Abstract
Background: Post-treatment contralateral hemiliver hypertrophy has created an interest in lobar liver radioembolization (RE) as a pre-surgery tool.
Methods: Liver and spleen volumes and function were studied in 83 patients submitted to partial liver volume RE at 4–8 weeks (T1), 10–26 weeks (T2), and >26 weeks (T3) after RE.
Results: More than half of the patients had cirrhosis with hepatocellular carcinoma. The main finding wasa progressive increase in the volume of the spared hemiliver (mean absolute increase at T3: 230 ml). The percentage of patients in whom the baseline ratio of spared volume to total liver volume was <40% dropped from 56.6% at baseline to 29.4% at T2 (P < 0.001). A significant and progressive increase in spleen volume but not in portal vein diameter was also observed. A small percentage of patients developed hypersplenism, mostly those without cirrhosis (16.0% at T2). Six patients (five with portal vein thrombosis, cirrhosisor both)developed signs of portal hypertension by T2.
Conclusions: The present results warrant further studies to better elucidate the mechanism underlying this phenomenon of spared hemiliver hypertrophy and to investigate its role as an alternative to portal vein embolizationin the management of patients with potentially resectable liver tumours.
This manuscript was presented at the 10th World IHPBA Congress, Paris, 1–5 July 2012.
Introduction
According to the last report of the International Agency for Research on Cancer, primary liver cancer is the sixth most common malignancy worldwide and the third most common cause of cancer-related mortality.1 The liver is also one of the organs most frequently affected by metastases from distant primary tumours. Curative treatment for liver tumours may consist of liver transplant, resection or percutaneous ablation. However, in many patients surgical treatment is contraindicated by inappropriate liver function, an extrahepatic tumour burden or thelack of a sufficient future liver remnant (FLR). When an excessive extent of disease precludes liver resection, downstaging strategies play an important role in rescuing patients for surgery.2 Similarly, when the FLR is expected to be insufficient, portal vein embolization (PVE) is used in preoperative management to induce selective hepatic hypertrophy.3
Liver radioembolization (RE) has been developed in recent years. It consists of the intra-arterial delivery of microspheres loaded with yttrium-90. Diverse evidence supports its use in different types of hepatic tumour, including both primary and metastatic disease, as well as hepatocellular carcinoma4 and liver metastases from colorectal cancer5 and neuroendocrine tumours.6 Although RE is commonly used as a palliative treatment, it has also been performed in some instances to downstage patients to salvage surgery, and to avoid progression until surgery (i.e. as treatment administered to a patient on the waiting list for resection or liver transplantation).7–10 Some authors have reported the occurrence of hypertrophy in the spared hemiliver after lobar, segmental or sequential lobar RE,11–14 raising the question of whether it could be deliberately used for this purpose in a manner similar to that of PVE. In this setting, the potential deleterious effects on the non-tumoral liver, including the development of portal hypertension and RE-induced liver disease, may pose technical difficulties in liver surgery.15 The purpose of this study was to analyse changes in liver and spleen volumes and function in patients treated with RE when only a partial volume is targeted.
Materials and methods
Patient cohort
The study population included all patients submitted to RE at the study institution between September 2003 and September 2010 in whom: (i) lobar or segmental treatment was performed, with or without flow redistribution; (ii) all image examinations [angiography, nuclear medicine scans, computed tomography (CT) and magnetic resonance imaging (MRI)] were performed in the same institution; (iii) screening angiography was performed no more than 15 days before treatment, and (iv) follow-up cross-sectional imaging studies were performed in weeks 4–8 (T1), weeks 10–26 (T2), and at >26 weeks (T3) after RE. In patients who received a second RE treatment to the treated or spared hemiliver or who were submitted to a major hepatic intervention after RE (hepatectomy, biliary drainage, trans jugular intrahepatic portosystemic stent shunt), those images obtained following this second event were excluded from analysis. The term ‘spared hemiliver’ will be used hereafter to distinguish the untreated hemiliver from the treated hemiliver (the entire hemiliver or one or more segments). General inclusion criteria for RE at this institution include an unequivocal diagnosis of unresectable primary or metastatic cancer with a liver-only or liver-dominant tumour burden, a life expectancy of >3 months and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, adequate pulmonary, haematology, hepatic and renal function, and the possibility to deliver to the lungs an estimated dose of radiation of < 30 Gy. Approval for this retrospective analysis was obtained from the local ethics committee.
