Abstract
Objectives: The aim of this study was to assess whether biological markers can provide prognostic information additional to that supplied by the clinical risk score (CRS) in patients with colorectal liver metastases.
Methods: A retrospective review of a prospectively maintained database was conducted. Patients selected for this study were treated between 1996 and 2011 with potentially curative liver surgery. Expressions of p53, Ki-67 and thymidylate synthase were assayed using immunohistochemical techniques on tissue microarrays.
Results: A total of 98 (24%) of 406 patients met the inclusion criteria. The median follow-up was 103 months. Analysis revealed a correlation between p53 protein overexpression and high CRS (P = 0.058). Following multivariate analysis, only high CRS remained as an independent negative prognostic predictor of survival (P = 0.018), as well as an indicator of early recurrence of disease (P = 0.010). Of the biological markers investigated, only Ki-67 overexpression was identified as a positive predictor of survival on multivariate analysis (P = 0.038).
Conclusions: Ki-67 overexpression was a positive predictor of survival. Only high CRS remained an independent negative prognostic predictor.
Introduction
Liver resection is the only treatment option providing longterm survival in patients with colorectal liver metastases (CLM).1 However, recurrent disease subsequently develops in approximately 75% of resection patients.2 Currently there are a number of criteria to identify patients who are at risk of developing recurrence after potentially curative liver resection for CLM. Many clinical prognostic markers have been investigated and several prognostic scoring systems have been proposed.3–5 The clinical risk score (CRS), published by Fong et al.,5 has been found to be a useful clinical tool in several studies.6,7
However, variations observed in the longterm survival of patients with similar prognostic factors suggest that other factors may also be at play in determining survival after resection of CLM. More recently, there has been increased interest in identifying molecular markers that may help to better define patients at risk for recurrence.8
The present study investigated the biological markers p53, Ki-67 and thymidylate synthase (TS). p53 is a tumour suppressor gene with a central role in controlling both the cell cycle and the apoptotic machinery.9 Ki-67 is associated with cell proliferation.10 Thymidylate synthase is an enzyme integral to DNA synthesis and is the target of fluorouracil (FU)-based chemotherapy.11 The presumed relationships among p53, Ki-67 and TS expression and prognosis have been studied previously, but the results are contradictory.8–12
The aim of this study was to evaluate the potential prognostic value of p53, Ki-67 and TS expression in predicting actual and disease-free survival after the radical resection of CLM.
Materials and methods
Clinical information
This study was approved by the National Medical Ethics Committee of the Republic of Slovenia. Study subjects were identified from a prospectively maintained database of 406 patients who underwent liver resections and/or local ablative procedures for CLM from January 1996 to December 2011 at the Department of Abdominal and General Surgery, University Medical Centre (UMC) Maribor. The database comprised data on preoperative assessment, surgical treatment, postoperative course, histopathology and longterm follow-up. Criteria for resectability required candidates to be medically fit for major surgery, to show no evidence of disseminated disease, and to be amenable to a resection strategy that encompassed all liver disease, leaving remnant liver adequate for recovery. The diagnosis of CLM was confirmed by histopathology.
All patients in the database who submitted to liver resection in the period from 1996 to 2006 were selected and included in the study if they fulfilled the inclusion criteria. Study inclusion criteria are shown in Fig. 1.
Figure 1.
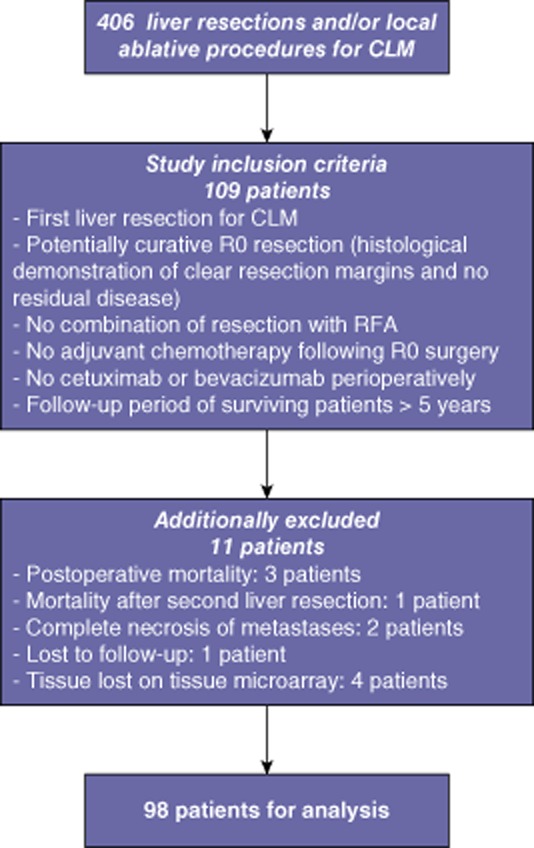
Study inclusion criteria and patient flow. CLM, colorectal liver metastasis; R0, negative margin; RFA, radiofrequency ablation
A total of 14 routinely available clinical variables were analysed. Preoperative clinical variables included patient demographics (age, sex), patient performance status [defined according to the American Society of Anesthesiologists (ASA) score], primary tumour site, synchronous or metachronous metastases, chemotherapy before liver resection, and CRS as defined by Fong et al.5 The CRS is derived by awarding one point for each of five factors: (i) node-positive primary colorectal cancer; (ii) a disease-free interval of <12 months; (iii) the presence of more than one tumour; (iv) a largest tumour size of >5 cm, and (v) a pre-hepatectomy carcinoembryonic antigen (CEA) level of >200 ng/ml. Liver surgery-related clinical variables included the temporal relationship of the surgical procedure (colorectal/liver surgery), the extent of liver resection, bilateral resection, and complications after liver resection. Histopathology-related clinical variables included the presence of extrahepatic disease, resection margin status, and grade of metastases.
