Abstract
Objectives:The timing of major elective operations is a potentially important but rarely examined outcome variable. This study examined elective pancreaticoduodenectomy (PD) timing as a perioperative outcome variable.
Methods:Consecutive patients submitted to PD were identified. Determinants of 90-day morbidity (prospectively graded and tracked), anastomotic leak or fistula, and mortality, including operation start time (time of day), day of week and month, were assessed in univariate and multivariate analyses. Operation start time was analysed as a continuous and a categorical variable.
Results:Of the 819 patients identified, 405 (49.5%) experienced one or more complications (total number of events = 684); 90-day mortality was 3.5%. On multivariate analysis, predictors of any morbidityincluded male gender (P = 0.009) and estimated blood loss (P = 0.017). Male gender (P = 0.002), benign diagnosis (P = 0.002), presence of comorbidities (P = 0.002), American Society of Anesthesiologists (ASA) score (P = 0.025), larger tumour size (P = 0.013) and positive resection margin status (P = 0.005) were associated with the occurrence of anastomotic leak or fistula. Cardiac and pulmonary comorbidities were the only variables associated with 90-day mortality. Variables pertaining to procedure scheduling were not associated with perioperative morbidity or mortality. Operation start time was not significant when analysed as a continuous or a categorical variable, or when stratified by surgeon.
Conclusions:Perioperative outcome after PD is determined by patient, disease and operative factors and does not appear to be influenced by procedure timing.
Introduction
In surgery, operator fatigue and experience, as well as the availability of appropriate supervision and assistance, may potentially influence perioperative outcomes. Concerns have been raised about possible variations in quality of care and increased complication rates as a result of the timing of surgical procedures. Several studies have suggested variability in the outcomes of operations based on the month of the year, day of the week and time of day at which they take place.
The ‘July effect’, used to describe the impact of the annual arrival of new housestaff in July, has been extensively researched, with conflicting results. A 2007 National Surgical Quality Improvement Program (NSQIP) study reported seasonal variation marked by significant increases in operative morbidity and mortality in the earlier months of the academic year,1 and a more recent study showed higher intraoperative complication and mortality rates in patients undergoing surgery for spinal metastases at teaching hospitals in the month of July.2 These data contrast with the results of several studies showing no differences in the outcomes of surgeries performed at the beginning of the academic year compared with those performed at other times.3–5 Data on outcomes according to the time of day at which procedures are performed have been similarly contradictory.6–9
Pancreaticoduodenectomy (PD) is among the more demanding and complex procedures in gastrointestinal surgery. Since Whipple et al.'s 1935 report,10 PD has evolved from a high-risk, resource-intensive endeavour to a commonly performed operation of which good outcomes are expected for most patients. Perioperative mortality has declined to <5%, although morbidity rates remain in the range of 30–65%.11 Infectious complications, particularly related to anastomotic leaks and fistulae, remain the major source of morbidity and are associated with intra-abdominal haemorrhage and sepsis.11 Perioperative morbidity is associated with greater hospital length of stay (LoS), higher costs and, in patients with malignant disease, delay in the initiation of adjuvant therapy and potentially reduced survival. Several studies have suggested that perioperative morbidity has an adverse impact on cancer-specific survival.12–14
Many factors contribute to perioperative morbidity, including patient-related (underlying diagnosis, pre-existing comorbidities), surgeon-related (experience) and institution-related (high-versus low-volume) variables. However, the impact of the timing of the procedure has not been fully investigated in outcome analyses.15 This study represents a single-institution analysis of consecutive patients undergoing PD and specifically addresses the impacts of operation scheduling (month of the year, day of the week and time of day) on perioperative morbidity and mortality.
Materials and methods
Subjects and data collection
This study was approved by the institutional review board at Memorial Sloan–Kettering Cancer Center (MSKCC); compliance with the Health Insurance Portability and Accountability Act (HIPAA) was assured. Data for patients submitted to PD for any diagnosis were analysed. Data were collected from electronic medical records and included clinical and pathological data, procedure performed (standard versus pylorus-preserving PD), pathological margin status, duration of operation, estimated blood loss (EBL), postoperative LoS, surgical schedule distribution, 90-day complications and 90-day mortality. The study population was analysed by age, gender, presence of a benign or malignant neoplasm, diagnosis, tumour size, presence of comorbidities [hypertension, cardiac disease, diabetes mellitus (DM), pulmonary disease, other comorbidities], body mass index (BMI) and American Society of Anesthesiologists (ASA) physical status score.16 Patients were divided by diagnosis into two groups represented by, respectively, malignant neoplasms [adenocarcinoma, gastrointestinal stromal tumour (GIST), endocrine tumours], and benign disease (cystic lesions and solid adenomas). It should be noted that the great majority of patients were referred to MSKCC after they had undergone biliary decompression.
Operative approach
All patients included in the study underwent either a standard or a pylorus-preserving PD at the discretion of the attending surgeon.17 Extended resections, including of adjacent organs or extensive retroperitoneal lymph node dissections, were not performed routinely; portal vein resection or reconstruction was performed as needed. Duct-to-mucosa end-to-side pancreaticojejunostomy was used to reconstruct the pancreatic remnant in nearly all patients. Patients operated by surgeons who performed fewer than 45 resections over the entire study period were excluded in order to exclude ‘the learning curve’ effect as a potential confounding variable. After surgery, patients recovered overnight in the post-anaesthesia care unit and were transferred to the regular surgical ward the following morning; transfer to the intensive care unit (ICU) was reserved for critically ill patients and was required in <5% of patients at any time in their course.
Surgical timing and scheduling
Surgical scheduling was categorized and analysed by month of the year, day of the week and time of day at which the procedure started (surgical start time). Surgical start time was analysed as a continuous variable and as a categorical variable (morning versus afternoon and in 4-h time periods). Surgeons whose patients were included in this study were assigned one or more full days of block time for surgery on the same day or days of the week and generally started between 07.30 h and 08.30 h and continued until the operating list had been completed. During the study period, operating room start time was generally 60–90 min later on Mondays and Wednesdays to accommodate educational conferences. The potential influence of surgical start time on perioperative outcome was analysed for all surgeons collectively and for each surgeon individually.
Complications
All postoperative complications were prospectively recorded in a departmental database and graded in severity using a score of 1–5, based on a grading system devised in 1999.18 The definition of postoperative pancreatic fistula (POPF) included pancreatic anastomotic leak (i.e. clinical signs and symptoms or radiologic confirmation of pancreatic anastomotic leak with amylase-rich drainage of >50 ml/day beyond postoperative day 5, without development of a fistula), pancreatic fistula (i.e. clinical signs and symptoms with amylase-rich drainage of >50 ml/day beyond postoperative day 10), and intra-abdominal abscess (i.e. clinical signs and symptoms or radiologic diagnosis of intra-abdominal abscess or peritonitis), as previously described.19 The POPF classification by the International Study Group on Pancreatic Fistula (ISGPF) was used to grade POPF.20
Statistical analysis
Values were expressed as medians (with interquartile ranges), or percentages, as appropriate. Statistical analyses were performed using Fisher's exact test and the Wilcoxon rank sum test to examine covariate differences between patients with and without complications, patients with and without leaks and fistulae, and patients deceased and alive at 90 days after surgery. Based on the sample size, a power calculation was performed to determine the level of difference detectable in complication rate. Subsequent to the univariate analysis, a logistic regression model was developed to determine the set of factors that independently predicted each of the three outcomes. Hospital LoS was analysed by Wilcoxon rank sum and Kruskal–Wallis tests regarding differences in operation time distribution. Factors that were significantly associated with complications in univariate analysis (inclusion criterion: P ≤ 0.1) were entered into a multivariate analysis to test for significant effects while adjusting for possible confounders. A P-value of <0.05 was considered to indicate statistical significance in univariate and multivariate analyses. Sample size calculation was performed to avoid type II error (β = 0.2), looking for expected proportional variation of 10% according to surgical start time of day (before and after 12.00 h) and day of week. All statistical analyses were conducted using stata Version 8.0 (StataCorp LP, College Station, TX, USA).