Pretreatment and treatment
Pretreatment investigations included cross-sectional imaging studies with CT or MRI, blood cell count and serum biochemistry. All previous images were carefully evaluated before angiographic mapping of the abdominal visceral and hepatic arteries was carried out. The techniques for the isolation of the hepatic vasculature and vascular redistribution have been well described16,17 and were followed in pre-treatment angiographic planning. Calculation of the lung-shunting fraction, pretreatment identification of infusion patterns, detection of any non-target infused liver volume and the unintentional delivery of radioactive particles to organs outside the liver were also evaluated after the infusion of technetium-99 m-labelled macroaggregated albumin (99mTc-MAA).
The treatment to be prescribed was calculated according to the body surface area formula or the compartmental model and injected within 15 days of the 99mTc-MAA scan. On the day of treatment, a new angiographic study was performed to exclude any changes in the vascular anatomy of the target volume and, in accordance with current recommendations, the delivery of yttrium-90-labeled resin microspheres (SIR-Spheres; Sirtex Medical Ltd, Sydney, NSW, Australia) was accomplished with a coaxially inserted microcatheter through one or two major afferent tumoral arteries, at the same injection site as the initial evaluation with 99mTc-MAA.
Follow-up and statistical analysis
All CT and MRI images were analysed by two radiologists with, respectively, 3 years and 5 years of experience on a Leonardo Workstation (Siemens AG Medical Solutions, Erlangen, Germany) and volumes were determined using 1-cm-thick reformatted images with standard Syngo software tools. The volumes of the right and left livers and the spleen were measured, as was the maximal diameter of the main portal vein before bifurcation. Total liver volume was calculated as the sum of the volumes of the right and left livers. All images were searched for the presence of portal vein thrombosis and indirect signs of portal hypertension including re-permeabilization of the umbilical vein, splenorenal shunt and gastro-oesophageal varices. Blood cell counts and liver function tests (serum transaminases, total bilirubin, albumin, prothrombin time) obtained at the time of cross-sectional imaging studies were used to evaluate changes in liver and spleen function.
Changes in the volumes of the treated and spared hemilivers, spleen volume, diameter of the main portal vein, platelet count, blood cell count and liver function tests between baseline and the three time intervals post-RE were studied using Student's t-test for paired samples. The association between changes in spared liver volume and baseline variables was studied using Student's t-test for unpaired samples. The correlation between the volume of the spared hemiliver before treatment and baseline total bilirubin levels and their change after RE were studied using analysis of variance (anova). All statistical analyses were performed using spss Version 10.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patients
A total of 83 patients submitted to RE between September 2003 and September 2010, in whom at least a single hemiliver was preserved from radiation, were found to fulfil all of the patient selection criteria. Their general characteristics are summarized in Table 1. Most patients had primary liver tumours (67.5%), usually with underlying cirrhosis (53.0%). Liver and haematological functions were basically preserved. Only 16.0% of patients were found to have hypersplenism as defined by a platelet count of <100·10e9/l. The right liver was targeted in 80.0% of patients. In 10.8% of patients, the target area involved one or more segments but not the entire right or left liver (segmental treatment). A total of 29 patients (34.9%) had been exposed to chemotherapy prior to RE, including 18 patients with liver metastases from colorectal or breast cancer who had received several lines of therapy. A total of 54 patients (65.1%) were chemo-naïve at the time of RE; these included 49 patients with hepatocellular carcinoma, three patients with cholangiocarcinoma, and two patients with liver metastases from neuroendocrine tumours who had received only somatostatin analogues and/or interferon.
Table 1.