Follow-up
Patients were followed at outpatient clinics at periodic intervals. Follow-up included clinical, biochemical (CEA test), thoracic X-ray, and liver ultrasound or computed tomography (CT) evaluations every 3 months for the first 2 years and every 6 months thereafter. Metastatic recurrence was diagnosed by CEA rise and CT. From 1999, positron emission tomography (PET) scans were performed in selected patients and from 2004 PET-CT scans were carried out in selected patients. In any uncertainty, histology was required. Follow-up data were obtained from outpatient follow-up and from the National Cancer Register of Slovenia. Patient follow-up data included details of dates of disease recurrence and death, site of recurrence, further therapy (e.g. systemic therapy, surgery), and cause of death. Recurrences were classified as hepatic, extrahepatic or combined. Recurrent disease was treated according to standard clinical practice and included surgery and/or chemotherapy whenever possible. The principles behind the selection criteria for resection of recurrent CLM were the same as those for the initial hepatectomy. Patients for whom no further information could be obtained were considered as lost to follow-up and excluded from this study. All patients were followed until their death or until December 2011.
Chemotherapy
Perioperative chemotherapy was not administered as a standard treatment protocol in patients with CLM. In general, patients with colorectal cancer and Union for International Cancer Control (UICC) stage III and IV disease were treated with adjuvant chemotherapy at the discretion of the medical oncologist.
Chemotherapy included fluoropyrimidine-based therapies alone or in combination with oxaliplatin or irinotecan. The different chemotherapy protocols included oral capecitabin administered as a single agent, and the MAYO (fluorouracil and leucovorin), FOLFOX (oxaliplatin, fluorouracil and leucovorin), XELOX (capecitabin and oxaliplatin), XELIRI (capecitabin and irinotecan) and FOLFIRI (irinotecan, fluorouracil and leucovorin) protocols, respectively.
In the last year of the period under study (2006), bevacizumab or cetuximab were added in selected patients; these patients were excluded from the current analyses.
Basically, no adjuvant chemotherapy was given after negative margin (R0) resection of CLM. Patients selected for this study received no adjuvant chemotherapy after liver resection.
Histopathology and construction of tissue microarrays
All liver metastases specimens were analysed routinely after liver resection for histology type, number of tumour nodules, size of the largest nodule and margin status. Histological grading (well, moderately or poorly differentiated) was performed according to the World Health Organization (WHO) classification.13 Additional immunohistochemistry (IHC) examinations were performed retrospectively on archived tumour specimens from these patients for the purposes of this study. Expressions of the p53, Ki-67 and TS markers were determined using IHC staining of constructed tissue microarrays.
Representative formalin-fixed and paraffin-embedded resection specimens of liver metastases were selected from the archive of the Department of Pathology, UMC Maribor. One representative tissue block of a metastasis from each patient was used. Firstly, a haematoxylin and eosin (H&E) stain of each specimen was reviewed by a pathologist and the areas of interest (i.e. viable, metastasized colorectal tumour cells in the liver) were marked. For each patient, representative tumour blocks were selected and used for the construction of tissue microarrays. To avoid possible intratumoral heterogeneity, three cores of different areas of the tumour from each donor tissue block were sampled. Three recipient tissue blocks were made in this way. Tumour samples were arrayed as previously described.14,15 The MTA-1 manual tissue arrayer (Beecher Instruments, Inc., Sun Prairie, WI, USA) was used to create the microarrays. The punch size was set at 0.6 mm. Sections of 2–3 μm in thickness were cut from each tissue microarray paraffin block and placed on SuperFrost/Plus object slides.