Results
During 2000–2008, 819 consecutive patients underwent PD performed by eight surgeons, each of whom performed at least 45 procedures during the study period. A total of 405 patients (49.5%) experienced one or more perioperative complications (total number of events = 684); the 90-day mortality for all causes (i.e. grade 5 complications) was 3.5%. Clinicopathological and operative data are outlined in Table 1. The population studied included 401 (49.0%) female and 418 (51.0%) male patients with a mean age at the date of operation of 66 ± 12 years (range: 29–92 years; median: 67 years). Significant differences in the presence of comorbidities between the groups with and without complications emerged only for cardiac disease (26.4% versus 19.8%, respectively; P = 0.025) and BMI (median: 27 kg/m2 versus 26 kg/m2, respectively; P = 0.002); no significant differences were noted for other comorbidities. Regarding the diagnoses, 701 (85.6%) lesions were malignant and 118 (14.4%) were benign. The mean tumour size was 3.1 ± 2.1 cm (range: 0–43.5 cm; median: 2.9 cm). Standard PD was performed in 84.1% of patients. The mean duration of surgery was 273 ± 73 min (range: 101–558 min; median: 266 min). The mean EBL during surgery was 727 ± 656 ml (range: 50–8500 ml; median: 600 ml). The mean LoS was 12.5 ± 10.6 days (range: 1–133 days; median: 9 days).
Table 1.
Clinicopathological and operative variables
| Variable | All patients (n = 819) | Complications |
P-value | |
|---|---|---|---|---|
| Yes (n = 405, 49.5%) | No (n = 414, 50.5%) | |||
| Age, years, median (IQR) | 67 (58–75) | 67 (59–76) | 68 (57–75) | 0.638 |
| Sex, n (%) | <0.001 | |||
| Female | 401 (49.0%) | 171 (42.2%) | 230 (55.5%) | |
| Male | 418 (51.0%) | 234 (57.8%) | 184 (44.5%) | |
| Body mass index, kg/m2, median (IQR) | 26.4 (23.4–29.7) | 27.0 (23.8–30.5) | 25.8 (22.8–29.2) | 0.002 |
| Hypertension, n (%) | 392 (47.9%) | 199 (49.1%) | 193 (46.6%) | 0.485 |
| Diabetes mellitus, n (%) | 157 (19.2%) | 76 (18.8%) | 81 (19.6%) | 0.790 |
| Cardiac disease, n (%) | 189 (23.1%) | 107 (26.4%) | 82 (19.8%) | 0.025 |
| Pulmonary disease, n (%) | 80 (9.8%) | 47 (11.6%) | 33 (8.0%) | 0.099 |
| Other comorbidities, n (%) | 533 (65.1%) | 258 (63.7%) | 275 (66.4%) | 0.421 |
| ASA physical status, n (%) | 0.106 | |||
| Class 1 | 17 (2.1%) | 6 (1.5%) | 11 (2.7%) | |
| Class 2 | 422 (51.5%) | 201 (49.6%) | 221 (53.4%) | |
| Class 3 | 370 (45.2%) | 190 (46.9%) | 181 (43.7%) | |
| Class 4 | 10 (1.2%) | 8 (2.0%) | 2 (0.5%) | |
| Greatest diameter of tumour, cm, median (IQR) | 782 (95.5%)a | 2.8 (2.0–3.6) | 3.0 (2.0–3.8) | 0.140 |
| Malignant tumoursb, n (%) | 701 (85.6%) | 342 (48.8%) | 359 (51.2%) | 0.319 |
| Diagnosis, n (%) | ||||
| Ampulla of Vater | ||||
| Adenocarcinoma | 139 (17.0%) | 74 (18.3%) | 65 (15.7%) | |
| Adenoma | 8 (1.0%) | 3 (0.7%) | 5 (1.2%) | |
| Neuroendocrine tumour | 3 (0.4%) | 1 (0.2%) | 2 (0.5%) | |
| Others | 4 (0.5%) | 2 (0.5%) | 2 (0.5%) | |
| Bile duct | ||||
| Adenocarcinoma | 55 (6.7%) | 33 (8.1%) | 22 (5.3%) | |
| Neuroendocrine tumour | 1 (0.1%) | 1 (0.2%) | 0 | |
| Others | 7 (0.9%) | 5 (1.2%) | 2 (0.5%) | |
| Duodenum | ||||
| Adenocarcinoma | 55 (6.7%) | 28 (6.9%) | 27 (6.5%) | |
| Adenoma | 9 (1.1%) | 5 (1.2%) | 4 (1.0%) | |
| GIST | 6 (0.7%) | 5 (1.2%) | 1 (0.2%) | |
| Neuroendocrine tumour | 4 (0.5%) | 2 (0.5%) | 2 (0.5%) | |
| Others | 1 (0.1%) | 1 (0.2%) | 0 | |
| Pancreas | ||||
| Adenocarcinoma | 437 (53.4%) | 197 (48.6%) | 240 (58.0%) | |
| Cystadenoma | 23 (2.8%) | 14 (3.5%) | 9 (2.2%) | |
| Neuroendocrine tumour | 36 (4.4%) | 22 (5.4%) | 14 (3.4%) | |
| Pancreatitis | 19 (2.3%) | 7 (1.7%) | 12 (2.9%) | |
| Others | 12 (1.5%) | 5 (1.2%) | 7 (1.7%) | |
| Procedure, n (%) | ||||
| Standard PD | 689 (84.1%) | 343 (49.8%) | 346 (50.2%) | 0.702 |
| Pylorus-preserving PD | 130 (15.9%) | 62 (47.7%) | 68 (52.3%) | |
| Duration of surgery, min, median (IQR) | 266 (221–322) | 276 (226–330) | 261 (217–313) | 0.005 |
| Estimated blood loss, ml, median (IQR) | 600 (400–900) | 600 (400–1000) | 500 (350–800) | 0.001 |
| Length of stay, days, median (IQR) | 9 (8–13) | 12 (9–18) | 9 (7–10) | |
| Any positive margins, n (%) | 135 (16.5%) | 58 (14.3%) | 77 (18.6%) | 0.109 |
The total number of the measureable tumours was 782.
Malignant tumours were confirmed by pathology in 701 patients.
ASA, American Society of Anesthesiologists; GIST, gastrointestinal stromal tumour; IQR, interquartile range; PD,pancreaticoduodenectomy.
The distribution of complications, by system, is presented in Table 2 (a), which shows the percentages of patients who experienced any complication, any high-grade complication (grades 3–5) and who died within 90 days stratified by organ system. Gastrointestinal complications were the most common, accounting for 32.6% (223 of 684 recorded events) of total morbidity, followed by infectious complications at 19.7% (135 of 684 recorded events). In patients with higher-grade complications, the gastrointestinal system was again the most common source, accounting for 34.5% (91 of 264) of recorded events, and cardiovascular or pulmonary events were most commonly associated with 90-day mortality (24.1% for each).
