Abstract
Objectives:Perioperative factors can affect outcomes of liver transplantation (LT) in recipients with hepatitis C virus (HCV) infection. This study was conducted to investigate whether the immunomodulatory effects of packed red blood cells (PRBC) and platelets administered in the perioperative period might affect immune responses to HCV and thus outcomes in LT recipients.
Methods:Data for a total of 257 HCV LT recipients were analysed. Data on clinical demographics including perioperative transfusion (during and within the first 24 h), serum cytokine concentration, HCV-specific interferon-γ (IFN-γ) and interleukin-17 (IL-17) producing cells, and outcomes including graft and patient survival were analysed.
Results:Patient survival was higher in HCV LT recipients who did not receive transfusions (Group 1, n = 65) than in those who did (Group 2, n = 192). One-year patient survival was 95% in Group 1 and 88% in Group 2 (P = 0.02); 5-year survival was 77% in Group 1 and 66% in Group 2 (P = 0.05). Group 2 had an increased post-transplant viral load (P = 0.032) and increased incidence of advanced fibrosis at 1 year (P = 0.04). After LT, Group 2 showed increased IL-10, IL-17, IL-1β and IL-6, and decreased IFN-γ, and a significantly increased rate of IL-17 production against HCV antigen. Increasing donor age (P = 0.02), PRBC transfusion (P < 0.01) and platelets administration were associated with worse survival.
Conclusions:Transfusion had a negative impact on LT recipients with HCV. The associated early increase in pro-HCV IL-17 and IL-6, with decreased IFN-γ, suggests that transfusion may be associated with the modulation of HCV-specific responses, increased fibrosis and poor transplant outcomes.
Introduction
The prevalence of hepatitis C virus (HCV) infection is high: an estimated 170–200 million people are affected worldwide.1 In the USA, it is estimated that 3.2 million people are infected with HCV1,2 and many develop complications of infection, including liver cirrhosis and failure, and hepatocellular carcinoma (HCC). These HCV-mediated end-stage liver diseases have become the leading indication for liver transplantation (LT) in the USA.3,4
Although LT is an available therapeutic option for patients with chronic HCV, the recurrence of HCV infection in the liver graft is nearly universal.5 Further, following HCV infection of the allograft liver, the progression of HCV-mediated liver injury and cirrhosis is rapid and severe compared with the natural progression of HCV disease in the native liver. This accelerated progression of liver fibrosis post-LT is seen in about 10–30% of LT recipients with HCV infection, and often results in advanced fibrosis and liver cirrhosis soon after LT.5
A number of risk factors have been associated with HCV recurrence after transplantation, including factors pertaining to the recipient, donor or the virus itself.6–10 Perioperative inflammation including inflammation caused by ischaemia–reperfusion injury has been proposed to facilitate viral persistence and replication. The replication of HCV and infection of the graft occur soon after reperfusion and viral loads return to pre-transplant levels within days after transplant.9
In addition, immunological factors in the recipient can control the course of HCV replication.4 The present group's studies have demonstrated that the development of a T-helper 17 type immune response against HCV antigens is associated with increased liver fibrosis.11 By contrast, a viral antigen-specific interferon-γ (IFN-γ) response helps to suppress the virus and prevent viral replication.11,12
In the early days of organ transplantation, a distinct immunosuppressive effect was noted when blood products were administered prior to transplantation. In canine models, prolonged kidney graft survival was seen with pre-transplant blood transfusion.13 Further, in 1973, a large study conducted in 148 cadaveric kidney transplant patients by Opelz et al. demonstrated that 1-year graft survival was significantly better (66% versus 29%) in patients who received a blood transfusion than in those who did not and this effect was directly proportional to the number of units of blood received.14 Similar findings were reported by others,15,16 and platelet transfusion was also noted to have a beneficial effect on allograft outcome.17–19 Although the exact mechanisms for these immunosuppressive properties were unknown, these studies led to the conclusion that blood products have immunomodulatory effects.20–23 However, a negative effect clearly apparent in all of these early studies was an increase in allo-sensitization in patients with transfusion. The presence of leukocytes was correlated with sensitization.24 Hence, strategies to deplete blood of the leukocyte fraction using washing, filtration and other methods were employed.25
Subsequent to the advent of newer and better immunosuppressive drugs, reports have demonstrated that blood transfusion may actually be detrimental to longterm allograft function.26 Blood transfusion was also noted to have a negative impact on postoperative outcomes in a variety of surgical settings.27–33 Various studies following lung, intestinal, cardiac, liver and other surgery demonstrated that an increase in blood transfusion can increase postoperative complications and the incidence of infections, as well as the recurrence of cancer.27–33
The effects of blood product administration and transfusion following kidney transplantation have been well studied.14,15,19,20,26,34–37 Although these findings cannot be directly translated to LT, various studies have suggested that the transfusion of blood products plays a controversial role in outcome following LT,38–40 particularly in terms of its effect on HCV recurrence after LT. Hence, in view of the immunomodulatory properties of blood products, the goal of this study was to determine the effects of perioperative blood transfusion (blood products administered during or within the first 24 h of transplant) on transplant outcome in HCV-infected LT recipients. The study demonstrated that perioperative blood transfusion was associated with decreased overall survival in HCV LT recipients. Further, blood transfusion was associated with the modulation of immune responses to HCV, including the development of Th17 with suppression of the HCV-specific IFN-γ response.
Materials and methods
Patient population
All adult LT patients submitted to transplant for HCV-mediated liver disease between 1 January 2002 and 31 December 2010 at Barnes Jewish Hospital, Washington University School of Medicine were included in this retrospective study. Infection with HCV was confirmed by an HCV-positive RNA quantitative polymerase chain reaction (qPCR) (detection limit of 50 IU/ml; Cobas® Amplicor; Roche Diagnostics, Indianapolis, IN, USA) and anti-HCV antibody enzyme-linked immunosorbent assay (ELISA) (Abbot Laboratories, Chicago, IL, USA). Viral load and genotype were determined for all patients. Liver transplant patients with hepatitis B virus (HBV) and/or human immunodeficiency virus (HIV) co-infection, those with liver disease of other aetiology and those aged < 18 years were excluded. Demographic and clinical data were obtained retrospectively from a prospective database on all LT patients maintained at the study centre. Details of blood products administered were obtained from patient charts and the blood bank. Liver transplant recipients with HCV for whom transfusion details were not available were excluded from the study. Pre-transplant laboratory parameters, including haemoglobin, bilirubin, platelet count and transaminases, were determined just prior to transplant. A pre-transplant laboratory Model for End-stage Liver Disease (MELD) score was calculated for all LT recipients. Donor liver quality (including extent of steatosis) was determined by a liver biopsy obtained from the left lateral segment either at the time of procurement or after reperfusion.
Blood samples were obtained pre-transplant and at 1 month, 6 months and 1 year post-transplant. Pre-transplant blood was obtained just before surgery (before any blood products were administered) and post-transplant blood was obtained during a scheduled outpatient visit. A protocol liver biopsy was performed in all patients at approximately 1 year (10–14 months) post-transplant. Biopsies were graded by pathologists for fibrosis and necroinflammatory activity using the modified Batts–Ludwig scoring system,41 which grades for fibrosis stage and inflammatory grade. Biopsies were categorized into those with advanced fibrosis (stages 3 and 4) and those without fibrosis (stages 0–2). Patients for whom biopsy details were not available were excluded from the study. Informed consent was obtained from all patients and the study was approved by the institutional review board at Washington University in St Louis.
At this centre, a standard three-drug immunosuppression regimen that includes tacrolimus (Prograf; Astellas Pharma US, Inc., Northbrook, IL, USA), corticosteroids and an antimetabolite (most commonly mycophenolate) is used. Methylprednisolone is administered intraoperatively (1000 mg) and is followed by oral prednisone, which is tapered over 3–6 months. Biopsy-confirmed acute rejection is treated with bolus steroids as first-line therapy and, in rare instances, with thymoglobulin or OKT3 in non-responders.
Blood product and transfusion policy
Blood products transfused included whole blood, packed red blood cells (PRBC), fresh frozen plasma (FFP), cryoprecipitates and platelets. Transfusion was delivered according to the judgement of the individual physician and based on, but not limited to, clinical parameters such as blood pressure, cardiac output, blood loss, coagulopathy and other laboratory findings. If blood products were utilized, they were mostly allogeneic; autotransfusion with salvaged blood was utilized only rarely.
