Abstract
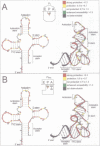
In vitro transcribed tRNA(Phe) analogues from Escherichia coli containing up to four randomly distributed A, G, U or C phosphorothioated nucleotides were used to investigate contact patterns with the ribosome in the A and P sites. The tRNAs were biologically active. Molecular iodine (I2) can trigger a break in the sugar-phosphate backbone at phosphorothioated positions of the ribosomal bound tRNAs if contacts with ribosomal components do not prevent access of the iodine. Highly differentiated protection patterns were found which were strikingly different in the A and P sites, respectively. Strong protections accumulated in the T psi C loop and no protection was seen in the extra-arm region in both sites, whereas the phosphates in the anticodon loop are more strongly protected in the A site. Strong common protections in both the A and P sites were found neighbouring universally or semi-universally conserved bases in prominent regions of the tertiary structure of tRNAs: Y11, Y32, U33, psi55, C56, A58 and Y60. These bases are therefore candidates for 'identity elements' in ribosomal tRNA recognition. The data further indicate that tRNAs change their conformations upon binding to either ribosomal site.
Full text
PDF
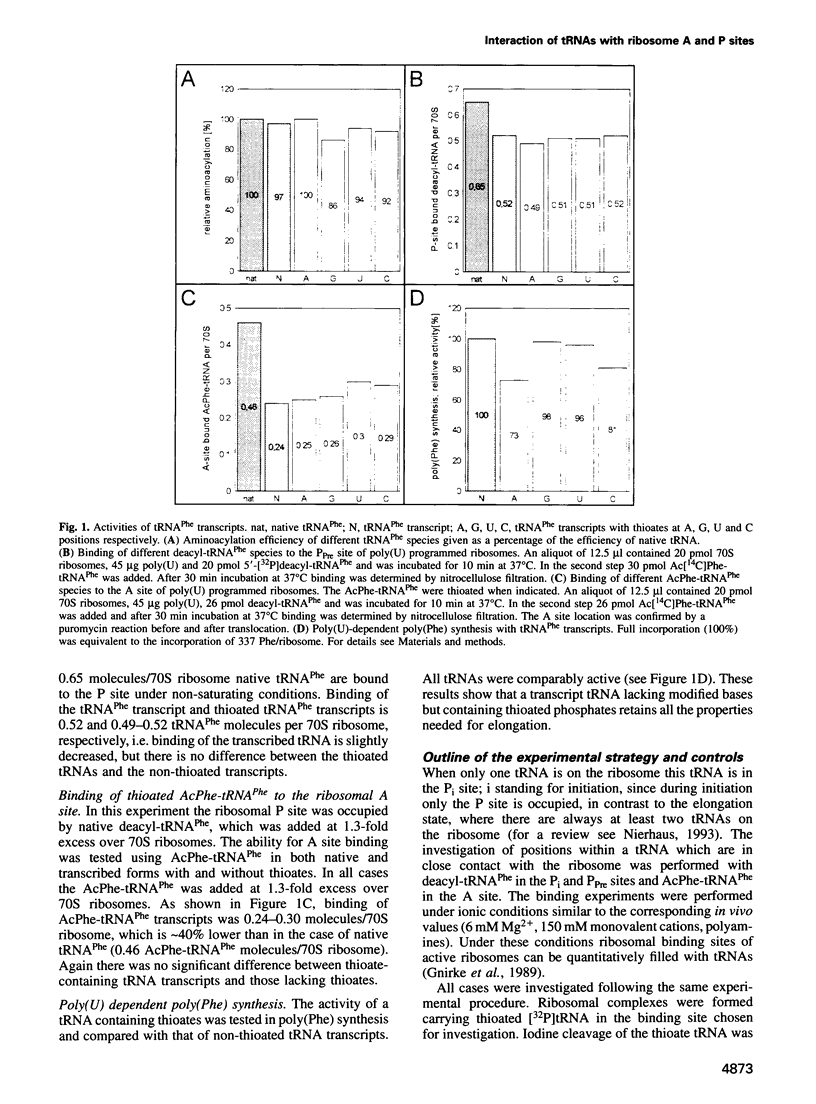





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Determination of tRNA nucleotide residues directly interacting with proteins in the post- and pretranslocated ribosomal complexes. FEBS Lett. 1990 Sep 3;269(2):398–401. doi: 10.1016/0014-5793(90)81202-y. [DOI] [PubMed] [Google Scholar]
- Bertram S., Göringer U., Wagner R. Structural investigation of Phe-tRNAPhe from E.coli bound to the ribosomal A-site. Nucleic Acids Res. 1983 Feb 11;11(3):575–589. doi: 10.1093/nar/11.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W. C., Horowitz J. 19F NMR of 5-fluorouracil-substituted transfer RNA transcribed in vitro: resonance assignment of fluorouracil-guanine base pairs. Nucleic Acids Res. 1989 Sep 25;17(18):7241–7252. doi: 10.1093/nar/17.18.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick W. B., Horowitz J. Probing structural differences between native and in vitro transcribed Escherichia coli valine transfer RNA: evidence for stable base modification-dependent conformers. Nucleic Acids Res. 1993 Oct 25;21(21):4948–4953. doi: 10.1093/nar/21.21.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnirke A., Geigenmüller U., Rheinberger H. J., Nierhaus L. H. The allosteric three-site model for the ribosomal elongation cycle. Analysis with a heteropolymeric mRNA. J Biol Chem. 1989 May 5;264(13):7291–7301. [PubMed] [Google Scholar]
- Harrington K. M., Nazarenko I. A., Dix D. B., Thompson R. C., Uhlenbeck O. C. In vitro analysis of translational rate and accuracy with an unmodified tRNA. Biochemistry. 1993 Aug 3;32(30):7617–7622. doi: 10.1021/bi00081a003. [DOI] [PubMed] [Google Scholar]