Table 2.
(a) Morbidity and 90-day mortality in the 405 patients with complications by organ system. (b) Rates of leaks and fistulae
| (a) System | All complications, n (%) | Grade 3–5 complications, n (%) | 90-day mortality, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Gastrointestinal | 223 (32.6%) | 91 (34.5%) | 4 (13.8%) | ||||
| Infection | 135 (19.7%) | 89 (33.7%) | 2 (6.9%) | ||||
| Wound or skin | 114 (16.7%) | 7 (2.7%) | 1 (3.5%) | ||||
| Haematologic/vascular | 52 (7.6%) | 19 (7.2%) | 0 | ||||
| Cardiovascular | 50 (7.3%) | 12 (4.5%) | 7 (24.1%) | ||||
| Pulmonary | 49 (7.2%) | 25 (9.5%) | 7 (24.1%) | ||||
| Generala | 25 (3.7%) | 13 (4.9%) | 4 (13.8%) | ||||
| Genitourinary | 15 (2.2%) | 3 (1.1%) | 0 | ||||
| Nervous system | 12 (1.8%) | 3 (1.1%) | 0 | ||||
| Metabolic | 5 (0.7%) | 2 (0.8%) | 0 | ||||
| Pain | 2 (0.3%) | 0 | 0 | ||||
| Endocrine | 1 (0.1%) | 0 | 0 | ||||
| Musculoskeletal | 1 (0.1%) | 0 | 0 | ||||
| Other causes | 0 | 0 | 4 (13.8%) | ||||
| Recorded events, n | 684 | 264 | 29 | ||||
| (b) Leaks and fistulae | |||||||
| Pancreatic | 104 (77.6%) | ||||||
| Biliary | 21 (15.7%) | ||||||
| Intestinal | 6 (4.5%) | POPF classification (ISGPF) | |||||
| Biliary/pancreatic | 2 (1.5%) | A | B | C | |||
| Chylous | 1 (0.7%) | 12 (11.5%) | 29 (27.9%) | 63 (60.6%) | |||
| Recorded events, n | 134 | 104 | |||||
The ‘General’ group includes non-infected intra-abdominal or thoracic collections (n = 11), dehydration (n = 7), death caused by progression of disease (n = 4), allergic reaction (n = 1), ascites (n = 1) and chyle leak (n = 1).
ISGPF, International Study Group on Pancreatic Fistula; POPF, postoperative pancreatic fistula.
Table 2 (b) summarizes the complications related to biliary, intestinal, chylous and pancreatic leaks and fistulae and the subgroup of pancreatic fistulae classified by ISGPF criteria. The overall incidence of POPF was 12.7% (104 in 819 patients); 34 (32.7%) occurred in men and 70 (67.3%) in women. The mean age of this group was 66 ± 18 years, and 78 (75.0%) patients had a malignant diagnosis compared with 26 (25.0%) with benign disease. The mean size of tumours was 2.7 ± 2.1 cm and the mean EBL during surgery was 787 ± 656 ml; median LoS for patients with pancreatic leaks or fistulae was 10 days (range: 7–122 days). In the subgroup of patients with pancreatic leaks or fistulae, 71 operations (68.3%) started before 12.00 h and 33 (31.7%) after 12.00 h. On univariate analysis, neither time of day (12.7% in each group starting before or after 12.00 h; P = 1.000), nor day of the week (Mondays, 12.8%; Tuesdays, 11.0%, Wednesdays, 21.2%; Thursdays, 13.2%; Fridays, 9.0%; P = 0.080), nor month of year (ranging from 5.7% to 18.6%; P = 0.570) had any impact on the incidence of this complication.
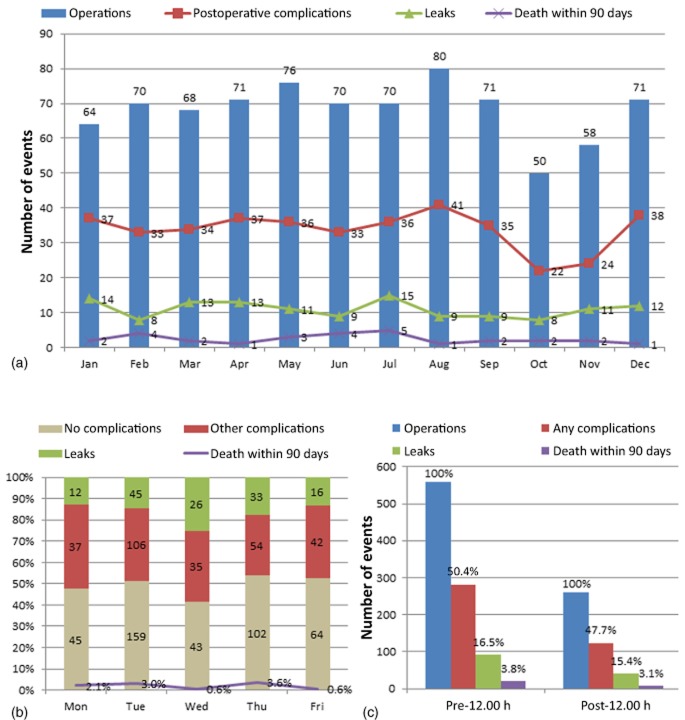
Surgical scheduling data for the complication and no-complication groups are shown in Table 3 and Fig. 1. Over the 9-year period, the mean number of operations per month was 7.6 ± 3.1 and the mean number of complications was 3.8 ± 2.1; the total number of resections for each calendar month (January–December × 9 years of the study period) ranged from 50 to 80 and the number of complications ranged from 22 to 42. Total complications, leak and fistula rates, and death within 90 days did not differ significantly in any particular month of the year when analysed as trends (P = 0.920, P = 0.715 and P = 0.736, respectively), as demonstrated in Fig. 1 (a). This was also true when data for each month of the year were analysed separately (P-values for each month of the year ranged from 0.190 to 1.000 for complications, 0.210 to 1.000 for leaks and fistulae, and 0.093 to 1.000 for death within 90 days). Of note, data for July and August did not differ from data for the other months: incidences of any complications were 51.3% and 49.0%, respectively (P = 0.652); incidences of high-grade complications (grades 3–5) were 24.0% and 21.2%, respectively (P = 0.445); incidences of leak or fistula were 16.0% and 16.1%, respectively (P = 1.000); procedure duration was 275 min and 265 min, respectively (P = 0.194); median EBL amounted to 600 ml and 580 ml, respectively (P = 0.053), and death within 90 days occurred in 4.0% and 3.4% of patients, respectively (P = 0.806).
Table 3.