Immunohistochemical staining of biological markers
Immunohistochemical staining of the tissue microarrays was performed with the Ventana BenchMark XT automated slide stainer (Ventana Medical Systems, Inc., Tucson, AZ, USA). The sections were deparaffinized, rehydrated and processed with the ultraView Universal DAB Detection Kit (Ventana Medical Systems, Inc.) for p53 and Ki-67. Thymidylate synthase was processed using the iVIEW DAB Detection Kit (Ventana Medical Systems, Inc.). Sections were submitted to antigen retrieval in the automated slide stainer for 60 min with Cell Conditioning 1 (CC1; Ventana Medical Systems, Inc.). The slides were then incubated with an appropriately diluted primary antibody. Mouse antihuman monoclonal antibodies to p53 (clone DO-7, 1 : 150) (Dako Denmark A/S, Glostrup, Denmark), Ki-67 (Mib-1, 1 : 100) (Dako Denmark A/S) and TS (clone TS106, 1 : 25) (Dako Denmark A/S) were used. Diaminobenzidine was used as the chromogen, and haematoxylin II, a bluing reagent, was used as the nuclear counterstain. Positive controls included tumour cells with high protein expression (for all antibodies tested). Negative controls were prepared by omitting the primary antibody.
Immunohistochemical scoring
Using a light microscope, patients represented by materials of which at least two of the three tissue cores showed analysable staining were selected for inclusion in the final analysis. Patients represented by materials of which only one or no cores showed analysable staining were regarded as lost from this study. All specimens were analysed by a pathologist and investigator.
Expressions of p53 and Ki-67 were evaluated by scoring the percentage of positively stained nuclei of the malignant cells in each tissue. Specimens were considered positive for p53 and Ki-67 when >50% of tumour cells were stained.
For TS, the intensity of cytoplasm staining of malignant cells in each tissue sample was taken into account because these proteins were expressed in almost all tumour cells. Definition of TS 106 staining intensity was based on a visual grading scale of 0–3. Intensity levels 0 and 1 were grouped together as representing low-intensity staining; levels 2 and 3 were grouped as representing intermediate or high-intensity staining.
Any scoring discrepancies (approximately 10% of cases) were resolved by consensus.
Outcome
Actual survival (AS) was defined as the interval between the date of resection of CLM and the date of death (or the date of the last follow-up in surviving patients). Disease-free survival (DFS) was calculated from the date of liver resection to the date of intra- and/or extrahepatic recurrence (or the date of the last follow-up in surviving patients without recurrence). Longterm survivors were considered to be those patients who remained alive at >5 years after first liver resection. Early death was defined as death within 2 years of liver surgery. Patients who achieved DFS of >5 years after first liver resection were considered to be longterm disease-free survivors. Early disease recurrence was represented by any disease recurrence within 2 years of liver surgery.
Statistical analysis
IBM spss for Windows Version 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical computations.
Differences in the frequency distributions of p53, Ki-67 and TS reactivity in relation to other clinical characteristics were tested using the chi-squared test for categorical variables (Pearson's or Fisher's exact test when appropriate, two-tailed in all instances). Continuous variables were analysed using Student's t-test for independent samples or the Mann–Whitney U-test if the criteria for parametric testing were not met. A difference with a P-value of <0.05 was considered statistically significant.
All survival probabilities were estimated using Kaplan–Meier survival analysis. The effect of each of the markers and 14 routinely available clinical characteristics on survival was evaluated using the log-rank test. As only specimens from patients operated before January 2007 were used, 5-year AS is provided.
Cox proportional hazards models were used for multivariate analysis. All three biological markers (p53, Ki-67 and TS), regardless of the results of bivariate analysis, and significant clinical variables from bivariate analysis (P < 0.10) were included. Model building was based on a backward stepwise algorithm with P < 0.10 as the exclusion criterion, in which the likelihood ratio was used as a test statistic.
Results
Study population
Of the 406 patients identified, 98 (24%) met the inclusion criteria. Their median age was 62 years (range: 27–78 years) and 56 (57%) patients were male. A total of 48 (49%) patients had concomitant systemic disease (ASA class 2 or 3). The primary tumour site was the colon in 48 (49%) and rectum in 50 (51%) patients; 41 patients (42%) had shown synchronous and 57 (58%) had shown metachronous metastases.
Fluoropyrimidine-based chemotherapy had been administered in the adjuvant setting following primary colorectal resection in 75 (76%) patients. Forty (41%) patients had received systemic chemotherapy, either for measurable metastatic disease within the 6 months prior to liver resection or in the adjuvant setting following primary resection. A total of 23 (23%) patients had been chemotherapy-naïve.
