Abstract
Background: Involvement of the 16b1 (interaortocaval) lymph node (LN) in gallbladder cancer (GBC) is considered to represent metastatic disease. Although this is universally accepted, the role of routine frozen-section histopathological examination (HPE) of the 16b1 LN in the management of GBC has not been previously reported.
Methods: A prospective study (August 2009–November 2011) using routine biopsy of 16b1 LNs and frozen-section HPE prior to radical resection in patients deemed operable on preoperative evaluation and staging laparoscopy was carried out.
Results: Of the 451 GBC patients assessed, 251 (55.7%) were deemed operable on preoperative imaging. Of these, 68 (27.1%) were found to have disseminated disease on staging laparoscopy/laparotomy. Of the 183 patients in whom 16b1 LN biopsy was performed, 34 (18.6%) had evidence of metastases on frozen-section HPE and the planned surgical resection was abandoned (Group A). Of the remaining 149 patients (Group B), 142 (95.3%) underwent curative resection and seven (4.7%) were found to be unresectable as a result of locoregionally advanced disease. A comparison of findings in Group A with those in Group B showed no significant difference in the clinical stage of the tumour. The proportions of patients with jaundice, elevated carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 levels were significantly higher in Group A than in Group B (P = 0.008, P = 0.012 and P = 0.023, respectively).
Conclusions: Routine 16b1 LN biopsy prevented non-therapeutic radical resection and its associated morbidity in 18.6% of patients deemed resectable on preoperative imaging and staging laparoscopy. The yield was higher in patients with jaundice and elevated preoperative tumour marker levels.
Introduction
Interaortocaval (16b1) lymph node (LN) involvement in gallbladder cancer (GBC) is a sign of advanced disease with a dismal prognosis equivalent to that of distant metastases.1–6 Hence, accurate preoperative assessment of the 16b1 LN is crucial in optimizing the management of patients with GBC. Although the morphological appearance (size >10 mm and heterogeneous internal architecture) of the 16b1 LN on computed tomography (CT) of the abdomen has been reported to be useful in predicting metastatic involvement in some studies, others have not found these factors to be good predictors of metastatic disease.7–9 Given the poor positive predictive value of cross-sectional imaging for detection of 16b1 LNs, intraoperative biopsy and frozen-section analysis of these nodes have been proposed.7,8 However, the impact of routine 16b1 LN biopsy on the surgical management of GBC has not been studied previously.
The aim of this study was to prospectively analyse the role of routine 16b1 LN biopsy and frozen-section histopathological examination (HPE) prior to radical surgery for GBC at a high-volume institution.
Materials and methods
This was a prospective study performed over a period of 28 months from August 2009 to November 2011. All patients suspected to have GBC and without evidence of distant metastases on clinical evaluation underwent ultrasound (US) examination of the abdomen. Patients without evidence of dissemination subsequently underwent dual-phase CT of the abdomen. Magnetic resonance cholangiopancreatography (MRCP) was carried out selectively in patients presenting with jaundice. Patients with incidental GBC (T-stage Ib or higher) underwent a similar staging workup. If preoperative imaging revealed an enlarged 16b1 LN (defined by a size >10 mm along the short axis or heterogeneous internal architecture), image-guided fine needle aspiration cytology (FNAC) was performed to confirm the diagnosis.9 Metastatic involvement of a 16b1 LN was considered to indicate advanced inoperable disease and only palliative treatment was offered. Levels of serum tumour markers [carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9] were obtained and tumours were staged according to the tumour–node–metastasis (TNM) method for staging GBC outlined by the American Joint Committee on Cancer.10
