Abstract
Background:Successful left lateral segment (sectionectomy) and right trisegmentectomy (trisectionectomy) split-liver transplantation (SLT) have been achieved. However, there are few reports of the use of true right/left splitting in SLT.
Methods:A single-centre retrospective review of true right/left ex vivo split-liver transplants performed during the period 1993–2010 was conducted. Nine cadaveric liver grafts underwent splitting and the resultant 18 allografts were used in transplants performed at the study centre.
Results:In the nine right lobe recipients, 10-year patient and graft survival rates were both 74%.There were no vascular complications, one biliary complication and one re-exploration. In the nine left lobe recipients,10-year patient and graft survival rates were 78% and 66%, respectively. Postoperative complications included six biliary complications, four of which required surgical revision and all of which occurred within 5 months of transplantation, and two vascular complications, including one early hepatic artery thrombosis (HAT) and one late HAT, one of which required retransplantation. Five left lobe recipients required re-exploration, and one patient developed small-for-size syndrome following SLT, which resolved with conservative measures.
Conclusions:True right/left ex vivo SLT remains a viable option for facilitating the expansion of the adult cadaver donor pool and allows for excellent patient and graft survival. Postoperative morbidity remains high, especially in recipients of the left lobe graft, and must be balanced with the benefits to be derived from transplant.
Introduction
The deficiency in donor organ availability is the most significant factor inhibiting the further application of liver transplantation in patients with end-stage liver disease.1 Split-liver transplantation (SLT), whereby a deceased donor liver allograft is divided to generate two allografts suitable for transplantation, was first reported by Pichlmayr et al. in 1988 as a potential method of expanding the adult donor organ pool to the paediatric population.2
Initially, SLT entailed splitting off the left lateral segment graft for the paediatric recipient and allocating the right trisegment (trisection) graft to a waitlisted adult patient. However, it is the true right lobe/left lobe SLT procedure that holds the greatest potential for addressing the severe organ shortage in the adult population because it allows for the allocation of both partial grafts to recipients on the adult waitlist. This procedure is technically more challenging and incurs a potential increase in postoperative recipient morbidity as a result of the splitting procedure in comparison with whole-organ transplantation, and there are few reports of true right/left allograft splitting.3–7
Previous reports have described the results obtained at this study centre with SLT in both adult and paediatric patient populations from 1993 to 2010.8 The current study was performed to further analyse outcomes in 18 recipients who underwent right lobe (RL) or left lobe (LL) transplantation following nine ex vivo liver splitting procedures, with specific attention to donor characteristics, as well as the recipients' operative course and surgical morbidity, and longterm survival of these partial liver allografts.
Materials and methods
The medical records of patients who underwent SLT from 18 September 1993 to 1 July 2010 at the University of California, San Francisco (UCSF) were retrospectively reviewed. This work was approved by the Committee for Human Research at UCSF. Longterm outcomes were assessed through office medical records; for cases in which this was not possible, survival was assessed via the Social Security Death Master File. Clinical medical records, operative notes and pathology reports were used to gather data. Additional recipient data, as well as donor data, were supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN), as of 30 July 2010.
Graft loss was defined as death or need for retransplantation. Complications were initially categorized as biliary, vascular, primary non-function, small-for-size syndrome (SFSS), incisional hernia or need for re-exploration. Biliary complications were characterized as leak, stricture or the combination of leak and stricture. The course of recipients with a biliary complication, including those with concomitant hepatic artery thrombosis, was further reviewed to determine the number of non-surgical and/or surgical interventions. Non-surgical interventions included the maintenance of a postoperative drain for continued bilious output, drainage of perihepatic bilious abscesses, endoscopic retrograde cholangiography (ERC) with or without stent placement, percutaneous transhepatic cholangiography with or without stent placement, dilation of biliary strictures, simple fistulograms, tube checks and tube removals. Vascular complications included stenosis or thrombosis, and were further characterized as early (within 1 month post-transplant) or late (>1 month post-transplant). The number and type of all surgical and/or non-surgical interventions necessary to treat a vascular complication (including retransplantation) were recorded. Kaplan–Meier curves were used to analyse patient and graft survival. Unless indicated, all means are expressed as the mean ± standard deviation.