All patients were found to have a CRS within the range of 0–4; no patient had a CRS of 5. Descriptive statistics for each of the individual clinical risk factors from which the CRS is derived were as follows: 57 (58%) patients had node-positive primary colorectal cancer; the median disease-free interval was 12 months (range: 0–164 months); the median number of hepatic metastases removed was one (range: one to eight); 46 (47%) patients had more than one metastasis; the median size of each patient's largest tumour was 4.0 cm (range: 0.8–12.0 cm), and the median pre-hepatectomy CEA level was 17 ng/ml (range: 0.3–1348 ng/ml).
Eleven (11%) patients underwent simultaneous liver and colorectal surgery. A total of 25 (26%) patients were operated during 1996–2001 and 73 (74%) were operated during 2002–2006.
To achieve a radical resection, extended hepatectomies were performed in six (6%) patients, formal hemi-hepatectomies in 12 (12%) patients, segment-oriented resections in 62 (63%) patients and non-anatomical resections in 18 (18%) patients. Major liver surgery (trisegmentectomy or greater) was performed in 39 (40%) patients and bilateral resection in 34 (35%) patients. Resections were performed by four different surgeons.
Complications after liver surgery occurred in 13 (13%) patients.
Eleven (11%) patients presented with extrahepatic disease, which was radically resected in addition to liver surgery with histological demonstration of no residual disease. The median resection margin in liver metastases resections was 2.0 mm (range: 0.1–20.0 mm). Liver metastases were graded as well differentiated in 27 (28%) patients, moderately differentiated in 69 (70%) patients, and poorly differentiated in two (2%) patients.
A total of 52 (53%) metastases stained positive for p53 protein and 27 (28%) stained positive for Ki-67 protein. Immunostaining for TS was low in 29 (30%) and high in 69 (70%) of the 98 metastatic samples.
The median follow-up in surviving patients was 103 months (range: 61–195 months). Rates of 5-year AS and 10-year overall survival (OS) were 34.5% and 24.3%, respectively, with median survival of 36 months. Actual 5-year DFS was 20.4%, with a median DFS of 13 months.
Recurrent disease
Of the 98 patients submitted to potentially curative liver resection, 72 (73%) developed recurrent disease and, of these, 33 (34%) patients underwent repeat hepatic resection (one to six repeat resections). In 59 (82%) of 72 patients, recurrence was diagnosed within 2 years of the first liver surgery of metastases. The pattern of recurrence in these 72 patients was as follows: in 20 (28%) patients, recurrence was confined to the liver; in 20 (28%) patients, recurrence was confined to an extrahepatic location only, and in 32 (44%) patients, intra- and extrahepatic metastases were present.
A total of 29 (30%) longterm survivors were identified. Another 29 (30%) patients were defined as belonging to the early death group. Sixteen (16%) longterm disease-free survivors were identified. A total of 59 (60%) patients were identified as suffering early disease recurrence.
Correlations of p53, Ki-67 and TS expression and survival
Chi-squared analysis revealed a relationship between p53 protein positive staining and high CRS (P = 0.058). There were no associations between Ki-67 or TS expression, and CRS or any of the other clinical variables investigated (data not shown). Analysis of the interrelationships among the three biological markers showed no associations (data not shown).
The results of analysis of p53, Ki-67 and TS overexpression in patients with differing outcomes following liver resection are shown in Table 1.
Table 1.
Proportions of patients with p53, Ki-67 and thymidiylate synthase (TS) overexpression in groups with differing outcomes following liver resection, showing actual survival and actual disease-free survival
| Variable | Earlya death, n | Longtermb survivors, n | P-value (χ2 test) | Earlya recurrence, n | Longtermb disease-free survivors, n | P-value (χ2 test) |
|---|---|---|---|---|---|---|
| p53 | 19/29 | 12/29 | 0.113 | 34/59 | 8/16 | 0.777 |
| Ki-67 | 6/29 | 11/29 | 0.248 | 12/59 | 7/16 | 0.101 |
| TS | 24/29 | 19/29 | 0.230 | 43/59 | 12/16 | 1.000 |
Within 2 years.
Over 5 years.
Data on AS and DFS in patients whose tumour samples stained positive or negative, respectively, for the biological markers are shown in Table 2.
Table 2.