Isolation of serum and peripheral blood mononuclear cells
Recipient serum was obtained from whole blood. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation. Following isolation, cells were used either immediately or were frozen in 10% dimethyl sulphoxide.
Hepatitis C virus antigens
Recombinant HCV core (Fitzgerald Industries International, Inc., Acton, MA, USA), HIV (Gp120 peptide; Biosynthesis, Inc., Lewisville, TX, USA) and phytohaemagglutinin (PHA) (Sigma-Aldrich, Inc., St Louis, MO, USA) peptide antigens were tested endotoxin-free by litmus amoebocyte limulus assay (Charles River Laboratories, Charleston, SC, USA). Samples of PBMC were stimulated with 5 μg/ml of each antigen overnight in 24-well plates (Corning, Inc., Corning, NY, USA) at 37 °C in 5% carbon dioxide (CO2).
Enzyme-linked immunospot assay to determine HCV-specific immune responses
Enzyme-linked immunospot assay (ELISpot) was carried out as described previously.11 Stimulated PBMC were cultured in triplicate (3 × 105 cells/200 μl) in immunospot plates (Millipore Corp., Billerica, MA, USA) in the presence of antigens (5 μg/ml) for 48–72 h in 5% CO2 at 37 °C. IFN-γ (BD Biosciences, Inc., San Jose, CA, USA) and interleukin-17 (IL-17) (eBioscience, Inc., San Diego, CA, USA) ELISpot was performed according to the manufacturer's instructions. The plates were washed and spots analysed in an ImmunoSpot Image Analyzer (Cellular Technology Ltd, Cleveland, OH, USA). Cells cultured in medium (Cellular Technology Ltd) and HIV Gp120 peptide were used as negative controls; PHA was used as a positive control. Counted spots were expressed as spots per million cells (spm).
Multiplex bead Luminex® assay to measure serum cytokines
Serum cytokines [IL-1β, IL-1ra, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17, tumour necrosis factor-α (TNF-α), IFN-α, IFN-γ, granulocyte macrophage colony-stimulating factor (GM-CSF)] and chemokines (MIP-1c, MIP-1b, IP-10, MIG, eotaxin, RANTES, MCP-1) were measured using multiplex bead immunoassays (Invitrogen, Inc., Carlsbad, CA, USA) performed as per the manufacturer's instructions. The Luminex xMAP™ system was used to read plates and to determine the mean fluorescence intensity (MFI) of experimental and standard wells. Cytokine and chemokine concentrations were obtained using a standard curve and expressed in pg/ml.
Statistical analysis
Analysis was performed using GraphPad Prism Version 5.0b (GraphPad Software, Inc., La Jolla, CA, USA) and IBM spss Statistics Version 21.0 (IBM Corp., Armonk, NY, USA). Data were checked for normality using the Shapiro–Wilk test and non-normal data were log-transformed. Clinical demographics and immunological parameters were compared using the Kruskal–Wallis test, chi-squared test and analysis of variance (anova) as appropriate. Kaplan–Meier survival curves and the log-rank test were used for allograft and patient survival analyses. A univariate logistic regression analysis stratified by log rank was performed to determine factors associated with patient and graft survival. Variables with a possible association (P < 0.10) were selected for a second multivariate regression model. These selected variables were analysed using Cox proportional hazard regression with forward stepwise selection to identify variables independently associated with poor graft and patient survival. A two-sided level of significance was set at P < 0.05.
Results
Patient demographics
A total of 702 LTs were performed between 1 January 2002 and 31 December 2010 at the study centre. Amongst these, 280 transplants were performed in adult HCV patients and were eligible to be included for analysis. Detailed demographic, transfusion and clinical data, with at least 1 year of follow-up, were available for 257 patients (74 women, 183 men). Perioperative transfusion was defined as any transfusion during LT surgery or within 24 h of LT. Patients were classified into two groups: Group 1 included patients who did not receive any perioperative transfusion of any blood product (n = 65), and Group 2 included patients who were transfused perioperatively with at least one unit of whole blood/PRBC/FFP/cryoprecipitate or platelets (n = 192). The clinical demographics of both groups are described in Table 1. Those who were transfused were younger (median age: 52 years). Fifty (76.9%) patients in Group 1 and 64 (33.3%) in Group 2 had HCC along with HCV (P = 0.001). In general, patients who were transfused had a higher pre-transplant MELD score, higher bilirubin, higher international normalized ratio (INR) and lower haemoglobin (Table 1). There were no differences in warm or cold ischaemia times or the degree of ischaemia–reperfusion injury. Donor liver quality was similar in both groups, as was the utilization of liver grafts donated after cardiac death. There was no difference in pre-transplant HCV viral load or genotype; HCV genotype 1a infection was the most prevalent in the study cohort. There was no difference in donor and recipient cytomegalovirus (CMV) status.
Table 1.
Clinical demographics of hepatitis C virus (HCV)-infected liver transplant (LT) recipients who did and did not receive perioperative (during LT and within the first 24 h post-LT) blood product transfusions
| Demographic characteristic | Group 1, non-transfused (n = 65) | Group 2, transfused (n = 192) | P-value |
|---|---|---|---|
| Recipient age at LT, years, median (range) | 55 (45–74) | 52 (27–75) | 0.009 |
| Recipient gender, n (%) | 0.554 | ||
| Female | 14 (21.5%) | 60 (31.3%) | |
| Male | 51 (78.5%) | 132 (68.8%) | |
| Recipient race, n (%) | 0.332 | ||
| White | 53 (81.5%) | 156 (81.3%) | |
| African-American | 11 (16.9%) | 24 (12.5%) | |
| Other | 1 (1.5%) | 12 (6.2%) | |
| Hepatocellular carcinoma, n (%) | 50 (76.9%) | 64 (33.3%) | 0.001 |
| Retransplant, n (%) | 0 | 8 (4.2%) | 0.063 |
| Donor age, years, median (range) | 47 (10–76) | 42 (10–76) | 0.028 |
| Donor race, n (%) | 0.582 | ||
| White | 54 (85.7%) | 145 (75.5%) | |
| African-American | 6 (9.5%) | 31 (16.1%) | |
| Other/unknown | 5 (7.8%) | 16 (8.4%) | |
| Donor (D)/recipient (R) CMV status, n (%) | |||
| D−/R− | 10 (15.3%) | 33 (17.2%) | 0.133 |
| D+/R− | 16 (24.6%) | 39 (20.3%) | |
| D−/R+ | 17 (26.2%) | 23 (12.0%) | |
| D+/R+ | 12 (18.4%) | 60 (31.2%) | |
| N/A | 10 (15.3%) | 37 (19.2%) | |
| Pre-LT bilirubin, mg/dl, median (range) | 2.68 (2–6) | 7.08 (3–58) | <0.001 |
| Pre-LT INR, median (range) | 1.31 (1.00–3.00) | 1.95 (1.02–5.06) | <0.001 |
| Pre-LT Hb, mg/dl, median (range) | 13.2 (8–16) | 10.9 (7–17) | <0.001 |
| Pre-LT platelet count, in 105, median (range) | 88.6 (30–351) | 84.1 (10–330) | 0.493 |
| Pre-LT MELD score, median (range) | 12 (6–24) | 21 (7–51) | <0.001 |
| Pre-LT creatinine, mg/dl, median (range) | 1.1 (0.6–4) | 1.2 (0.3–8) | 0.435 |
| Pre-LT HCV viral load, 106 copies/ml, median (range) | 0.42 (0.1–1.01) | 0.31 (0.3–1.14) | 0.334 |
| Post-LT HCV viral load, 106 copies/ml, median (range) | 5.6 (0.6–10.1) | 10.3 (0.3–15.4) | 0.032 |
| HCV genotype, n (%) | 1–14 (21.5%) | 1–30 (15.6%) | 0.275 |
| 1a: 20 (30.8%) | 1a: 72 (38%) | ||
| 1b: 10 (15.4%) | 1b: 40 (20.8%) | ||
| 2a: 3 (4.7%) | 3: 6 (3.1%) | ||
| N/A: 18 (27.6%) | N/A: 44 (22.9%) | ||
| Cold ischaemia time, min, median (range) | 345.5 (85–748) | 370.4 (79–878) | 0.251 |
| Warm ischaemia time, min, median (range) | 35.8 (20–80) | 34.9 (15–69) | 0.513 |
N/A, not available; CMV, cytomegalovirus; INR, international normalized ratio; Hb, haemoglobin; MELD, Model for End-stage Liver Disease.