True right/left ex vivo SLT procedure
All grafts were split utilizing the ex vivo technique previously described.8 In brief, the ex vivo split-liver procedure at UCSF was performed in the following fashion. Care was taken to maintain the liver allograft submerged in an ice bath at all times. The biliary anatomy and arterial anatomy were initially delineated with contrast radiography (Fig. 1) prior to proceeding with liver splitting. These images, combined with careful anatomic inspection, not only allowed an assessment of the liver's suitability for splitting, but also provided a blueprint for the splitting procedure. Once the allograft had been deemed suitable for splitting, the hilar structures were approached in a posterior fashion, in which the left portal vein was divided from the main portal trunk, leaving the main portal vein in continuity with the right portal vein branch in all but two cases. The left portal vein was left in continuity with the main portal vein as a result of anatomic concerns in these two recipients (patient 5-LL was undergoing a third liver transplant; patient 9-LL had maple syrup urine disease and was donating a domino graft). If necessary, biliary and arterial anatomy were further defined during liver splitting with the use of dilute methylene blue injected through the open end of the common bile duct (CBD) or hepatic artery. The right hepatic artery was divided from the proper hepatic artery, leaving the left graft with the coeliac trunk in all patients but one (patient 8-LL: this donor allograft had an early bifurcation of the right and left hepatic arteries at the level of the coeliac axis). The left bile duct was taken off the main duct, leaving the right hepatic duct in continuity with the CBD in all cases. The left and middle hepatic veins were dissected from the vena cava in four patients (patients 1-LL, 6-LL, 7-LL and 8-LL), leaving the vena cava with the right-sided graft. In the remaining LL recipients, the vena cava was preserved with the left-sided graft. The decision on which lobe should retain the vena cava was based upon surgical and anatomic considerations in both RL and LL recipients. The parenchymal division was accomplished using the fracture technique and a combination of ties, clips and vascular staplers. The cut surface was inspected for leaks by flushing the hilar structures, and stasis was achieved using silk ties or sutures. The cut surface was treated with argon beam coagulation and covered with topical sealant prior to reperfusion. Transplants were performed in the usual fashion without veno–venous bypass or T-tubes.
Figure 1.
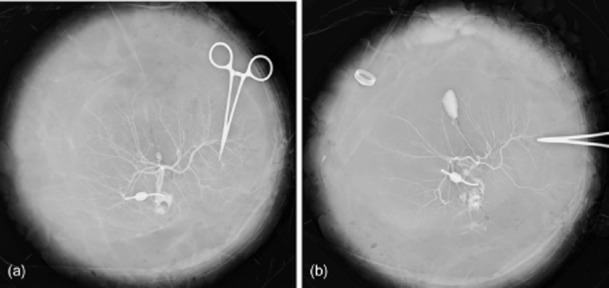
Prior to ex vivo splitting, backtable (a) arteriography and (b) cholangiography are performed to assist in delineation of the relevant anatomy
Results
From 1993 to 2010, a total of 107 SLTs in 107 patients were carried out at UCSF. This cohort included 10 true RL (liver segments V–VIII) transplants and nine true LL transplants (liver segments I–IV). Data for one RL recipient were excluded from the final analysis because the graft was imported. The remaining 18 patients were recipients of nine RL and nine LL grafts generated after nine donor livers had been split ex vivo at UCSF (Table 1).
Table 1.
