Abstract
Objective: A right and left hepatic trisectionectomy and an extended trisectionectomy are the largest liver resections performed for malignancy. This report analyses a series of 23 patients who had at least one repeat resection after a hepatic trisectionectomy for colorectal liver metastasis (CRLM).
Methods: A retrospective analysis of a single-centre prospective liver resection database from May 1996 to April 2009 was used for patient identification. Full notes, radiology and patient reviews were analysed for a variety of factors with respect to survival.
Results: Twenty-three patients underwent up to 3 repeat hepatic resections after 20 right and 3 left hepatic trisectionectomies. In 18 patients the initial surgery was an extended trisectionectomy. Overall 1-, 3- and 5-year survival rates after a repeat resection were 100%, 46% and 32%, respectively. No factors predictive for survival were identified.
Conclusion: A repeat resection after a hepatic trisectionectomy for CRLM can offer extended survival and should be considered where appropriate.
Keywords: trisectionectomy, repeat resection, hepatectomy, colorectal liver metastases, extended trisectionectomy, metastasectomy
Introduction
A hepatic trisectionectomy entails a large liver resection involving removal of up to 80% of the liver parenchyma.1–6 These procedures are used in highly specialized units primarily for the treatment of extensive and advanced hepatic or biliary disease. A hepatic trisectionectomy remains the most challenging of the major anatomic hepatectomies with higher complication rates than other hepatic resections.4,5 There have been few published series with these procedures and long-term follow-up has rarely been considered.4,5,7–12 Recently reported experience with a right and left hepatic trisectionectomy, and described extensions of these operations, regarded as extended trisectionectomy, have been analysed in an attempt to define the current role for these challenging operations.4,5,9
For hepatic colorectal metastases (CRLM), a liver resection has greatly improved survival rates, with 5-year survival reported to be 30% to 60%.13–16 However, after the initial liver resection, recurrence occurs in up to 75% of patients and in 20–40% of these patients, the liver is the only site of recurrence.16–20 At present there are no clear practice guidelines for the management of recurrent hepatic metastasis. A repeat liver resection is increasing in popularity, but there are few long-term studies assessing the outcome. Further, most of the available data relates to patients who have undergone only a minor liver resection initially.13,15,18,19,21–23
After an extensive hepatic resection, recurrence is more likely owing to the aggressive nature of the initial disease.24 As there is a significantly reduced liver volume after the initial resection, although liver regeneration occurs, important anatomy is altered. Thus, a repeat liver resection after a hepatic trisectionectomy is more challenging. This study reports a cohort of patients who underwent a trisectionectomy or an extended trisectionectomy and had a repeat hepatectomy with the aim of describing the clinical course and surgical techniques used in selected patients in order to illustrate the difficulties with this patient population.
Patients and methods
A retrospective analysis of a prospective database was undertaken. The study period was from May 1996 and April 2009. During this period, a total of 427 patients underwent right and left hepatic trisectionectomies for a variety of aetiologies. Twenty-three (5.3%) patients with CRLM subsequently underwent a further liver resection and were included in this study. The technique for a trisectionectomy and an extended trisectionectomy has previously been described.4,5,9 Portal vein embolization was not routinely used. Future liver remnant function was assessed as previously described.25 After recurrence of a tumour, patients were assessed prior to resection with a computed Tomography (CT) of the thorax, abdomen and pelvis to exclude extrahepatic disease. More recently, positron emission tomogrophy (PET) CT has been used to assess any suspicious extrahepatic lesions that were not fully characterized by CT alone. Patients were only subjected to re-resection if extrahepatic disease was felt to be resectable (most often lung metastases). All patients underwent magnetic resonance imaging (MRI) of the liver to delineate the anatomy clearly and to characterize the tumour. It has been the unit policy never to biopsy malignant liver lesions when considering surgery because of the fear of tumour seeding. All resections were performed using a Cavi-Pulse Ultrasonic Surgical Aspirator (CUSA, Model 200T; Valley Lab., Boulder, CO, USA), with a Pringle manoeuvre (portal triad clamping) applied only in selected patients to minimize blood loss and total vascular exclusion (portal triad and hepatic vein or inferior vena cava clamping) was used when necessary for tumours at the hepatocaval confluence. Figures 1 and 2 represent two of the 23 patients, and demonstrate the complexities of the types of cases described.