Scheduling of operations by month, day of the week and time of day and impacts on morbidity
| Variable | All patients (n = 819) | Complications |
P-value | |
|---|---|---|---|---|
| Yes (n = 405, 49.5%) | No (n = 414, 50.5%) | |||
| Operations by month, mean ± SD | 7.6 ± 3.1 | 3.8 ± 2.1 | 3.8 ± 2.0 | 0.920 |
| January, n (%) | 64 (7.8%) | 37 (57.8%) | 27 (42.2%) | |
| February, n (%) | 70 (8.5%) | 33 (47.1%) | 37 (52.9%) | |
| March, n (%) | 68 (8.3%) | 34 (50.0%) | 34 (50.0%) | |
| April, n (%) | 71 (8.7%) | 37 (52.1%) | 34 (47.9%) | |
| May, n (%) | 76 (9.3%) | 36 (47.4%) | 40 (52.6%) | |
| June, n (%) | 69 (8.4%) | 32 (46.4%) | 37 (53.6%) | |
| July, n (%) | 70 (8.5%) | 36 (51.4%) | 34 (48.6%) | |
| August, n (%) | 80 (9.8%) | 41 (51.3%) | 39 (48.8%) | |
| September, n (%) | 71 (8.7%) | 35 (49.3%) | 36 (50.7%) | |
| October, n (%) | 50 (6.1%) | 22 (44.0%) | 28 (56.0%) | |
| November, n (%) | 59 (7.2%) | 24 (40.7%) | 35 (59.3%) | |
| December, n (%) | 71 (8.7%) | 38 (53.5%) | 33 (46.5%) | |
| Operations by working day, n (%) | 0.279 | |||
| Monday | 94 (11.5%) | 49 (52.1%) | 45 (47.9%) | |
| Tuesday | 309 (37.7%) | 150 (48.5%) | 159 (51.5%) | |
| Wednesday | 104 (12.7%) | 61 (58.7%) | 43 (41.3%) | |
| Thursday | 190 (23.2%) | 87 (45.8%) | 103 (54.2%) | |
| Friday | 122 (14.9%) | 58 (47.5%) | 64 (52.5%) | |
| Operation start time, n (%) | ||||
| Before 12.00 h | 559 (68.3%) | 281 (50.3%) | 278 (49.7%) | 0.500 |
| 12.00 h and later | 260 (31.7%) | 124 (47.7%) | 136 (52.3%) | |
| 07.00–11.00 h | 499 (60.9%) | 260 (52.1%) | 239 (47.9%) | 0.063 |
| 11.01–15.00 h | 274 (33.5%) | 122 (44.5%) | 152 (55.5%) | 0.054 |
| 15.01 h and later | 46 (5.6%) | 23 (50.0%) | 23 (50.0%) | 1.000 |
SD, standard deviation.
Figure 1.
Distribution of 819 pancreaticoduodenectomies according to surgical scheduling in the 9-year period studied(2000–2008). (a) Distribution of operations, related postoperative complications, leaks and 90-day mortality stratified by month (P = 0.920, P = 0.715 and P = 0.736, respectively). (b) Frequency of postoperative complications, leaks and fistulae, and 90-day mortality stratifiedby day of the week (P = 0.279, P = 0.097 and P = 0.114, respectively). (c) Number of operations, associated complications, leaks or fistulae, and 90-day mortality stratified by start time (prior to or after 12.00 h) (P = 0.057, P = 0.760 and P = 0.690, respectively)
A median of two operations were performed per week (range: 1–7 operations) and a median of one operation was performed per surgical day (Mondays–Fridays; range: 1–4 operations). Over the study period, the number of operations per specific day of the week ranged from 94 to 309 (Mondays–Fridays over 9 years), and numbers of complications ranged from 49 to 150. The median LoS subsequent to surgeries performed on Mondays–Wednesdays, and surgeries performed on Thursdays and Fridays was 9 days in both cases (P = 0.626); an analysis of weekdays individually showed that median LoS varied between 9 days and 10 days (P = 0.012). The median LoS after surgery performed in July and August was 1 day longer than that after surgery performed in September–June (10 days versus 9 days; P = 0.024); however, when all months were analysed as multi-categorical variables, no significant difference in LoS was noted (P = 0.270). Day of the week had no impact on perioperative outcome, including overall complication rate (52.1%, 48.6%, 58.7%, 45.8% and 47.5% for Mondays to Fridays, respectively; P = 0.279), high-grade (grades 3–5) complication rate (21.3%, 21.4%, 26.9%, 21.1% and 19.7% for Mondays to Fridays, respectively; P = 0.736), pancreatic leak or fistula rate (12.8%, 14.5%, 25.0%, 17.4% and 13.1% for Mondays to Fridays, respectively; P = 0.097), median EBL (600 ml, 600 ml, 500 ml, 600 ml and 500 ml for Mondays to Fridays, respectively; P = 0.469), or 90-day mortality (3.2%, 4.5%, 0.6%, 5.3% and 0.6% for Mondays to Fridays, respectively; P = 0.114) (Fig. 1b). The procedure duration was longer by a median of 17 min for operations starting later in the day (262 min before 12.00 h and 279 min after 12.00 h; P = 0.021), and there was also some variation by day of the week (280 min, 261 min, 291 min, 230 min and 305 min for Mondays to Fridays, respectively; P < 0.001); however, procedure duration did not differ according to month of the year (medians ranged from 244 min to 290 min; P = 0.589).
The majority of operations were started before 12.00 h (n = 559, 68.3%) rather than at or after 12.00 h (n = 260, 31.7%). Overall morbidity did not differ between operations started in the morning and operations started in the afternoon (P = 0.500): high-grade (grades 3–5) complication rates were 23.6% and 17.7%, respectively (P = 0.057); rates of leak or fistula were 16.5% and 15.4%, respectively (P = 0.760), and rates of operative mortality were 3.8% and 3.1%, respectively (P = 0.690) (Fig. 1c). Operation start time was stratified into three time periods (07.00–11.00 h, 11.01–15.00 h, 15.01 h and later). The majority (60.9%) of operations were found to start early in the day. Despite some slight variations in morbidity rates, no significant differences in overall morbidity were seen (07.00–11.00 h: P = 0.063; 11.01–15.00 h: P = 0.054; 15.01 h and later: P = 1.000) (Table 3). Of particular note, operations starting late in the day (i.e. after 15.00 h) were not associated with greater morbidity, including presence of any complications (50.0% versus 49.2%; P = 1.000), presence of high-grade complications (17.4% versus 22.0%; P = 0.582), rates of leak or fistula (17.4% versus 16.0%; P = 0.836), EBL (600 ml versus 500 ml; P = 0.056), and death within 90 days (6.5% versus 3.4%; P = 0.219).