Patients with potentially resectable tumours on preoperative imaging underwent a staging laparoscopy (SL) as previously described.11 Patients with an enlarged 16b1 LN on preoperative imaging and in whom image-guided FNAC was not possible or was negative proceeded to surgery. Patients without evidence of metastatic disease on SL underwent laparotomy. A thorough evaluation for evidence of occult liver and peritoneal metastases was performed; positive findings were confirmed by frozen-section HPE. Before proceeding with radical resection, routine biopsy of the 16b1 LN was performed. Duodenal kocherization was performed to adequately expose the interaortocaval area. All LNs in the interaortocaval region below the level of the left renal vein were excised and sent for frozen-section examination. Lymph nodes sent for frozen-section examination were counted and dissected by the pathologist. Each LN was bisected and processed whole. Three frozen sections from each LN were examined after haematoxylin and eosin staining for the presence of metastases. A 16b1 LN found to be positive for disease on frozen-section analysis was considered to indicate metastatic disease and the planned surgical resection was abandoned. Palliative procedures were performed if indicated. In patients with resectable disease, the type of resection performed was determined by the locoregional extent of the tumour (i.e. liver and adjacent organ infiltration).12
Clinicopathological features in patients with 16b1 LN metastases (Group A) were compared with those in patients without 16b1 LN metastases (Group B) to determine factors predictive of 16b1 LN metastases. For the purpose of this analysis, early GBC was defined by the clinical presence of stage T1 or T2 tumours irrespective of LN involvement; stage T3 and T4 tumours were classified as indicating locally advanced disease. Clinical staging rather than pathological staging was used because a final histopathological confirmation of the T-stage was not available in patients found to have 16b1 LN metastases.
Statistical analysis was performed using GraphPad instat Version 4 (GraphPad Software, Inc., La Jolla, CA, USA). Median values were compared using the Mann–Whitney test. Proportions were compared using Fisher's exact test. A P-value of <0.05 was considered to indicate statistical significance.
Results
During the study period, 451 patients with GBC were assessed for operability (Fig. 1). Of these, 183 (40.6%) patients underwent intraoperative biopsy followed by frozen-section examination of 16b1 LNs. The median number of 16b1 nodes sampled was three (range: one to six). Resection of the tumour was not performed in 34 (18.6%) patients with evidence of 16b1 nodal metastases on frozen-section examination (Group A). This was subsequently confirmed in a final HPE by paraffin section in all patients; there were no false positive results in frozen-section examination. Of the remaining 149 (81.4%) patients without evidence of metastases on frozen-section examination of 16b1 LNs (Group B), 142 (95.3%) underwent curative resection and seven (4.7%) were found to be unresectable as a result of locoregionally advanced disease.
Figure 1.
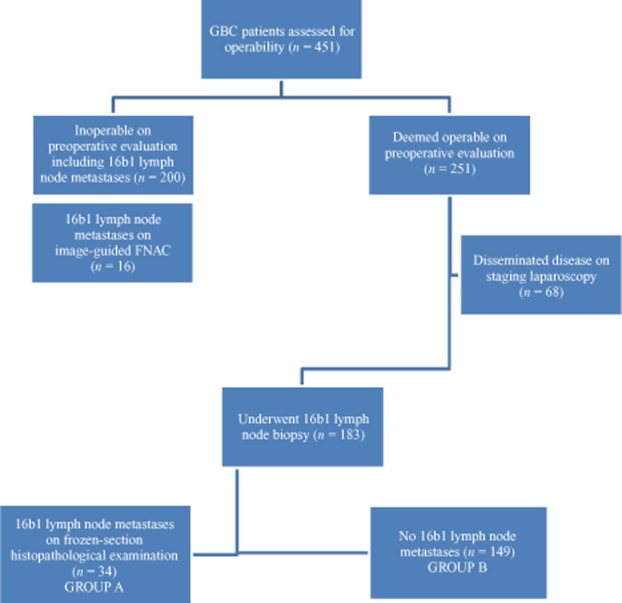
Flow chart showing the process of evaluation in 451 gallbladder cancer (GBC) patients assessed for operability during the period from August 2009 to November 2011. FNAC, fine needle aspiration cytology
The median age of patients in Group A was 54 years (range: 23–66 years) and the majority were female (female : male ratio: 2.1:1). There was no significant difference between Groups A and B in the age and gender distribution of patients (P = 0.128 and P = 0.282, respectively). Of the 183 patients, 25 (13.7%) had early GBC and the other 158 (86.3%) had locally advanced disease. Jaundice was identified in 29 (15.8%) patients and gastric outlet obstruction in nine (4.9%) patients. There was no significant difference between the groups in prevalences of abdominal pain, weight loss, vomiting and palpable gallbladder mass (P = 1.000, P = 0.098, P = 0.063 and P = 0.343, respectively). The proportion of patients with jaundice was significantly higher in Group A than in Group B (P = 0.008) (Table 1). Twenty-six (76.5%) patients in Group A had elevated tumour marker levels (CEA and CA 19-9) compared with 81 (54.4%) in Group B (P = 0.021).