Demographic data for recipients of left and right lobe split-liver transplants (n = 18)
| Year of transplant | Gender | Age, years | Height, cm | Weight, kg | Cause of disease | |
|---|---|---|---|---|---|---|
| Right lobe (RL) recipients | ||||||
| 1-RL | 1998 | Male | 49 | 167 | 62 | HBV |
| 2-RL | 2006 | Male | 55 | 142 | 36 | HCV |
| 3-RL | 2008 | Male | 60 | 160 | 53 | HCV |
| 4-RL | 2009 | Male | 46 | 178 | 119 | NASH |
| 5-RL | 2009 | Male | 54 | 170 | 75 | HCV/HCC |
| 6-RL | 2009 | Female | 55 | 168 | 91 | HCV/HCC |
| 7-RL | 2009 | Male | 66 | 168 | 52 | HBV |
| 8-RL | 2009 | Male | 57 | 183 | 68 | HCV/HPS |
| 9-RL | 2010 | Male | 40 | 163 | 73 | HBV/HCC |
| Left lobe (LL) recipients | ||||||
| 1-LL | 1998 | Female | 62 | 155 | 55 | HCV |
| 2-LL | 2006 | Male | 18 | 175 | 36 | PSC |
| 3-LL | 2008 | Female | 48 | 173 | 81 | PBC |
| 4-LL | 2009 | Female | 65 | 157 | 53 | PBC |
| 5-LL | 2009 | Female | 16 | 119 | 25 | Autoimmune |
| 6-LL | 2009 | Male | 14 | 130 | 26 | MMA |
| 7-LL | 2009 | Female | 2 | 88 | 14 | PFIC |
| 8-LL | 2009 | Female | 13 | 127 | 28 | CHF |
| 9-LL | 2010 | Female | 10 | 137 | 29 | MSUD |
CHF, congenital hepatic fibrosis; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HPS, hepatopulmonary syndrome; MMA, methylmalonate aciduria; MSUD, maple syrup urine disease; NASH, non-alcoholic steatohepatitis; PBC, primary biliary cirrhosis; PFIC, progressive familial intrahepatic cholestasis; PSC, primary sclerosing cholangitis.
Donors
The criteria used at UCSF for optimal donor selection include the following: brain death of the donor; donor age of <40 years; donor body mass index (BMI) of <30 kg/m2; a sodium level of <155 mEq/l (to convert to millimoles per litre, multiply by 1.0); no more than single-agent vasopressor requirements; minimal elevations in transaminases; donor hospitalization of <7 days, and organ downtime of <30 min. The demographics of the true right/left split-liver donors and causes of death are given in Table 2. The median age of donors was 19 years (range: 16–40 years). In donors, median height was 183 cm (range: 175–191 cm), median weight was 83 kg (range: 53–120 kg) and mean BMI was 26.6 kg/m2.
Table 2.
Demographics and cause of death in liver donors
| Donor | Gender | Age, years | Height, cm | Weight, kg | Cause of death |
|---|---|---|---|---|---|
| 1 | Male | 16 | 183 | 105 | CVA/stroke |
| 2 | Male | 40 | 188 | 99 | CVA/stroke |
| 3 | Male | 19 | 183 | 87 | Head trauma |
| 4 | Male | 20 | 191 | 120 | Head trauma |
| 5 | Male | 17 | 181 | 83 | Head trauma |
| 6 | Male | 22 | 183 | 83 | Head trauma |
| 7 | Male | 17 | 175 | 53 | Head trauma |
| 8 | Female | 20 | 180 | 76 | Anoxia |
| 9 | Male | 17 | 169 | 82 | Head trauma |
CVA, cerebrovascular accident.