P-values in bold indicate statistically significant differences (P < 0.05).
Transfusion characteristics of HCV LT recipients
Among the 192 HCV LT recipients who were transfused, 20 (10.4%) patients received whole blood in the perioperative period. PRBC were administered to 180 (93.8%) patients. Fresh frozen plasma was utilized in 163 (84.8%) patients and platelets in 72 (37.5%) patients. Cryoprecipitates were mostly administered during transplant and were utilized in 95 (49.5%) patients. A total of 72 (37.5%) patients received all three of FFP, platelets and PRBC. Data on the number of units of blood products (i.e. whole blood, PRBC, FFP, cryoprecipitate, platelets) and time of administration are described in Table 2.
Table 2.
Blood product utilization during and 24 h after liver transplantation (LT) in hepatitis C virus (HCV)-infected recipients
| Blood product | Group 1, non-transfused (n = 65) | Group 2, transfused (n = 192) | P-value |
|---|---|---|---|
| Whole blood, units, median (range) | 0 | 1 (0–4) | 0.04 |
| PRBC during LT, units, median (range) | 0 | 3 (0–102) | <0.001 |
| PRBC within 24 h of LT, units, median (range) | 0 | 2 (0–28) | <0.001 |
| FFP during LT, units, median (range) | 0 | 5 (0–70) | <0.001 |
| FFP within 24 h of LT, units, median (range) | 0 | 1 (0–21) | 0.008 |
| Cryoprecipitate during LT, units, median (range) | 0 | 1 (0–10) | <0.001 |
| Cryoprecipitate within 24 h of LT, units, median (range) | 0 | 1 (0–2) | 0.037 |
| Platelets during LT, units, median (range) | 0 | 1 (0–12) | 0.001 |
| Platelets within 24 h of LT, units, median (range) | 0 | 1 (0–5) | 0.002 |
PRBC, packed red blood cells; FFP, fresh frozen plasma.
Pre-transplant bilirubin, MELD score and haemoglobin are predictive of PRBC transfusion in HCV LT recipients
To determine variables predictive of PRBC administration in the perioperative period, odds ratios (ORs) were calculated. As Table 3 shows, high pre-transplant bilirubin [(OR) = 1.52, 95% confidence interval (CI) 0.023–2.253; P = 0.037] and a high MELD score (OR = 1.173, 95% CI 1.032–1.333; P = 0.015) were predictive of PRBC transfusion. Haemoglobin (OR = 0.662, 95% CI 0.521–0.842; P = 0.001) was also predictive of PRBC transfusion [i.e. the lower the haemoglobin, the greater the likelihood of transfusion (as noted by an OR of < 1)]. Other variables, including platelet count, creatinine and donor age, were not predictive of PRBC transfusion (Table 3).
Table 3.
Regression analysis to determine predictors of peri-liver transplant (LT) packed red blood cell transfusion requirement
| Variable | Odds ratio (95% CI) | P-value |
|---|---|---|
| Recipient age | 1.023 (0.957–1.093) | 0.507 |
| Donor age | 0.995 (0.971–1.019) | 0.685 |
| Pre-LT bilirubin | 1.52 (1.025–2.253) | 0.037 |
| Pre-LT INR | 0.688 (0.254–1.865) | 0.462 |
| Pre-LT MELD score | 1.173 (1.032–1.333) | 0.015 |
| Pre-LT haemoglobin | 0.662 (0.521–0.842) | 0.001 |
| Pre-LT creatinine | 1.033 (0.887–1.334) | 0.559 |
| Pre-LT platelet count | 1.008 (1–1.016) | 0.056 |
| Cold ischaemia time | 1.002 (0.999–1.005) | 0.115 |
95% CI, 95% confidence interval; INR, international normalized ratio; MELD, Model for End-stage Liver Disease.
P-values in bold indicate statistical significance (P < 0.05).
Increased postoperative intensive care unit and hospital stay in transfused patients
All LT recipients were taken to the intensive care unit (ICU) postoperatively for at least 1 day; the decision to transfer a patient out of the ICU varied according to the clinical situation. The duration of ICU stay was calculated as the number of postoperative days spent in the ICU, and the duration of hospital stay as the number of days post-transplant spent in the hospital. As shown in Fig. 1, HCV LT recipients who were transfused spent more time in both the ICU and the hospital post-transplant than those who were not transfused. Amongst the patients who were not transfused, most went home within 6 days of transplant, whereas those who were transfused remained in hospital for an average of 13 days.
Figure 1.
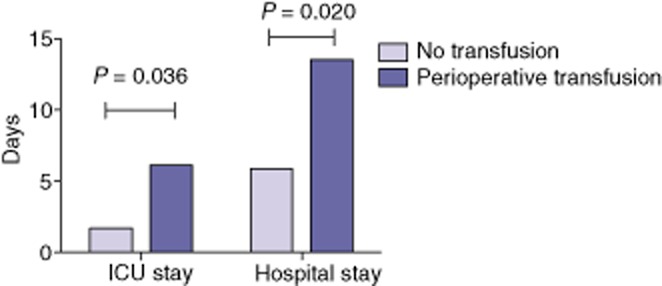
Median duration of intensive care unit (ICU) and hospital stay in liver transplant patients with hepatitis C virus infection who did and did not receive perioperative transfusion with any blood product
Increased post-transplant HCV recurrence and increased incidence of advanced liver fibrosis 1 year post-transplant in transfused HCV LT recipients
In all patients, HCV viral load was determined by qPCR (Cobas® Amplicor; Roche Diagnostics). HCV viral load is known to drop immediately after transplant and to increase soon afterwards.9 To account for these changes, HCV viral load was determined pre-transplant and at 3 months post-transplant (range: 2–6 months in both groups). As Table 1 shows, post-transplant HCV viral load was 10–40-fold higher than pre-transplant load in all patients. Post-transplant viral load was significantly higher in LT recipients who received perioperative blood transfusions than in the non-transfused cohort, although pre-transplant levels had been similar between the groups (P = 0.032). Further, the change in HCV viral load (from pre-to post-transplant) was significantly greater in transfused than in non-transfused patients [mean ± standard error (SE) fold change: 35.2 ± 3.4 in Group 2 and 11.4 ± 5.6 in Group 1; P = 0.001]. This suggests a possible relationship between blood transfusion and increased HCV replication.
Additionally, all HCV LT recipients underwent a 1-year protocol liver biopsy post-LT to determine the status of HCV infection in the liver. Pathologists scored liver biopsies for fibrosis stage and inflammatory activity using the modified Batts–Ludwig scoring system.41 All patients demonstrated some changes as a result of HCV re-infection of the graft. Among the 180 patients who had received PRBC transfusions, 66 (36.7%) had advanced fibrosis (Batts–Ludwig stage 3–4), whereas only 14 (18.2%) of the 77 patients who did not receive PRBC did so (P = 0.04). There were no differences in the groups of patients to whom FFP, cryoprecipitates or platelets were administered in the incidence of advanced fibrosis (data not shown).
Effect on acute rejection following LT
Biopsy-confirmed acute rejection episodes in the first year post-transplant were present in 21 patients in the entire cohort. Most of these rejection episodes occurred within the first month after transplantation. In the group to whom PRBC were administered, 5.7% of patients had at least one episode of acute rejection, whereas 15.3% of patients in the non-transfused group did so. However, this difference was not statistically significant (P = 0.061). It has been suggested that a ‘small’ number of units of blood may actually have a protective effect on the graft.26 Hence, incidences of acute rejection were compared among patients who received 1–3 units of PRBC, patients who did not receive any transfusions, and patients who received > 3 units of PRBC. Of the 52 patients who received 1–3 units of blood, only one (1.9%) patient experienced acute rejection, whereas 12.3% of patients who received > 3 units and 15.3% of non-transfused patients experienced acute rejection (P = 0.001). The distinction between rejection and HCV recurrence in liver biopsy can be difficult to make; however, the decision to treat rejection at the study centre depends on close discussion among the centre's pathologists. In the present patient population, the distinction between rejection and recurrence could not be established in six patients (including two who received > 3 units of blood and four non-transfused patients), but all of these patients were treated for rejection and demonstrated clinical improvement as indicated by reductions in transaminases and other parameters.
