Postoperative complications were graded according to the Clavien–Dindo scale for classifying complications in pancreatic surgery.9 Pancreatic leak or fistula was defined according to the definition established by the International Study Group on Pancreatic Fistula (ISGPF),10 in which an anastomotic leak is defined by an amylase-rich output (amylase content three times the upper limit of normal serum amylase) on or after postoperative day 3. Pancreatic fistulae were further graded into Grades A (biochemical fistula with no clinical consequence), B (fistula that requires a change in clinical management, including the adjustment or insertion of a drain) and C (clinically significant fistula requiring reoperation), respectively. Delayed gastric emptying (DGE) was defined as an inability to return to standard diet by the end of the first week after surgery and the consequent reinsertion of the nasogastric tube as established by the International Study Group of Pancreas Surgery (ISGPS).11 Postoperative bleeding was defined as intra-abdominal or gastrointestinal bleeding in the postoperative period that was significant enough to require blood transfusions and/or reoperation. Complications of Clavien–Dindo Grade III or higher, any DGE and any postoperative bleeding were considered to represent major complications.
Statistics
The chi-squared test was used for categorical variables of interest. Student's t-test was used for continuous variables. The Kaplan–Meier product-limit method was used to obtain survival rates over time. The log-rank test was used to compare overall survival between patients with and without an aRHA. A P-value of <0.05 was considered to indicate statistical significance.
Results
Classification of an aRHA
As illustrated in Table 1, the classification of aRHA anatomy was based on that described by Michels.1 Of 289 patients, 40 were found to have aRHA anatomy; this manifested as a fully replaced aRHA arising from the SMA in 31 (77.5%) subjects, an accessory aRHA in three (7.5%) patients, and a replaced aRHA with a concomitant replaced LHA in two (5.0%) patients. Total replacement of the hepatic arterial circulation in which the CHA arose from the SMA was found in two (5.0%) patients. In another two (5.0%) patients, the RHA arose directly from the coeliac artery, immediately distal to its take-off from the aorta, coursed behind the head of the pancreas and the portal vein and ran along the posterior lateral aspect of the bile duct. This variant was not described in the original classification by Michels1, but, because it is similar to a replaced RHA originating from the SMA, it was included in this series.
Table 1.
Demographics, preoperative staging and management of patients who did and did not undergo pancreaticoduodenectomy (PD)
| Variable | Unresected group (n = 40) | Resected group (n = 249) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 61 ± 10 | 64 ± 10 | 0.1 |
| Sex, male : female | 27:13 | 131:118 | 0.08 |
| Size, cm, mean ± SD | 3.2 ± 1.1 | 3.2 ± 1.2 | 1.0 |
| Aberrant RHA, n (%) | 3 (7.5%) | 37 (14.9%) | 0.2 |
| Types of aberrant RHA, n (%) | |||
| Replaced RHA from SMA | 3 (100%) | 28 (75.7%) | |
| Accessory RHA | 0 | 3 (8.1%) | |
| Replaced RHA and LHA | 0 | 2 (5.4%) | |
| Coeliomesenteric trunk | 0 | 2 (5.4%) | |
| RHA from coeliac artery | 0 | 2 (5.4%) | |
| NCCN staging, n (%) | |||
| Resectable | 29 (72.5%) | 222 (89.2%) | 0.004 |
| Borderline | 11 (27.5%) | 27 (10.8%) |
SD, standard deviation; RHA, right hepatic artery; SMA, superior mesenteric artery; LHA, left hepatic artery; NCCN, National Comprehensive Cancer Network.
Pancreaticoduodenectomy in patients with an aRHA
As illustrated in Fig. 2, of 289 patients with PA who underwent exploratory laparotomy with intent for PD, 40 patients underwent palliative procedures and 249 patients underwent PD (overall resection rate: 86.2%). Of the 40 patients with an aRHA, 37 underwent PD (resection rate: 92.5%), and of the patients with standard arterial anatomy, 212 underwent PD (resection rate: 85.1%).
Figure 2.