Figure 1.
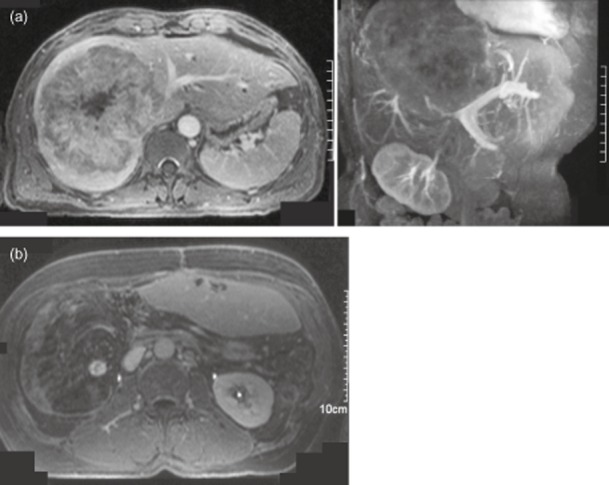
A 53-year-old male who had resection of a T1 N0 sigmoid colon cancer in 1993 presented with deranged liver function tests (LFTs). A cross-section and a coronal oblique magnetic resonance scan (MRI) scan (Fig. 1a) in July 2000 demonstrated a large liver metastasis obliterating the right lobe and invading the left lobe. The tumour surrounded much of the inferior vena cava (IVC), with obliteration of the right and middle hepatic veins and it was in close apposition to the left portal triad structures. A surgical resection was by a right hepatic trisectionectomy, caudate lobectomy (peeling the tumour off the IVC and left hepatic vein), with bile duct excision and a hepaticojejunostomy. Histopathology of the 2.0-kg resection specimen showed a 14-cm-diameter tumour comprised of moderately differentiated adenocarcinoma consistent with colorectal origin, with a clear resection margin of 0.5 mm. The patient made an uncomplicated recovery, with discharge on day 8. He received adjuvant chemotherapy with 5-Fluorouracil (5-FU) for 6 months. In June 2001, a routine follow-up computed tomography (CT) scan detected further liver metastases and MRI showed three tumours in the liver remnant (Fig. 1b). It was possible to remove these tumours by metastasectomy – anterior segment 2, anterior segment 3, posterior segment 3. Histopathology demonstrated the tumours to be 3.3, 2.7 and 2.0 cm in diameter and composed of moderately differentiated adenocarcinoma, with vascular invasion into portal vein branches. A tumour was seen microscopically at the resection margins in two of the three specimens (R1). No further chemotherapy was offered by his local oncology team. Follow-up was routine until May 2006 when a CT scan showed an 8-mm pulmonary metastasis, which was resected with a clear margin, confirmed to be metastatic adenocarcinoma. No chemotherapy was offered at this stage. Further follow to January 2013 up has been uneventful
Figure 2.