Incidence of other complications
Postoperative bleeding during the first week after transplantation was noted to be higher in the transfused (15.3%) than in the non-transfused (9.3%) cohort (P = 0.04). However, it is possible that this reflects the increased transfusion needs post-LT of coagulopathic patients who have already received perioperative transfusions. None of the patients in the non-transfused cohort received any transfusions in the later postoperative period. Of the 192 patients who were transfused perioperatively, 153 (79.7%) did not receive further transfusions. The remaining 39 (20.3%) patients received transfusions (range: 1–8 units) within the first week of transplant.
Incidences of other surgical complications, including vascular and biliary complications, were similar in both groups. Donor (D) and recipient (R) CMV status was similar in both groups and CMV reactivation among the 214 patients at risk (D+/R− or D−/R+ or D+/R+) occurred in 5.4% of patients in the non-transfused cohort and 11.3% in the transfused cohort in the first year after transplantation (P = 0.06).
Antiviral therapy for HCV at this institution consists of IFN-α and ribavirin. None of the patients had a sustained virological response (SVR) to treatment prior to transplant and all transplanted patients had a detectable viral load prior to transplant. In general, most patients are not offered antiviral therapy within the first year of transplant; however, in some instances of clinical worsening of liver disease without evidence of rejection and with an increasing viral load, specific antiviral therapy is initiated. Further continuation of therapy depends on tolerance and treatment response. In the first year after LT, five (7.7%) patients in the non-transfused cohort and 28 (14.6%) patients in the transfused cohort received antiviral therapy. Of these, 33.3% failed to complete therapy as a result of significant side-effects. Incidences of SVR in the patients who completed therapy were similar in both groups (approximately 30%). None of the patients analysed in the current study received the newer protease inhibitors as antiviral therapy.
Increased serum IL-17, IL-1β, IL-6, IL-4, IL-5, IL-10 cytokines and suppression of IFN-γ in transfused HCV LT patients
To determine the serum concentrations of cytokines and chemokines pre-transplant and at 1 month, 6 months and 1 year post-LT, serum was analysed by 25-plex Luminex®. In order to eliminate any potential confounding factors, only patients who had received perioperative transfusions and no further transfusions in the postoperative period were included. Patients who received antiviral therapy (pre-or post-transplant), had HCC, had rejection episodes, had CMV reactivation or any other illness (respiratory infection, diarrhoea, etc.) 1 month before or after sample collection were also excluded. According to these criteria and the availability of serial samples, only 30 (46.2%) patients in the non-transfused cohort and 85 (44.3%) in the transfused cohort were included for analysis. The demographics and clinical profiles of these groups of LT recipients did not differ from those of the entire non-transfused and transfused cohorts, respectively (data not shown). As Table 4 shows, there were no differences in the pre-transplant cytokine profiles of these patient groups. However, patients who were transfused had significantly higher serum concentrations of IL-17, IL-1β, IL-6, IL-4, IL-5 and IL-10 as early as 1 month post-transplant. These levels continued to be higher in the transfused cohort at 6 months and at 1 year post-LT. Similarly, concentrations of IFN-γ in patients who were transfused were significantly lower than those in patients who were not transfused. This demonstrates that patients who were transfused had an associated increase in IL-17 and pro-Th17 (IL-6, IL-1β) and Th2 cytokines (IL-4, IL-10, IL-5) and decreased Th1 cytokine (IFN-γ). Concentrations of other cytokines and chemokines did not vary among the groups (data not shown).
Table 4.
Serum cytokine concentration measured by 25-plex Luminex® pre-liver transplantation (LT), 1 month post-LT, 6 months post-LT and 1 year post-LT in hepatitis C virus (HCV)-infected patients who were or were not perioperatively transfused
| Cytokine | Pre-LT |
1 month post-LT |
6 months post-LT |
1 year post-LT |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1, non-transfused | Group 2, transfused | P-value | Group 1, non-transfused | Group 2, transfused | P-value | Group 1, non-transfused | Group 2, transfused | P-value | Group 1, non-transfused | Group 2, transfused | P-value | |
| IL-17, pg/ml | 64.3 ± 11.2 | 70.2 ± 20.2 | 0.10 | 13.4 ± 8.71 | 44.1 ± 11.5 | 0.03 | 24.5 ± 15.6 | 88.9 ± 20.1 | 0.014 | 33.3 ± 5.5 | 100.3 ± 22.2 | 0.011 |
| IL-1β, pg/ml | 45.4 ± 16.1 | 40.5 ± 15.3 | 0.22 | 10.2 ± 7.33 | 40.4 ± 7.9 | 0.01 | 34.5 ± 9.3 | 70.5 ± 6.5 | 0.00 | 29.4 ± 11.1 | 88.3 ± 18.5 | 0.002 |
| IL-6, pg/ml | 50.4 ± 14.9 | 60.1 ± 21.5 | 0.54 | 12.1 ± 6.21 | 64.3 ± 11.3 | 0.01 | 27.5 ± 13.8 | 80.1 ± 20.1 | 0.009 | 30.3 ± 9.9 | 94.4 ± 25.3 | 0.005 |
| IL-4, pg/ml | 70.1 ± 17.3 | 79.5 ± 16.4 | 0.44 | 43.2 ± 10.5 | 98.5 ± 20.1 | 0.02 | 39.3 ± 8.1 | 120.3 ± 17.9 | 0.003 | 44.8 ± 13.9 | 111.3 ± 12.2 | 0.002 |
| IL-5, pg/ml | 55.6 ± 20.2 | 70.9 ± 28.4 | 0.21 | 15.3 ± 4.4 | 52.2 ± 11.6 | 0.02 | 50.6 ± 20.3 | 89.4 ± 18.9 | 0.010 | 55.5 ± 25.2 | 100.3 ± 21.7 | 0.020 |
| IL-10, pg/ml | 47.9 ± 11.4 | 54.3 ± 17.7 | 0.69 | 13.3 ± 6.5 | 39.2 ± 10.1 | 0.03 | 40.5 ± 4.9 | 68.8 ± 5.7 | 0.040 | 44.5 ± 18.3 | 74.3 ± 19.2 | 0.010 |
| IFN-γ, pg/ml | 20.4 ± 5.6 | 19.9 ± 6.1 | 0.8 | 95.3 ± 17.7 | 45.6 ± 18.9 | 0.01 | 124.9 ± 29.4 | 30.1 ± 10.3 | 0.001 | 100.9 ± 8.0 | 23.1 ± 11.2 | 0.001 |
| TGF-β, pg/ml | 145.7 ± 21.9 | 120.3 ± 24.3 | 0.32 | 24.5 ± 3.7 | 60.5 ± 11.9 | 0.009 | 40.5 ± 18.9 | 166.4 ± 31.4 | 0.002 | 50.3 ± 23.4 | 188.9 ± 31.5 | 0.005 |
IL, interleukin; IFN, interferon; TGF, transforming growth factor.
Transfused HCV LT patients have an increased frequency of HCV-specific IL-17 and decreased frequency of IFN-γ secreting cells 1 year post-LT
In order to determine the cellular responses specific to HCV, PBMC from transfused and non-transfused patients, respectively, were stimulated with HCV core antigen and cells secreting IL-17 or IFN-γ were enumerated by ELISpot. As Fig. 2 shows, patients who were transfused in the perioperative period had an associated increase in HCV core-specific IL-17 producing cells compared with non-transfused patients (P = 0.003). However, there was a significant suppression of HCV-specific IFN-γ producing cells in patients who were transfused (P = 0.002).
Figure 2.