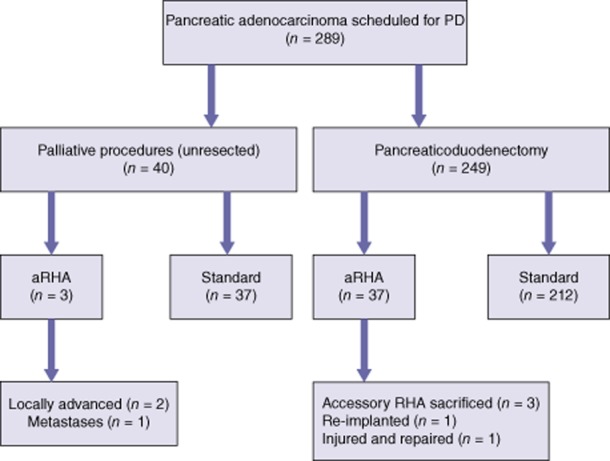
An outline of the management of patients with pancreatic adenocarcinoma with or without an aberrant right hepatic artery (aRHA) who underwent laparotomy with intent for pancreaticoduodenectomy (PD)
Of the 40 patients submitted to palliative procedures, three were found to have an aRHA. The presence of an aRHA did not ultimately determine resectability. In two of these patients, PD was aborted because of the involvement of the SMA in addition to the presence of aRHA anatomy. One patient with an aRHA did not undergo PD as a result of diffuse metastases.
As Table 1 shows, there was no difference in the incidence of an aRHA between patients who underwent PD and those who did not (14.9% versus 7.5%; P = 0.2). Although tumour size was similar in both groups, patients who did not undergo resection had a higher proportion of borderline resectable tumours according to the NCCN preoperative staging system (27.5% versus 10.8%; P = 0.004).
Of 249 patients who underwent PD, 37 patients had aRHA anatomy (aRHA group) and 212 had standard hepatic arterial anatomy (standard PD group) (Table 2). Operative variables were similar between the two groups. Mean ± standard deviation (SD) operative times in the aRHA group and the standard PD group were, respectively, 479 ± 85 min and 439 ± 128 min (P = 0.05). Median estimated blood loss in the aRHA group and the standard PD group was, respectively, 950 ml and 650 ml (P = 0.5). Incidences of vascular (portal vein) resections in the aRHA and standard PD groups were, respectively, 18.9% and 27.8% (P = 0.3). There was a slightly higher rate of postoperative complications in the standard PD group (Table 2), but when different types of complication were compared, no differences emerged between the two groups.
Table 2.
Perioperative details of patients who underwent pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma with an aberrant right hepatic artery (PD + aRHA) and with standard hepatic arterial anatomy (standard PD)
| Variable | PD + aRHA (n = 37) | Standard PD (n = 212) | P-value |
|---|---|---|---|
| Age, years, mean ± SD | 63 ± 10 | 64 ± 10 | 0.3 |
| Sex, male : female | 22:15 | 109:103 | 0.4 |
| Surgery time, min, mean ± SD | 479 ± 85 | 439 ± 128 | 0.05 |
| Estimated blood loss, ml, median (range) | 950 (100–4000) | 650 (100–6500) | 0.5 |
| Length of stay, days, median (range) | 9 (5–39) | 10 (5–90) | 0.2 |
| Portal vein resection, n (%) | 7 (18.9%) | 59 (27.8%) | 0.3 |
| Type of portal vein resection, n (%) | 0.4 | ||
| Circumferential | 5 (13.5%) | 34 (16.0%) | |
| Partial | 2 (5.4%) | 25 (11.8%) | |
| None | 30 (81.1%) | 153 (72.2%) | |
| Complications (CDC), n (%) | 9 (24.3%) | 88 (41.5%) | 0.04 |
| Grade of complication | 0.6 | ||
| I | 0 | 19 (21.6%) | |
| II | 5 (55.6%) | 37 (42.0%) | |
| IIIa | 3 (33.3%) | 18 (20.0%) | |
| IIIb | 1 (11.1%) | 9 (10.2%) | |
| IV | 0 | 4 (4.5%) | |
| V | 0 | 1 (1.1%) | |
| Pancreatic leak, n (%) | 1 (2.7%) | 13 (6.1%) | 0.7 |
| Type A | 0 | 1 (7.7%) | |
| Type B | 1 (100%) | 9 (69.2%) | |
| Type C | 0 | 3 (23.1%) | |
| Biliary leak, n (%) | 0 | 1 (0.5%) | 1.0 |
| Delayed gastric emptying, n (%) | 4 (10.8%) | 16 (7.6%) | 0.5 |
| Postoperative bleed, n (%) | 1 (2.7%) | 16 (7.6%) | 0.5 |
| Type of bleeding, n (%) | 0.06 | ||
| Intra-abdominal | 0 | 9 (56.3%) | |
| Gastrointestinal | 0 | 7 (43.8%) | |
| Retroperitoneal | 1 (100%) | 0 | |
| Intra-abdominal sepsis, n (%) | 4 (10.8%) | 26 (12.3%) | 1.0 |
| Postoperative mortality, n (%) | 0 | 1 (0.5%) | 1.0 |
SD, standard deviation; CDC, Clavien–Dindo classification.