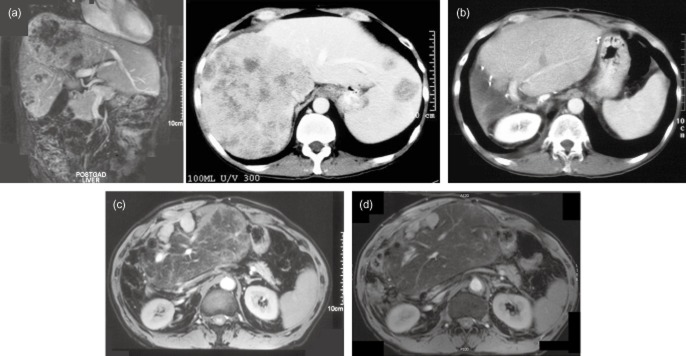
A 50-year-old male presented with synchronous colon cancer and liver metastases in August 2000 and he underwent a left hemicolectomy in, followed by chemotherapy with 5-Fluorouracil (5-FU). In March 2001, he was assessed for hepatic surgery and imaging confirmed tumours in every liver segment (Fig. 2a). There was a large right-sided tumour enveloping the inferior vena cava (IVC) and obliterating the right and middle hepatic veins and the hepatocaval confluence of the left hepatic vein. The portal triad structures to the left side appeared clear. In June 2001, he underwent a right hepatic trisectionectomy with a caudate lobectomy and resection of parts of segments 2 and 3 (an extended right trisectionectomy) using total vascular exclusion with in situ hypothermic perfusion and veno-veno bypass for a 90-min period. Intra-operative ultrasound confirmed tumours in segments 1, 4, 5, 6, 7, 8 and further metastases in segments 2 and 3. The LHV was involved and was partially resected and subsequently reconstructed using a flap created from IVC. The right hemi-diaphragm was involved and was resected en bloc with the liver and then reconstructed. Histology confirmed adenocarcinoma of a colorectal origin and showed clear margins from the trisectionectomy, but R1 from the segment 2/3 metastasis. A computed tomography (CT) scan 6 months post liver surgery revealed a 6-mm deposit in segment 2, which was confirmed on magnetic resonance imaging (MRI) (Fig. 2b) and he subsequently underwent a repeat metastasectomy in January 2002. Histology confirmed clear margins (R0). He remained disease free until March 2004 when he required 2 right lung metastasectomies. He then remained well until October 2005, where he was found to have a large lesion in the left upper lobe, abutting the subclavian artery and another lesion in the same lobe. Both were resected successfully. In February 2006, follow-up imaging revealed a new liver lesion in the anterior aspect of the remnant. He was recommended to undergo further chemotherapy, but after 3 cycles of oxaliplatin and 5-FU, treatment ceased owing to significant side effects. MRI and PET CT in May 2006 revealed 2 small liver metastases in the remnant but there was also marked uptake in the left upper thorax near the trachea and oesophagus. He underwent further thoracic surgery in August 2006, with resection of a 3.0 × 2.5 cm nodal mass of adenocarcinoma next to subclavian artery. A further MRI of the liver to re-asses the liver metastasis and this confirmed two new tumours, with an overall size of 5.3 cm (Fig. 2c). He had a repeat hepatic resection and histology confirmed adenocarcinoma, R1 owing to proximity to important vascular structures at time of hepatectomy. Owing to the aggressive nature of the disease, the patient was offered further chemotherapy, but declined due to poor experiences previously. Interim CT scans again remained normal. However, at a clinical review in October 2009, now 8.5 years after a trisectionectomy, he was noted to have a mass in the anterior abdominal wall with ascites. An MRI scan revealed a 3.7-cm liver metastasis in the anterior part of the liver remnant and it also demonstrated left hepatic vein stenosis (Fig. 2d). He went on to have left hepatic vein endovascular stenting and the ascites improved. PET CT and MRI scan in February 2010 confirmed the lesions in the remnant liver with no extrahepatic disease. He went on to have his third repeat hepatectomy in March 2010, this time R0. This patient remains well at present with no evidence of recurrent disease on CT in November 2012
During the 14-year study period, emerging evidence relating to the use of neoadjuvant and adjuvant chemotherapy has meant that the policies for the use of these treatments have evolved. In recent years, 5-flurouracil (5-FU) has been used in combination with Oxaliplatin or Irinotecan after a liver recection, where in the past patients only received 5-FU. However, since the beginning of the study period adjuvant chemotherapy was offered to all liver resection patients who had not received chemotherapy within the previous 2 years. It has been this unit's preference not to use neo-adjuvant chemotherapy prior to a redo liver resection because of worries about future liver remnant function. Several patients in this series received chemotherapy adjuvant to the primary liver surgery; this is detailed in the results. No patients were treated with intra-arterial chemotherapy, transarterial chemoembolization (TACE) or radiolabelled microspheres.