General characteristics of patients in the present series treated with radioembolization
| Patients, n | 83 |
| Age, years, median ± IQR | 66 ± 13 |
| Sex, male, n (%) | 61 (73.5%) |
| Type of tumour, n (%) | |
| • Hepatocellular carcinoma | 52 (62.7%) |
| • Cholangiocarcinoma | 4 (4.8%) |
| • Colorectal cancer | 13 (15.7%) |
| • Other | 14 (16.8%) |
| Prior chemotherapy, n (%) | 29 (34.9%) |
| Cirrhosis, n (%) | 44 (53.0%) |
| Portal vein thrombosis, n (%) | 8 (9.6%) |
| Haemoglobin, g/dl, median ± IQR | 12.80 ± 3.05 |
| Leukocytes, 109/l, median ± IQR | 5.9 ± 3.3 |
| Platelets, 109/l, median ± IQR | 163 ± 145 |
| Albumin, g/dl, median ± IQR | 3.73 ± 0.80 |
| Prothrombin time, s, median ± IQR | 12.5 ± 2.1 |
| AST, UI/l, median ± IQR | 30.0 ± 35.5 |
| ALT, UI/l, median ± IQR | 23.0 ± 32.5 |
| Alkaline phosphatase, UI/l, median ± IQR | 168.5 ± 140.0 |
| γ-GTP, UI/l, median ± IQR | 85.5 ± 120.5 |
| Total bilirubin, mg/dl, median ± IQR | 0.92 ± 0.86 |
| Treated volume, n (%) | |
| • Right liver | 60 (72.3%) |
| • Left liver | 14 (16.9%) |
| • One or more right segments | 6 (7.2%) |
| • One or more left segments | 3 (3.6%) |
| Sites of microsphere infusion, n (%) | |
| • Right liver artery | 56 (67.4%) |
| • Left liver artery | 11 (13.2%) |
| • Segmental arteries | 13 (15.6%) |
| • Other | 3 (3.6%) |
γ-GTP, γ-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IQR, inter-quartile range.
Changes in liver volume and function
The main finding was a progressive and statistically significant increase in the volume of the spared hemiliver, which was already significant at T1, but continued to rise until maximal follow-up (Fig. 1). The mean ± standard deviation (SD) absolute increase was 73.1 ± 137.7 ml at T1, 134.7 ± 193.9 ml at T2, and 229.7 ± 303.0 ml at T3 (P < 0.001 for each time period). This was paralleled by a statistically significant decrease in the volume of the treated hemiliver, although the magnitude of this change was not as large as for the spared hemiliver. The mean ± SD absolute decrease was 64.5 ± 198.3 ml at T1, 218.6 ± 287.4 ml at T2, and 321.9 ± 375.6 ml at T3 (P < 0.001 for each period). These results remained significant when patients with progression of existing tumours or development of new tumours in the spared hemiliver were excluded from analysis. In addition, the results remained consistent irrespective of whether the left or right hemiliver was treated. Globally, the mean ± SD total liver volume did not change significantly from baseline (2008 ± 801 ml) to T1 (2081 ± 972 ml; P = 0.841), T2 (1848 ± 950 ml; P = 0.081) or T3 (1649 ± 658 ml; P = 0.335) because the hypertrophy of the spared hemiliver compensated for the atrophy of the treated hemiliver.
Figure 1.
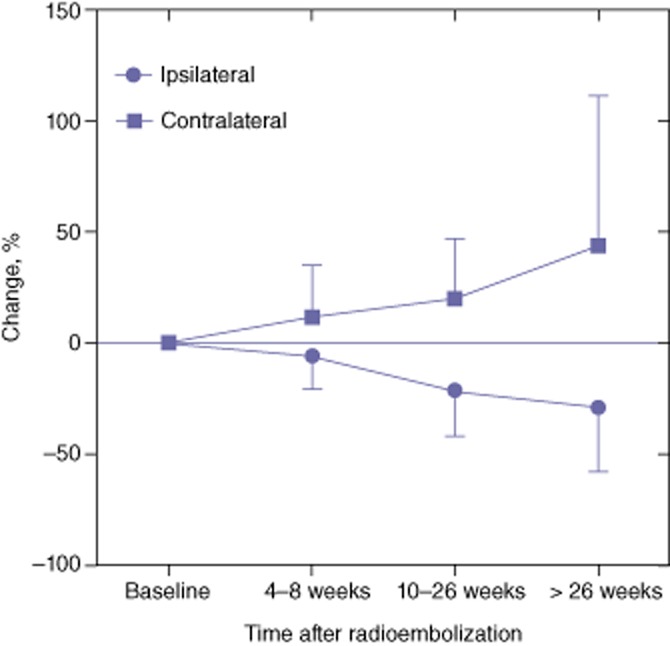
Changes in the volume of the spared hemiliver following partial liver volume radioembolization
The ratio between the volume of the spared hemiliver and the volume of the total liver was also calculated. In patients in whom this ratio was found to be <40%, the mean ± SD ratio rose from 29 ± 6% at baseline to 32 ± 8% at T1, 40 ± 12% at T2 and 50 ± 13% at T3 (P < 0.001 for each time interval). Even more importantly, the percentage of patients with a ratio of <40% declined from 56.6% at baseline to 47.6% at T1 (P < 0.001), 29.4% at T2 (P < 0.001) and 15.6% at T3 (P = 0.155).
