Key Points
A novel TKI is discovered with potent and selective activity against FLT3-mutant cell lines and primary patient samples.
TTT-3002 is effective in vivo in several mouse tumor models of FLT3/ITD-associated AML with minimal toxicity.
Abstract
More than 35% of acute myeloid leukemia (AML) patients harbor a constitutively activating mutation in FMS-like tyrosine kinase-3 (FLT3). The most common type, internal tandem duplication (ITD), confers poor prognosis. We report for the first time on TTT-3002, a tyrosine kinase inhibitor (TKI) that is one of the most potent FLT3 inhibitors discovered to date. Studies using human FLT3/ITD mutant leukemia cell lines revealed the half maximal inhibitory concentration (IC50) for inhibiting FLT3 autophosphorylation is from 100 to 250 pM. The proliferation IC50 for TTT-3002 in these same cells was from 490 to 920 pM. TTT-3002 also showed potent activity when tested against the most frequently occurring FLT3-activating point mutation, FLT3/D835Y, against which many current TKIs are ineffective. These findings were validated in vivo by using mouse models of FLT3-associated AML. Survival and tumor burden of mice in several FLT3/ITD transplantation models is significantly improved by administration of TTT-3002 via oral dosing. Finally, we demonstrated that TTT-3002 is cytotoxic to leukemic blasts isolated from FLT3/ITD-expressing AML patients, while displaying minimal toxicity to normal hematopoietic stem/progenitor cells from healthy blood and bone marrow donors. Therefore, TTT-3002 has demonstrated preclinical potential as a promising new FLT3 TKI in the treatment of FLT3-mutant AML.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by myeloproliferation and a block in cellular differentiation, leading to expansion of immature blasts in the bone marrow (BM) and peripheral blood (PB).1 This disease causes approximately 7000 deaths in the United States annually.1 FMS-like tyrosine kinase-3 (FLT3) is a receptor tyrosine kinase expressed in hematopoietic stem/progenitor cells.2 Upon binding FLT3 ligand, the receptor undergoes dimerization and activation of its kinase domain resulting in autophosphorylation and phosphorylation of substrate proteins with subsequent stimulation of downstream signaling cascades via the STAT5, PI3K/AKT, and RAS/MAPK pathways.3,4 Approximately 35% of AML patients harbor a mutation in the FLT3 gene leading to constitutive activation.5,6 The most commonly observed mutation (25% of AML patients) occurs in the juxtamembrane domain as an internal tandem duplication (FLT3/ITD) of variable-length sequence repeats.7 Activating point mutations (FLT3/PM) are also observed in 7% to 10% of patients.8 Constitutive activation of FLT3 in a ligand-independent manner provides a survival and proliferative advantage to cells.9 ITD mutations in FLT3 in particular confer a poor prognosis, and many studies are directed at developing and testing novel FLT3 inhibitors for the treatment of AML.10-12
Several clinical trials are now underway for novel tyrosine kinase inhibitors (TKIs) that target FLT3, such as sorafenib (developed as an inhibitor of Raf-1 kinase and approved for advanced renal cell carcinoma and hepatocellular carcinoma),13-16 lestaurtinib (CEP-701; completed phase 3 trials in AML),17,18 quizartinib (AC220; completed phase 2 trials for relapsed/refractory AML),19 and midostaurin (PKC412; currently in phase 3 trials).19,20 While some interim results are encouraging, overall there have been limitations in the responses observed in patients on these trials, often related to insufficient achievement of FLT3 inhibition and the emergence of resistance mutations in FLT3/ITD.21-25 Therefore, the search for novel FLT3 TKIs with improved potency against FLT3/ITD- and FLT3/PM-induced phosphorylation is necessary to improve the cure rate of this disease.
In this study, we demonstrate that TTT-3002 is the most potent FLT3 inhibitor to date, with picomolar half maximal inhibitory concentrations (IC50s) for autophosphorylation of FLT3/ITD and FLT3/PM mutations. This compound is also well tolerated and effective in vivo in a variety of xenograft mouse models of FLT3-mutant AML. Further studies reveal that TTT-3002 is active against FLT3-expressing blasts from primary patient samples while showing minimal toxicity to normal BM mononuclear cells and CD34+ progenitors at biochemically relevant doses, making it a promising new candidate for the treatment of AML.
Methods
Compounds
TTT-3002 was a generous gift of TauTaTis, Inc. (San Diego, CA). CEP-701, AC220, and sorafenib were purchased from LC Laboratories. Compounds were dissolved in 100% dimethylsulfoxide (DMSO) and prepared as 10 µM stock solutions in RPMI with 0.1% DMSO and stored at −80°C.
Growth inhibition
Cells were seeded at a density of 1 to 2.5 × 105 cells per milliliter in the presence or absence of inhibitor for the indicated times. Viable cell counts were measured every 24 hours by Trypan blue exclusion assay. Cell proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide (MTT) assay according to the manufacturer’s instructions (Roche Applied Science). IC50s were calculated by linear regression analysis of optical density measured at 570 nm, relative to DMSO control (CalcuSyn; Biosoft). For the colony-forming unit (CFU) assay, cells were plated at a density of 5 × 102 cells per milliliter to 2 × 104 cells per milliliter in methylcellulose (MethoCult H4230 or M3434; Stem Cell Technologies) and incubated at 37°C. Colony counts (total and/or burst-forming unit erythroid, CFU-granulocyte-macrophage, CFU-granulocyte, and CFU-macrophage) were obtained 7 to 14 days after plating.
Immunoprecipitation and western blotting
Cells were cultured in the presence of inhibitor for 1 hour at 37°C, and FLT3 and phospho-FLT3 (pFLT3) expression were analyzed by performing immunoprecipitation of whole-cell extracts for FLT3 (S-18), followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting. Proteins were transferred to a polyvinyl difluoride membrane (Millipore) and probed for FLT3 and pFLT3 by using S-18 and 4G10 phosphotyrosine antibodies, respectively, followed by fluorescent anti-rabbit and anti-mouse secondary antibodies. FLT3 was then detected on a LI-COR Biosciences imager using Odyssey software. Other proteins were detected in whole-cell lysates by using the indicated antibodies and a horseradish peroxidase–conjugated goat anti-rabbit secondary antibody followed by enhanced chemiluminescence.