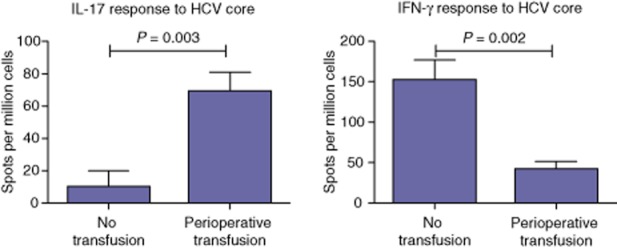
Increased hepatitis C virus (HCV)-specific interleukin-17 (IL-17) and decreased interferon-γ (IFN-γ) secretion in HCV-infected liver transplant patients who received perioperative blood transfusions, determined by enzyme-linked immunospot (ELISpot) comparing IL-17 and IFN-γ responses to HCV core antigen. Peripheral blood mononuclear cells (PBMC) at 3 × 105 per well were stimulated with HCV core antigen of human immunodeficiency virus (HIV) Gp120 peptide (negative control) or PHA (positive control) at 5 μg/ml concentration, and IL-17 and IFN-γ spots enumerated. Data are presented as the mean ± standard error number of spots per million cells and compared using the Mann–Whitney U-test
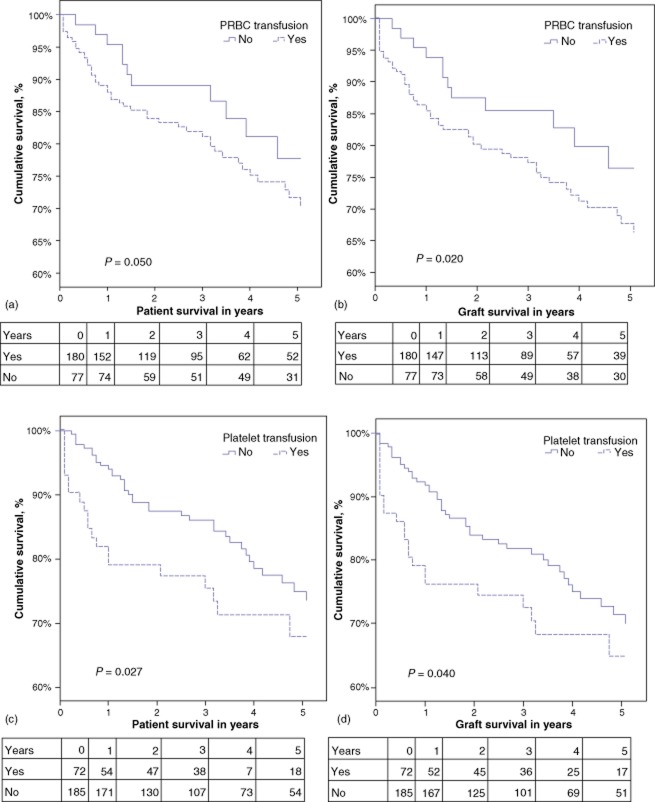
HCV LT patients administered with PRBC or platelets have decreased patient and graft survival rates
Patient and graft survival were analysed using Kaplan–Meier survival curves and log-rank tests. A comparison of transfused and non-transfused patients showed survival to be significantly lower in the transfused group (n = 192) at 1 year (88% versus 95%; P = 0.02) and at 5 years (66% versus 77%; P = 0.05). In particular, as Fig. 3 (a, b) shows, LT patients who received PRBC had significantly decreased 1-year and 5-year patient and graft survival compared with those who did not. Similarly, HCV LT recipients who received platelets had significantly lower survival than those who did not (Fig. 3c, d) at both 1 year and 5 years.
Figure 3.
Kaplan–Meier survival curves depicting overall patient and graft survival in hepatitis C virus (HCV)-infected liver transplant patients who were or were not perioperatively transfused with (a, b) packed red blood cells (PRBC) or (c, d) platelets
Patients transfused with PRBC were further stratified into subgroups of patients who received, respectively, 1–3 units, 4–6 units or ≥ 7 units of blood. As Fig. 4 shows, patients who received ≥ 7 units of blood had the lowest 1-and 5-year patient survival of all the groups. There was no effect of FFP, cryoprecipitate or whole blood transfusion on overall transplant outcomes.
Figure 4.
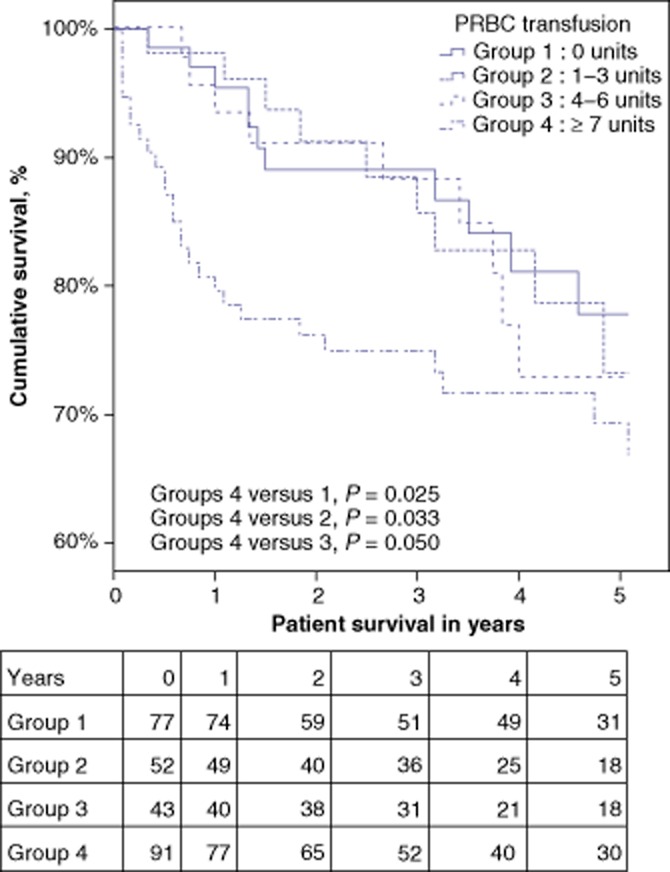
Kaplan–Meier survival curves for hepatitis C virus (HCV)-infected liver transplant patients stratified by number of perioperative units of packed red blood cells (PRBC) transfused. Group 1: 0 units; Group 2: 1–3 units; Group 3: 4–6 units; Group 4: ≥ 7 units
Multivariate regression analysis demonstrates that donor age and transfusion of platelets or PRBC are independent predictors of post-LT survival in HCV LT patients
To determine variables affecting post-LT survival, a univariate regression analysis was performed. All factors that were found to differ significantly between the cohorts (Table 1) were included in the univariate regression model. Donor age, pre-transplant platelet count, warm ischaemia time, transfusion of platelets, FFP or PRBC caused a higher hazard to patient survival (P < 0.1) (Table 5). Variables such as MELD score and bilirubin level were not associated with patient survival. These variables were then selected and analysed in a multivariate Cox regression model. As Table 6 shows, donor age [hazard ratio (HR) = 1.019, 95% CI 1.004–1.034; P = 0.003], transfusion of platelets (HR = 1.069, 95% CI 1.008–1.134; P = 0.026) and PRBC transfusion (HR = 1.364, 95% CI 1.116–1.666; P = 0.002) were independently associated with poor patient survival. The P-value for the constructed Cox model comparing survival differences between the transfused and non-transfused cohorts was < 0.0001.
Table 5.
Univariate regression analysis of variables affecting overall survival after liver transplantation (LT) in hepatitis C virus (HCV)-infected patients. Variables associated with mortality (P < 0.1) were utilized to construct a multivariate Cox regression model (Table 6)
| Variable | HR (95% CI) | P-value |
|---|---|---|
| Recipient age | 0.982 (0.947–1.018) | 0.323 |
| Recipient race (African-American) | 3.09 (0.334–4.537) | 0.633 |
| Recipient sex (male) | 1.04 (0.507–1.579) | 0.834 |
| Donor age | 1.019 (1.004–1.034) | 0.011 |
| Donor sex (male) | 1.003 (0.956–1.225) | 0.524 |
| Pre-LT PT/INR | 0.972 (0.706–1.337) | 0.861 |
| Pre-LT MELD score | 1.012 (0.985–1.039) | 0.4 |
| Pre-LT serum bilirubin | 0.996 (0.964–1.029) | 0.825 |
| Pre-LT haemoglobin | 1.006 (0.901–1.123) | 0.919 |
| Pre-LT platelet count | 1.004 (1.000–1.008) | 0.067 |
| Cold ischaemia time | 0.999 (0.997–1.001) | 0.213 |
| Warm ischaemia time | 0.968 (0.935–1.002) | 0.067 |
| Perioperative platelet transfusion | 1.049 (1.03–1.068) | <0.01 |
| Perioperative FFP transfusion | 1.065 (1.036–1.094) | <0.01 |
| Perioperative cryoprecipitate transfusion | 1.089 (0.940–1.262) | 0.254 |
| Perioperative PRBC transfusion | 1.339 (1.182–1.518) | <0.01 |
| Rejection | 1.003 (0.879–1.122) | 0.774 |
| Complications | 1.045 (0.344–4.557) | 0.559 |
HR, hazard ratio; 95% CI, 95% confidence interval; PT, prothrombin time; INR, international normalized ratio; MELD, Model for End-stage Liver Disease; FFP, fresh frozen plasma; PRBC, packed red blood cells.