Bivariate analysis of clinical variables and biological markers in relation to 5-year actual survival (AS) and 5-year actual disease-free survival (DFS)
| Variable | Patients (n = 98), n (%) | Median AS, days | Actual 5-year survival, % | P-value (log-rank test)a | Median DFS, days | Actual 5-year DFS, % | P-value (log-rank test)a |
|---|---|---|---|---|---|---|---|
| Age | 0.108 | 0.829 | |||||
| <62 years | 47 (48%) | 1412 | 42.6% | 420 | 17.0% | ||
| ≥62 years | 51 (52%) | 809 | 26.8% | 382 | 23.5% | ||
| CRS | 0.041 | 0.007 | |||||
| Low | 62 (63%) | 1295 | 38.5% | 568 | 24.2% | ||
| High | 36 (37%) | 794 | 24.3% | 279 | 13.9% | ||
| ED | 0.257 | 0.011 | |||||
| Absent | 87 (89%) | 1239 | 35.3% | 423 | 23.0% | ||
| Present | 11 (11%) | 764 | 27.3% | 297 | 0% | ||
| RM | 0.174 | 0.093 | |||||
| ≤1 mm | 29 (30%) | 850 | 27.6% | 306 | 13.8% | ||
| >1 mm | 69 (70%) | 1295 | 37.2% | 568 | 23.2% | ||
| Grade of metastases | 0.058 | 0.099 | |||||
| I | 27 (28%) | 1318 | 40.4% | 790 | 25.9% | ||
| II or III | 71 (72%) | 914 | 30.6% | 342 | 18.3% | ||
| p53 | 0.195 | 0.317 | |||||
| + | 52 (53%) | 869 | 26.8% | 344 | 19.2% | ||
| − | 46 (47%) | 1460 | 43.1% | 476 | 21.7% | ||
| Ki-67 | 0.159 | 0.166 | |||||
| + | 27 (28%) | 1460 | 48.1% | 790 | 29.6% | ||
| − | 71 (72%) | 881 | 29.3% | 350 | 16.9% | ||
| TS | 0.825 | 0.935 | |||||
| + | 69 (70%) | 1076 | 34.3% | 350 | 23.8% | ||
| − | 29 (30%) | 1274 | 34.5% | 529 | 13.8% |
Values in bold are significant at P < 0.05.
CRS, clinical risk score; ED, extrahepatic disease; RM, resection margin; TS, thymidiylate synthase.
Clinical variables associated with significant (P < 0.05) or marginally significant (P ≤ 0.10) differences in AS or DFS in bivariate analysis are shown in Table 2.
The following clinical variables showed no significant (P > 0.2) associations with any of the study endpoints: sex; ASA class; primary tumour site; synchronous or metachronous metastases; chemotherapy before liver resection; temporal relationship of surgical procedure; extent of liver resection; bilateral resection; complications after liver resection, and resection margin (2-mm cut-off). These data are not shown.
Multivariate results
The following non-correlated factors were included in multivariate survival analyses: age; CRS; grade of metastases; extrahepatic disease; resection margin (1-mm cut-off), and all investigated biological markers. Findings of the multivariate analysis for each endpoint (AS and DFS) are summarized in Table 3. Values for non-significant variables were taken from the last step in a stepwise regression model in which they were still included.
Table 3.
Cox regression analyses. Variables that remained in the final model with P < 0.05 were identified as predictors of survival
| HR | 95% CI for HR | P-valuea | |
|---|---|---|---|
| Actual survival | |||
| Age ≥62 years | 1.54 | 0.95–2.48 | 0.075 |
| High CRS (≥3) | 1.78 | 1.10–2.89 | 0.018 |
| ED present | 1.15 | 0.55–2.43 | 0.699 |
| RM of ≤ 1 mm | 1.31 | 0.79–2.17 | 0.285 |
| Grade of metastases II or III | 1.73 | 0.98–3.03 | 0.055 |
| p53+ | 1.35 | 0.81–2.22 | 0.239 |
| Ki-67 + | 0.82 | 0.68–0.98 | 0.038 |
| TS+ | 1.07 | 0.63–1.80 | 0.789 |
| Disease-free survival | |||
| Age ≥62 years | 1.11 | 0.70–1.76 | 0.650 |
| High CRS (≥3) | 1.81 | 1.15–2.86 | 0.010 |
| ED present | 2.11 | 1.09–4.07 | 0.025 |
| RM of ≤ 1 mm | 1.26 | 0.76–2.09 | 0.371 |
| Grade of metastases II or III | 1.54 | 0.92–2.56 | 0.096 |
| p53+ | 1.18 | 0.74–1.88 | 0.492 |
| Ki-67 + | 0.88 | 0.73–1.05 | 0.161 |
| TS+ | 0.97 | 0.59–1.60 | 0.927 |
Values in bold are significant at P < 0.05.
HR, hazard ratio; 95% CI, 95% confidence interval; CRS, clinical risk score; ED, extrahepatic disease; RM, resection margin; TS, thymidiylate synthase.