Transplantation experiments
Transplantation of Ba/F3-ITD luciferase (Luc) –expressing cells was performed as described previously.26 For engraftment, 2 × 106 cells were injected by tail vein into BALB/C mice (day 0). Starting 7 days later, mice were treated with 6 mg/kg of TTT-3002 hydrochloride suspended by brief sonication 1 hour prior to dosing in 1 mM HCl twice daily by oral gavage for 2 to 4 weeks. Mice were imaged by intraperitoneally injecting d-luciferin (3 mg) and visualizing on an IVIS Spectrum imager (Caliper LifeSciences) using Living Image software for analysis on day 7 postinoculation to monitor engraftment and once weekly thereafter to assess response to drug.
Transplantation of leukemic NUP98-HOXD13 (NHD13)/ITD BM was performed as described previously.27 In brief, 5 × 105 BM donor cells (CD45.2+) from leukemic mice, together with 5 × 105 CD45.1+ wild-type (WT) BM cells as helper cells, were injected into the lateral tail vein of sublethally irradiated (750 rads) C57BL/6 CD45.1+ recipient mice. PB was obtained from recipient mice every 2 weeks for assessment of complete PB counts and expression of CD45.1 and CD45.2 on the cell surface. Mice were treated with 6 mg/kg of TTT-3002 as described above or 10 mg/kg of sorafenib suspended in 30% (weight/volume) Cremophor EL, 30% weight/volume polyethylene glycol 400, 10% ethanol absolute proanalysis, and 10% glucose, as described previously (all Sigma-Aldrich) once daily by oral gavage.28 Disease progression was measured by white blood cell (WBC) counts, spleen weight, CD45.2 engraftment, and histopathology.
For histopathologic analysis, PB, femur, tibia, hips, and spine were isolated from NHD13/ITD or Ba/F3-ITD Luc+ transplant recipients. BM cells were isolated by crushing and cytospin preparation. BM and PB smears were stained with Wright-Giemsa stain (Richard-Allan Scientific). Slides were imaged on an Olympus BX46 microscope with an Olympus DP72 camera at ×20 and ×100 magnifications with 0.5 and 1.3 apertures, respectively. Olympus cellSens Standard 1.5 image acquisition software was used.
Patient samples
Human AML and normal BM samples were collected under an institutionally approved protocol with informed patient consent in accordance with the Declaration of Helsinki. Samples were obtained prior to therapy or at relapse. Mononuclear cells were isolated by Ficoll centrifugation and cryopreserved in liquid nitrogen until use. All studies used freshly thawed cells. CD34+ stem/progenitor cells were isolated from human cord blood (100 mL) by using the CD34 MicroBead Kit (Miltenyi Biotech) following the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with Student t test and log-rank test by using the GraphPad software analysis program (Prism). P values <.05 were considered to be statistically significant. All data are presented as the mean ± standard deviation (SD).
Results
Activity of TTT-3002 in FLT3-dependent leukemia cell lines
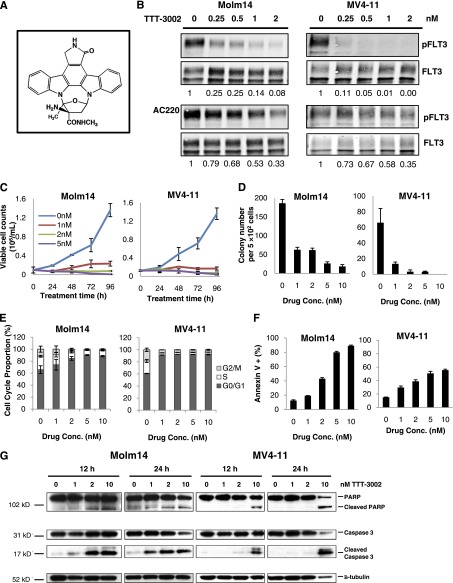
TTT-3002 is a small molecule kinase inhibitor of the indolocarbazole class with a molecular weight of 465 g/mol (Figure 1A).29 To determine whether TTT-3002 targets FLT3 kinase activity and inhibits autophosphorylation, we studied the effect of TTT-3002 treatment on the FLT3/ITD-expressing human leukemia cell lines Molm14 and MV4-11. Cells were subjected to 1 hour of treatment with increasing concentrations of TTT-3002 or AC220, previously the most potent published FLT3 TKI. Western blotting analysis showed that FLT3 phosphorylation was downregulated in a dose-dependent manner (Figure 1B). The IC50 for FLT3 phosphorylation in both cell lines was six- to sevenfold lower for TTT-3002 compared with AC220 at 0.2 vs 1.3 nM, respectively, making TTT-3002 the most potent FLT3 inhibitor investigated to date.
Figure 1.
TTT-3002 is a potent inhibitor of FLT3/ITD in AML cell lines. (A) Structure of TTT-3002. (B) Inhibition of pFLT3 in Molm14 and MV4-11 cells treated with TTT-3002 or AC220; fraction of pFLT3/FLT3 relative to DMSO control is indicated below each western blot. (C) Viable cell counts by Trypan blue exclusion assay; error bars represent average ± standard deviation (SD). (D) Inhibition of colony formation by TTT-3002; error bars represent average ± SD. (E) Cell cycle arrest at 24 hours following treatment with TTT-3002 (0 to 10 nM); error bars represent average ± SD. (F) Annexin V binding at 48 hours; data represent average percentage of Annexin V–positive cells ± SD. (G) Expression of proapoptotic markers, cleaved poly ADP ribose polymerase (PARP), and cleaved caspase-3 was increased at 12 and 24 hours by western blotting of Molm14 and MV4-11 cell lysates following treatment with TTT-3002 at the indicated concentrations.
We next studied the effect of TTT-3002 treatment on the viability of the Molm14 and MV4-11 cells. Exposure to this compound potently inhibited the rate of cell proliferation in culture at concentrations of 1 nM or greater and greatly decreased colony formation ability of these cells (Figure 1C-D). Cell cycle arrest followed by marked induction of apoptosis was observed by propidium iodide and Annexin V binding analysis at low concentrations of TTT-3002, along with concurrent activation of caspase 3 and poly ADP ribose polymerase cleavage (Figure 1E-G). Cytotoxic effects of TTT-3002, but not AC220, were also observed in the HB11;19 cell line, harboring an FLT3/D835H mutation, which renders it insensitive to treatment with AC220 (supplemental Figure 1A-C on the Blood Web site). No cytotoxic effects were observed in HL60 cells, an AML cell line that does not express FLT3 (supplemental Figure 2A-D).