Table 1.
Clinicopathological characteristics in gallbladder cancer (GBC) patients with 16b1 lymph node (LN) metastases (Group A) and without 16b1 LN metastases (Group B)
| Characteristics | Group A (n = 34) | Group B (n = 149) | P-value |
|---|---|---|---|
| Clinical stage, n (%) | |||
| Early GBC | 2 (5.9%) | 23 (15.4%) | 0.175 |
| Locally advanced GBC | 32 (94.1%) | 126 (84.6%) | |
| Jaundice | 11 (32.4%) | 18 (12.1%) | 0.008 |
| Gastric outlet obstruction | 4 (11.8%) | 5 (3.4%) | 0.063 |
| Incidental GBC, n (%) | |||
| Stage T1b | – | 6 (4.0%) | 1.000 |
| Stages T2 and T3 | 2 (5.9%) | 23 (15.4%) | |
| Tumour markers | |||
| CEA, ng/ml, median (range) | 21.7 (2.3–189) | 10.6 (2.7–105) | 0.012 |
| CA 19-9, U/ml, median (range) | 181.5 (10.3–1081) | 79.3 (12.6–313) | 0.023 |
CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9.
Of the 183 patients, 15 (8.2%) fulfilled the radiological criteria suggestive of 16b1 LN metastases (Table 2). The sensitivity and positive predictive value of these radiological criteria to detect 16b1 LN metastases were 14.7% and 33.3%, respectively. Image-guided FNAC of enlarged 16b1 LNs showed a false negative rate of 30% (Table 2). The radiological criteria used to identify 16b1 LN metastases were applied for LNs in stations 8, 9, 12, 13 and 14 to determine whether these enlarged proximal LNs correlated with 16b1 LN metastases. Although the proportion of patients with proximal LN metastases was higher in Group A (79.4%, 27 of 34 patients) than in Group B (65.8%, 98 of 149 patients), the difference was not statistically significant (P = 0.154).
Table 2.
Outcomes of fine needle aspiration cytology (FNAC) in gallbladder cancer patients in whom enlarged 16b1 lymph nodes (n = 15) were identified on computed tomography of the abdomen
| Parameter | Group A (n = 5) | Group B (n = 10) |
|---|---|---|
| Image-guided FNAC technically not feasible, n = 5 | 2 | 3 |
| Image-guided FNAC negative for malignancy, n = 10 | 3 | 7 |
Discussion
Gallbladder cancer is characterized by early LN metastases; overall 45–85% of patients with GBC have LN involvement.1,2,13 The collecting trunks from the lymphatic plexuses in the wall of the gallbladder terminate in the cystic and pericholedochal LNs and follow one of three pathways to converge at the 16b1 LN in the interaortocaval location (Fig. 2).14 Although this is the most common arrangement of lymphatic spread from the gallbladder, extensive connections exist between the various lymphatic channels, including a direct spread to the 16b1 node from the pericholedochal node. However, the 16b1 node is considered as the final abdominal nodal station for GBC and from here tumour cells enter the systemic circulation through the thoracic duct.14 Although extended lymphadenectomy (including para-aortic lymphadenectomy) was recommended to achieve longterm cure in some early reports, Kondo et al. and others reported that patients with 16b1 LN metastases had poor survival similar to that in patients with hepatic or peritoneal metastases.4–6,15 Hence, although intraoperative biopsy and frozen-section analysis of the 16b1 node before radical surgery have been proposed by various authors,7,8 the impact of routine 16b1 LN biopsy on the surgical management of GBC has not been analysed previously.