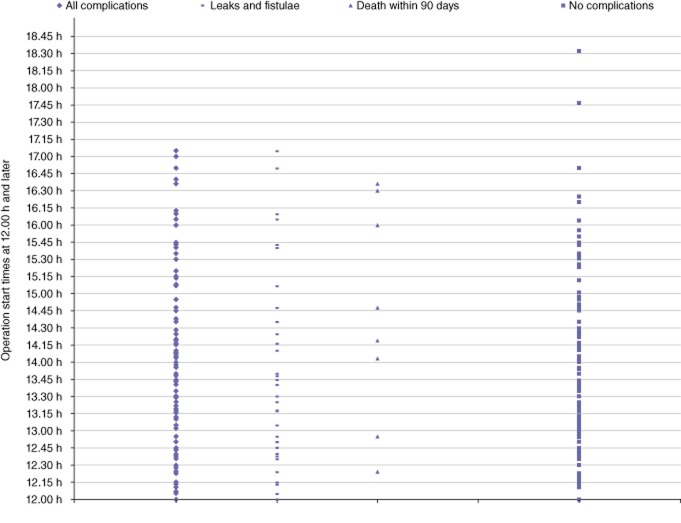
Clinicopathological and operative parameters stratified by operation start time are shown in Table 4. The procedure duration was slightly shorter for operations started prior to 12.00 h (median: 262 min; range: 134–470 min) compared with those started after 12.00 h (median: 279 min; range: 121–452 min) (P = 0.020). Hypertension was more common in patients who underwent surgery after 12.00 h (53.1%) compared with those in whom surgery began before 12.00 h (45.4%) (P = 0.043). However, all other variables, including age, gender, BMI, DM, cardiac disease, pulmonary disease, tumour size, margin status and EBL did not differ between the groups. Median LoS was 1 day longer after operations started after 12.00 h (9 days versus 10 days; P = 0.002). When the surgical start time as a continuous variable was analysed for all complications, leaks and fistulae, and 90-day mortality, no significant differences were found (P = 0.905, P = 0.405 and P = 0.695, respectively). Furthermore, the operation start time (before or after 12.00 h) had no impact on overall complication rate, rate of leak or fistula, or 90-day mortality when analysed for individual surgeons. The number of events in patients in whom surgery commenced after 12.00 h, analysed as a continuous variable, is demonstrated in Fig. 2, which shows a homogeneous distribution.
Table 4.
Clinicopathological and operative characteristics stratified by start time (before and after 12.00 h)
| Characteristic | Before 12.00 h (n = 559, 68.3%) | 12.00 h and later (n = 260, 31.7%) | Total (n = 819) | P-value |
|---|---|---|---|---|
| Age, years, median (IQR) | 67 (58–75) | 68 (58–76) | 0.292 | |
| Sex, n (%) | 0.261 | |||
| Female | 266 (47.6%) | 135 (51.9%) | 401 (49.0%) | |
| Male | 293 (52.4%) | 125 (48.1%) | 418 (51.0%) | |
| Body mass index, kg/m2, median (IQR) | 26.3 (23.4–29.7) | 26.8 (23.4–29.2) | 26.4 (23.4–29.7) | 0.792 |
| Hypertension, n (%) | 254 (45.4%) | 138 (53.1%) | 392 (47.9%) | 0.043 |
| Diabetes mellitus, n (%) | 97 (17.4%) | 60 (23.1%) | 157 (19.2%) | 0.057 |
| Cardiac disease, n (%) | 123 (22.0%) | 66 (25.4%) | 189 (23.1%) | 0.286 |
| Pulmonary disease, n (%) | 49 (8.8%) | 31 (11.9%) | 80 (9.8%) | 0.165 |
| Other comorbidities, n (%) | 373 (66.7%) | 160 (61.5%) | 533 (65.1%) | 0.157 |
| ASA physical status, n (%) | 0.432 | |||
| Class 1 | 10 (1.8%) | 7 (2.7%) | 17 (2.1%) | |
| Class 2 | 296 (53.0%) | 126 (48.5%) | 422 (51.5%) | |
| Class 3 | 245 (43.8%) | 125 (48.1%) | 370 (45.2%) | |
| Class 4 | 8 (1.4%) | 2 (0.8%) | 10 (1.2%) | |
| Greatest diameter of tumour, cm, median (IQR) | 2.9 (2.0–3.8) | 2.9 (2.0–3.6) | 2.9 (2.0–3.7) | 0.959 |
| Neoplasm, n (%) | 0.285 | |||
| Malignant | 473 (84.6%) | 228 (87.7%) | 701 (85.6%) | |
| Benign and others | 86 (15.4%) | 32 (12.3%) | 118 (14.4%) | |
| Procedure, n (%) | 0.918 | |||
| PD | 471 (84.3%) | 218 (83.8%) | 689 (84.1%) | |
| Pylorus-preserving PD | 88 (15.7%) | 42 (16.1%) | 130 (15.9%) | |
| Duration of operation, min, median (IQR) | 262 (218–317) | 279 (205–330) | 266 (221–322) | 0.021 |
| Estimated blood loss, ml, median (IQR) | 600 (400–900) | 600 (350–900) | 600 (400–900) | 0.671 |
| Length of stay, days, median (IQR) | 9 (8–12) | 10 (8–14) | 9 (8–13) | 0.002 |
| Any positive margins, n (%) | 93 (16.6%) | 42 (16.2%) | 135 (16.5%) | 0.920 |
| Highest grade complications, n (%) | 0.267 | |||
| No complications | 278 (49.7%) | 136 (52.3%) | 414 (50.5%) | |
| Grade 1 | 85 (15.2%) | 40 (15.4%) | 125 (15.3%) | |
| Grade 2 | 64 (11.4%) | 38 (14.6%) | 102 (12.5%) | |
| Grade 3 | 110 (19.7%) | 37 (14.2%) | 147 (17.9%) | |
| Grade 4 | 3 (0.5%) | 3 (1.2%) | 6 (0.7%) | |
| Grade 5 | 19 (3.4%) | 6 (2.3%) | 25 (3.1%) |
ASA, American Society of Anesthesiologists; IQR, interquartile range.
Figure 2.
Perioperative outcomes of 260 pancreaticoduodenectomies with surgical start times after 12.00 h. Patients are stratified by perioperative outcomes of any complication, leaks or fistulae, mortality within 90 days, and no complications (P = 0.731, P = 0.489 and P = 0.345, respectively)
Univariate and multivariate analyses of morbidity and mortality are shown in Table 5. Several significant differences were noted between patients with and without any complications, including in gender (P < 0.001), BMI of >25 kg/m2 (P = 0.017), cardiac disease (P = 0.025), duration of operation (P = 0.005), and EBL (P = 0.001); however, only male gender [odds ratio (OR) = 1.48, 95% confidence interval (CI) 1.10–1.97; P = 0.009)] and EBL (OR = 1.03, 95% CI 1.01–1.06; P = 0.017) remained significant on multivariate analysis. Of note, none of the variables pertaining to the timing of surgery (month of the year, day of the week, time of day) showed any trend toward significance. With respect to anastomotic leaks and fistulae, independent predictors included male gender (OR = 2.0, 95% CI 1.29–3.10; P = 0.002), other comorbidities (OR = 0.51, 95% CI 0.34–0.79; P = 0.002), ASA score (OR = 0.63, 95% CI 1.06–2.50; P = 0.025), tumour diameter (OR = 0.83, 95% CI 0.72–0.96; P = 0.013), malignant diagnosis (OR = 0.44, 95% CI 0.26–0.74; P = 0.002), and resection margin status (OR = 0.13, 95% CI 0.03–0.53; P = 0.005). A BMI of >25 kg/m2 (P = 0.707), duration of operation (P = 0.492) and EBL (P = 0.084), although significant on univariate analysis, did not emerge as predictive factors in the multivariate model and, once again, variables related to surgical scheduling played no role. Factors associated with 90-day mortality were related to age (P = 0.013), previous cardiac (P = 0.003) and pulmonary (P = 0.055) disease, and ASA score (P = 0.001). In the multivariate model, only the presence of cardiac disease (OR = 2.03, 95% CI 0.89–4.58; P = 0.09) showed borderline significance for increased risk for death. Body mass index as a continuous variable was significantly associated with any complication (P = 0.002) and anastomotic leaks and fistulae (P = 0.006) on univariate analysis, but not in the multivariate model (P = 0.870 and P = 0.830, respectively).
Table 5.