A clinically irrelevant but statistically significant increase in mean ± SD serum total bilirubin was observed from baseline (0.92 ± 0.86 mg/ml) to T1 (0.93 ± 1.16 mg/ml; P = 0.015), T2 (1.13 ± 1.57 mg/ml; P = 0.000) and T3 (1.59 ± 1.22 mg/ml; P < 0.001). However, no significant changes related to synthetic liver function (prothrombin time and albumin) were seen.
Different baseline conditions were analysed in an attempt to identify factors that might determine the magnitude of the spared hemiliver hypertrophy (Fig. 2). The degree of hypertrophy was not significantly associated with sex, prior chemotherapy or an abnormal platelet count, transaminase level or prothrombin time at baseline. However, there was a trend to reduced hypertrophy among patients with cirrhosis or an abnormal baseline total bilirubin (>1.2 mg/dl), although the difference did not reach statistical significance (P = 0.08). No correlation between the estimated dose of radiation delivered to the treated hemiliver (calculated using the partition model) and changes in volume at T1, T2 and T3 were seen.
Figure 2.
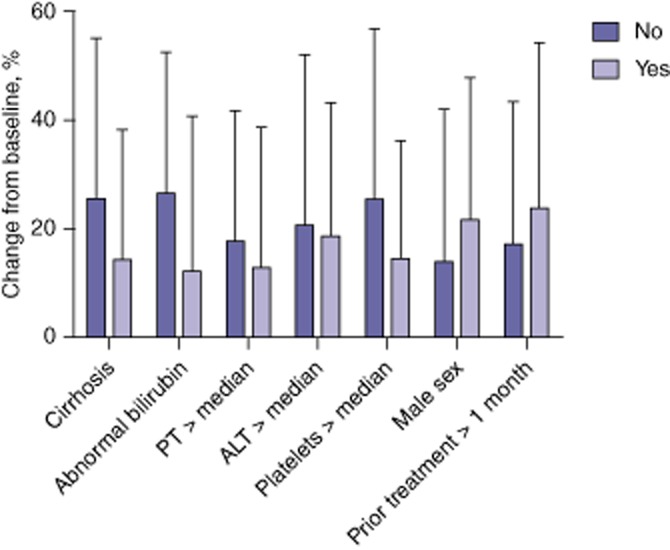
Factors related to the magnitude of spared hemiliver hypertrophy following partial liver volume radioembolization. ALT, alanine aminotransferase; PT, prothrombin time
Finally, there was no significant correlation between the degree of hypertrophy of the spared hemiliver at any time-point and the volumes of the spared and ipsilateral hemi livers before RE. However, a correlation emerged between baseline total bilirubin level and the degree of hypertrophy of the spared hemiliver (Fig. 3).
Figure 3.