Values in bold indicate statistical significance (P < 0.05).
Table 6.
Multivariate Cox proportional hazard model determining variables that are independently associated with post-transplant mortality in hepatitis C virus (HCV)-infected liver transplant patients
| Variable | Adjusted HR (95% CI) | P-value |
|---|---|---|
| Donor age | 1.019 (1.004–1.034) | 0.003 |
| Pre-LT platelet count | 1.005 (0.993–1.010) | 0.067 |
| Warm ischaemia time | 0.965 (0.930–1.002) | 0.061 |
| Perioperative platelet transfusion | 1.069 (1.008–1.134) | 0.026 |
| Perioperative FFP transfusion | 1.080 (0.930–1.254) | 0.314 |
| Perioperative PRBC transfusion | 1.364 (1.116–1.666) | 0.002 |
HR, hazard ratio; 95% CI, 95% confidence interval; FFP, fresh frozen plasma; PRBC, packed red blood cells.
Values in bold indicate statistical significance (P < 0.05).
Discussion
Blood transfusion is commonly used in LT recipients and critically ill patients to increase the oxygen-carrying capacity of blood and for volume replacement. Fresh frozen plasma, platelets and cryoprecipitates also help to correct coagulopathies.42 However, their utilization is associated with risk for transfusion reactions ranging from hypersensitivity and serum sickness to anaphylaxis and transfusion-associated lung injury. Currently, it is not clear whether blood transfusion during the perioperative period affects longterm outcomes in LT recipients, especially patients with HCV. This is of relevance because other peri-transplant factors, including ischaemia–reperfusion injury, have been associated with the worsening of HCV recurrence following LT.9 This study evaluated the role of perioperative blood product administration in overall transplant outcome, as well as HCV-specific immune responses in HCV LT recipients. These findings agree with those published by others demonstrating a significant effect of PRBC and platelet transfusion on survival in LT patients.38,39 Ramos et al. demonstrated that transfusion of > 6 units of PRBC was not only associated with poor survival, but also resulted in an increased hospital stay (OR = 3.06; P = 0.032).39 Similarly, in the present study, transfusion was associated with increased ICU and hospital stay, as well as with poor post-transplant survival, especially in patients receiving ≥ 7 units of blood products. Nacoti et al.43 found PRBC transfusion to be significantly associated with poor 1-year survival in a cohort of 243 paediatric LT patients (HR = 3.15; P = 0.033) and confirmed this finding with an independent risk-adjusted analysis similar to the multivariate regression model used in the present study. Although it can be argued that sicker patients do worse, have an increased hospital stay and transfusion requirement and hence a poorer outcome, the multivariate regression and the propensity score-adjusted models were used to account for such differences. As Tables 5 and 6 show, PRBC remains an independent predictor of poor outcome even when variables such as a high MELD score, which are reflective of degree of sickness or coagulopathy in LT recipients, are accounted for.
Historically, in animal models of transplantation, preoperative blood transfusions were described as significantly improving graft outcome.13,19 This was also noted in clinical renal transplantation, especially in large studies by Opelz et al. of cadaveric kidney transplants, and in many other independent reports.14–16,34,37 It was hypothesized that blood products result in immunosuppression and that leukocytes expressing major histocompatibility complex (MHC) antigens partly mediate this effect.36,44 Terasaki postulated that the beneficial effect of transfusion is attributable to clonal deletion.45 This was particularly relevant in instances of peri-and postoperative transfusion. According to this theory, transplantation resulted in an immune response affecting memory cells that led to the activation of clones directed towards the graft. Blood transfusion was thought to counteract this by hyperimmunizing the patient to multiple antigens, and engaging and inactivating memory responses, and protecting the graft.
However, a major concern in the use of random transfusion was that patients receiving transfusions had a higher predisposition to develop antibodies against human leukocyte antigen (anti-HLA) and thereby become sensitized against potential donors (although this was more relevant in kidney transplantation).14 This sensitization occurred as a result of HLA class I and II expressed on leukocytes in transfused blood46 and, therefore, leukocyte-depleting strategies were employed to prevent allo-sensitization.
Although these data are not conclusive, this immunosuppressive benefit of blood transfusion was seen in part in the present study, in which HCV LT recipients who received 1–2 units of blood seemed to have a reduced incidence of acute rejection. However, the overall incidence of acute rejection in patients who received > 2 units of blood was similar to that in the non-transfused LT patient cohort. Further, there was no distinct survival benefit conferred by the receipt of < 2 units of blood (Fig. 4). Hence, because of the small number of patients included in the present sample, it was difficult to conclude a definite correlation between acute rejection and transfusion.
Sensitization to HLA can occur following transfusion, which is relevant in kidney transplantation. However, recent prospective research from the Mayo Clinic, by Taner et al., demonstrated that donor-specific antibodies (DSA) against donor HLA may not have important clinical impact following LT and that even patients with pre-LT DSA clear it soon after transplant.47 In this centre, pre-and post-transplant detection of sensitization to donor HLA is not routinely performed in LT recipients and liver donors are not usually typed for HLA; hence, this study was unable to evaluate rates of allo-sensitization in the present patient cohorts, especially after transfusion. This may represent an important avenue of research in future studies.
It is important to note that most of the studies demonstrating beneficial effects of blood transfusion on allograft outcome were carried out before the use of cyclosporine became prevalent. Studies in the late 1980s and 1990s demonstrated that this apparent effect of blood transfusion may not necessarily be useful.48,49 Lundgren et al. reported no demonstrable difference in patient or graft survival among patients who were or were not transfused among 928 kidney transplant recipients receiving calcineurin inhibitor-based immunosuppression.48 Additional reports established a detrimental effect of blood transfusion on longterm graft function and patient outcomes.35,49 Hence, this concept was largely abandoned as newer and better immunosuppressive drugs became available.
Although most of these studies investigating blood transfusion and transplant outcomes were conducted in the field of kidney transplantation, studies in liver transplantation in both the adult and paediatric settings have demonstrated that PRBC and platelet administration can have significant deleterious effects on transplant outcome.38,39,43 Further, higher incidences of postoperative infection in transfused patients have been reported in a variety of surgical settings.27,28,33,42,50 Intraoperative transfusion has also been associated with an increase in the incidence of cancer recurrence in colon cancer patients.50 In the present series, although the non-transfused cohort included more patients with HCC attributable to HCV, incidences of recurrent HCC were similar in both groups (approximately 2%). Similarly, there were no differences in postoperative infection/complication rates, including the reactivation of viral infections such as CMV.
Several laboratories have reported that transfusion can exert immunomodulatory effects. Blood products directly inhibit cellular responses by suppressing antigen presentation and natural killer cell activity.51,52 More importantly, both in vitro and in vivo studies have demonstrated that allogeneic blood product administration can alter the cytokine milieu and mediate a switch of Th1 (producing IFN-γ) cells to Th2 (producing IL-10, IL-4 and IL-5) cells.53,54 These effects have important implications in relation to HCV-specific immune responses. Natural killer cells are an important first line of host defence, especially in the early period of HCV infection.55 Infection of the new graft liver with HCV occurs soon after reperfusion9 and thus perioperative transfusion may affect early innate immune responses directed towards HCV. In addition, the current study found that patients who were transfused showed suppressed Th1 responses in association with a decrease in serum IFN-γ. It is important to note that there was no difference in the immunological profiles of these patients pre-transplant. However, post-transplant, even as early as 1 month, a significant difference in the cytokine profiles of the transfused and non-transfused cohorts emerged. This effect was apparent after any potential confounders, such as presence of HCC, rejection episodes, infections, antiviral therapy and further transfusions, were eliminated.