Predictors of overall presence of any complications, leaks and fistulae, and 90-day mortality
| Characteristics | Any complications (n = 405) |
Any leaks or fistulae (n = 132) |
Death within 90 days (n = 29) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||
| P-value | OR | 95% CI | P-value | P-value | OR | 95% CI | P-value | P-value | OR | 95% CI | P-value | |
| Age | 0.638 | – | – | – | 0.474 | – | – | – | 0.013 | 1.03 | 0.98–1.07 | 0.180 |
| Sex (male versus female) | <0.001 | 1.48 | 1.10–1.97 | 0.009 | <0.001 | 2.00 | 1.29–3.10 | 0.002 | 0.708 | – | – | – |
| BMI (>25 kg/m2 versus ≤25 kg/m2) | 0.017 | 1.23 | 0.91–1.66 | 0.174 | 0.024 | 1.09 | 0.69–1.71 | 0.707 | 0.239 | – | – | – |
| Hypertension | 0.485 | – | – | – | 0.635 | – | – | – | 0.708 | – | – | – |
| Diabetes mellitus | 0.790 | – | – | – | 0.398 | – | – | – | 0.474 | – | – | – |
| Cardiac disease | 0.025 | 1.31 | 0.93–1.84 | 0.119 | 0.431 | – | – | – | 0.003 | 2.03 | 0.89–4.58 | 0.090 |
| Pulmonary disease | 0.099 | 1.45 | 0.89–2.34 | 0.134 | 0.015 | 1.71 | 0.95−3.10 | 0.074 | 0.055 | 2.11 | 0.81–5.50 | 0.124 |
| Other comorbidities | 0.421 | – | – | – | 0.022 | 0.51 | 0.34–0.79 | 0.002 | 0.240 | – | – | – |
| ASA class (3 or 4 versus 1 or 2) | 0.208 | – | – | – | 0.028 | 1.63 | 1.06–2.50 | 0.025 | 0.001 | 2.09 | 0.77–5.62 | 0.144 |
| Greatest diameter of tumour | 0.140 | – | – | – | 0.002 | 0.83 | 0.72–0.96 | 0.013 | 0.818 | – | – | – |
| Malignant neoplasm (yes versus no) | 0.319 | – | – | – | 0.002 | 0.44 | 0.26–0.74 | 0.002 | 0.415 | – | – | – |
| Duration of operation | 0.005 | 1.00 | 0.99–1.00 | 0.231 | 0.010 | 1.00 | 0.99–1.00 | 0.492 | 0.446 | – | – | – |
| Estimated blood loss (100 ml) | 0.001 | 1.03 | 1.01–1.06 | 0.017 | <0.001 | 1.03 | 1.0–1.050 | 0.084 | 0.662 | – | – | – |
| Any positive margin | 0.109 | – | – | – | <0.001 | 0.13 | 0.03–0.53 | 0.005 | 0.803 | – | – | – |
| Operation month | 0.920 | – | – | – | 0.715 | – | – | – | 0.736 | – | – | – |
| Operation day (Mon–Wed versus Thurs, Fri) | 0.195 | – | – | – | 0.845 | – | – | – | 1.000 | – | – | – |
| Operation start time | ||||||||||||
| Before versus after 12.00 h | 0.500 | – | – | – | 0.760 | – | – | – | 0.690 | – | – | – |
| 07.00–11.00 h | 0.063 | 1.03 | 0.55–1.90 | 0.937 | 0.497 | – | – | – | 0.441 | – | – | – |
| 11.01–15.00 h | 0.054 | 0.72 | 0.38–1.36 | 0.313 | 0.422 | – | – | – | 0.163 | – | – | – |
| After 15.00 h | 1.000 | – | – | – | 0.836 | – | – | – | 0.219 | – | – | – |
95% CI, 95% confidence interval; ASA, American Society of Anesthesiologists; BMI, body mass index; OR, odds ratio.
Given the sample size of 819 patients and a 50% complication rate, this analysis had an 80% power to detect a difference of ≥10% between groups defined by the surgical start time. For example, if the true underlying complication rates were 45% for morning operations and 55% for afternoon operations, this study had sufficient power to find them to be significantly different. The sample size also provided an 80% power to detect a difference of ≥8% in complication rates by day of the week. Power analysis for month of the year was not possible because of the number of categories (12 months of the year).
Discussion
Work hours and workers' schedules, and their impact on quality and safety in the workplace, are major societal concerns. In a study of 7577 German workers, Hänecke et al. described the rate of work-related errors and suggested an association with duration of work day and time of day.21 Other studies have suggested that the performances of airline pilots22,23 and night shift engineers24 are related not only to hours worked, but also to the circadian cycle. Limiting work hours to meet health and safety requirements has also been applied to the practice of medicine and the training of resident physicians. Concerns regarding medical error were a major reason for the reduction in resident work hours mandated by the Accreditation Council for Graduate Medical Education (ACGME). The effect on patient safety of restricting residents' work hours remains controversial, with little clear evidence of a positive impact. Naylor et al. evaluated 275 patients undergoing emergency cholecystectomy before and after duty hour regulations instituted by the ACGME and suggested that duty hour restrictions might not have a measurable influence on the surgical complication rate.25
The explanation for the ‘July phenomenon’ described by Englesbe et al. referred to the cyclic influx of inexperienced trainees each July and the lack of oversight by attending physicians.1 Whether this phenomenon is real or not remains the subject of debate, as the published data are contradictory. In 1993, Rich et al. analysed 240 467 hospital admissions in two metropolitan areas during 1983–1987 and showed that in the subset of patients with internal medicine diagnoses, a ‘July phenomenon’ was observed, whereby significant decreases in costs for diagnostic tests and medications in teaching hospitals occurred over the academic year; however, in patients with surgical diagnoses, the opposite trend was observed.26 More recently, van Walraven et al. analysed the influence of housestaff experience on teaching hospital mortality by studying 164 318 patients in a single academic institution and found no association between the arrival of new housestaff and the adjusted risk for death in hospital.27 Similarly, two 2003 studies in the ICU and obstetric settings found no such evidence, possibly as a result of the presence of round-the-clock staffing.28,29 In 2006, two neurosurgical studies showed that there was either a negligible effect of placing shunts during the summer months, or no effect at all.30,31 A 2008 study by Dhaliwal et al. also showed no increase in adverse outcomes following cardiac surgery at the beginning of the academic cycle.32 More recently, however, Dasenbrock et al. analysed a nationwide sample of 2920 patients admitted with spinal metastases and found significantly higher inpatient mortality and operative complications in patients admitted during the month of July.2
Variation in postoperative outcomes has been evaluated by not only time of year, but also by day of the week and time of day. Zare et al. described the differences in morbidity and mortality among different days of the week, showing increased mortality in operations performed on Fridays, compared with those performed on Mondays–Thursdays.33 The authors suggested that in patients admitted to regular hospital floors after non-emergent major surgery, mortality was increased if surgery was performed on a Friday rather than earlier in the week. By contrast, in patients admitted to ICUs or discharged home after surgery, 30-day mortality was not influenced by day of the week.33 Variation in the outcomes of procedures performed during the day versus during the night was studied by Uyarel et al., who found that percutaneous cardiac interventions can be safely performed at night in high-volume centres.34 Similarly, in a large study of cardiac surgical procedures, postoperative morbidity and mortality were not influenced by attending surgeon sleep hours.7
Pancreaticoduodenectomy is a procedure with multiple potential causes of morbidity, and in which pancreatic leaks and fistulae have represented a major persistent source of complications since the operation was first described.11,35 However, the mortality rate has declined as a result of advances in surgical technique and perioperative management, increased volume and improved patient selection. Pancreatic resections are now performed more frequently in older patients with acceptable morbidity and mortality rates and acceptable longterm survival in comparison with younger patients. Obesity, as defined by BMI, has been considered an important predictor of surgical outcome.36,37 Ramsey and Martin published a meta-analysis of 17 retrospective studies conducted during 1990–2010 and concluded that although BMI increases the complexity of the operation and postoperative morbidity, it does not affect mortality.38 In the present study, BMI was not a factor in operative morbidity or mortality; however, it should be highlighted that the interquartile range of BMI did not vary much across the present cohort.