Figure 2.
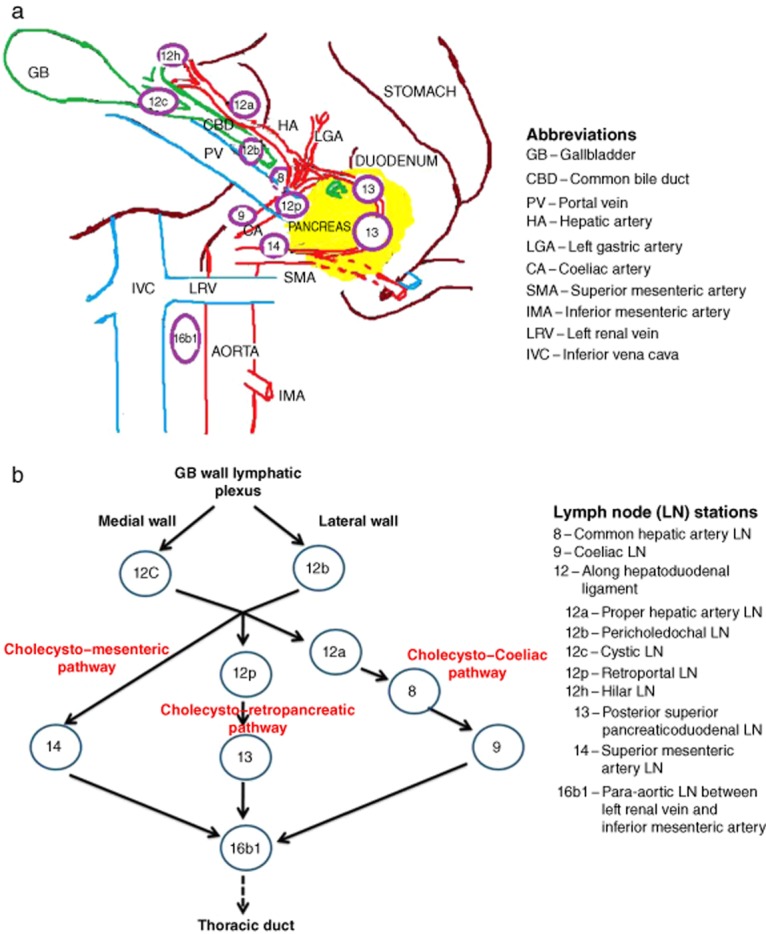
The collecting trunks from the lymphatic plexuses in the gallbladder wall terminate in the cystic and pericholedochal lymph nodes (LNs) and follow one of three pathways to converge at the 16b1 LN in the interaortocaval location
Preoperative assessment of 16b1 LN metastases is essential to prevent non-therapeutic surgical exploration and resection. The criteria proposed as diagnostic of metastatic LN involvement on CT of the abdomen are: size of >10 mm; a round shape, and a heterogeneous internal architecture.9 The poor sensitivity (14.7%) and positive predictive value (33.3%) reported in the present series highlight the fact that a significant number of patients without these radiological characteristics were found to have 16b1 LN metastatic involvement; this is consistent with previous reports.7,16 Although 18FDG-positron emission tomography (PET) has been used to identify metastatic LNs in patients with biliary cancer, its sensitivity is low.17 High-resolution magnetic resonance imaging (MRI) using lymphotropic superparamagnetic nanoparticles has been shown to detect metastatic LNs of <5 mm in diameter in prostate cancer.18 However, its role in evaluating LN involvement in GBC remains to be determined. As current imaging modalities are less accurate in detecting 16b1 LN metastases, histopathological confirmation is essential.