Right lobe recipients
General demographics, causes of end-stage liver disease and year of transplant in RL recipients are listed in Table 1. In these recipients, median age at transplant was 55 years (range: 40–66 years), median wait time was 128 days (range: 16–2550 days), median height was 168 cm (range: 142–183 cm), median weight was 68 kg (range: 52–119 kg) and mean BMI was 25.2 kg/m2. Right lobe recipients had a median match Model for End-stage Liver Disease (MELD) or Paediatric End-stage Liver Disease (PELD) score of 29 (range: 15–33), and a median laboratory MELD/PELD score of 20 (range: 8–33). Four RL recipients had MELD exception points: these referred to hepatocellular carcinoma in three patients (patients 5-RL, 6-RL, 9-RL), and to hepatopulmonary syndrome in one (patient 8-RL). The relevant liver-splitting anatomy is detailed in Table 3. The median warm ischaemic time was 40 min (range: 32–61 min), median cold ischaemic time was 9.8 h (range: 6.1–13.4 h), and median estimated blood loss (EBL) was 1750 ml (range: 700–6000 ml). Ten-year patient survival and 10-year graft survival were both 74%. There were no vascular complications in RL transplants, and there was one biliary complication, which consisted of concomitant anastomotic stricture and cut edge leak and was managed by endoscopic retrograde cholangiopancreatography (Table 4). Additional complications in the RL recipient group included one requirement for re-exploration (patient 1-RL). One subject (patient 4-RL, at 119 kg the largest recipient transplanted) required splenic artery ligation followed by portocaval shunt creation at the time of SLT as a result of a high portal systemic pressure gradient after implantation and thus potential concern for the development of SFSS. Splenic artery ligation alone was insufficient to reduce the portal pressure gradient and thus portocaval shunt creation was performed, and the patient recovered without evidence of SFSS in the postoperative period. Recipient 7-RL received a combined liver and kidney transplant (CLKT). There were no cases of primary non-function. Median hospital length of stay (LoS) was 8 days (range: 6–44 days).
Table 3.
Operative course and relevant anatomy in recipients of right and left lobe split-liver transplants (n = 18)
| Caval drainage | Biliary drainage | Portal anatomy | Arterial anatomy | |
|---|---|---|---|---|
| Right lobe (RL) recipients | ||||
| 1-RL | Bicaval | Duct-to-duct | Main portal | Right hepatic artery |
| 2-RL | Piggyback | Duct-to-duct | Main portal | Right hepatic artery |
| 3-RL | Piggyback | Duct-to-duct | Main portal | Right hepatic artery |
| 4-RL | Piggyback | Duct-to-duct | Main portal | Right hepatic artery |
| 5-RL | Piggyback | Duct-to-duct | Right portal | Right hepatic artery |
| 6-RL | Bicaval | Duct-to-duct | Main portal | Right hepatic artery |
| 7-RL | Bicaval | Duct-to-duct | Main portal | Right hepatic artery |
| 8-RL | Bicaval | Duct-to-duct | Main portal | Right hepatic artery |
| 9-RL | Piggyback | Duct-to-duct | Right portal | Right hepatic artery |
| Left lobe (LL) recipients | ||||
| 1-LL | Piggyback | Roux-en-Y | Left portal | Coeliac axis |
| 2-LL | Bicaval | Roux-en-Y | Left portal | Coeliac axis |
| 3-LL | Bicaval | Roux-en-Y | Left portal | Coeliac axis |
| 4-LL | Bicaval | Roux-en-Y | Left portal | Coeliac axis |
| 5-LL | Bicaval | Roux-en-Y | Main portal | Coeliac axis |
| 6-LL | Piggyback | Roux-en-Y | Left portal | Coeliac axis |
| 7-LL | Piggyback | Roux-en-Y | Left portal | Coeliac axis |
| 8-LL | Piggyback | Roux-en-Y | Left portal | Left hepatic artery |
| 9-LL | Bicaval | Roux-en-Y | Main portal | Coeliac axis |
Table 4.