Pancreaticoduodenectomy is a highly technical operation performed largely in specialized centres and although some randomized controlled trials regarding technical aspects of the procedure have been performed, data on outcomes specific to the timing of the operation (i.e. time of day, day of the week, month of the year) are scant. The strengths of the current study include the homogeneity of its population, which was consecutively managed in a single high-volume centre with a consistent surgical oncology group, and the careful tracking of postoperative complications. The endpoints were postoperative morbidity and 90-day mortality, and the study sought to identify variations according to operation time, day or month.
Intraoperative and clinicopathological data analysis offered interesting insights. Male gender was associated with increased risks for complications, leaks and fistulae. Higher EBL was associated with increased complications, probably reflecting greater technical difficulty of the procedure. Interestingly, for every 1-cm increase in tumour diameter, the presence of malignancy and positive resection margins, the risk was significantly decreased, possibly with reference to the degree of ductal dilatation, which allows for a technically easier pancreatic anastomosis. The 1-day extension in LoS subsequent to procedures performed after 12.00 h is likely to relate to the delay in initiating the standard postoperative recovery regimen. Regarding 90-day mortality, the multivariate analysis showed no association with gender, tumour size, malignancy or EBL. The presence of cardiac and pulmonary comorbid conditions was associated with borderline significance to 90-day mortality, which correlates with the observation that complications related to these two organ systems were associated with the highest operative mortality rate. The duration of surgery had no significance on rates of complications or mortality.
The major finding of this analysis was the lack of any association between the timing of PD and perioperative morbidity of any type, pancreatic leaks or fistulae, or 90-day mortality. The analysis of perioperative outcome by month showed no evidence to support the existence of a ‘July phenomenon’ with respect to this operation performed at MSKCC. Furthermore, neither the surgical start time nor the day of the week had any significant impact on the three outcome variables, and this was also true when cases were stratified by individual surgeon. Although some variability in morbidity and mortality rates was noted, and indeed was expected, none of these differences were significant.
The results of the current study no doubt reflect the contributions and complex interplay of several factors. At the study institution, the surgical services are staffed with fellows who have completed general surgical training, in addition to junior-level housestaff. Whereas this somewhat greater experience of the trainees helped to ensure consistency of patient care, other variables are very likely to have made greater and more profound contributions, including the high-volume nature of the surgical practice, the high degree of attending surgeon oversight, an infrastructure of highly experienced nursing (floor nurses and nurse practitioners) and ancillary personnel accustomed to managing these patients after surgery, and appropriate patient selection. It is important to recognize that the latter variables represent changes that can be implemented at most centres.
The results of this study should not be misinterpreted as endorsing the performance of an operation of this magnitude at any time of the day. On the contrary, most of the operations in this study were performed during the normal courseof the operating day and it is possible that resections that were anticipated to be more difficult were purposely scheduled earlier. Nevertheless, the results suggest that in selected patients, and in the hands of an experienced surgical team, the timing of the operation does not appear to affect perioperative outcome.
The limitations of the study are those associated with all retrospective studies. Additionally, although operative morbidity, in-hospital and 90-day mortality, and LoS measures are widely used perioperative outcome variables, these measures may not fully assess other aspects of the care rendered, such as cost, longterm functional outcome and patient satisfaction. Furthermore, complications may have been underestimated if they occurred after discharge, although major efforts were made to capture these events and such ‘missed’ complications are likely to have represented a small fraction of the total morbidity. Although the sample size was adequate to detect relatively small differences, and patient-, operative-and disease-related variables were generally evenly distributed between the complication and no-complication groups, it is likely that case scheduling involved some degree of prioritization based on the technical difficulty anticipated or associated comorbid medical conditions. Although this must be recognized as a potential confounding influence, it should also be recognized as a reflection of reasonable judgement by the operating surgeon. The factthat the current study is derived from a specialized, tertiary care referral centre should not be viewed as limiting its message. On the contrary, as the nature of surgical training continues to evolve and complex surgical procedures are increasingly limited to high-volume centres, this landscape will become the norm and fluctuations in the quality of care based on the time of day, day of the week or month of the year in which care is delivered should not be expected.
In summary, the present study identified no association between the timing of PD with respect to time of day, day ofthe week or month of the year, and perioperative outcome. Operations performed later in the day resulted in complication rates similar to those found in operations performed earlier, as did those performed at the end (i.e. on Fridays) compared with earlier in the working week. Similarly, there was no evidence of a greater incidence of complications at thebeginning of the academic year (i.e. in July and August) compared with the other months. These findings are contrary tothose of some previous studies that have shown differences in the outcomes of operations based on timing and scheduling,but some of these reports have suffered from a heterogeneous patient mix, which makes the data difficult to interpret.The present study was adequately powered to detect relatively small differences and the results are therefore unlikely to reflect a type II error.
Conflicts of interest
None declared.