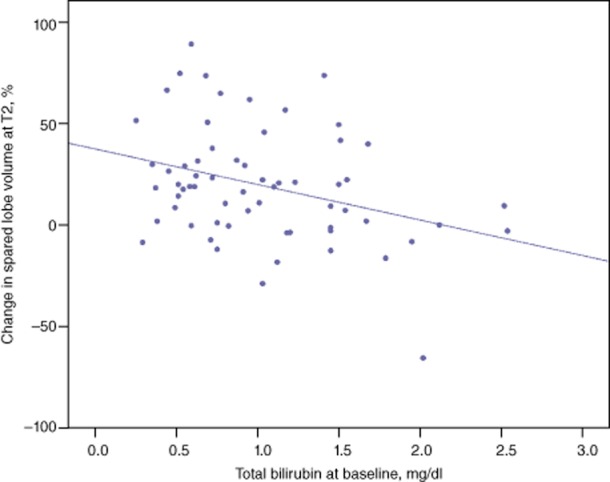
Correlation between baseline bilirubin level and spared hemiliver hypertrophy following partial liver volume radioembolization
Changes in spleen volume and function, and signs of portal hypertension
As shown in Fig. 4, a significant and progressive increase in mean ± SD spleen volume was observed from baseline (447 ± 283 ml) to T1 (484 ± 349 ml; P = 0.009), T2 (497 ± 303 ml; P = 0.003) and T3 (576 ± 281 ml; P = 0.031). By contrast, platelet counts remained basically stable over time, except at T2, when a mild but statistically significant decrease in platelet count was observed. However, the percentage of patients with hypersplenism was 16.0% at baseline, 17.5% at T1, 26.2% at T2, and 36.7% at T3. Most of the patients who developed hypersplenism did not have cirrhosis and thus the percentage of non-cirrhotic patients with hypersplenism was 0% at baseline and 16.0% at T2. These changes in spleen size and function did not differ between patients with and without cirrhosis, or between those exposed and not exposed to chemotherapy before RE.
Figure 4.
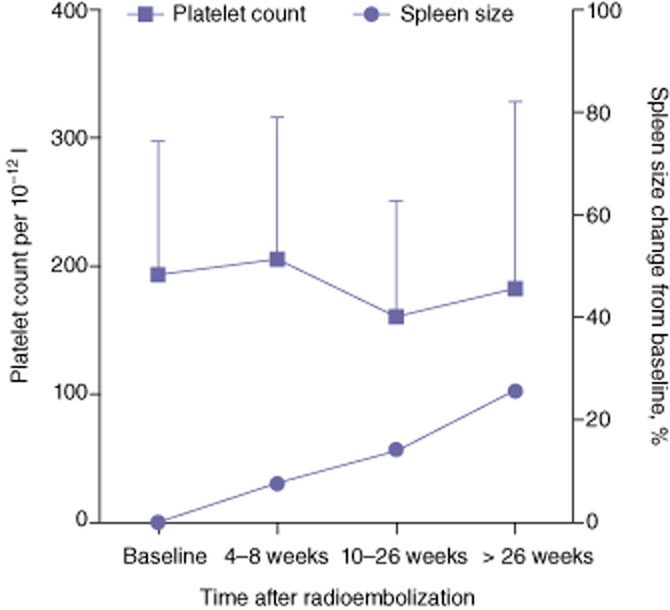
Changes in spleen volume and platelet count following partial liver volume radioembolization
The increase in spleen size was not accompanied by changes in the diameter of the main portal vein. The mean ± SD diameter of the main portal vein was 1.42 ± 0.33 cm at baseline, 1.39 ± 0.33 cm (P = 0.474) at T1, 1.45 ± 0.30 cm (P = 0.344) at T2, and 1.52 ± 0.34 cm (P = 0.895) at T3. At baseline, 26 patients (31.3%) had signs of portal hypertension. Six additional patients (7.2%) developed such signs by T2 (indicated by the re-permeabilization of the umbilical vein in four patients and the appearance of varices in two). All but one of these six patients had other conditions at baseline that might cause portal hypertension, including portal vein thrombosis, cirrhosis or both, and portal vein tumour invasion progressed after RE in two of these patients. The average increase in spleen volume was 53.3% in these six patients and 9.9% in patients who did not develop signs of portal hypertension (P = 0.127).