In four patients with aRHA anatomy who underwent PD, the aRHA was intimately involved or encased with the tumour. Intraoperative assessment by Doppler ultrasound (US) of intact arterial flow to the right lobe of the liver was performed prior to any decision on whether an aRHA could be sacrificed. In three patients, the aRHA was deemed to be an accessory artery (indicated by an intact arterial Doppler signal to the right lobe after temporary clamping) and was sacrificed to achieve a negative oncologic margin. In one patient, the aRHA was deemed to be a replaced RHA and was re-implanted to the stump of the gastroduodenal artery (GDA) after the involved segment had been resected (Fig. 2). All four patients who underwent PD with an involved aRHA had R0 resection. One additional patient had an injured aRHA (replaced RHA) that was not involved by the tumour and was repaired primarily.
Oncologic outcomes
Tumour characteristics were similar between the PD + aRHA and standard PD groups (Table 3). Mean ± SD tumour size was 3.4 ± 1.4 cm and 3.1 ± 1.1 cm (P = 0.2) in the aRHA and standard arterial anatomy groups, respectively. The TNM stage was similar in both groups. The rate of lymphovascular invasion was significantly higher in the standard PD group (65.6% versus 48.7%; P = 0.009). Rates of positive margin (R1) resection were similar in the PD + aRHA and standard PD groups (10.8% versus 16.0%; P = 0.4). When positive margins were analysed by location, the most common site was found to be the retroperitoneal margin (SMA margin).
Table 3.
Oncologic details in patients who underwent pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma with an aberrant right hepatic artery (PD + aRHA) and standard hepatic arterial anatomy (standard PD)
| Variables | PD + aRHA (n = 37) | Standard PD (n = 212) | P-value |
|---|---|---|---|
| Neoadjuvant therapy, n (%) | 1 (2.7%) | 13 (6.1%) | 0.7 |
| Size, cm, mean ± SD | 3.4 ± 1.4 | 3.1 ± 1.1 | 0.2 |
| Grade (differentiation), n (%) | (n = 35) | (n = 208) | 0.3 |
| Good | 10 (28.6%) | 39 (18.8%) | |
| Moderate | 19 (54.3%) | 139 (66.8%) | |
| Poor | 6 (17.1%) | 30 (14.4%) | |
| Perineural invasion, n (%) | 27 (73.0%) | 162 (76.4%) | 0.09 |
| Lymphovascular invasion, n (%) | 18 (48.6%) | 139 (65.6%) | 0.009 |
| T-stage 1, n (%) | 1 (2.7%) | 9 (4.2%) | 0.9 |
| T-stage 2, n (%) | 3 (8.1%) | 14 (6.6%) | |
| T-stage 3, n (%) | 33 (89.2%) | 183 (86.3%) | |
| T-stage 4, n (%) | 0 | 4 (1.9%) | |
| N1, n (%) | 27 (73.0%) | 159 (75.0%) | 0.8 |
| Number of positive LN | 3 ± 3 | 3 ± 3 | 0.6 |
| Total number of LN | 17 ± 7 | 17 ± 8 | 0.8 |
| LN ratioa | 0.21 ± 0.18 | 0.24 ± 0.17 | 0.4 |
| R1 resection, n (%) | 4 (10.8%) | 34 (16.0%) | 0.4 |
| Type of R1 resection, n (%) | 1.0 | ||
| Retroperitoneal | 3 (75.0%) | 19 (55.9%) | |
| Pancreas | 1 (25.0%) | 13 (38.2%) | |
| Common bile duct | 0 | 1 (2.9%) | |
| Portal vein | 0 | 1 (2.9%) |
Defined as the ratio between the number of lymph nodes examined and the number of positive lymph nodes.