Postoperative complications in recipients of right and left lobe split-liver transplants (n = 18)
| Vascular Cx | Vascular intervention | Biliary Cx | Biliary intervention | Day and cause of graft failure | |
|---|---|---|---|---|---|
| Right lobe recipients | |||||
| 1-RL | No | None | No | None | N/A |
| 2-RL | No | None | No | None | N/A |
| 3-RL | No | None | No | None | N/A |
| 4-RL | No | None | No | None | N/A |
| 5-RL | No | None | No | None | N/A |
| 6-RL | No | None | No | None | 346 days; cause of death unknown |
| 7-RL | No | None | Leak and stricture | GI-ERCP stent and angioplasty | N/A |
| 8-RL | No | None | No | None | 44 days; death of cardiopulmonary cause |
| 9-RL | No | None | No | None | N/A |
| Left lobe recipients | |||||
| 1-LL | No | None | No | None | 4255 days; cause of death unknown |
| 2-LL | No | None | No | None | N/A |
| 3-LL | No | None | Leak | IR: stent; surgical revision (1 month)b | N/A |
| 4-LL | No | None | Leak | IR: drain; surgical revision (0 months)b | 93 days; death of sepsis |
| 5-LL | Early HAT (0 months)a | IR: thrombectomy, angioplasty, tPA | Stricture | IR: stent and drain | N/A |
| 6-LL | No | None | No | None | N/A |
| 7-LL | No | None | Stricture | IR: stent; surgical revision (5 months)b | N/A |
| 8-LL | No | None | Leak | IR: drain | 20 days; death of sepsis |
| 9-LL | Late HAT (2 months)a | Retransplantation | Leak | IR: drain; surgical revision (2 months)b | 80 days; late HAT |
Timing of vascular complication.
Timing of biliary surgical revision.
Cx, complication; ERCP, endoscopic retrograde cholangiopancreatography; GI, gastrointestinal; HAT, hepatic artery thrombosis; IR, interventional radiology; N/A, not applicable; tPA, tissue plasminogen activator.
Left lobe recipients
General demographics, causes of end-stage liver disease and year of transplant in LL recipients are listed in Table 1. Left lobe recipients had a median age at transplant of 16 years (range: 2–65 years), median wait time of 74 days (range: 18–1057 days), median height of 137 cm (range: 88–175 cm), median weight of 29 kg (range: 14–81 kg) and a mean BMI of 19.7 kg/m2. Their median match MELD/PELD score was 28 (range: 10–40) and median laboratory MELD/PELD score was 21 (range: 0–40). No LL recipients had MELD exception points. The relevant liver-splitting anatomy is detailed in Table 3. In LL recipients, median warm ischaemic time was 36 min (range: 27–65 min), median cold ischaemic time was 9.7 h (range: 3.6–15.2 h) and median EBL was 1450 ml (range: 500–10000 ml). Ten-year patient survival was 78% and 10-year graft survival was 66%. Vascular and biliary complications in LL recipients are depicted in Table 4. Five of the nine patients required re-exploration (patients 1-LL, 2-LL, 3-LL, 5-LL, 6-LL). The largest LL recipient (patient 3-LL, 81 kg) developed postoperative SFSS, which resolved with conservative management. Recipients 5-LL and 8-LL underwent CLKT. The LL graft represented the second liver transplant in recipient 6-LL and the third in recipient 7-LL. There were no cases of primary non-function. The median hospital LoS was 40 days (range: 8–148 days).
Discussion
The technical evolution of SLT has largely addressed the needs of the paediatric population awaiting liver transplantation. The benefits of SLT to the paediatric population are evident, as demonstrated by decreases in wait time to transplant and in subsequent waitlist mortality.1 In addition, SLT, unlike reduced-size liver transplantation, does not deprive an adult patient awaiting transplantation of the opportunity for transplantation. Despite the higher risk for morbidity to the adult recipient associated with SLT in comparison with whole-organ transplantation, the application of SLT results in a net gain in life-years and successful transplantation in a greater number of recipients.9 Data suggest that, with proper donor and recipient selection, results similar to those of whole-organ liver allografts can be obtained in recipients of split-liver transplants.10 However, SLT currently accounts for approximately 4% of liver transplants performed each year in the USA,11 although it is estimated that up to 25% of donor allografts are suitable for splitting.1
The use of true right/left SLT for two adult recipients (or an adult and an adolescent recipient) has yet to gain wider acceptance, and still holds potential as a method to address the growing waitlisted adult population. The increased technical demands associated with true right/left SLT, concern for the potential of inadequate functional hepatic mass for the adult recipient post-transplant (SFSS), as well as the recognition of an increased rate of vascular or biliary complications, are likely to have contributed to scepticism on the application of true right/left SLT. Consideration of a variance by the OPTN which allows the splitting centre to make the decision on the second recipient of the SLT has the potential to increase the utilization of this technique and these organs.