Discussion
Candidates for liver surgery with a supposedly insufficient FLR can be treated with PVE to induce selective liver hypertrophy and minimize the risk for liver insufficiency after surgery. The methods and materials used for PVE are not fully standardized.18 The conventional approach is transparietohepatic and different embolic materials have been used.19 Although the time needed to achieve significant hypertrophy after PVE depends on the procedure and material used, it does not usually take <4–5 weeks. In recent years, new experimental embolization techniques and materials have been developed in an attempt to obtain more hypertrophy with fewer complications. These new techniques have been explored only in animal models and include transarterial embolization20 and transinusoidal PVE.21
Radioembolization has been used to downstage patients in order to salvage them for surgery and to bridge patients for liver transplantation.7–10 Some authors have recently reported hypertrophy of the spared hemiliver after RE when only a partial liver volume is targeted. Jakobs et al. analysed volumetric liver and spleen changes occurring 4–6 weeks after the treatment of the right liver in 17 patients receiving sequential bilobar RE, and 30 days after treatment in 15 patients receiving lobar (right or left) RE.12 Median hypertrophy in the spared hemiliver was 4% after sequential RE, 9% after lobar RE and a significant 17% after right lobar RE (in 10 patients). This concept was further explored in a subsequent analysis by the same group in 20 patients treated with right lobar RE. After an average of 18 months (range: 2–49 months), the authors observed atrophy of 52% in the treated hemiliver, compensated by hypertrophy of 40% in the spared hemiliver.14 These results are in agreement with the hypertrophy of almost 45% achieved at >26 weeks after RE in the present study.
In general, most surgical teams would agree that the minimum volume of the FLR required to allow safe resection is 20% in patients with no underlying liver disease, 30% in patients who have received chemotherapy prior to surgery, and 40% in patients with chronic liver disease.22,23 In this regard, the finding in the present series of a significant decline in the number of patients with a ratio of spared hemi liver volume to total liver volume of <40% at weeks 10–26 is remarkable. Tumour progression may occur after PVE in both the embolized and the non-embolized hemilivers. Whether this represents the natural history of the tumour or whether PVE may foster this progression is not clear,24 although some studies have suggested that tumour growth in the embolized hemiliver may be accelerated after PVE.25,26 On the basis of this information, some authors have proposed the use of anticancer chemotherapy after PVE27 or transarterial chemoembolization (TACE) before PVE28,29 to control tumour growth, and have obtained apparently good results in terms of hypertrophy, complications and prognosis. Gulec et al.13 first reported on the deliberate use of chemotherapy and RE to increase the FLR in a patient with a history of treated cervical cancer and a voluminous tumour mass in the right liver in which a right hepatectomy was later safely performed. As observed in the present series, spared hemiliver hypertrophy after RE is an early yet evolving phenomenon and the most clinically relevant changes usually occur after 10 weeks post-RE. This is a longer period than that usually observed after PVE. In patients with liver metastases from colorectal cancer, this may necessitate the concomitant use of chemotherapy, whereas in patients with primary liver tumours, it may allow for the natural selection of candidates without progression in the untreated hemiliver. Given that PVE is associated with adverse reactions in up to 15% of patients,19 partial volume RE may represent an appealing alternative. Whether increasing the dose of radiation to deliberately produce liver damage in the treated hemiliver may further enhance hypertrophy is not known. The present results indicate that hypertrophy may occur when the dose is only therapeutic.
By contrast, the reduced amount of radiation delivered to the non-tumoral liver can cause tissue damage that occasionally translates into clinical findings, such as altered liver function tests, portal hypertension or subacute sinusoidal obstruction syndrome.30–32 In agreement with previous series,12 the authors observed a significant increase in spleen volume, but no increase in portal vein diameter after lobar RE, as has been described after whole-liver RE.11 However, only sporadically was this increase in spleen volume associated with the development or aggravation of hypersplenism, which raises the possibility that alternative causes may explain such RE-induced splenomegaly. Interestingly, patients without cirrhosis developed hypersplenism more frequently than those with cirrhosis. Whether or not minor changes in portal pressure may occur after RE, such changes rarely pose a surgical problem. In fact, 15 of the present patients underwent liver surgery (liver transplantation and major hepatectomy) after RE without major complications.