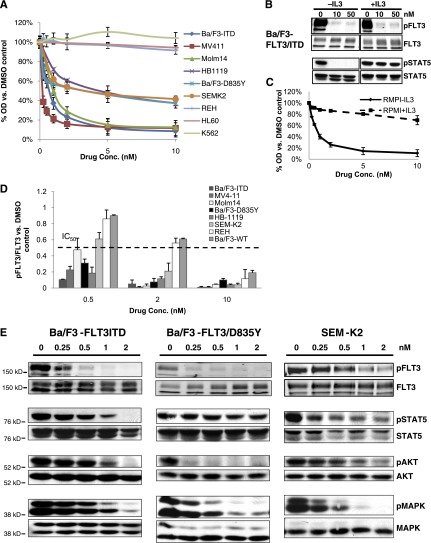
To further examine whether TTT-3002 was a relatively selective FLT3 inhibitor, we tested whether the drug would have a greater effect on FLT3-dependent cells (cells with FLT3-activating mutations or high levels of WT expression with autocrine activation) than on FLT3-independent cells (cells with low or no levels of WT FLT3 expression or autocrine activation). A panel of FLT3/ITD, FLT3/PM, and FLT3/WT cell lines were plated in increasing concentrations of TTT-3002 (0 to 200 nM), and cell proliferation was measured by MTT assay (Figure 2A). The mutation status and IC50 values of each cell line are summarized in Table 1. In cell lines expressing FLT3/ITD, TTT-3002 had IC50 values of <1 nM, which is similar to the activity of AC220 with regard to cell proliferation when compared side by side (supplemental Table 1). IC50s of 1 to 5 nM were noted against the FLT3/PM lines, and a highly expressed autocrine-activated FLT3/WT cell line had an IC50 of 3.5 nM. By comparison, AC220 had an IC50 of >100 nM against the FLT3/PM cell line HB11;19 (supplemental Table 1). In contrast, in the cell lines without FLT3 activation, TTT-3002 showed IC50s of 112 to >200 nM. Rescue of inhibition and cell viability was achieved in Ba/F3-FLT3/ITD cells through activation of parallel downstream signaling proteins such as interleukin-3 receptor stimulation of STAT5 signaling, providing further evidence that selective FLT3 inhibition by TTT-3002 caused the observed decreases in cell proliferation (Figure 2B-C).
Figure 2.
TTT-3002 is a kinase inhibitor of FLT3 signaling. (A) Proliferation of leukemia cell lines at 48 hours following treatment with TTT-3002, measured by MTT assay. Error bars represent average ± SD. Data are representative of 3 independent experiments. (B) STAT5 signaling is rescued by interleukin-3 (IL-3) stimulation. Expression of pFLT3 and pSTAT5 by western blotting of Ba/F3-FLT3/ITD cell lysates following treatment with TTT-3002 ± IL-3 (10 ng/mL). (C) Ba/F3-FLT3/ITD cell proliferation is rescued by IL-3 stimulation. Ba/F3-FLT3/ITD cells were plated in quadruplicate with TTT-3002 (0 to 10 nM) with and without IL-3 (10 ng/mL), and proliferation was measured at 48 hours by MTT assay. Error bars represent average of 3 independent experiments ± SD. (D) FLT3 phosphorylation is inhibited by TTT-3002 in FLT3/ITD, FLT3/PM, and FLT3/WT cell lines. Expression of FLT3 and pFLT3 was measured by western blotting following 1 hour of treatment with TTT-3002. Data from 3 independent experiments are represented as a fraction of pFLT3/FLT3 relative to DMSO control ± SD. IC50 is indicated on the graph. (E) FLT3/ITD (Ba/F3-FLT3/ITD), FLT3/PM (Ba/F3-FLT3/D835Y), and overexpressed FLT3/WT (SEM-K2) cells were treated with TTT-3002 for 1 hour, and expression of pFLT3, FLT3, pSTAT5, STAT5, pAKT, AKT, pMAPK, and MAPK were measured by western blotting. Data are representative of 3 independent experiments.
Table 1.
FLT3 mutational status and proliferation IC50 for TTT-3002 treatment of leukemia cell lines
| Cell line | FLT3 status | IC50 (nM) |
|---|---|---|
| Ba/F3-ITD | ITD | 0.62 |
| MV4-11 | ITD | <0.25 |
| Molm14 | ITD | 0.72 |
| HB-11;19 | PM | 4.24 |
| Ba/F3-D835Y | PM | 1.74 |
| SEM-K2 | WT (overexpressed with autocrine activation) | 3.45 |
| REH | WT | 112 |
| HL60 | No expression | 138.3 |
| K562 | No expression | >200 |
Proliferation IC50 (nM) measured after 48 hours of drug exposure (0 to 200 nM) in RPMI-1640 with 10% fetal bovine serum, relative to DMSO control, as in Figure 2A.
To assess the activity of TTT-3002 in inhibiting FLT3 phosphorylation, the leukemia cell line panel was subjected to treatment with TTT-3002 followed by western blotting analysis, which confirmed that FLT3 phosphorylation was potently downregulated in a dose-dependent manner (Figure 2D-E). We observed IC50 values of ∼100 to 250 pM for FLT3/ITD and FLT3/PM, and 500 pM for highly expressed autocrine-activated FLT3/WT cell lines. The IC50 was ∼10-fold higher (>2 nM) for REH and Ba/F3-FLT3/WT cells. We also observed that TTT-3002 treatment inhibited phosphorylation/activation of downstream targets of FLT3 signaling, including STAT5, AKT, and MAPK, in Ba/F3-FLT3/ITD, Ba/F3-FLT3/D835Y, and SEM-K2 cells (Figure 2E).