Follow-up included a general review at 6 weeks post procedure, followed by CT of the thorax, abdomen and pelvis at 3, 6, 12, 18, 24 months post last resection and blood studies including liver function tests (LFTs) and tumour markers (CEA, CA19-9). Blood tests and clinical examinations were then repeated yearly to 10 years, with CT scanning yearly to 5 years and again at 7 and 10 years.
Statistical analysis
Statistical analysis focused on a variety of factors including: survival time, chemotherapy, the size of tumours, resection margin status and CEA. Statistical analysis involved Kaplein–Mier survival plots, the log-rank test and the Mann U-Whitney tests, performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Demographic data
Twenty-three patients underwent a hepatic trisectionectomy or an extended trisectionectomy for CRLM: 20 right and 3 left. There were 16 males and 7 females with an age range of 39 to 77 years at initial surgery.
Technical aspects
In addition to the hepatic trisectionectomy, a caudate lobectomy was necessary in six patients (five right and one left). Eighteen patients had an extended trisectionectomy, with a non-anatomical resection for tumours in the remaining segments en bloc or separately. Of the 20 right trisectionectomies, 15 were extended and all of the 3 left trisesectionectomies involved resection into segment 6 and/or 7. Total vascular exclusion was applied in five patients, with four patients requiring hepatic vein reconstruction. All 23 patients had at least one repeat resection, with 4 patients undergoing further liver resections at a later date (Fig. 3). Nine patients had lung resections, with two patients undergoing a second lung resection and one a third thoracic resection.
Figure 3.
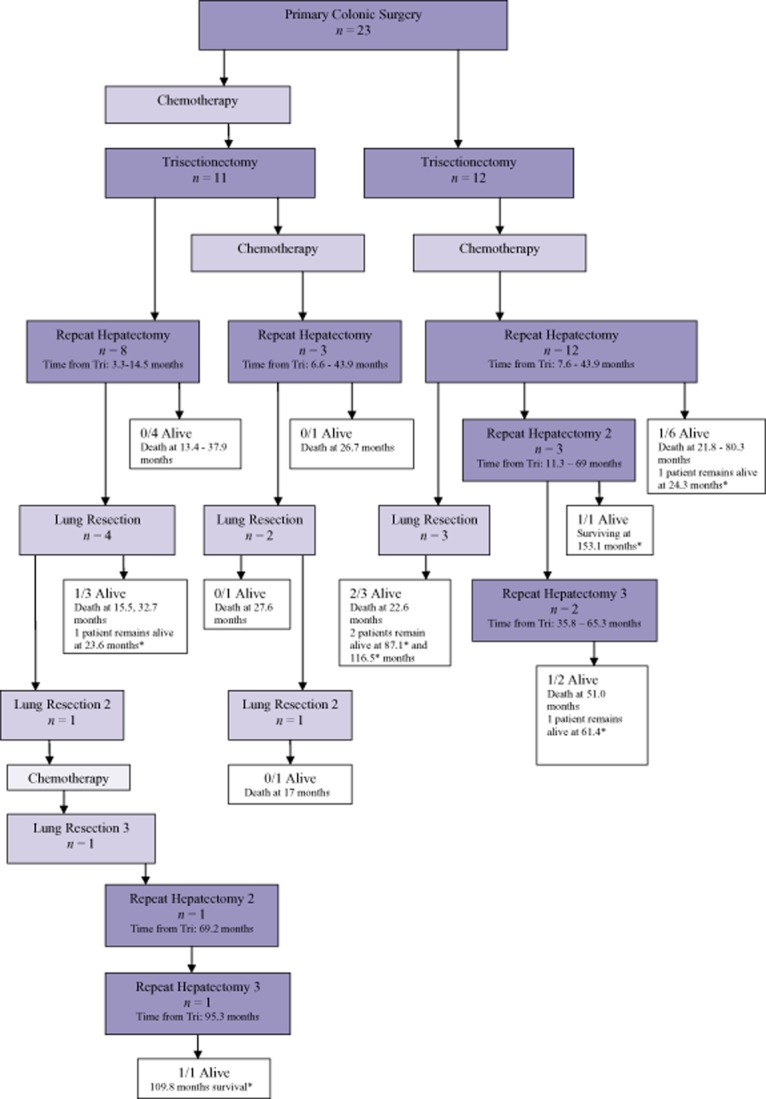
Flow chart detailing colorectal liver metastasis patients (n = 23). Survival times are from first repeat resection after a trisectionectomy or an extended trisectionectomy. *disease free at last assessment
Survival
After a hepatic trisectionectomy, the overall 1-, 3-, and 5-year survival was 100%, 87% and 55%, respectively, after a trisectionectomy, with a median survival of 69 months. After the first repeat resection 1-, 3, and 5-year survival was 100%, 46% and 32% with a median survival of 32.5 months (Fig. 4). Four patients had a second repeat resection with a median survival time of 85.6 months after the first repeat resection, 3 of whom remain alive. Three patients went on to have a third redo hepatectomy with a median survival of 64.1 months (from first repeat hepatectomy), with two of these patients remaining alive and disease free. The median follow-up for the survivors was 87.1 months (n = 7).