The mechanism by which lobar RE induces contralateral liver hypertrophy is not known. The consistent association with ipsilateral atrophy strongly suggests that it may well be a compensatory effect. Whole-liver RE can cause liver atrophy11,12,33 and different types of histopathological liver damage have been described in asymptomatic patients treated with RE, including periportal fibrosis,13 portal ‘triaditis’34 and necrosis followed by fibrosis and regenerative activity at the periphery of the tumour where the level of radiation is maximal.35,36 The atrophy–hypertrophy complex and the proliferating signalling pathways involved in hepatocyte proliferation after PVE37,38 may also be triggered after lobar RE as the radiation emitted by microspheres embolized into the terminal hepatic arteries certainly may reach the terminal portal venules. Importantly, this microscopic damage rarely produces clinical problems in lobar RE. No significant increases in liver transaminases or synthetic liver function tests were seen in the present study, although a mild and clinically irrelevant elevation in bilirubin was observed, as reported previously.14 Further prospective studies in animal models and patients may help to elucidate these mechanisms.
Linked to its retrospective nature, the main weakness of this study concerns the reduction in its sample size over the period of study. However, this weakness is partly offset by the number of patients (it is to date the largest series reporting on changes in liver and spleen volumes) and the consistency of the main results (progressive hypertrophy of the spared hemiliver and increased spleen size with minor hypersplenism) across analyses of different subsets of patients.
In summary, the present study demonstrates that partial liver volume RE induces significant hypertrophy in the spared hemiliver and the magnitude of this increase tends to be lower in patients with cirrhosis and an abnormal baseline bilirubin level. Although spleen size increases after lobar RE, the lack of increase in portal vein diameter and the only occasional development of hypersplenism or radiological signs of portal hypertension prevent the conclusion that lobar RE invariably provokes portal hypertension. Further studies are required to better elucidate the mechanism underlying this phenomenon and to investigate the role of RE as an alternative to PVE in the management of patients with potentially resectable liver tumours.
Conflicts of interest
Jose I. Bilbao, Fernando Pardo and Bruno Sangro have received lecture and consulting fees from Sirtex Medical.
References
- 1.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 4.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012;56:464–473. doi: 10.1016/j.jhep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol. 2009;6:687–697. doi: 10.1038/nrclinonc.2009.165. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Integrating radioembolization into the treatment paradigm for metastatic neuroendocrine tumours in the liver. Am J Clin Oncol. 2012;35:393–398. doi: 10.1097/COC.0b013e3182005768. [DOI] [PubMed] [Google Scholar]
- 7.Kulik LM, Mulcahy MF, Hunter RD, Nemcek AA, Jr, Abecassis MM, Salem R. Use of yttrium-90 microspheres (TheraSphere) in a patient with unresectable hepatocellular carcinoma leading to liver transplantation: a case report. Liver Transpl. 2005;11:1127–1131. doi: 10.1002/lt.20514. [DOI] [PubMed] [Google Scholar]
- 8.Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 9.Kim DY, Kwon DS, Salem R, Ma CK, Abouljoud MS. Successful embolization of hepatocellular carcinoma with yttrium-90 glass microspheres prior to liver transplantation. J Gastrointest Surg. 2006;10:413–416. doi: 10.1016/j.gassur.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299–305. doi: 10.1097/01.sla.0000133123.11932.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidensticker R, Seidensticker M, Damm R, Mohnike K, Schutte K, Malfertheiner P, et al. Hepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35:1109–1118. doi: 10.1007/s00270-011-0295-7. [DOI] [PubMed] [Google Scholar]
- 12.Jakobs TF, Saleem S, Atassi B, Reda E, Lewandowski RJ, Yaghmai V, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90-yttrium microspheres. Dig Dis Sci. 2008;53:2556–2563. doi: 10.1007/s10620-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 13.Gulec SA, Pennington K, Hall M, Fong Y. Preoperative Y-90 microsphere selective internal radiation treatment for tumour downsizing and future liver remnant recruitment: a novel approach to improving the safety of major hepatic resections. World J Surg Oncol. 2009;7:6. doi: 10.1186/1477-7819-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 15.Ayav A, Habib N, Jiao LR. Portal hypertension secondary to 90-yttrium microspheres: an unknown complication. J Clin Oncol. 2005;23:8275–8276. doi: 10.1200/JCO.2005.03.7820. [DOI] [PubMed] [Google Scholar]