The present experience with ex vivo true right/left SLT demonstrates that excellent longterm patient and graft survival can be obtained. However, this form of liver transplantation must be undertaken on the understanding that, in its current state, it is associated with increased postoperative morbidity, especially in recipients of the LL graft. Indeed, although only seven of the present 18 recipients experienced a biliary complication, six of these were recipients of LL grafts. Two patients receiving LL grafts also experienced vascular complications. These complications are not unique to SLT: incidences of biliary strictures are reported to be 5–15% following deceased donor liver transplantation (DDLT) and 28–32% following living donor liver transplantation (LDLT).12 Although the biliary complication rate associated with LL transplants could potentially be minimized by maintaining the CBD with the LL graft, this is likely to result in the transplantation of an RL graft with multiple ducts. Given the likely reduction in the risk for biliary complications with the graft containing the CBD, during the splitting procedure consideration should be given to whether the CBD should be retained with the liver segment to be transplanted into the recipient who drew the initial liver allocation. The latter is akin to the maintenance of the coeliac artery with the left lateral segment in an adult/child SLT.
No recipients in the present series developed primary non-function, a once feared complication of ex vivo liver splitting.13 Efforts were made to retain a graft weight : recipient weight ratio of >1.0% for all SLTs by carefully selecting recipients according to body weight. Indeed, the average weight of an RL recipient in this series was 70 kg and that of an LL recipient was 39 kg. The only recipient to develop SFSS was the largest (81 kg) recipient of an LL graft and this patient was amenable to conservative management with full symptom resolution. The largest transplant recipient in this series (recipient 4-RL, 119 kg) received an RL graft and underwent splenic artery ligation followed by portocaval shunt at the time of liver transplant to serve as inflow modulation for the prevention of SFSS. Indeed, it has been previously demonstrated that living donor LL grafts with a graft : recipient weight ratio of <0.8 can be safely used if a hemi-portocaval shunt is constructed to attenuate the associated portal hyperperfusion.14 The principle of portal inflow modulation used in LDLT may need to be applied in recipients of SLT grafts in order to minimize the risk for SFSS and to facilitate the expanded use of SLT grafts to larger recipients.
Although significant morbidity is associated with SLT, this should be considered in the context of the survival benefit to be derived by transplantation in the setting of an expanding demand that greatly outweighs current supply. The latter serves to justify the pursuit of living liver donation, which in the USA is performed at the same rate as SLT, and appears to be focused on the areas of greatest need.15 Indeed, it should be noted that recipient morbidity following LDLT is similar to that seen in SLT. Results from the Adult-to-Adult Living Donor Liver Transplantation (A2ALL) Cohort Study demonstrated that recipients of LDLT had a 31.8% incidence of biliary leak, a 26.2% incidence of unplanned re-exploration, a 6.5% incidence of hepatic artery thrombosis, and a 2.9% incidence of portal vein thrombosis, all of which were higher than in recipients of whole-organ deceased donor allografts.16 Furthermore, SLT, unlike LDLT, carries no risk to a healthy donor. Indeed, results from the A2ALL cohort have demonstrated that up to 40% of living liver donors experience postoperative complications.17 If the intended adult or adolescent recipient requires partial liver transplantation as a result of donor size considerations, he or she may assume a higher risk for surgical morbidity by requesting SLT for the benefit of expediting the transplant rather than remaining on the waitlist. However, if the intended recipient is facilitating SLT, in lieu of accepting a whole organ, this recipient is assuming a higher risk for surgical morbidity for the benefit of allowing an additional patient to undergo expedited liver transplantation. The latter is akin to the risk a living liver donor must assume. In this situation, the intended recipient should be provided with the biliary and vascular anatomy that minimizes this risk. The recipient of the other portion of the liver should be told of this decision and must be willing to accept the potential for a higher surgical risk along with the benefit of expedited transplantation.