Figure 4.
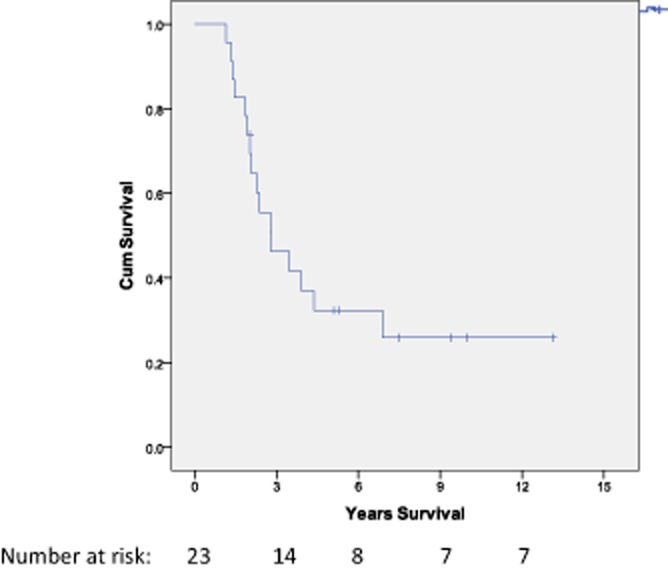
Survival data (overall survival from a repeat resection for the CRLM group (n = 23). 1-, 3- and 5-year survival was 100%, 46% and 32%. The median survival was 32.5 months (2.7 years)
The time to redo surgery in the CRLM group was a range of 3.3 to 66.9 months, with a median interval of 14.5 months. All patients received chemotherapy, eleven patients prior to trisectionectomy and 15 patients had it after a trisectionectomy (all the patients who did not have it previously and three patients who had it prior to trisectionectomy). Patients who had chemotherapy prior to the trisectionectomy had a median time to redo surgery of 8.2 months to redo compared with 25.5 months for patients that only received it after the trisectionectomy. There was no statistical difference in survival between the two groups.
After a repeat resection, the median survival in the R0 group was 45.8 months and in the R1 group was 26.7 months (P = 0.465). Tumours were analysed by size based on previous studies of a repeat hepatectomy for CRLM demonstrating prognostic significance for tumours <3 cm or <5 cm.19,21 Tumour size did not significantly influence survival. Plasma CEA levels did not correlate with survival.
Complications
Recognizing that the re-resection cohort described in this paper is selected from patients who survived a trisectionectomy, these patients were fortunate in experiencing few complications. In the short term, after the initial right or left hepatic trisectionectomy or extended trisectionectomy, there was one bile leak (managed non-operatively) and one patient required a laparotomy for bleeding. One patient developed hepatorenal syndrome after the trisectionectomy, but recovered. There were no significant long-term complications, although two patients developed incisional hernias. After a repeat resection, there were no significant short-term or long-term complications except for patient 10 who required endovascular stenting for left hepatic vein stenosis.