Discussion
There is an ongoing effort to translate basic knowledge about biological markers into clinical use. An important advantage of this study is its inclusion of a homogeneous group of patients. All patients were uniformly treated with liver resection and selected for the study appropriately in order to avoid any potential biases in defining the prognostic values of biological markers. Moreover, because all patients were operated before January 2007, a minimal follow-up period of 5 years was obtained, with a median follow-up of 103 months. Therefore, the survival analysis is based on actual data rather than on estimations that form the basis of actuarial survival. To maximize the potential biological difference, two groups of patients with opposite outcomes following liver resection were evaluated, as proposed previously by D'Angelica et al.16
The actual 5-year and actuarial 10-year survival of patients included in this study were 34.5% and 24.3%, respectively, with median survival of 36 months. Although all patients underwent a potentially curative resection, 73% of them subsequently developed recurrent disease. Reported 5-year OS data in some modern series reaches 60% in selected patients.17–20 However, the current study presents actual rather than actuarial survival data. Moreover, patients with prognostic factors known to be adverse (extrahepatic disease) were included in the analysis. Finally, these patients underwent resection of CLM before the introduction of modern targeted therapy with bevacizumab or cetuximab.
Other than CRS, the present study found none of the variables investigated had any consistent influence on AS or DFS. Patients with a high CRS (≥3) fared worse after resection than those with a low CRS (0–2), as evidenced by a lower median AS (26 months versus 43 months; P = 0.041), as well as a lower DFS (9 months versus 19 months; P = 0.007).
For the construction of tissue microarrays, three cores of different areas of the metastasis from each donor tissue block were sampled. It should be noted that only a single metastasis from each patient was investigated, although 47% of patients in the current series had multiple tumours. However, in a series of 82 patients and 143 investigated CLM, Menon et al. found a very high concordance of biological markers in those patients in whom multiple metastases were available.21
In the present series, a correlation between p53 protein overexpression and high CRS was found (P = 0.058). The number of patients with p53 overexpression in the early death group (65%) was higher than that in the longterm survival group (41%); this difference was marginally significant (P = 0.113). Patients with overexpression of p53 had shortened survival as well as DFS, but the difference was not statistically significant. Subjectivity in scoring and the use of different antibodies across trials reported in the literature make it difficult to compare the findings of separate trials; findings on the role of p53 protein in CLM in relation to prognosis are variable.21–29 The confusion on the prognostic importance of p53 is no less in published series examining the association of TP53 gene mutation status in CLM.12,30–33
The most important and unexpected finding of the present study was the identification of Ki-67 overexpression as a positive predictor of survival. This contradicts findings reported in several published series, in which a high Ki-67 score is associated with a worse outcome.34–37 In the study reported by Petrowsky et al., patients with high Ki-67 scores (≥50%) had significantly shorter median survival compared with those with low scores.35 However, measures of proliferation have not always demonstrated a significant association with clinical outcome.16,27 Moreover, recently a correlation between slow proliferation and high aggressiveness of colorectal cancer was demonstrated.38
The current study used a 50% cut-off for defining Ki-67 protein positive phenotypes, as proposed by several previous authors.34–37 There were more Ki-67 positive patients among the longterm survivors, but no correlation between Ki-67 score and CRS was found. The proportion of Ki-67 positive patients was higher in longterm disease-free survivors (P = 0.101). Moreover, patients with Ki-67 overexpression lived longer, as demonstrated by the results of multivariate analysis (P = 0.038). As Ki-67 positivity was a predictor of AS, but not a predictor of DFS, the present authors speculate that Ki-67 positivity may have some impact on further therapies administered subsequent to disease recurrence. However, no correlation was found between the administration of repeat liver procedures and/or chemotherapy for recurrence and Ki-67 status (data not shown).
Findings on TS expression provided no clinical information. This result contradicts the findings of prior reports which have demonstrated a significantly worse outcome in CLM patients whose tumours have shown high TS expression.22,32,39 However, several reports have found no association between TS scores and clinical outcome.16,33,40 In the current study, evaluation of the interrelationships among p53, Ki-67 and TS expression showed no associations.
The present study is subject to a number of limitations. The first involves its sample size. Although the study included 98 patients, numbers were relatively low when the patients were divided into subgroups. The inclusion of larger cohorts of patients might improve the strength of further studies. Secondly, IHC is a semi-quantitative assay. For all marker expressions, cut-offs for positive phenotypes were determined according to the literature. Using different cut-off values may alter the conclusions of any study using IHC. A standardized method of assessing the degree of positivity or negativity of staining would facilitate the comparison of results across different studies. Thirdly, an important factor that did not influence the outcome was the use of chemotherapy. There were no standard protocols for chemotherapy across the study population, although 76% of the study patients had received fluoropyrimidine-based chemotherapy prior to liver surgery (either for measurable metastatic disease or in the adjuvant setting following primary resection). Finally, 34% of patients in this series underwent repeat hepatic resection. The inclusion of these patients may skew statistical analysis regarding AS because these patients are likely to achieve better survival as a result of treatable recurrences.