TTT-3002 is effective against FLT3/ITD mutations in vivo
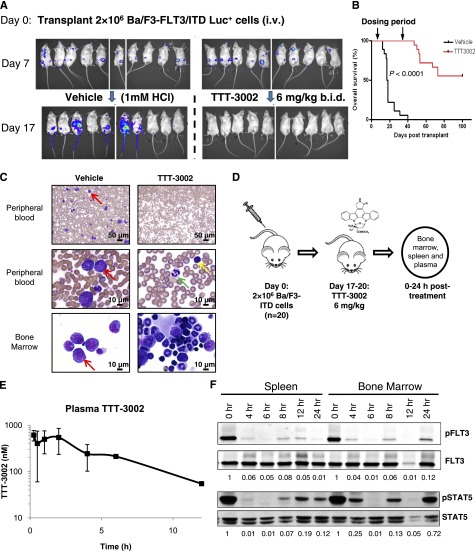
To assess the efficacy of TTT-3002 in vivo, we used a mouse model of leukemia in which transplantation of luciferase-expressing Ba/F3-FLT3/ITD cells (Ba/F3-ITD Luc+) was monitored with bioluminescence imaging, enabling us to measure the response of the leukemia cells in vivo at multiple time points. The Ba/F3-ITD Luc+ cell line maintained drug sensitivity in vitro by MTT and western blotting analyses with subnanomolar IC50 values (supplemental Figure 3A-B). The cells engrafted by day 7 following tail vein injection of 2 × 106 cells in BALB/c mice and tumor burden were measured by bioluminescence imaging periodically during a 4-week course of treatment. An oral dose of 6 mg/kg TTT-3002 twice per day was well-tolerated in BALB/c mice with no significant changes in animal weight and was sufficient to eliminate the presence of Ba/F3-ITD Luc+ cells by day 17 (10 days of treatment; Figure 3A). Mice treated with vehicle (1 mM HCl) alone succumbed to disease at a median of 17.5 days posttransplantation, whereas survival of TTT-3002–treated mice was significantly prolonged (P < .0001; Figure 3B). BM and PB samples revealed a predominance of leukemic blasts in vehicle-treated mice. In contrast, there was no evidence of disease in TTT-3002–treated mice at the end of treatment, and normal trilineage hematopoiesis was observed in the BM samples from these mice (Figure 3C). Relapse occurred in 44% of the mice in the TTT-3002–treated cohort after cessation of dosing at 4 weeks, with reappearance of luciferase-expressing cells. A small number of these mice were treated with TTT-3002 (6 mg/kg), and we observed decreases in tumor burden yet again, indicating that the cause of relapse was unlikely to be selection for resistance mutations, but rather that a minute population of leukemic cells was protected from drug exposure and allowed to expand upon cessation of treatment (data not shown). However, there was long-term survival (greater than 6 months posttransplantation) in all other cases.
Figure 3.
FLT3/ITD is an in vivo target of TTT-3002. BALB/c mice (female, age 6 to 8 weeks) received 2 × 106 Ba/F3-ITD Luc+ cells by tail vein injection on day 0. Engraftment was confirmed on day 7 by luminescence imaging, and mice were treated with TTT-3002 (6 mg/kg twice per day) or vehicle control (1 mM HCl twice per day) for 28 days. (A) Tumor cell abundance on days 7 and 17 by luminescence imaging; representative mice (n = 10) from each treatment cohort shown. (B) Overall survival of vehicle-treated (n = 18; median survival, 17.5 days) and TTT-3002–treated (n = 18; median survival, >100 days) mice at day 100 (P < .0001). (C) Histopathology of PB and BM preparations (Wright-Giemsa stain) of representative mice from each cohort euthanized at day 18 (vehicle) or day 35 (TTT-3002). Red arrows represent examples of leukemic blasts, yellow arrow represents a normal lymphocyte, and green arrow indicates a normal neutrophil. Slides were imaged on an Olympus BX46 microscope with an Olympus DP72 camera at ×20 and ×100 magnifications with 0.5 and 1.3 apertures, respectively; Olympus cellSens Standard 1.5 image acquisition software was used. (D) BALB/C mice (female, age 6 to 8 weeks) received 2 × 106 Ba/F3-ITD Luc+ cells by tail vein injection, and mice were administered a single dose of TTT-3002 (6 mg/kg) anywhere from day 17 to day 20 as they were succumbing to disease when they had high tumor burden. Spleen and BM cells and plasma samples were harvested from these mice at the indicated time points. (E) Total plasma concentrations of TTT-3002 as measured by liquid chromatography/tandem mass spectrometry; n = 3 mice per time point, represented as average ± SD. (F) pFLT3 and pSTAT5 are inhibited in vivo by the earliest time point (4 hours postdosing) and maintained for 12 to 24 hours. Fraction of pFLT3/FLT3 and pSTAT5/STAT5 relative to time 0 hours is indicated below each blot. Western blot is representative of 3 independent experiments.
Pharmacokinetics and pharmacodynamics of TTT-3002
The Ba/F3-ITD Luc+ transplant model was also used to study the pharmacodynamics of TTT-3002 in BALB/c mice. Because plasma inhibitory assay is used in human clinical trials of FLT3 TKIs, we wanted to determine whether the dose of 6 mg/kg was sufficient to achieve trough plasma concentrations capable of inhibiting FLT3 phosphorylation by >90%, a level of inhibition which has been shown to correlate with improved clinical outcome.18,21,22 We treated leukemic engrafted mice (confirmed by luminescence imaging) with a single 6-mg/kg dose of TTT-3002 and harvested spleen and BM cells and plasma at 0, 4, 6, 8, 12, and 24 hours (Figure 3D). Plasma was analyzed for total drug level by liquid chromatography/tandem mass spectrometry. After oral administration, TTT-3002 was rapidly absorbed with a biphasic maximum serum concentration (Cmax) followed by a monoexponential decay. The Cmax and area under the concentration-time curve from time 0 to infinity (AUC0→∞) were 613 nM and 3127 nM⋅h, respectively. The half-life, apparent volume of distribution, and apparent clearance were 3.6 hours, 21 L/kg, and 4.1 L/h per kilogram, respectively (Figure 3E). In parallel, total cell lysates from BM and spleen from mice were analyzed by western blotting to determine the extent of FLT3 inhibition in vivo (Figure 3F). Phosphorylation of FLT3 was inhibited >90% by the first time point at 4 hours posttreatment, and this level of inhibition was maintained for up to 24 hours. Potent inhibition of phosphorylation of STAT5, an important downstream target of FLT3, was also observed (Figure 3F). These studies provide evidence that free drug levels achieved in the mice were sufficient to disrupt FLT3 signaling and thus prevent cell proliferation.