Discussion
In recent years, a repeat liver resection has been increasing in popularity as the morbidity and mortality rates from a hepatectomy are low.26 However, a repeat liver resection is technically demanding because of peri-hepatic adhesions, altered anatomy in the remnant liver owing to regeneration and often fragile liver parenchyma because of chemotherapy.13,19 There are several series reporting a repeat liver resection with good survival rates.14,18,19,21,22,27 Some of these repeat resections are major, but the majority remain minor resections (single metastasectomies). A repeat liver resection is the most challenging after an initial major liver resection. There has been no series published to date describing a repeat resection after a hepatic trisectionectomy or extended trisectionectomy, operations that involve removing up to 80% of the liver parenchyma. With any repeat resection, the aim is to improve survival, and this series demonstrates that a continual aggressive approach achieves this.
It is suggested that in untreated CRLM patients, a median survival is 4.5 to 6.5 months or up to 24 months for patients treated with systemic chemotherapy alone.21 In a previous study, this unit looked at 54 repeat resections for patients with CRLM and demonstrated a median survival of 50.3 months after a repeat resection. The 3- and 5-year survival rates after a resection were 53% and 46%, respectively.19 Other previous studies have been based on repeat resections after smaller primary resections than in our series but have reported similar results, with 5 year survivals of 31–38%.13,18,21,22 The data from the current series shows that the median survival after a hepatic trisectionectomy in patients who have gone on to develop resectable recurrent disease was 69 months and a repeat hepatectomy gained a median extension of 32.5 months, suggesting that operating for recurrence after a trisectionectomy improves survival. After a second repeat resection, patients survived for a median of 85.6 months and all the patients who underwent three or more repeat resections remain alive.
In this series, the median time to a repeat resection was 14.5 months (range, 3.3 to 66.9) and this is similar to studies reported by De Jong et al. (19.1 months) and Imamura et al. (11 months).13,26 The prognosis of patients who underwent a repeat hepatic resection at 1 year or less after the initial hepatectomy was no worse than the prognosis of patients who underwent a repeat resection with an interval of 1 year or more (P = 0.747). This is contrary to other previous studies that suggested a repeat hepatectomy in less than 1 year leads to a poorer prognosis.18,26
There is a general consensus that a positive or involved (R1) resection is associated with early disease recurrence and shorter long-term survival.18,28 Owing to the scale of resection required for patients in this series a high R1 resection rate is expected. At repeat resection, patients who underwent a R0 compared with a R1 resection had slightly higher survival rates, but not significantly. It has previously been demonstrated that with larger numbers of patients these differences become significant.19 Owing to the small number of patients in this cohort, the value of chemotherapy could not be established.
De Jong et al. found the only significant associated factor for survival from the time of the second resection was the presence of extra-hepatic disease (P < 0.001).13 Patients with extra-hepatic disease at the time of second resection had a median survival of 27 vs. 50 months for those who had intrahepatic disease only (P < 0.001).13 In the current series, nine patients underwent lung resections for metastases from colorectal cancer. Two of these patients had a second lung resection.
In summary, this series demonstrates that that a repeat liver resection after a right and left hepatic trisectionectomy and an extended trisectionectomy can lead to improved survival for patients with CRLM. Further, this series illustrates the surgical challenges in dealing with this complex patient group. Figure 5 is offered as an algorithm for the management of this select patient group.
Figure 5.