SD, standard deviation; LN, lymph nodes.
Although the rate of lymphovascular invasion was higher in the standard PD group, this did not appear to have an impact on longterm survival, which was similar in both groups of resected patients (Fig. 3). Median survival in patients with an aRHA was 17 months, whereas median survival in patients with standard hepatic arterial anatomy was 23 months (P = 0.1). Rates of 1-, 2-and 5-year overall survival in patients in the aRHA and standard arterial anatomy groups were 68%, 36% and 11%, and 70%, 47% and 20%, respectively.
Figure 3.
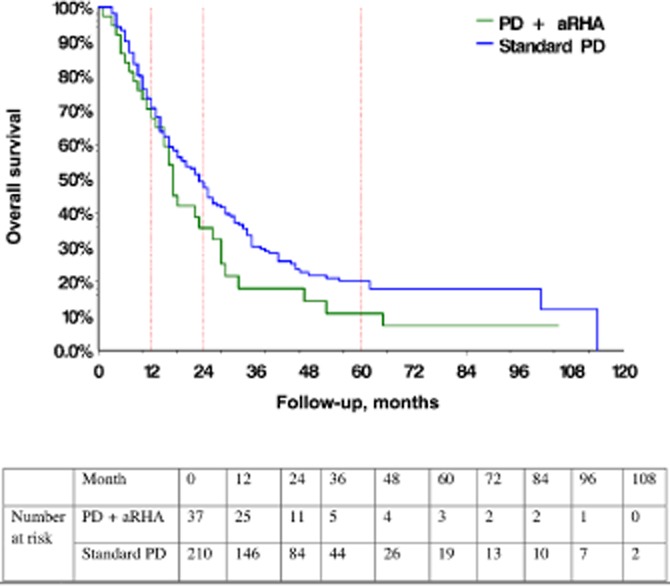
Overall survival in pancreatic adenocarcinoma patients with an aberrant right hepatic artery (PD + aRHA group) and standard hepatic arterial anatomy (standard PD group) undergoing pancreaticoduodenectomy (PD) (P = 0.1)
Univariate and multivariate analyses of the variables found that size, grade, lymph node involvement, lymphovascular invasion and positive margin resection were associated with survival (Tables 4 and 5).
Table 4.
Univariate analysis of variables associated with survival in patients with pancreatic adenocarcinoma who underwent pancreaticoduodenectomy
| Variable | HR | 95% CI for HR |
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Size | 1.3 | 1.2 | 1.5 | <0.0001 |
| Grade | <0.0001 | |||
| Moderate | 1.2 | 0.8 | 1.8 | |
| Good | 2.7 | 1.7 | 4.5 | |
| Perineural invasion | 1.0 | 0.7 | 1.5 | 0.9 |
| Lymphovascular invasion | 1.4 | 1.0 | 2.0 | 0.03 |
| Positive lymph nodes | 2.1 | 1.5 | 3.1 | <0.0001 |
| T-stage | 0.1 | |||
| T1 | 0.2 | 0.07 | 0.8 | |
| T2 | 0.3 | 0.09 | 0.8 | |
| T3 | 0.3 | 0.1 | 0.9 | |
| Lymph node involvement | 2.0 | 1.4 | 3.0 | 0.0002 |
| R1 resection | 2.0 | 1.4 | 2.9 | 0.0003 |
| Age | 1.0 | 1.0 | 1.0 | 0.5 |
| Gender (female) | 1.0 | 0.7 | 1.3 | 1.0 |
| Aberrant right hepatic artery | 1.4 | 0.9 | 2.0 | 0.1 |
HR, hazard ratio; 95% CI, 95% confidence interval.