As the present group has previously reported, appropriate donor and recipient selection are essential to optimize the results of SLT.8 The laboratory MELD scores of 21 in both LL and RL recipients represent an institutional preference for the use of SLT in patients with low true MELD scores (which precludes these patients from contending for DDLT within this study's donor service area) and/or in patients who require expedited transplantation. The selection of a potential SLT recipient is based on the patient's size, severity of disease, risk for waitlist death and risk for waitlist dropout. Suitable adult recipients for SLT are evaluated and consented by the transplant surgical team in advance of transplant in order to ensure they obtain a full understanding of the risks and benefits of partial liver transplantation. The grafts transplanted into the four RL recipients for whom exception points were listed were initially allocated to the LL recipients, but the RL recipients pursued SLT as a form of expediting transplantation.
It has been demonstrated that adult split-liver recipients include a higher proportion of women than do adult whole-organ recipients.9 Furthermore, women have significantly increased odds of undergoing LDLT in comparison with male transplant recipients.15 The greater need for partial liver transplantation in women, whether it be through SLT or LDLT, probably reflects a combination of: (i) a disparity in MELD score-based liver allocation caused by an underestimation of the degree of renal dysfunction in female patients (as a result of the inclusion of the serum creatinine level rather than the glomerular filtration rate in the calculation of the MELD score), and (ii) the smaller size of female recipients, which hampers appropriate donor–recipient organ matching at the time of initial organ offer. Partial liver transplantation, using SLT or LDLT, not only allows for expedited transplantation to the female recipient, but also allows for an appropriately sized, smaller graft. Indeed, in the present series 78% of LL grafts were transplanted to female recipients, whereas 89% of RL grafts were transplanted to male recipients.
This study represents an observational, retrospective review of a single-centre experience with ex vivo true right/left SLT. The population of interest was represented by a small number of patients, which represents a limitation of this study. Although this series includes only 18 patients, it represents one of the larger series of true right/left SLT, especially with utilization of the ex vivo technique. Humar et al. reported the first North American series of six adult/adult SLT procedures, performed in situ, demonstrating patient and graft survival of 83% at a mean follow-up of 9 months.6 An updated report on the use of in situ splitting in 31 adult patients documented graft and patient survival of 74% at 3 years post-transplant, but without sub-classifying LL and RL graft and patient survival.10 The Paul Brousse group has extensive experience with ex vivo SLT and in 2001 reported a series of 34 recipients of SLT with 2-year patient and graft survival of 74% and 74%, respectively, for RL split grafts, and 64% and 43%, respectively, for LL split grafts.7 It should be noted that only eight of the 34 recipients received true right/left ex vivo SLT as the vast majority of LL grafts in this series were transected through segment IV and contained solely the left hepatic vein.7 Broering et al. reported their results with a combination of in situ and ex vivo right/left SLT in 35 patients using a technique of vena caval and middle hepatic vein splitting.18 They reported an overall biliary complication rate of 29%, with patient and graft survival at 27 months of 87.5% and 75.0%, respectively, for RL grafts, and 89.5% and 84.0%, respectively, for LL grafts.
In summary, this experience with ex vivo true right/left SLT demonstrates that excellent longterm patient and graft survival can be obtained, but that significant morbidity occurs in recipients of LL split grafts. Optimization of the technical aspects of liver splitting, with attention to the left-side biliary system, is warranted to reduce postoperative morbidity. Although a proportion of the LL recipients in the present series were adolescents, further understanding of various techniques for portal inflow modulation for the prevention of SFSS, such as the application of hemi-portocaval shunts and/or splenic artery ligation, will potentially allow for the expansion of both RL and LL SLT to an adult population that includes larger patients.
Conflicts of interest
None declared.