TTT-3002 improves survival and other measurements of disease in a transgenic murine model of leukemia
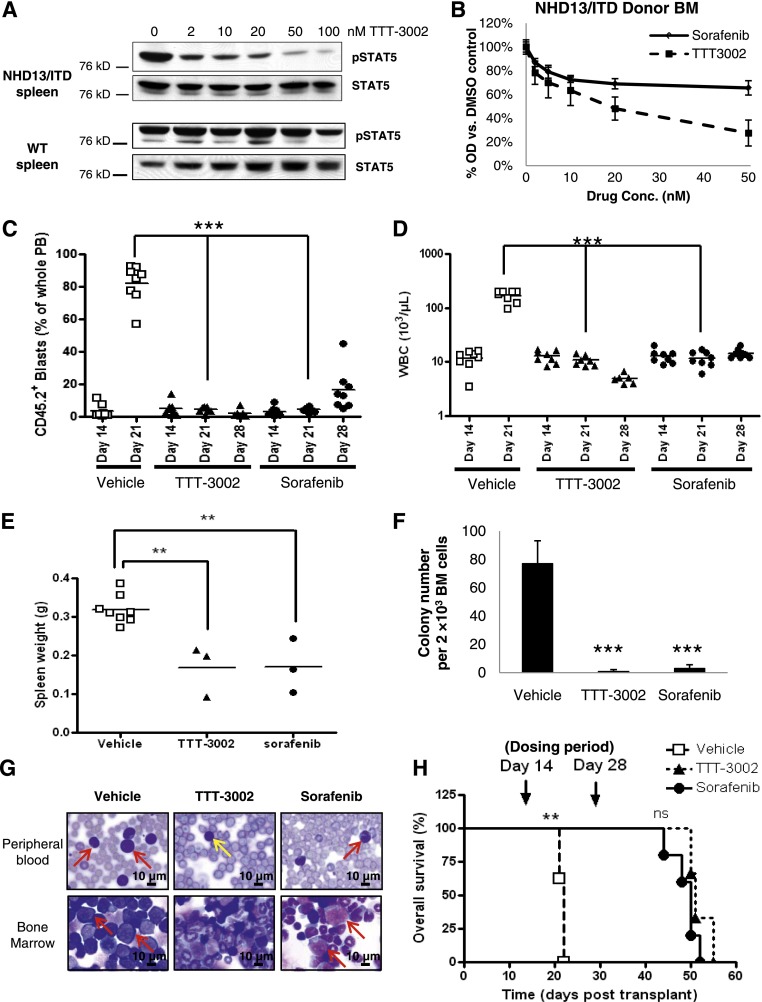
Aside from testing the leukemia generated by an FLT3/ITD-transfected Ba/F3 cell line, we also wanted to test the therapeutic potential of TTT-3002 in vivo against a fully transformed, spontaneous murine myeloid leukemia that results from a genetically engineered mouse model that recapitulates many features of human disease. Our laboratory has previously characterized an AML that is generated in 4 to 5 months with 100% penetrance when NHD13 fusion transgenic mice are bred to FLT3/ITD knockin mice (hereafter referred to as NHD13/ITD).27 These leukemic mice are sensitive to treatment with sorafenib at the maximum-tolerated dose in C57BL/6 mice of 10 mg/kg daily,28 and we sought to determine whether TTT-3002 would also be effective against this FLT3/ITD-associated leukemia. We also performed ex vivo drug sensitivity studies on leukemic cells harvested from the spleens of primary leukemic NHD13/ITD mice to assess the effects of TTT-3002 on cell signaling. Activation of STAT5, a major downstream target of the FLT3 pathway, was substantially reduced in TTT-3002–treated NHD13/ITD leukemic cells in a dose-dependent manner, with an IC50 of <2 nM (Figure 4A). In contrast, there was no effect on STAT5 signaling in the spleen of normal mice, where it is reflective of B-cell pathways activated via normal growth factors. Whole BM harvested from primary leukemic NHD13/ITD mice engrafts in sublethally irradiated recipient mice with reconstitution of leukemia in as little as 2 weeks. CD45.2+ whole BM cells isolated from an NHD13/ITD leukemic donor mouse were sensitive to TTT-3002 and sorafenib ex vivo by MTT assay (Figure 4B). To study the effects of TTT-3002 on NHD13/ITD leukemia in vivo, sublethally irradiated CD45.1+ recipients received 5 × 105 CD45.2+ cells by tail vein injection, and PB engraftment (blasts >95% CD45.2+) was confirmed by flow cytometry analysis of CD45.2+ and CD45.1+ cells at day 14. In PB, ∼10% of cells were CD45.2+ (range, 0.5% to 14.6%), and the mice were found to have slightly elevated WBC counts (range, 10 to 20 × 103/µL) (Figure 4C-D). A 2-week course of treatment (TTT-3002 6 mg/kg twice daily, sorafenib 10 mg/kg once per day, or vehicle 1 mM HCl) was initiated at day 14. By day 21, we observed profound effects on WBC counts and engraftment of CD45.2+ cells in the PB of drug-treated mice (P < .001) (Figure 4C-D). The vehicle cohort succumbed to disease on days 21 to 22 with elevated WBC counts of >100 ×103/µL and an average of >80% engraftment (range, 56% to 92%) of CD45.2+ cells in the PB. The mice were processed for histopathology of the BM and spleen, measurement of spleen weight, and assay of BM colony formation ability (Figure 4E-G). Drug treatment was ceased at day 28, and specimens from the TTT-3002– and sorafenib-treated cohorts were processed for analysis (n = 3). Two-week treatment with TTT-3002 or sorafenib had significant effects on engraftment (P < .001), WBC counts (P < .001), spleen weight (P < .01), and colony formation ability (P < .001) (Figure 4C-F). Histopathologic analysis demonstrated that TTT-3002 treatment resulted in clearance of leukemic blasts from the BM and PB compared with vehicle control, with normal WBC counts at the end of treatment (Figure 4G). Sorafenib also reduced evidence of disease by histopathology, although to a lesser extent since leukemic blasts were still present along with atypical monocytes. A subset of drug-treated mice was monitored for signs of relapse. Survival of both drug-treated groups was significantly prolonged compared with vehicle-treated mice (P < .01), indicating that TTT-3002 was as effective as sorafenib in an FLT3/ITD-associated leukemia in vivo (Figure 4H). The TTT-3002–treated (n = 3) and sorafenib-treated (n = 5) cohorts eventually relapsed at days 44 to 55 (16 to 27 days after stopping drug) with elevated WBC counts at the time of euthanasia (data not shown).
Figure 4.