In conclusion, Ki-67 overexpression was identified as a positive predictor of survival in a homogeneous group of patients. More extensive studies will be required to determine the clinical relevance of biological markers, which should be assessed singly and in combinations.
Conflicts of interest
None declared.
References
- Pulitanò C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, et al. What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB. 2010;12:244–249. doi: 10.1111/j.1477-2574.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- Rees M, Tekkis PP, Welsh FKS, O'Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanecz A, Potrc S, Horvat M, Jagric T, Gadzijev E. The validity of clinical risk score for patients undergoing liver resection for colorectal metastases. Hepatogastroenterology. 2009;56:1452–1458. [PubMed] [Google Scholar]
- Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, et al. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–3288. doi: 10.1245/s10434-009-0654-7. [DOI] [PubMed] [Google Scholar]
- Pawlik TM, Choti MA. Shifting from clinical to biologic indicators of prognosis after resection of hepatic colorectal metastases. Curr Colorectal Cancer Rep. 2006;2:85–93. doi: 10.1007/s11912-007-0021-4. [DOI] [PubMed] [Google Scholar]
- Munro AJ, Lain S, Lane DP. p53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer. 2005;92:434–444. doi: 10.1038/sj.bjc.6602358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lentz F, Tran A, Rey E, Pons G, Tréluyer JM. Pharmacogenomics of fluorouracil, irinotecan, and oxaliplatin in hepatic metastases of colorectal cancer. Am J Pharmacogenomics. 2005;5:21–33. doi: 10.2165/00129785-200505010-00002. [DOI] [PubMed] [Google Scholar]
- De Jong KP, Gouw ASH, Peeters PMJG, Bulthuis M, Menkema L, Porte RJ, et al. p53 mutation analysis of colorectal liver metastases: relation to actual survival, angiogenic status, and p53 overexpression. Clin Cancer Res. 2005;11:4067–4073. doi: 10.1158/1078-0432.CCR-04-2389. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Aaltonen LA, editors. WHO Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- Packeisen J, Buerger H, Krech R, Boecker W. Tissue microarrays: a new approach for quality control in immunohistochemistry. J Clin Pathol. 2002;55:613–615. doi: 10.1136/jcp.55.8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumour specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- D'Angelica M, Ammori J, Gonen M, Klimstra DS, Low PS, Murphy L, et al. Folate receptor-α expression in resectable hepatic colorectal cancer metastases: patterns and significance. Mod Pathol. 2011;24:1221–1228. doi: 10.1038/modpathol.2011.82. [DOI] [PubMed] [Google Scholar]
- Adam R, Bhangui P, Poston G, Mirza D, Nuzzo G, Barroso E, et al. Is preoperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252:774–787. doi: 10.1097/SLA.0b013e3181fcf3e3. [DOI] [PubMed] [Google Scholar]
- Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L, et al. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases. Ann Surg. 2012;256:772–779. doi: 10.1097/SLA.0b013e3182734423. [DOI] [PubMed] [Google Scholar]
- Jamal MH, Hassanain M, Chaudhury P, Tran TT, Wong S, Yousef Y, et al. Stage hepatectomy for bilobar colorectal hepatic metastases. HPB. 2012;14:782–789. doi: 10.1111/j.1477-2574.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Taylor MA, McWilliams B. The role of cetuximab as first-line treatment of colorectal liver metastases. HPB. 2013;15:11–17. doi: 10.1111/j.1477-2574.2012.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon AG, Tollenaar R, Velde van de C, Putter H, Janssen-van Rhijn CM, Keijzer R, et al. p53 and HLA class-I expression are not downregulated in colorectal cancer liver metastases. Clin Exp Metastasis. 2004;21:79–85. doi: 10.1023/b:clin.0000017206.08931.42. [DOI] [PubMed] [Google Scholar]
- Gonen M, Hummer A, Zervoudakis A, Sullivan D, Fong Y, Banerjee D, et al. Thymidylate synthase expression in hepatic tumours is a predictor of survival and progression in patients with resectable metastatic colorectal cancer. J Clin Oncol. 2003;21:406–412. doi: 10.1200/JCO.2003.06.060. [DOI] [PubMed] [Google Scholar]
- Belluco C, Guillem JG, Kemeny N, Huang Y, Klimstra D, Berger MF, et al. p53 nuclear protein overexpression in colorectal cancer: a dominant predictor of survival in patients with advanced hepatic metastases. J Clin Oncol. 1996;14:2696–2701. doi: 10.1200/JCO.1996.14.10.2696. [DOI] [PubMed] [Google Scholar]