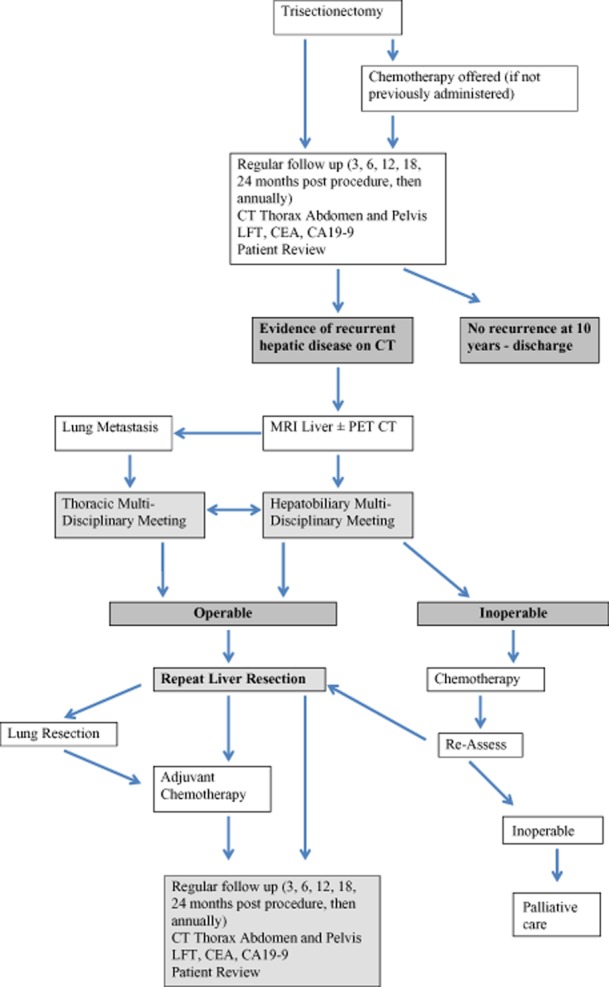
Flow chart detailing the management algorithm for this complex group of patients
Conflicts of interest
None declared.
References
- Lortat-Jacob JL, Robert HG, Henry C. [Excision of the right lobe of the liver for a malignant secondary tumor.] Arch Mal Appar Dig Mal Nutr. 1952;41:662–667. Hepatectomie lobaire droite reglee pour tumeur maligne secondaire. [PubMed] [Google Scholar]
- Starzl TE, Iwatsuki S, Shaw BW, Jr, Waterman PM, Van Thiel D, Diliz HS, et al. Left hepatic trisegmentectomy. Surg Gynecol Obstet. 1982;155:21–27. [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Koep LJ, Weil R, 3rd, Lilly JR, Putnam CW, Aldrete JA. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet. 1980;150:208–214. [PMC free article] [PubMed] [Google Scholar]
- Halazun KJ, Al-Mukhtar A, Aldouri A, Malik HZ, Attia MS, Prasad KR, et al. Right hepatic trisectionectomy for hepatobiliary diseases: results and an appraisal of its current role. Ann Surg. 2007;246:1065–1074. doi: 10.1097/SLA.0b013e3181492795. [DOI] [PubMed] [Google Scholar]
- Nishio H, Hidalgo E, Hamady ZZ, Ravindra KV, Kotru A, Dasgupta D, et al. Left hepatic trisectionectomy for hepatobiliary malignancy: results and an appraisal of its current role. Ann Surg. 2005;242:267–275. doi: 10.1097/01.sla.0000171304.70678.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IHBPA TCo. The brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;3:333–339. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1268–1274. doi: 10.1007/s11605-008-0503-1. [DOI] [PubMed] [Google Scholar]
- Lang H, Sotiropoulos GC, Brokalaki EI, Radtke A, Frilling A, Molmenti EP, et al. Left hepatic trisectionectomy for hepatobiliary malignancies. J Am Coll Surg. 2006;203:311–321. doi: 10.1016/j.jamcollsurg.2006.05.290. [DOI] [PubMed] [Google Scholar]
- Lodge JP, Menon KV, Fenwick SW, Prasad KR, Toogood GJ. In-contiguity and non-anatomical extension of right hepatic trisectionectomy for liver metastases. Br J Surg. 2005;92:340–347. doi: 10.1002/bjs.4830. [DOI] [PubMed] [Google Scholar]
- Blumgart LH, Baer HU, Czerniak A, Zimmermann A, Dennison AR. Extended left hepatectomy: technical aspects of an evolving procedure. Br J Surg. 1993;80:903–906. doi: 10.1002/bjs.1800800735. [DOI] [PubMed] [Google Scholar]
- Povoski SP, Fong Y, Blumgart LH. Extended left hepatectomy. World J Surg. 1999;23:1289–1293. doi: 10.1007/s002689900664. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–730. doi: 10.1097/01.sla.0000124385.83887.d5. discussion 30–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong de MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–2151. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- Yan TD, Sim J, Black D, Niu R, Morris DL. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2069–2077. doi: 10.1245/s10434-007-9388-6. [DOI] [PubMed] [Google Scholar]