Table 5.
Multivariate analysis of variables associated with survival in patients with pancreatic adenocarcinoma who underwent pancreaticoduodenectomy
| Variable | HR | 95% CI for HR | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Size | 1.3 | 1.2 | 1.5 | <0.0001 |
| Grade | 0.008 | |||
| Moderate | 1.3 | 0.9 | 2.0 | |
| Poor | 2.2 | 1.3 | 3.8 | |
| Positive lymph nodes | 2.2 | 1.5 | 3.3 | <0.0001 |
| R1 resection | 2.3 | 1.5 | 3.4 | <0.0001 |
| Aberrant right hepatic artery | 1.2 | 0.8 | 1.8 | 0.4 |
HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
The findings of this study show that the presence of an aRHA does not significantly affect resectability in patients undergoing PD for PA. When an aRHA was present, the reason for unresectability did not usually reflect the isolated involvement of the aRHA, but, rather, the presence of metastases or locally advanced disease involving major arterial structures (i.e. the SMA). In addition, the finding that patients submitted to palliative procedures (unresected patients) did not show an increased incidence of aRHA further supports the suggestion that an aRHA does not directly affect resectability. In patients who underwent resection, the presence of an aRHA did not affect postoperative or oncologic outcomes, including margin status and overall survival.
In patients in whom the isolated involvement of an aRHA was identified, R0 resection could be achieved through adherence to technical and oncologic principles during the resection: an accessory RHA was sacrificed for R0 resection, and a replaced RHA was resected and re-implanted to achieve an R0 resection.
The importance of preserving a replaced RHA arising from the SMA during PD has been well described by others. The inadvertent division of the aRHA has been reported to result in ischaemic breakdown of the bilioenteric anastomosis and subsequent biliary fistula.12 The common bile duct obtains its blood supply from the RHA and the retroduodenal artery, a branch of the GDA.13 The latter is taken during PD and, as a result, inadvertent division of the RHA renders the common bile duct ischaemic.
Another challenging RHA anatomic anomaly is a replaced RHA arising from the GDA.14 This variant poses challenges to achieving an R0 resection because its origin is close to the head of the pancreas and it can be inadvertently ligated if the GDA is divided close to its origin on the CHA.
The main surgical principles of PD in a patient with an aRHA are two-fold: the blood supply to the liver and the common bile duct must be preserved, and the resection must be conducted in an oncologically sound manner. The present results show that both principles can be maintained. When resection of the aRHA was required to achieve an R0 margin, it was sacrificed (resected and not re-implanted) and deemed to be an accessory RHA. This action was taken based on the review of preoperative imaging and the confirmation by Doppler US of an intact arterial blood flow to the right side of the liver. If the aRHA was thought to be a replaced RHA, the involved segment of the artery was resected and re-implanted to maintain the blood supply to the liver and bile duct. No biliary fistulae were observed in the aRHA group. In fact, the rate of postoperative complications was slightly higher in the standard PD group. Although this was not statistically significant, a trend toward a slightly longer operative time in the aRHA group was apparent and probably reflected the extra time spent on dissecting and constructing the aRHA.
The origin of the aRHA from the SMA occurs at the point at which the inferior pancreaticoduodenal artery (IPDA) would arise in PD without an aRHA. Intuitively, if an aRHA is preserved, it may compromise the oncologic dissection on the SMA, which is usually facilitated by dividing the IPDA. In the present series, the presence of an aRHA did not compromise oncologic margins in that R0 resection rates were similar in patients with and without an aRHA. Furthermore, there was no difference in survival between the two groups of patients. The issue of margin may be more relevant and challenging in patients with an uncinate tumour that is intimately involved with the aRHA. Four possible scenarios may arise in this situation: (i) an accessory RHA may be sacrificed to achieve R0 resection (as in three patients in the present series); (ii) a replaced RHA may be preserved with a high risk for R1 resection; (iii) the tumour may be deemed unresectable because of the concomitant involvement of the SMA (as in two patients in the present series), and (iv) the involved portion of the replaced RHA may be resected and reconstructed (as in one patient in the present series).