- 16.Bilbao JI, Garrastachu P, Herraiz MJ, Rodriguez M, Inarrairaegui M, Rodriguez J, et al. Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumours. Cardiovasc Intervent Radiol. 2010;33:523–531. doi: 10.1007/s00270-009-9717-1. [DOI] [PubMed] [Google Scholar]
- 17.Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA, Jr, Kulik L, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol. 2007;30:571–592. doi: 10.1007/s00270-007-9064-z. [DOI] [PubMed] [Google Scholar]
- 18.den Baere T, Denys A, Madoff DC. Preoperative portal vein embolization: indications and technical considerations. Tech Vasc Interv Radiol. 2007;10:67–78. doi: 10.1053/j.tvir.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 20.Madoff DC, Gupta S, Pillsbury EP, Kan Z, Tinkey PT, Stephens LC, et al. Transarterial versus transhepatic portal vein embolization to induce selective hepatic hypertrophy: a comparative study in swine. J Vasc Interv Radiol. 2007;18:79–93. doi: 10.1016/j.jvir.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Smits ML, Vanlangenhove P, Sturm EJ, van den Bosch MA, Hav M, Praet M, et al. Transsinusoidal portal vein embolization with ethylene vinyl alcohol copolymer (Onyx): a feasibility study in pigs. Cardiovasc Intervent Radiol. 2012;35:1172–1180. doi: 10.1007/s00270-011-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision making in resectional surgery for hepatic tumours. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 24.de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumour growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423–430. doi: 10.1245/s10434-008-0222-6. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumour growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721–727. doi: 10.1080/02841850701424514. [DOI] [PubMed] [Google Scholar]
- 26.Elias D, De Baere T, Roche A, Ducreux M, Lecere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784–788. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 27.van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, et al. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436–444. doi: 10.1159/000184735. [DOI] [PubMed] [Google Scholar]
- 28.Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]
- 29.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 30.Moroz P, Anderson JE, Van Hazel G, Gray BN. Effect of selective internal radiation therapy and hepatic arterial chemotherapy on normal liver volume and spleen volume. J Surg Oncol. 2001;78:248–252. doi: 10.1002/jso.1162. [DOI] [PubMed] [Google Scholar]
- 31.Goin JE, Salem R, Carr BI, Dancey JE, Soulen MC, Geschwind JF, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol. 2005;16:205–213. doi: 10.1097/01.rvi.00001142592.89564.f9. [DOI] [PubMed] [Google Scholar]
- 32.Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, et al. Liver disease induced by radioembolization of liver tumours: description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 33.Nosher JL, Ohman-Strickland PA, Jabbour S, Narra V, Nosher B. Changes in liver and spleen volumes and liver function after radioembolization with yttrium-90 resin microspheres. J Vasc Interv Radiol. 2011;22:1706–1713. doi: 10.1016/j.jvir.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 35.Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, et al. Radiologic–pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009;49:1185–1193. doi: 10.1002/hep.22747. [DOI] [PubMed] [Google Scholar]
- 36.Nalesnik MA, Federle M, Buck D, Fontes P, Carr BI. Hepatobiliary effects of 90-Yttrium microsphere therapy for unresectable hepatocellular carcinoma. Hum Pathol. 2009;40:125–134. doi: 10.1016/j.humpath.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Schweizer W, Duda P, Tanner S, Balsiger D, Hoflin F, Blumgart LH, et al. Experimental atrophy/hypertrophy complex (AHC) of the liver: portal vein, but not bile duct obstruction, is the main driving force for the development of AHC in the rat. J Hepatol. 1995;23:71–78. doi: 10.1016/0168-8278(95)80313-0. [DOI] [PubMed] [Google Scholar]
- 38.Kim RD, Kim JS, Watanabe G, Mohuczy D, Behrns KE. Liver regeneration and the atrophy–hypertrophy complex. Semin Interv Radiol. 2008;25:92–103. doi: 10.1055/s-2008-1076679. [DOI] [PMC free article] [PubMed] [Google Scholar]