- Mise Y, Imamura H, Hashimoto T, Seyama Y, Aoki T, Hasegawa K, et al. Cohort study of the survival benefit of resection for recurrent hepatic and/or pulmonary metastases after primary hepatectomy for colorectal metastases. Ann Surg. 2010;251:902–909. doi: 10.1097/SLA.0b013e3181c9868a. [DOI] [PubMed] [Google Scholar]
- Shah SA, Bromberg R, Coates A, Rempel E, Simunovic M, Gallinger S. Survival after liver resection for metastatic colorectal carcinoma in a large population. J Am Coll Surg. 2007;205:676–683. doi: 10.1016/j.jamcollsurg.2007.06.283. [DOI] [PubMed] [Google Scholar]
- Sharma S, Camci C, Jabbour N. Management of hepatic metastasis from colorectal cancers: an update. J Hepatobiliary Pancreat Surg. 2008;15:570–580. doi: 10.1007/s00534-008-1350-x. [DOI] [PubMed] [Google Scholar]
- Sa Cunha A, Laurent C, Rault A, Couderc P, Rullier E, Saric J. A second liver resection due to recurrent colorectal liver metastases. Arch Surg. 2007;142:1144–1149. doi: 10.1001/archsurg.142.12.1144. discussion 50. [DOI] [PubMed] [Google Scholar]
- Nishio H, Hamady ZZ, Malik HZ, Fenwick S, Rajendra Prasad K, Toogood GJ, et al. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol. 2007;33:729–734. doi: 10.1016/j.ejso.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- Brachet D, Lermite E, Rouquette A, Lorimier G, Hamy A, Arnaud JP. Prognostic factors of survival in repeat liver resection for recurrent colorectal metastases: review of sixty-two cases treated at a single institution. Dis Colon Rectum. 2009;52:475–483. doi: 10.1007/DCR.0b013e31819d12bc. [DOI] [PubMed] [Google Scholar]
- Thelen A, Jonas S, Benckert C, Schumacher G, Lopez-Hanninen E, Rudolph B, et al. Repeat liver resection for recurrent liver metastases from colorectal cancer. Eur J Surg Oncol. 2007;33:324–328. doi: 10.1016/j.ejso.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB. 2011;13:774–782. doi: 10.1111/j.1477-2574.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts DA, Haas de RJ, Andreani P, Ariche A, Salloum C, Pascal G, et al. Short- and long-term results of extended left hepatectomy for colorectal metastases. HPB. 2011;13:536–543. doi: 10.1111/j.1477-2574.2011.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge JP. Assessment of hepatic reserve for the indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:4–9. doi: 10.1007/s00534-004-0948-x. [DOI] [PubMed] [Google Scholar]
- Imamura H, Seyama Y, Kokudo N, Aoki T, Sano K, Minagawa M, et al. Single and multiple resections of multiple hepatic metastases of colorectal origin. Surgery. 2004;135:508–517. doi: 10.1016/j.surg.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Jong de MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- Welsh FK, Tekkis PP, O'Rourke T, John TG, Rees M. Quantification of risk of a positive (R1) resection margin following hepatic resection for metastatic colorectal cancer: an aid to clinical decision-making. Surg Oncol. 2008;17:3–13. doi: 10.1016/j.suronc.2007.12.003. [DOI] [PubMed] [Google Scholar]