Various studies have described the safety of occlusion of aberrant hepatic arteries in the setting of hepatic arterial infusion pump insertions.15–18 It is difficult to apply the lessons learned from these studies to the present study in which PD was carried out and bilioenteric anastomoses performed.
To the knowledge of the present authors, this is the first published study to specifically address the impact of an aRHA on resectability in PD for pancreatic cancer. The present study is limited by its failure to capture patients with an aRHA and locally advanced disease, who were initially deemed to be unresectable. Therefore, no comment on the impact of an aRHA in locally advanced PA can be made. In addition, the relatively small numbers of patients with aRHA anatomy in the unresected and resected groups may have masked a potentially significant difference in impact on resectability and survival. A larger series that includes more patients with an aRHA may help to further elucidate the impact of an aRHA on resectability and postoperative outcome.
In summary, the present findings indicate that the presence of an aRHA does not appear to affect resectability, or perioperative and oncologic outcomes in patients with PA who were initially deemed to have otherwise resectable tumours. If technical and oncologic principles are observed, PD in patients with an aRHA can be performed with results similar to those achieved in patients with standard hepatic arterial anatomy.
Conflicts of interest
None declared.
References
- Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. 1994;220:50–52. doi: 10.1097/00000658-199407000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Lee YJ, Kim CW, Moon KM, Kim MW. Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Surg. 2009;33:1727–1732. doi: 10.1007/s00268-009-0063-x. [DOI] [PubMed] [Google Scholar]
- Eshuis WJ, Olde Loohuis KM, Busch OR, Gulik van TM, Gouma DJ. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB. 2011;13:161–167. doi: 10.1111/j.1477-2574.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jah A, Jamieson N, Huguet E, Praseedom R. The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today. 2009;39:669–674. doi: 10.1007/s00595-009-3947-3. [DOI] [PubMed] [Google Scholar]
- Turrini O, Wiebke EA, Delpero JR, Viret F, Lillemoe KD, Schmidt CM. Preservation of replaced or accessory right hepatic artery during pancreaticoduodenectomy for adenocarcinoma: impact on margin status and survival. J Gastrointest Surg. 2010;14:1813–1819. doi: 10.1007/s11605-010-1272-1. [DOI] [PubMed] [Google Scholar]
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- Sobin LHGM, Wittekind C. TNM Classification of Malignant Tumours. 7th edn. Oxford: Wiley & Sons; 2010. [Google Scholar]
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. discussion 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Traverso LW, Freeny PC. Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg. 1989;55:421–426. [PubMed] [Google Scholar]
- Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- Yang SH, Yin YH, Jang JY, Lee SE, Chung JW, Suh KS, et al. Assessment of hepatic arterial anatomy in keeping with preservation of the vasculature while performing pancreatoduodenectomy: an opinion. World J Surg. 2007;31:2384–2391. doi: 10.1007/s00268-007-9246-5. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Stojadinovic A, Ben-Porat L, Gonen M, Kooby D, Blumgart L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol. 2002;9:875–880. doi: 10.1007/BF02557524. [DOI] [PubMed] [Google Scholar]
- Cohen AM, Higgins J, Waltman AC, Athanasoulis C, McKusick K. Effect of ligation of variant hepatic arterial structures on the completeness of regional chemotherapy infusion. Am J Surg. 1987;153:378–380. doi: 10.1016/0002-9610(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Curley SA, Chase JL, Roh MS, Hohn DC. Technical considerations and complications associated with the placement of 180 implantable hepatic arterial infusion devices. Surgery. 1993;114:928–935. [PubMed] [Google Scholar]
- Rayner AA, Kerlan RK, Stagg RJ, Price DC, Hohn DC. Total hepatic arterial perfusion after occlusion of variant lobar vessels: implications for hepatic arterial chemotherapy. Surgery. 1986;99:708–715. [PubMed] [Google Scholar]