Histological confirmation can be obtained preoperatively through image-guided (using US or CT) FNAC of the enlarged 16b1 LN. However, as in the present study, image-guided FNAC may not be possible in all patients. The absence of a safe window as a result of adjacent vessels is the most common cause of technical failure. Percutaneous image-guided FNAC was found to have a false negative rate of 30% in the present series. Possible reasons for this include failure to target the metastatic node and inadequate aspiration. This highlights the importance of intraoperative 16b1 LN biopsy and frozen-section examination, even in patients without evidence of metastases on percutaneous image-guided FNAC. In the present series, which represents the largest prospective study to analyse the role of routine 16b1 LN biopsy in GBC reported to date, radical surgery was found to be avoidable in 18.6% of patients with potentially resectable tumours on preoperative evaluation. The majority (94.1%) of patients with 16b1 LN metastases had locally advanced tumours (clinical stages T3 and T4). However, 16b1 LN involvement was also present in two (8%) patients with early GBC (clinical stages T1 and T2). These findings suggest that although the yield of routine 16b1 LN biopsy is low in stage T1 and T2 tumours, it may still have a role. In the present study, GBC patients with jaundice secondary to bile duct infiltration had a higher incidence of 16b1 LN metastases. Although skip metastases to the 16b1 LN can occur, in the present study all 34 patients with 16b1 LN metastases also had enlarged regional LNs. Because surgical resection was not performed in patients with 16b1 LN metastases, histological confirmation of regional LN involvement could not be obtained. Recently, biopsies of regional LNs in patients with 16b1 LN metastases have been performed to ascertain the incidence of skip metastases. However, in the study by Kondo et al., all patients with 16b1 LN metastases (n = 23) were also found to have regional LN metastases, which suggests that skip metastases to the 16b1 LN are rare.6
Nanashima et al. assessed the utility of combining CT findings and serum CA 19-9 levels in the diagnosis of LN metastases and reported raised CA 19-9 levels in the absence of typical CT findings of a metastatic 16b1 LN in nine of 20 patients with 16b1 LN metastases.19 In the present study, patients with 16b1 LN metastases had significantly higher tumour marker (CEA and CA 19-9) levels, which suggests that elevated levels of tumour markers may help to predict 16b1 LN metastases in patients with potentially resectable disease on preoperative evaluation. Future strategies should seek to improve preoperative detection and to avoid non-therapeutic laparotomy secondary to 16b1 LN metastases. Endoscopic US (EUS) is a promising method of assessing the 16b1 LN and of performing image-guided FNAC.19 However, there are no studies on the role of EUS-guided FNAC in detecting 16b1 LN metastases in GBC and a prospective study is currently underway to ascertain whether the addition of EUS (with guided FNAC in patients with enlarged nodes) could obviate false negative results associated with percutaneous FNAC. Staging laparoscopy has high sensitivity for detecting surface liver and peritoneal metastases; however, it is generally not used as a tool for detecting nodal metastases.11 Laparoscopic 16b1 LN biopsy as part of SL has been performed recently in selected patients and has the potential to improve the yield of SL and obviate non-therapeutic laparotomy.
Conclusions
Routine 16b1 LN biopsy avoided radical surgery in 18.6% of patients with potentially resectable GBC after preoperative evaluation. The yield of 16b1 LN biopsy was higher in patients with jaundice, locally advanced GBC and elevated preoperative serum tumour marker levels. Whether the addition of EUS and laparoscopic 16b1 LN biopsy can improve detection and obviate non-therapeutic laparotomy should be further evaluated in future prospective studies.
Conflicts of interest
None declared.