References
- 1.Busuttil RW, Goss JA. Split liver transplantation. Ann Surg. 1999;229:313–321. doi: 10.1097/00000658-199903000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. Transplantation of a donor liver to two recipients (splitting transplantation): a new method in the further development of segmental liver transplantation. [In German.] Langenbecks Arch Chir. 1988;373:127–130. [PubMed] [Google Scholar]
- 3.Colledan M, Segalin A, Andorno E, Corno V, Lucianetti A, Spada M, et al. Modified splitting technique for liver transplantation in adult-sized recipients. Technique and preliminary results. Acta Chir Belg. 2000;100:289–291. [PubMed] [Google Scholar]
- 4.Sommacale D, Farges O, Ettorre GM, Lebigot P, Sauvanet A, Marty J, et al. In situ split liver transplantation for two adult recipients. Transplantation. 2000;69:1005–1007. doi: 10.1097/00007890-200003150-00060. [DOI] [PubMed] [Google Scholar]
- 5.Kilic M, Seu P, Stribling RJ, Ghalib R, Goss JA. In situ splitting of the cadaveric liver for two adult recipients. Transplantation. 2001;72:1853–1858. doi: 10.1097/00007890-200112150-00028. [DOI] [PubMed] [Google Scholar]
- 6.Humar A, Ramcharan T, Sielaff TD, Kandaswamy R, Gruessner RW, Lake JR, et al. Split liver transplantation for two adult recipients: an initial experience. Am J Transplant. 2001;1:366–372. doi: 10.1034/j.1600-6143.2001.10413.x. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay D, Casating D, Adam R, Savier E, Delvart V, Karam V, et al. Split-liver transplantation for two adult recipients: feasibility and longterm outcomes. Ann Surg. 2001;233:565–574. doi: 10.1097/00000658-200104000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vagefi PA, Parekh J, Ascher NL, Roberts JP, Freise CE. Outcomes with split liver transplantation in 106 recipients: the University of California, San Francisco experience from 1993 to 2010. Arch Surg. 2011;146:1052–1059. doi: 10.1001/archsurg.2011.218. [DOI] [PubMed] [Google Scholar]
- 9.Merion RM, Rush SH, Dykstra DM, Goodrich N, Freeman RB, Jr, Wolfe RA. Predicted lifetimes for adult and paediatric split liver versus adult whole liver transplant recipients. Am J Transplant. 2004;4:1792–1797. doi: 10.1111/j.1600-6143.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- 10.Humar A, Beissel J, Crotteau S, Kandaswamy R, Lake J, Payne W. Whole liver versus split liver versus living donor in the adult recipient: an analysis of outcomes by graft type. Transplantation. 2008;85:1420–1424. doi: 10.1097/TP.0b013e31816de1a3. [DOI] [PubMed] [Google Scholar]
- 11.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5:133–142. doi: 10.5009/gnl.2011.5.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renz JF, Yersiz H, Reichert PR, Hisatake GM, Farmer DG, Emond JC, et al. Split-liver transplantation: a review. Am J Transplant. 2003;3:1323–1335. doi: 10.1046/j.1600-6135.2003.00254.x. [DOI] [PubMed] [Google Scholar]
- 14.Botha JF, Langnas AN, Campos BD, Grant WJ, Freise CE, Ascher NL, et al. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2010;16:649–657. doi: 10.1002/lt.22043. [DOI] [PubMed] [Google Scholar]
- 15.Vagefi PA, Ascher NL, Freise CE, Dodge JL, Roberts JP. The use of living donor liver transplantation varies with the availability of deceased donor liver transplantation. Liver Transpl. 2012;18:160–165. doi: 10.1002/lt.22455. [DOI] [PubMed] [Google Scholar]
- 16.Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8:2569–2579. doi: 10.1111/j.1600-6143.2008.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abecassis MM, Fisher RA, Olthoff KM, Freise CE, Rodrigo DR, Samstein B, et al. Complications of living donor hepatic lobectomy – a comprehensive report. Am J Transplant. 2012;12:1208–1217. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broering DC, Wilms C, Lenk C, Schulte am Esch J, 2nd, Schönherr S, Mueller L, et al. Technical refinements and results in full-right full-left splitting of the deceased donor liver. Ann Surg. 2005;242:802–812. doi: 10.1097/01.sla.0000189120.62975.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]