Transfused patients also had significantly higher serum levels of IL-6, IL-1β and transforming growth factor-β (TGF-β). These cytokines favour the differentiation of Th17 cells.11 This may also explain the significantly higher HCV-specific IL-17 response at 1 year post-LT (Fig. 2). Early events in the post-LT period are known to affect the longterm post-LT course of HCV infection.7 Because the changes in serum cytokines were noted in the period soon after transplant (Table 4), it is possible that these early effects on the immune response can skew the immunological milieu to favour HCV replication as seen in the significant increase in viral load post-transplant, especially in the transfused cohort. In addition, most patients received transfusions either during or immediately after transplantation. None of the patients in the non-transfused cohort received any transfusions in the later postoperative period, especially when the cytokine profile of HCV viral load was assessed. Although some patients were transfused in the first week post-transplant, these additional transfusions in a small number of patients did not affect overall results in the present study.
Investigations conducted in the study centre laboratory have indicated that the increase in IL-17 and suppression of IFN-γ, particularly to HCV antigens, favours the progression of HCV associated with the development of liver fibrosis post-transplantation.11 Hence, the increase in proinflammatory cytokines (IL-17, TGF-β, IL-4, etc.) following transfusion may favour persistent replication of the virus that may lead to liver injury and the development of fibrosis. By contrast with those of Rice et al.,40 the present findings indicate that blood transfusion is associated with increased HCV recurrence and advanced liver fibrosis. The recurrence of HCV itself post-transplant is universal,56 but incidences of progression of advanced liver fibrosis within 1 year of transplant vary and can reach 30–40% post-LT.5,56,57 These are similar to the rates observed in the present study and reflect a variety of factors, including the viral genotype, immunosuppression and the immunological milieu.5,11,56 The current study determined an association of blood transfusion with worsened HCV recurrence, which may be partly explained by the upregulation of IL-17 seen in these patients.
Given the present findings, it would appear that transfusion practices in HCV LT recipients should be judicious and unnecessary transfusion should be avoided as much as possible. Factors such as a high MELD score, low haemoglobin and evidence of coagulopathy (Table 3) are predictive of blood product requirement during transplant. In such patients, strategies such as pre-transplant nutritional build-up to raise iron stores and haematocrit, the use of erythropoietin, the minimizing of intraoperative blood loss and the use of autotransfusion methods may be considered to avoid the deleterious effects of allogeneic blood transfusion.
The limitations of this study include its retrospective nature. The effect of pre-LT transfusion on outcomes could not be determined as a result of insufficient data. Comorbid conditions, including diabetes, hypertension and CMV infection, and the use of antiviral therapy, were accounted for in the multivariate analysis. Blood samples and biopsy tissue were not available universally at different time-points, and thus immunological analysis was not performed in all patients. Additionally, as there are no widely accepted and standardized guidelines for transfusion policy in LT, differences attributable to the clinical judgements of the various clinicians involved in the decision for transfusion (anaesthesiologists, critical care physicians and surgeons) could not be included in the analysis. This study established clear associations of PRBC and platelet transfusion with transplant outcome, but did not show any effect of FFP, cryoprecipitate or whole blood transfusion. Nacoti et al. demonstrated that FFP transfusion in paediatric LT patients was associated with worse survival.43 The effect of whole blood was not apparent in the present study because its usage was relatively low (10% of patients).
In summary, this study demonstrates that blood product administration in the perioperative period is associated with a worsened outcome in HCV LT recipients. Perioperative transfusion may be associated with an early modulation of immune responses that sets up an inflammatory milieu leading to a suppression of HCV-specific IFN-γ and activation of IL-17. These early changes may alter the progression of HCV in the allograft and may lead to advanced fibrosis, as well as a poor outcome following LT in HCV recipients. Hence, perioperative transfusion in HCV LT recipients should be judiciously utilized to improve longterm outcomes in HCV LT patients.
Acknowledgments
The authors would like to thank Billie Glasscock, Department of Surgery, Washington University School of Medicine, for assistance in the preparation of this manuscript.
Conflicts of interest
None declared.
Funding: TM and WCC are supported by the BJC Foundation.This manuscript was presented at the 10th World IHPBA Congress, Paris,1–5 July 2012.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55(Suppl. 1):10–15. doi: 10.1093/cid/cis361. [DOI] [PubMed] [Google Scholar]
- 3.McCashland TM. Management of liver transplant recipients with recurrent hepatitis C. Curr Opin Organ Transplant. 2009;14:221–224. doi: 10.1097/MOT.0b013e32832ade76. [DOI] [PubMed] [Google Scholar]
- 4.Chinnadurai R, Velazquez V, Grakoui A. Hepatic transplant and HCV: a new playground for an old virus. Am J Transplant. 2012;12:298–305. doi: 10.1111/j.1600-6143.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973–978. doi: 10.1038/nature04083. [DOI] [PubMed] [Google Scholar]
- 6.Rubin A, Aguilera V, Berenguer M. Liver transplantation and hepatitis C. Clin Res Hepatol Gastroenterol. 2011;35:805–812. doi: 10.1016/j.clinre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian V, Seetharam AB, Vachharajani N, Tiriveedhi V, Angaswamy N, Ramachandran S, et al. Donor graft steatosis influences immunity to hepatitis C virus and allograft outcome after liver transplantation. Transplantation. 2011;92:1259–1268. doi: 10.1097/TP.0b013e318235a1ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesner RH, Sorrell M, Villamil F. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9((Suppl):1–9. doi: 10.1053/jlts.2003.50268. [DOI] [PubMed] [Google Scholar]
- 9.Gruener NH, Jung MC, Schirren CA. Recurrent hepatitis C virus infection after liver transplantation: natural course, therapeutic approach and possible mechanisms of viral control. J Antimicrob Chemother. 2004;54:17–20. doi: 10.1093/jac/dkh297. [DOI] [PubMed] [Google Scholar]
- 10.Ghobrial RM, Steadman R, Gornbein J, Lassman C, Holt CD, Chen P, et al. A 10-year experience of liver transplantation for hepatitis C: analysis of factors determining outcome in over 500 patients. Ann Surg. 2001;234:384–393. doi: 10.1097/00000658-200109000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basha HI, Subramanian V, Seetharam A, Nath DS, Ramachandran S, Anderson CD, et al. Characterization of HCV-specific CD4+ Th17 immunity in recurrent hepatitis C-induced liver allograft fibrosis. Am J Transplant. 2011;11:775–785. doi: 10.1111/j.1600-6143.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat A, Barros F, Narayanan K, Borg B, Lisker-Melman M, Shenoy S, et al. Characterization of virus-specific T-cell immunity in liver allograft recipients with HCV-induced cirrhosis. Am J Transplant. 2008;8:1214–1220. doi: 10.1111/j.1600-6143.2008.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halasz NA, Orloff MJ, Hirose F. Increased survival of renal homografts in dogs after injection of graft donor blood. Transplantation. 1964;2:453–458. doi: 10.1097/00007890-196407000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–259. [PubMed] [Google Scholar]
- 15.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 16.Werner-Favre C, Jeannet M, Harder F, Montandon A. Blood transfusions, cytotoxic antibodies, and kidney graft survival. Preliminary results of a systematic transfusion protocol. Transplantation. 1979;28:343–346. doi: 10.1097/00007890-197910000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Claas FH, Blankert JJ, Ruigrok R, Moerel L. Platelet transfusions can induce transplantation tolerance. Immunol Lett. 1982;5:35–39. doi: 10.1016/0165-2478(82)90088-8. [DOI] [PubMed] [Google Scholar]
- 18.Oh JH, McClure HM. Non-specific immunosuppressive effect of platelet transfusion in rhesus monkeys. Transplantation. 1987;43:304–305. [PubMed] [Google Scholar]