References
- Tsukada K, Kurosaki I, Uchida K, Shirai Y, Oohashi Y, Yokoyama N, et al. Lymph node spread from carcinoma of the gallbladder. Cancer. 1997;80:661–667. [PubMed] [Google Scholar]
- Todoroki T, Kawamoto T, Takahashi H, Takada Y, Koike N, Otsuka M, et al. Treatment of gallbladder cancer by radical resection. Br J Surg. 1999;86:622–627. doi: 10.1046/j.1365-2168.1999.01085.x. [DOI] [PubMed] [Google Scholar]
- Shimada H, Endo I, Fujii Y, Kamiya N, Masunari H, Kunihiro O, et al. Appraisal of surgical resection of gallbladder cancer with special reference to lymph node dissection. Langenbecks Arch Surg. 2000;385:509–514. doi: 10.1007/s004230000163. [DOI] [PubMed] [Google Scholar]
- Chijiiwa K, Yamaguchi K, Tanaka M. Clinicopathologic differentiation between longterm and short-term postoperative survivors with advanced gallbladder carcinoma. World J Surg. 1997;21:98–102. doi: 10.1007/s002689900200. [DOI] [PubMed] [Google Scholar]
- Kaneoka Y, Yamaguchi A, Isogai M, Harada T, Suzuki M. Hepatoduodenal ligament invasion by gallbladder carcinoma: histologic patterns and surgical recommendation. World J Surg. 2003;27:260–265. doi: 10.1007/s00268-002-6702-0. [DOI] [PubMed] [Google Scholar]
- Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–422. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- Noji T, Kondo S, Hirano S, Tanaka E, Ambo Y, Kawarada Y, et al. CT evaluation of para-aortic lymph node metastasis in patients with biliary cancer. J Gastroenterol. 2005;40:739–743. doi: 10.1007/s00535-005-1618-8. [DOI] [PubMed] [Google Scholar]
- Kondo S, Hirano S, Tanaka E, Tsuchikawa T, Kato K, Matsumoto J, et al. Two types of extended liver resection for advanced gallbladder cancer: how to do it. Dig Surg. 28:148–153. doi: 10.1159/000323826. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Shirai Y, Tsukada K, Muto T, Hatakeyama K. Spread of gallbladder carcinoma: CT evaluation with pathologic correlation. Abdom Imaging. 2011;21:195–201. doi: 10.1007/s002619900045. 1996. [DOI] [PubMed] [Google Scholar]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th edn. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. Role of staging laparoscopy in primary gallbladder cancer – an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2012 doi: 10.1097/SLA.0b013e318271497e. Epub ahead of print 2012/10/10. [DOI] [PubMed] [Google Scholar]
- Kalayarasan R, Javed A, Puri AS, Puri SK, Sakhuja P, Agarwal AK. A prospective analysis of the preoperative assessment of duodenal involvement in gallbladder cancer. HPB. 2013;15:203–209. doi: 10.1111/j.1477-2574.2012.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sons HU, Borchard F, Joel BS. Carcinoma of the gallbladder: autopsy findings in 287 cases and review of the literature. J Surg Oncol. 1985;28:199–206. doi: 10.1002/jso.2930280311. [DOI] [PubMed] [Google Scholar]
- Fahim RB, McDonald JR, Richards JC, Ferris DO. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg. 1962;156:114–124. doi: 10.1097/00000658-196207000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Itoh H, Ambiru S, Shimizu H, Togawa A, Gohchi E, et al. Radical surgery for advanced gallbladder carcinoma. Br J Surg. 1996;83:478–481. doi: 10.1002/bjs.1800830413. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Ajiki T, Ueda T, Sawa H, Fujita T, Matsumoto I, et al. Histological features of lymph node metastasis in patients with biliary tract cancer. J Surg Oncol. 2008;97:423–427. doi: 10.1002/jso.20963. [DOI] [PubMed] [Google Scholar]
- Kluge R, Schmidt F, Caca K, Barthel H, Hesse S, Georgi P, et al. Positron emission tomography with [18F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029–1035. doi: 10.1053/jhep.2001.23912. [DOI] [PubMed] [Google Scholar]
- Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, Kaa van de CH, et al. Non-invasive detection of clinically occult lymph node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- Nanashima A, Sakamoto I, Hayashi T, Tobinaga S, Araki M, Kunizaki M, et al. Preoperative diagnosis of lymph node metastasis in biliary and pancreatic carcinomas. Evaluation of the combination of multi-detector CT and serum CA 19-9 level. Dig Dis Sci. 2010;55:3617–3626. doi: 10.1007/s10620-010-1180-y. [DOI] [PubMed] [Google Scholar]