- Hüttenhofer A., Noller H. F. Hydroxyl radical cleavage of tRNA in the ribosomal P site. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7851–7855. doi: 10.1073/pnas.89.17.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Robertus J. D., Ladner J. E., Brown R. S., Finch J. T. Conservation of the molecular structure of yeast phenylalanine transfer RNA in two crystal forms. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3711–3715. doi: 10.1073/pnas.71.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lührmann R., Eckhardt H., Stöffler G. Codon-anticodon interaction at the ribosomal peptidyl-site. Nature. 1979 Aug 2;280(5721):423–425. doi: 10.1038/280423a0. [DOI] [PubMed] [Google Scholar]
- Nazarenko I. A., Harrington K. M., Uhlenbeck O. C. Many of the conserved nucleotides of tRNA(Phe) are not essential for ternary complex formation and peptide elongation. EMBO J. 1994 May 15;13(10):2464–2471. doi: 10.1002/j.1460-2075.1994.tb06531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus K. H. Solution of the ribosome riddle: how the ribosome selects the correct aminoacyl-tRNA out of 41 similar contestants. Mol Microbiol. 1993 Aug;9(4):661–669. doi: 10.1111/j.1365-2958.1993.tb01726.x. [DOI] [PubMed] [Google Scholar]
- Noren C. J., Anthony-Cahill S. J., Suich D. J., Noren K. A., Griffith M. C., Schultz P. G. In vitro suppression of an amber mutation by a chemically aminoacylated transfer RNA prepared by runoff transcription. Nucleic Acids Res. 1990 Jan 11;18(1):83–88. doi: 10.1093/nar/18.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Herr W. Chemical probing of the tRNA--ribosome complex. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2273–2277. doi: 10.1073/pnas.78.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M., Yarus M. Transfer RNA selection at the ribosomal A and P sites. J Mol Biol. 1979 Nov 5;134(3):471–491. doi: 10.1016/0022-2836(79)90364-4. [DOI] [PubMed] [Google Scholar]
- Rheinberger H. J., Geigenmüller U., Wedde M., Nierhaus K. H. Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol. 1988;164:658–670. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- Rheinberger H. J., Nierhaus K. H. Allosteric interactions between the ribosomal transfer RNA-binding sites A and E. J Biol Chem. 1986 Jul 15;261(20):9133–9139. [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Borén T., Johansen T. I., Lustig F. Properties of a transfer RNA lacking modified nucleosides. J Biol Chem. 1988 Sep 25;263(27):13692–13699. [PubMed] [Google Scholar]
- Schatz D., Leberman R., Eckstein F. Interaction of Escherichia coli tRNA(Ser) with its cognate aminoacyl-tRNA synthetase as determined by footprinting with phosphorothioate-containing tRNA transcripts. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6132–6136. doi: 10.1073/pnas.88.14.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach P., Nierhaus K. H. Codon-anticodon interaction at the ribosomal P (peptidyl-tRNA)site. Proc Natl Acad Sci U S A. 1979 May;76(5):2143–2147. doi: 10.1073/pnas.76.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]