References
- 1.Englesbe MJ, Pelletier SJ, Magee JC, Gauger P, Schifftner T, Henderson WG, et al. Seasonal variation in surgical outcomes as measured by the American College of Surgeons–National Surgical Quality Improvement Program (ACS-NSQIP) Ann Surg. 2007;246:456–462. doi: 10.1097/SLA.0b013e31814855f2. discussion 463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasenbrock HH, Clarke MJ, Thompson RE, Gokaslan ZL, Bydon A. The impact of July hospital admission on outcome after surgery for spinal metastases at academic medical centres in the United States, 2005 to 2008. Cancer. 2012;118:1429–1438. doi: 10.1002/cncr.26347. [DOI] [PubMed] [Google Scholar]
- 3.Highstead RG, Johnson LS, Street JH, 3rd, Trankiem CT, Kennedy SO, Sava JA. July – as good a time as any to be injured. J Trauma. 2009;67:1087–1090. doi: 10.1097/TA.0b013e3181b8441d. Erratum in:J Trauma 2010; 69:1637. [DOI] [PubMed] [Google Scholar]
- 4.Bakaeen FG, Huh J, LeMaire SA, Coselli JS, Sansgiry S, Atluri PV, et al. The July effect: impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients. Ann Thorac Surg. 2009;88:70–75. doi: 10.1016/j.athoracsur.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Yaghoubian A, Kaji AH, Putnam B, de Virgilio C. Trauma surgery performed by ‘sleep deprived’ residents: are outcomes affected? J Surg Educ. 2010;67:449–451. doi: 10.1016/j.jsurg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lonze BE, Parsikia A, Feyssa EL, Khanmoradi K, Araya VR, Zaki RF, et al. Operative start times and complications after liver transplantation. Am J Transplant. 2010;10:1842–1849. doi: 10.1111/j.1600-6143.2010.03177.x. [DOI] [PubMed] [Google Scholar]
- 7.Chu MW, Stitt LW, Fox SA, Kiaii B, Quantz M, Guo L, et al. Prospective evaluation of consultant surgeon sleep deprivation and outcomes in more than 4000 consecutive cardiac surgical procedures. Arch Surg. 2011;146:1080–1085. doi: 10.1001/archsurg.2011.121. [DOI] [PubMed] [Google Scholar]
- 8.Ellman PI, Kron IL, Alvis JS, Tache-Leon C, Maxey TS, Reece TB, et al. Acute sleep deprivation in the thoracic surgical resident does not affect operative outcomes. Ann Thorac Surg. 2005;80:60–64. doi: 10.1016/j.athoracsur.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Turrentine FE, Wang H, Young JS, Calland JF. What is the safety of non-emergent operative procedures performed at night? A study of 10,426 operations at an academic tertiary care hospital using the American College of Surgeons National Surgical Quality Improvement Program database. J Trauma. 2010;69:313–319. doi: 10.1097/TA.0b013e3181e49291. [DOI] [PubMed] [Google Scholar]
- 10.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of Vater. Ann Surg. 1935;102:763–779. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai M, Yamaue H. Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery. Surg Today. 2010;40:1011–1017. doi: 10.1007/s00595-009-4245-9. [DOI] [PubMed] [Google Scholar]
- 12.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai S, Choti MA, Assumpcao L, Cameron JL, Gleisner AL, Herman JM, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg. 2010;14:1143–1150. doi: 10.1007/s11605-010-1201-3. [DOI] [PubMed] [Google Scholar]
- 14.Rueth NM, Parsons HM, Habermann EB, Groth SS, Virnig BA, Tuttle TM, et al. The longterm impact of surgical complications after resection of stage I non-small cell lung cancer: a population-based survival analysis. Ann Surg. 2011;254:368–374. doi: 10.1097/SLA.0b013e31822150fe. [DOI] [PubMed] [Google Scholar]
- 15.Traverso LW. The state of the highest level of evidence: an overview of systematic reviews of pancreaticobiliary disease customized for the gastroenterologist and GI surgeon. J Gastrointest Surg. 2008;12:617–619. doi: 10.1007/s11605-007-0450-2. [DOI] [PubMed] [Google Scholar]
- 16.Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Fischer M, Matsuo K, Gönen M, Grant F, DeMatteo RP, D'Angelica MI, et al. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: results of a prospective randomized trial of acute normovolemic haemodilution compared with standard intraoperative management. Ann Surg. 2010;252:952–958. doi: 10.1097/SLA.0b013e3181ff36b1. [DOI] [PubMed] [Google Scholar]
- 18.Grobmyer SR, Pieracci FM, Allen PJ, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204:356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, et al. Management and outcomes of post-pancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207:490–498. doi: 10.1016/j.jamcollsurg.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Hänecke K, Tiedemann S, Nachreiner F, Grzech-Sukalo H. Accident risk as a function of hour at work and time of day as determined from accident data and exposure models for the German working population. Scand J Work Environ Health. 1998;24(Suppl. 3):43–48. [PubMed] [Google Scholar]
- 22.Smith L, Mason C. Reducing night shift exposure: a pilot study of rota, night shift and age effects on sleepiness and fatigue. J Hum Ergol (Tokyo) 2001;30:83–87. [PubMed] [Google Scholar]
- 23.Lamond N, Dorrian J, Roach GD, Burgess HJ, Holmes AL, McCulloch K, et al. Performance, sleep and circadian phase during a week of simulated night work. J Hum Ergol (Tokyo) 2001;30:137–142. [PubMed] [Google Scholar]
- 24.Folkard S, Hill J. Can we predict perceived risk? J Hum Ergol (Tokyo) 2001;30:89–95. [PubMed] [Google Scholar]
- 25.Naylor RA, Rege RV, Valentine RJ. Do resident duty hour restrictions reduce technical complications of emergency laparoscopic cholecystectomy? J Am Coll Surg. 2005;201:724–731. doi: 10.1016/j.jamcollsurg.2005.06.271. [DOI] [PubMed] [Google Scholar]
- 26.Rich EC, Hillson SD, Dowd B, Morris N. Specialty differences in the ‘July phenomenon’ for Twin Cities teaching hospitals. Med Care. 1993;31:73–83. doi: 10.1097/00005650-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 27.van Walraven C, Jennings A, Wong J, Forster AJ. Influence of housestaff experience on teaching-hospital mortality: the ‘July phenomenon’ revisited. J Hosp Med. 2011;6:389–394. doi: 10.1002/jhm.917. [DOI] [PubMed] [Google Scholar]
- 28.Barry WA, Rosenthal GE. Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals. J Gen Intern Med. 2003;18:639–645. doi: 10.1046/j.1525-1497.2003.20605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myles TD. Is there an obstetric July phenomenon? Obstet Gynecol. 2003;102(5 Pt. 1):1080–1084. doi: 10.1016/j.obstetgynecol.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Kestle JR, Cochrane DD, Drake JM. Shunt insertion in the summer: is it safe? J Neurosurg. 2006;105(Suppl. 3):165–168. doi: 10.3171/ped.2006.105.3.165. [DOI] [PubMed] [Google Scholar]
- 31.Smith ER, Butler WE, Barker FG., 2nd Is there a ‘July phenomenon’ in paediatric neurosurgery at teaching hospitals? J Neurosurg. 2006;105(Suppl):169–176. doi: 10.3171/ped.2006.105.3.169. [DOI] [PubMed] [Google Scholar]
- 32.Dhaliwal AS, Chu D, Deswal A, Bozkurt B, Coselli JS, Lemaire SA, et al. The July effect and cardiac surgery: the effect of the beginning of the academic cycle on outcomes. Am J Surg. 2008;196:720–725. doi: 10.1016/j.amjsurg.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Zare MM, Itani KM, Schifftner TL, Henderson WG, Khuri SF. Mortality after non-emergent major surgery performed on Friday versus Monday through Wednesday. Ann Surg. 2007;246:866–874. doi: 10.1097/SLA.0b013e3180cc2e60. [DOI] [PubMed] [Google Scholar]
- 34.Uyarel H, Ergelen M, Akkaya E, Ayhan E, Demirci D, Gul M, et al. Impact of day versus night as intervention time on the outcomes of primary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2009;74:826–834. doi: 10.1002/ccd.22154. [DOI] [PubMed] [Google Scholar]
- 35.Orr RK. Outcomes in pancreatic cancer surgery. Surg Clin North Am. 2010;90:219–234. doi: 10.1016/j.suc.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995;222:426–434. doi: 10.1097/00000658-199522240-00002. discussion 434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodul P, Tansey J, Golts E, Oh D, Pickleman J, Aranha GV. Age is not a contraindication to pancreaticoduodenectomy. Am Surg. 2001;67:270–275. doi: 10.1016/s0016-5085(00)81967-8. discussion 275–276. [DOI] [PubMed] [Google Scholar]
- 38.Ramsey AM, Martin RC. Body mass index and outcomes from pancreatic resection: a review and meta-analysis. J Gastrointest Surg. 2011;15:1633–1642. doi: 10.1007/s11605-011-1502-1. [DOI] [PubMed] [Google Scholar]