TTT-3002 improves survival and measures of disease in the NHD13/ITD transgenic mouse model of AML. (A) Cells were harvested from the spleens of age-matched WT and leukemic NHD13/ITD mice (age 10 weeks). Expression of pSTAT5 relative to total STAT5 of WT and NHD13/ITD cells following 1 hour of treatment with TTT-3002 (0 to 100 nM) at equivalent protein loading and exposure time. (B) Whole BM was harvested from a CD45.2+ NHD13/ITD leukemic donor mouse (age 10 weeks) and used in cell proliferation studies following treatment with 0 to 50 nM TTT-3002 or sorafenib in vitro for 24 hours; error bars represent average ± SD. (C-H) In all, 5 × 105 CD45.2+ donor NHD13/ITD leukemic cells and 5 × 105 CD45.1+ WT helper cells were transplanted by tail vein injection into sublethally irradiated CD45.1+ WT recipients. Engraftment of CD45.2+ cells in PB was confirmed on day 14 by flow cytometry, and mice were divided into 3 treatment cohorts: TTT-3002 (n = 6; 6 mg/kg twice per day), sorafenib (n = 8; 10 mg/kg once per day), or vehicle (n = 8; 1 mM HCl twice per day). (C) Percentage of engrafted CD45.2+ leukemic cells in the PB on days 14, 21, and 28. (D) WBC counts on days 14, 21, and 28 (WBC, 103/µL) ***P < .001. (E) Splenomegaly is decreased in FLT3 TKI–treated mice. Spleens were harvested and weighed from vehicle-treated mice as they succumbed to disease, and spleens were harvested from mice in the TTT-3002–treated (n = 3; **P < .01) and sorafenib-treated (n = 3; **P < .01) cohorts on day 28 posttransplantation, corresponding with cessation of treatment. (F) TKI treatment impairs leukemic colony formation of whole BM harvested from vehicle-, TTT-3002–, and sorafenib-treated mice (n = 3 per cohort) at time points described in (E). Data represent average of 3 mice per cohort ± SD; ***P < .001. (G) Histopathology of PB and BM preparations (Wright-Giemsa stain) of representative mice from each cohort. PB and BM were harvested from mice at time points described in (E). Arrows indicate examples of leukemic blasts or atypical monocytes (red) in the BM and PB of vehicle- and sorafenib-treated mice. The PB image of the TTT-3002 representative mouse contains normal mature lymphocytes (yellow arrow), but blasts and atypical monocytes are virtually absent. Slides were imaged on an Olympus BX46 microscope with an Olympus DP72 camera at ×100 magnification with 1.3 aperture. Olympus cellSens Standard 1.5 image acquisition software was used. (H) Overall survival of vehicle- (n = 8), TTT-3002– (n = 3) and sorafenib- (n = 5) treated cohorts, with median time to death of 22, 51, and 50 days, respectively; **P < .01; ns, not significant.
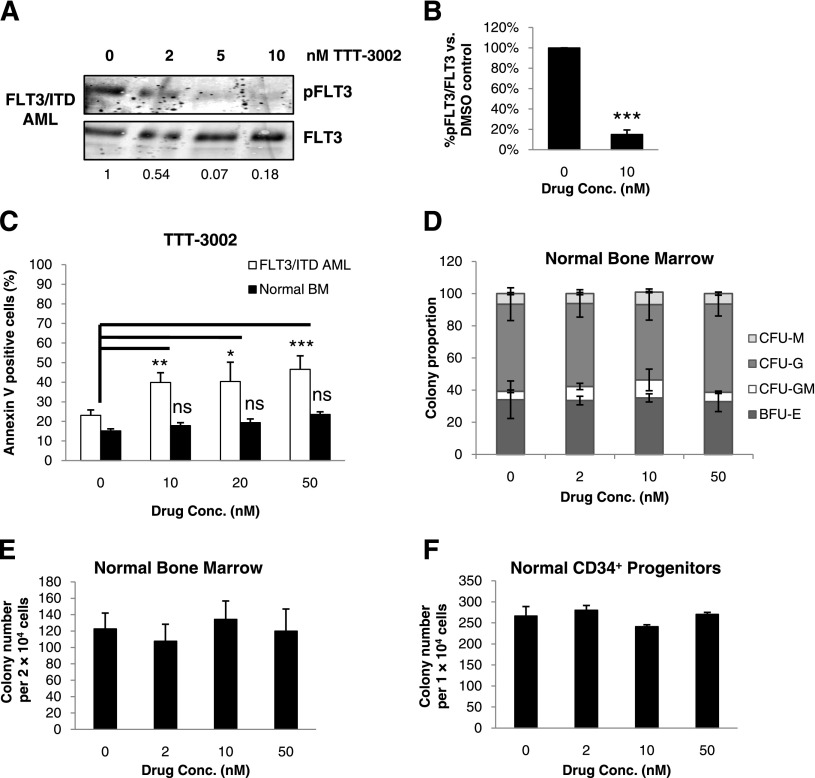
TTT-3002 is cytotoxic to human AML patient blasts but not normal BM
We further characterized the effects of this new FLT3 TKI on samples from newly diagnosed and relapsed human AML patients with FLT3/ITD mutations (Table 2). We observed dose-dependent inhibition of FLT3/ITD phosphorylation (Figure 5A-B). A significant induction of apoptosis was observed in TTT-3002–treated FLT3/ITD+ AML blasts; conversely, there was no significant cytotoxicity induced by TTT-3002 treatment of normal whole BM cells (Figure 5C). Furthermore, treatment of normal whole BM and CD34+ progenitors obtained from healthy donors did not reduce hematopoietic colony-forming ability despite treatment with up to 50 nM TTT-3002 (Figure 5D-F). These data suggest the potential for a broad therapeutic window for this compound.
Table 2.
AML patient characteristics
| Patient | Age (y) | Sex | Status | Cytogenetics | Length of ITD (bp) |
|---|---|---|---|---|---|
| 1 | 52 | F | Diagnosis | Normal | 48 |
| 2 | 59 | M | Diagnosis | Normal | 96 |
| 3 | 27 | F | Relapse | Normal | 159 |
| 4 | 54 | F | Relapse | Normal | 51 |
| 5 | 81 | M | Relapse | Normal | 33 |
| 6 | 30 | M | Relapse | t(6;9) +8 | 81 |
| 7 | 74 | M | Diagnosis | Normal | 60 |
Figure 5.