- Heisterkamp J, Bommel van J, Hop WC, Tilanus HW, Zondervan PE, Ijzermans JNM. p53 overexpression in colorectal metastases confined to the liver and outcome of liver resection. Hepatogastroenterology. 1999;46:3109–3114. [PubMed] [Google Scholar]
- Crowe PJ, Phil D, Yang JL, Berney CR, Erskine C, Ham JM, et al. Genetic markers of survival and liver recurrence after resection of liver metastases from colorectal cancer. World J Surg. 2001;25:996–1001. doi: 10.1007/s00268-001-0069-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Shimada H, Miura M, Fujii Y, Yamaguchi S, Endo I, et al. Metastatic tumour doubling time: most important prehepatectomy predictor of survival and non-recurrence of hepatic colorectal cancer metastasis. World J Surg. 2004;28:263–270. doi: 10.1007/s00268-003-7088-3. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Nakanishi Y, Fukasawa Y, Sato Y, Yoshimura K, Moriya Y, et al. A new formula for predicting liver metastasis in patients with colorectal cancer: immunohistochemical analysis of a large series of 439 surgically resected cases. Oncology. 2008;75:32–41. doi: 10.1159/000151667. [DOI] [PubMed] [Google Scholar]
- Backus HHJ, Groeningen van CJ, Vos W, Dukers DF, Bloemena E, Wouters D, et al. Differential expression of cell cycle and apoptosis-related proteins in colorectal mucosa, primary colon tumours, and liver metastases. J Clin Pathol. 2002;55:206–211. doi: 10.1136/jcp.55.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosari S, Viale G. The clinical significance of p53 aberrations in human tumours. Virchows Arch. 1995;427:229–241. doi: 10.1007/BF00203389. [DOI] [PubMed] [Google Scholar]
- Yang Y, Forslund A, Remotti H, Lönroth C, Andersson M, Brevinge H, et al. p53 mutations in primary tumours and subsequent liver metastases are related to survival in patients with colorectal carcinoma who undergo liver resection. Cancer. 2001;91:727–736. [PubMed] [Google Scholar]
- Molleví DG, Serrano T, Ginestà MM, Valls J, Torras J, Navarro M, et al. Mutations in TP53 are a prognostic factor in colorectal hepatic metastases undergoing surgical resection. Carcinogenesis. 2007;28:1241–1246. doi: 10.1093/carcin/bgm012. [DOI] [PubMed] [Google Scholar]
- Etienne MC, Chazal M, Laurent-Puig P, Magné N, Rosty C, Formento JL, et al. Prognostic value of tumoral thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol. 20:2832–2843. doi: 10.1200/JCO.2002.09.091. [DOI] [PubMed] [Google Scholar]
- Saw RP, Koorey D, Painter D, Gallagher PJ, Solomon MJ. p53, DCC and thymidylate synthase as predictors of survival after resection of hepatic metastases from colorectal cancer. Br J Surg. 2002;89:1409–1415. doi: 10.1046/j.1365-2168.2002.02222.x. 2002. [DOI] [PubMed] [Google Scholar]
- Weber JC, Nakano H, Bachellier P, Oussoultzoglou E, Inoue K, Shimura H, et al. Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases? Am J Surg. 2001;182:81–88. doi: 10.1016/s0002-9610(01)00656-0. [DOI] [PubMed] [Google Scholar]
- Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, et al. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27:80–87. doi: 10.1053/ejso.2000.1029. [DOI] [PubMed] [Google Scholar]
- Smith DL, Soria JC, Morat L, Yang Q, Sabatier L, Liu DD, et al. Human telomerase reverse transcriptase (hTERT) and Ki-67 are better predictors of survival than established clinical indicators in patients undergoing curative hepatic resection for colorectal metastases. Ann Surg Oncol. 2004;11:45–51. doi: 10.1007/BF02524345. [DOI] [PubMed] [Google Scholar]
- Nash GM, Gimbel M, Shia J, Nathanson DR, Ndubuisi MI, Zeng ZS, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572–578. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- Anjomshoaa A, Nasri S, Humar B, McCall JL, Chatterjee A, Yoon HS, et al. Slow proliferation as a biological feature of colorectal cancer metastasis. Br J Cancer. 2009;101:822–828. doi: 10.1038/sj.bjc.6605229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi DC, Ciaparrone M, Zannoni G, Mancini M, Cassano A, Specchia M, et al. Predictive value of thymidylate synthase expression in resected metastases of colorectal cancer. Eur J Cancer. 2002;38:527–534. doi: 10.1016/s0959-8049(01)00402-6. [DOI] [PubMed] [Google Scholar]
- Lassmann S, Tang L, Capanu M, Brabletz T, Schöpflin A, Hausen AZ, et al. Predictive molecular markers for colorectal cancer patients with resected liver metastases and adjuvant chemotherapy. Gastroenterology. 2007;133:1831–1839. doi: 10.1053/j.gastro.2007.08.075. [DOI] [PubMed] [Google Scholar]