- 19.Martin DC, Hewitt CW, Osborne JG, Dowdy SF, Fristoe TL, Russell LA, et al. Enhanced kidney graft survival in rats by single or multiple blood transfusion(s) and various blood products. Transplant Proc. 1982;14:407–409. [PubMed] [Google Scholar]
- 20.Opelz G, Vanrenterghem Y, Kirste G, Gray DW, Horsburgh T, Lachance JG, et al. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–967. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 21.Blajchman MA. Immunomodulation and blood transfusion. Am J Ther. 2002;9:389–395. doi: 10.1097/00045391-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Clark DA, Gorczynski RM, Blajchman MA. Transfusion-related immunomodulation due to peripheral blood dendritic cells expressing the CD200 tolerance signalling molecule and alloantigen. Transfusion. 2008;48:814–821. doi: 10.1111/j.1537-2995.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 23.Landers DF, Hill GE, Wong KC, Fox IJ. Blood transfusion-induced immunomodulation. Anesth Analg. 1996;82:187–204. doi: 10.1097/00000539-199601000-00035. [DOI] [PubMed] [Google Scholar]
- 24.Blumberg N, Triulzi DJ, Heal JM. Transfusion-induced immunomodulation and its clinical consequences. Transfus Med Rev. 1990;4((Suppl. 1):24–35. doi: 10.1016/s0887-7963(90)70239-8. [DOI] [PubMed] [Google Scholar]
- 25.Diepenhorst P, Engelfriet CP. Removal of leukocytes from whole blood and erythrocyte suspensions by filtration through cotton wool. V. Results after transfusion of 1820 units of filtered erythrocytes. Vox Sang. 1975;29:15–22. doi: 10.1111/j.1423-0410.1975.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 26.Chavers BM, Sullivan EK, Tejani A, Harmon WE. Pre-transplant blood transfusion and renal allograft outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant. 1997;1:22–28. [PubMed] [Google Scholar]
- 27.Perisanidis C, Dettke M, Papadogeorgakis N, Schoppmann A, Mittlbock M, Kyzas PA, et al. Transfusion of allogenic leukocyte-depleted packed red blood cells is associated with postoperative morbidity in patients undergoing oral and oropharyngeal cancer surgery. Oral Oncol. 2012;48:372–378. doi: 10.1016/j.oraloncology.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Lujan JJ, Nemeth ZH, Barratt-Stopper PA, Bustami R, Koshenkov VP, Rolandelli RH. Factors influencing the outcome of intestinal anastomosis. Am Surg. 2011;77:1169–1175. [PubMed] [Google Scholar]
- 29.Tuinman PR, Vlaar AP, Cornet AD, Hofstra JJ, Levi M, Meijers JC, et al. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: a case control study. Crit Care. 2011;15:59. doi: 10.1186/cc10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napolitano LM. Blood transfusion and the lung: first do no harm? Crit Care. 2011;15:152. doi: 10.1186/cc10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraris VA, Davenport DL, Saha SP, Bernard A, Austin PC, Zwischenberger JB. Intraoperative transfusion of small amounts of blood heralds worse postoperative outcome in patients having non-cardiac thoracic operations. Ann Thorac Surg. 2011;91:1674–1680. doi: 10.1016/j.athoracsur.2011.01.025. discussion 1680. [DOI] [PubMed] [Google Scholar]
- 32.Shander A, Fink A, Javidroozi M, Erhard J, Farmer SL, Corwin H, et al. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232–246. doi: 10.1016/j.tmrv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Shander A, Goodnough LT. Why an alternative to blood transfusion? Crit Care Clin. 2009;25:261–277. doi: 10.1016/j.ccc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Brynger H, Frisk B, Sandberg L, Gelin LE. Renal graft rejection and blood transfusion before and during the transplant operation. Scand J Urol Nephrol. 1978;12:271–273. doi: 10.3109/00365597809179729. [DOI] [PubMed] [Google Scholar]
- 35.Bucin D. Adverse effect of blood transfusion on the longterm outcome of kidney transplantation. Exp Clin Immunogenet. 1988;5:39–47. [PubMed] [Google Scholar]
- 36.George CD, Morello PJ. Immunologic effects of blood transfusion upon renal transplantation, tumour operations, and bacterial infections. Am J Surg. 1986;152:329–337. doi: 10.1016/0002-9610(86)90269-2. [DOI] [PubMed] [Google Scholar]
- 37.Hourmant M, Soulillou JP, Bui-Quang D. Beneficial effect of blood transfusion. Role of the time interval between the last transfusion and transplantation. Transplantation. 1979;28:40–43. [PubMed] [Google Scholar]
- 38.de Boer MT, Christensen MC, Asmussen M, van der Hilst CS, Hendriks HG, Slooff MJ, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106:32–44. doi: 10.1213/01.ane.0000289638.26666.ed. [DOI] [PubMed] [Google Scholar]
- 39.Ramos E, Dalmau A, Sabate A, Lama C, Llado L, Figueras J, et al. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003;9:1320–1327. doi: 10.1016/jlts.2003.50204. [DOI] [PubMed] [Google Scholar]
- 40.Rice MJ, Wendling A, Firpi RJ, Hemming AW, Nelson DR, Schwab WK, et al. Transfusion has no effect on recurrence in hepatitis C after liver transplantation. Acta Anaesthesiol Scand. 2010;54:1224–1232. doi: 10.1111/j.1399-6576.2010.02313.x. [DOI] [PubMed] [Google Scholar]
- 41.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 42.McCrossan L, Masterson G. Blood transfusion in critical illness. Br J Anaesth. 2002;88:6–9. doi: 10.1093/bja/88.1.6. [DOI] [PubMed] [Google Scholar]
- 43.Nacoti M, Cazzaniga S, Lorusso F, Naldi L, Brambillasca P, Benigni A, et al. The impact of perioperative transfusion of blood products on survival after paediatric liver transplantation. Pediatr Transplant. 2012;16:357–366. doi: 10.1111/j.1399-3046.2012.01674.x. [DOI] [PubMed] [Google Scholar]
- 44.Woodruff MF, van Rood JJ. Possible implications of the effect of blood transfusion on allograft survival. Lancet. 1983;1:1201–1203. doi: 10.1016/s0140-6736(83)92476-5. [DOI] [PubMed] [Google Scholar]
- 45.Terasaki PI. The beneficial transfusion effect on kidney graft survival attributed to clonal deletion. Transplantation. 1984;37:119–125. doi: 10.1097/00007890-198402000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Balasubramaniam GS, Morris M, Gupta A, Mesa IR, Thuraisingham R, Ashman N. Allosensitization rate of male patients awaiting first kidney grafts after leuko-depleted blood transfusion. Transplantation. 2012;93:418–422. doi: 10.1097/TP.0b013e3182419864. [DOI] [PubMed] [Google Scholar]
- 47.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 48.Lundgren G, Groth CG, Albrechtsen D, Brynger H, Flatmark A, Frodin L, et al. No difference in outcome between 314 non-transfused and 614 transfused cadaveric renal transplant recipients: the Scandinavian experience. Clin Transpl. 1987:249–255. [PubMed] [Google Scholar]
- 49.Ross WB, Yap PL. Blood transfusion and organ transplantation. Blood Rev. 1990;4:252–258. doi: 10.1016/0268-960x(90)90005-d. [DOI] [PubMed] [Google Scholar]
- 50.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–310. [PubMed] [Google Scholar]
- 52.Ghio M, Contini P, Negrini S, Mazzei C, Zocchi MR, Poggi A. Downregulation of human natural killer cell-mediated cytolysis induced by blood transfusion: role of transforming growth factor-beta(1), soluble Fas ligand, and soluble Class I human leukocyte antigen. Transfusion. 2011;51:1567–1573. doi: 10.1111/j.1537-2995.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 53.Pandey P, Chaudhary R, Aggarwal A, Kumar R, Khetan D, Verma A. Transfusion-associated immunomodulation: quantitative changes in cytokines as a measure of immune responsiveness after one time blood transfusion in neurosurgery patients. Asian J Transfus Sci. 2010;4:78–85. doi: 10.4103/0973-6247.67021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Mast BJ, Vietor HE, van der Meer-Prins EM, van Bree SP, Brand A, van den Elsen PJ, et al. Modulation of the T cell compartment by blood transfusion. Effect on cytotoxic and helper T lymphocyte precursor frequencies and T cell receptor Vbeta usage. Transplantation. 1997;63:1145–1154. doi: 10.1097/00007890-199704270-00015. [DOI] [PubMed] [Google Scholar]
- 55.Bozzano F, Marras F, Biassoni R, Maria AD. Natural killer cells in hepatitis C virus infection. Expert Rev Clin Immunol. 2012;8:775–788. doi: 10.1586/eci.12.71. [DOI] [PubMed] [Google Scholar]
- 56.Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9((Suppl):28–34. doi: 10.1053/jlts.2003.50248. [DOI] [PubMed] [Google Scholar]
- 57.Cameron AM, Ghobrial RM, Hiatt JR, Carmody IC, Gordon SA, Farmer DG, et al. Effect of non-viral factors on hepatitis C recurrence after liver transplantation. Ann Surg. 2006;244:563–571. doi: 10.1097/01.sla.0000237648.90600.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]