TTT-3002 inhibits FLT3 signaling and reduces cell viability in primary FLT3/ITD AML patient blasts. (A) Inhibition of pFLT3 in leukemic cells from FLT3/ITD AML patients treated ex vivo with TTT-3002. Fraction of pFLT3/FLT3 relative to DMSO control is indicated below each blot; representative example of FLT3/ITD AML. (B) Quantitation of (A), representing results from 3 unique patient samples ± SD; ***P < .001. (C) Percentage of Annexin V-positive cells of leukemic BM from FLT3/ITD AML patients or BM from healthy donors at 48 hours following treatment with increasing concentrations of TTT-3002. Normal BM (n = 2), FLT3/ITD AML (n = 4); error bars represent average of triplicate samples ± SD; *P < .05; **P < .01; ***P < .001; ns, not significant. (D) Colony proportions of burst-forming unit erythroid (BFU-E), CFU-granulocyte-macrophage, CFU-granulocyte, and CFU-macrophage colonies from BM mononuclear cells of healthy donors treated with increasing concentrations of TTT-3002 (representative example shown). Error bars represent average ± SD. (E) Total colony counts from BM mononuclear cells from healthy donors treated with increasing concentrations of TTT-3002 (representative example shown). Error bars represent average ± SD. (F) Total colony counts from 1 × 104 CD34+ stem/progenitor cells from healthy donors treated with increasing concentrations of TTT-3002 (representative example shown). Error bars indicate average ± SD.
Discussion
One of the obstacles to successfully treating FLT3/ITD-expressing leukemia patients with FLT3 TKIs is the failure to achieve adequate FLT3 inhibition. This is thought to be primarily due to insufficient potency and/or low free-drug availability in the plasma.21,22 Therefore, the search for novel FLT3 TKIs with increased activity against FLT3 phosphorylation is necessary to be able to test whether FLT3 TKIs can improve the cure rate of FLT3-mutant AML. In this study, we demonstrate the potent activity of TTT-3002 against FLT3-activating mutations, including both FLT3/ITD and FLT3/PM, as well as FLT3/WT cells with overexpression of autocrine-activated FLT3. These data provide strong evidence that TTT-3002 is the most potent FLT3 TKI reported to date, even exceeding the activity of AC220 against autophosphorylation of FLT3/ITD.30 Interestingly, unlike TTT-3002, the IC50 of AC220 for cell proliferation by MTT assay is lower than its IC50 for inhibiting FLT3 phosphorylation in MV4-11 and Molm14 cells. This finding has also been reported previously for AC22031 and suggests that AC220 may have off-target effects even at low nanomolar concentrations, given that there are changes in cell proliferation without inhibiting significant levels of FLT3 activity. TTT-3002 activity against FLT3 phosphorylation correlates strongly with its activity in proliferation and cytotoxicity assays, implying that its action is most likely due to its activity against FLT3. Thus, it may be a fairly selective FLT3 inhibitor under a realistic clinical dosing regimen. Indeed, when profiled against a panel of 140 kinases by Yao et al,29 TTT-3002 has a rather high degree of selectivity in vitro. In our studies with TTT-3002, we observed an IC90 of 1 nM against FLT3/ITD phosphorylation. Of the 140 kinases in the Yao et al panel, there was only 1 kinase (MKK1) with an IC90 of ≤1 nM, giving this compound a selectivity score of S(90) = 0.007 at 1 nM. At 10 nM, which is 10-fold higher than the IC90 for FLT3, the selectivity score is S(90) = 0.11, with 16 of the 140 kinases inhibited by more than 90% vs control. Thus, given the observation that <1% of kinases in this panel are targeted at the IC90 of FLT3/ITD, and only about 11% of kinases are targeted at a concentration 10-fold higher than this IC90 concentration, we feel that this compound is relatively selective for activity against FLT3.29
While mice with complete genetic knock-out of Flt3 have only subtle deficits in hematopoietic stem cell activity, combination with partial inactivating c-Kit mutation results in severe hematopoietic stem cell defects.32 Thus, it is desirable that FLT3 TKIs not have significant activity against c-Kit because combination trials with chemotherapy would likely result in delayed hematopoietic recovery. We directly tested the activity of TTT-3002 against WT c-KIT phosphorylation and found the IC50 for TTT-3002 was 100-fold lower for FLT3/ITD than for WT c-KIT (supplemental Figure 4).30 Several FLT3 TKIs have shown evidence of delayed count recovery when used in combination with chemotherapy and some require a several-week break from TKI treatment to allow for recovery. The selectivity and potency of TTT-3002 is somewhat surprising because it is structurally related to CEP-701 and PKC412, being derivatives of K252a and staurosporine, respectively. This exemplifies the large impact on potency and specificity profiles that can result from relatively minor changes to a lead compound and demonstrates that the general bias against the indolocarbazole scaffold may be unjustified.
Seven percent to 10% of AML patients harbor an FLT3/PM activating mutation, and these mutations are completely resistant to inhibition by sorafenib and AC220.8,23,33-36 Furthermore, AML patients often develop secondary point mutations in FLT3 that lead to TKI resistance and relapse.24,37-39 TTT-3002 potently inhibits FLT3 phosphorylation by activating mutations at residue D835. We have preliminary data showing that TTT-3002 also has a broad spectrum of activity against a variety of FLT3/PMs and mutations conferring resistance to several FLT3 TKIs that have been selected for in recent clinical trials. This suggests that TTT-3002 is a candidate to overcome some of these mechanisms of primary and secondary drug resistance.
Oral dosing of TTT-3002 was well tolerated in mice, with no significant changes in animal weight over the course of treatment (supplemental Figure 5A). Spleen and liver weights of drug-treated animals were within the normal range (supplemental Figure 5B). Further toxicity studies were performed to determine whether there were any myelosuppressive effects at the dose of 6 mg/kg twice per day. Analysis of PB and BM of healthy mice treated for 2 weeks with TTT-3002 or vehicle indicated that blood counts and BM differentiation were normal, with the presence of normal lymphocytes, monocytes, granulocytes, and platelets in the PB and normal megakaryocytes and myeloid and erythroid precursors in the BM. Tissue examination of brain, BM, esophagus, stomach, small bowel, colon, pancreas, liver, heart, kidney, lung, spleen, thymus, ovary, and skin was histologically unremarkable on hematoxylin and eosin–stained sections and revealed no evidence of lesions or other signs of drug-related toxicity (supplemental Figure 5C-D).
Pharmacodynamic studies revealed that a single dose causes >90% inhibition of FLT3 signaling that is maintained over the course of 12 hours. This level of FLT3 inhibition has been shown to correlate with improved outcome in human clinical trials and has not always been achieved.21,22 The pSTAT5 signal reemerges in the BM cells at 24 hours, just as the pFLT3 signal increases to 12% of the basal level. Thus, given these pharmacodynamic data, a twice daily dosing schedule in mice is appropriate for this compound in order to inhibit the survival and proliferation pathways driven by FLT3 signaling.
Taken together, these data illustrate the promising activity of TTT-3002 against FLT3-dependent cells both in vitro and in vivo and promote the further exploration of this compound as a next-generation FLT3 inhibitor in the treatment of AML.
Supplementary Material
Acknowledgments
This work was supported by the Alex’s Lemonade Stand Foundation for Childhood Cancer, National Cancer Institute (CA90668, CA70970), Giant Food Pediatric Cancer Research Fund, and the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (National Institutes of Health Center Core grant P30 CA006973 and Cooperative Agreement grant UL1 RR025005). D.S. is also supported by the Kyle Haydock Professorship.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.M. designed and performed experiments, analyzed data, and wrote the manuscript; B.N., L.L., S.G., A.W., and M.Z. performed experiments; M.R. and A.D. designed experiments and analyzed data; M.L. provided patient samples; and D.S. designed experiments and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donald Small, Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University, CRBI Room 251, 1650 Orleans St, Baltimore, MD 21231; e-mail: donsmall@jhmi.edu.
References
- 1.McKenzie SB. Advances in understanding the biology and genetics of acute myelocytic leukemia. Clin Lab Sci. 2005;18(1):28–37. [PubMed] [Google Scholar]
- 2.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci USA. 1994;91(2):459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng R, Bailey E, Nguyen B, et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene. 2011;30(38):4004–4014. doi: 10.1038/onc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Fukuda S, Lee Y, et al. Essential role of signal transducer and activator of transcription (Stat)5a but not Stat5b for Flt3-dependent signaling. J Exp Med. 2000;192(5):719–728. doi: 10.1084/jem.192.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stirewalt DL, Kopecky KJ, Meshinchi S, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97(11):3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- 6.Kiyoi H, Towatari M, Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12(9):1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 7.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. [PubMed] [Google Scholar]
- 8.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 9.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14(10):1766–1776. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 10.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 11.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 12.Levis M, Small D. FLT3 tyrosine kinase inhibitors. Int J Hematol. 2005;82(2):100–107. doi: 10.1532/IJH97.05079. [DOI] [PubMed] [Google Scholar]
- 13.Auclair D, Miller D, Yatsula V, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21(3):439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 14.Borthakur G, Kantarjian H, Ravandi F, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62–68. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100(3):184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 16.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28(11):1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–3301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 19.Fathi AT, Chabner BA. FLT3 inhibition as therapy in acute myeloid leukemia: a record of trials and tribulations. Oncologist. 2011;16(8):1162–1174. doi: 10.1634/theoncologist.2011-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapper S, Mills KI, Gilkes AF, Austin SJ, Walsh V, Burnett AK. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108(10):3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg E, Sattler M, Ray A, Griffin JD. Drug resistance in mutant FLT3-positive AML. Oncogene. 2010;29(37):5120–5134. doi: 10.1038/onc.2010.273. [DOI] [PubMed] [Google Scholar]
- 24.Moore AS, Faisal A, Gonzalez de Castro D, et al. Selective FLT3 inhibition of FLT3-ITD+ acute myeloid leukaemia resulting in secondary D835Y mutation: a model for emerging clinical resistance patterns. Leukemia. 2012;26(7):1462–1470. doi: 10.1038/leu.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cools J, Mentens N, Furet P, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64(18):6385–6389. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- 26.Williams AB, Nguyen B, Li L, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia. 2013;27(1):48–55. doi: 10.1038/leu.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenblatt S, Li L, Slape C, et al. Knock-in of a FLT3/ITD mutation cooperates with a NUP98-HOXD13 fusion to generate acute myeloid leukemia in a mouse model. Blood. 2012;119(12):2883–2894. doi: 10.1182/blood-2011-10-382283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Zhang L, Fan J, et al. Defective nonhomologous end joining blocks B-cell development in FLT3/ITD mice. Blood. 2011;117(11):3131–3139. doi: 10.1182/blood-2010-05-286070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao C, Johnson WM, Gao Y, et al. Kinase inhibitors arrest neurodegeneration in cell and C. elegans models of LRRK2 toxicity. Hum Mol Genet. 2013;22(2):328–344. doi: 10.1093/hmg/dds431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114(14):2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunawardane RN, Nepomuceno RR, Rooks AM, et al. Transient exposure to quizartinib mediates sustained inhibition of FLT3 signaling while specifically inducing apoptosis in FLT3-activated leukemia cells. Mol Cancer Ther. 2013;12(4):438–447. doi: 10.1158/1535-7163.MCT-12-0305. [DOI] [PubMed] [Google Scholar]
- 32.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3(1):147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 33.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong SA, Mabon ME, Silverman LB, et al. FLT3 mutations in childhood acute lymphoblastic leukemia. Blood. 2004;103(9):3544–3546. doi: 10.1182/blood-2003-07-2441. [DOI] [PubMed] [Google Scholar]
- 35.Fröhling S, Schlenk RF, Breitruck J, et al. AML Study Group Ulm. Acute myeloid leukemia. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 36.Stirewalt DL, Meshinchi S, Kussick SJ, et al. Novel FLT3 point mutations within exon 14 found in patients with acute myeloid leukaemia. Br J Haematol. 2004;124(4):481–484. doi: 10.1111/j.1365-2141.2004.04808.x. [DOI] [PubMed] [Google Scholar]
- 37.Man CH, Fung TK, Ho C, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood. 2012;119(22):5133–5143. doi: 10.1182/blood-2011-06-363960. [DOI] [PubMed] [Google Scholar]
- 38.Alvarado T, Kantarjian H, Ravandi F, et al. FLT3 inhibitor treatment in FLT3-mutated AML is associated with development of secondary FLT3-TKD mutations. [abstract] Blood. 2008;118:1493. [Google Scholar]
- 39.Zhang W, Konopleva M, Jacamo RO, et al. Acquired point mutations of TKD are responsible for sorafenib resistance in FLT3-ITD mutant AML [abstract]. Blood. 2011;118(21):3505. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.