Key Points
Genome-wide RNAi screen provides the first comprehensive list of putative hepatic hepcidin regulators.
Hepcidin suppression is linked to the control of mitogen stimulation and nutrient status via components of Ras/RAF MAPK and mTOR signaling.
Abstract
The hepatic hormone hepcidin is a key regulator of systemic iron metabolism. Its expression is largely regulated by 2 signaling pathways: the “iron-regulated” bone morphogenetic protein (BMP) and the inflammatory JAK-STAT pathways. To obtain broader insights into cellular processes that modulate hepcidin transcription and to provide a resource to identify novel genetic modifiers of systemic iron homeostasis, we designed an RNA interference (RNAi) screen that monitors hepcidin promoter activity after the knockdown of 19 599 genes in hepatocarcinoma cells. Interestingly, many of the putative hepcidin activators play roles in signal transduction, inflammation, or transcription, and affect hepcidin transcription through BMP-responsive elements. Furthermore, our work sheds light on new components of the transcriptional machinery that maintain steady-state levels of hepcidin expression and its responses to the BMP- and interleukin-6–triggered signals. Notably, we discover hepcidin suppression mediated via components of Ras/RAF MAPK and mTOR signaling, linking hepcidin transcriptional control to the pathways that respond to mitogen stimulation and nutrient status. Thus using a combination of RNAi screening, reverse phase protein arrays, and small molecules testing, we identify links between the control of systemic iron homeostasis and critical liver processes such as regeneration, response to injury, carcinogenesis, and nutrient metabolism.
Introduction
Hepcidin is a hepatic peptide hormone that orchestrates systemic iron homeostasis by adjusting systemic iron availability to body iron requirements.1 Hepcidin binds to ferroportin, the iron efflux channel predominantly expressed on iron-exporting cells, causing its internalization and degradation.2 Conditions of high iron load or inflammation activate hepcidin production in the liver to reduce serum iron levels, whereas iron deficiency, hypoxia, and augmented erythropoiesis inhibit hepcidin expression to increase systemic iron availability.1
Most iron-related disorders are attributed to excessive or inadequate liver hepcidin expression. Hereditary hemochromatosis (HH) is caused by inappropriately low hepcidin levels1 and has been linked to loss-of-function mutations in the hepcidin-activating genes HFE,3 transferrin receptor 2 (TfR2),4 and hemojuvelin (HJV),5 or in hepcidin itself.6 Despite the high prevalence of mutations in the HFE gene, the clinical penetrance of HH is low, and disease severity may vary substantially among patients, suggesting a significant contribution of genetic and/or environmental modifiers of systemic iron levels.7-9 Iron also accumulates in several subtypes of hereditary anemias (eg, thalassemias) that are characterized by deficient hepcidin expression despite progressive iron loading.10 In addition, there is increasing awareness in the medical field that tissue iron accumulation exacerbates the pathologies of common acquired diseases such as chronic liver disease or diabetes.11,12 By contrast, high levels of hepcidin transcription in response to inflammatory cytokines or as a result of genetic defects of the TMPRSS6 gene are implicated in the pathogenesis of the anemia of inflammation and iron-refractory iron-deficiency anemia, respectively.13-15
Two major signaling pathways were identified thus far to control hepcidin expression. Proinflammatory cytokines activate hepcidin expression via the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway that operates via a conserved STAT binding motif proximal to the transcription start site.14,16 Hepcidin is however most potently induced by bone morphogenetic proteins (BMPs), which bind to their receptors and to the coreceptor protein HJV and trigger SMAD signaling.17 Two sequence motifs (the proximal BMP-RE1 and the distal BMP-RE2) within the hepcidin promoter are critical for the stimulation of hepcidin via the BMP/HJV/SMAD4 signaling axis.18 The BMP pathway communicates systemic iron levels19,20 and, by still largely unknown mechanism, contributes to the activation of hepcidin by inflammatory stimuli.18,21,22 Furthermore, reduced hepcidin expression in HFE and TfR2-related hemochromatosis is linked to an impairment of SMAD signaling.23-26 However, it remains to be defined how the cross-talk among HFE, TfR2, and the BMP signaling pathway is established.
In addition, several mechanisms of hepcidin suppression have been identified and involve either the protease TMPRSS6 (matriptase-2),15,27,28 the inhibitory SMAD7,29 stabilization of hypoxia-inducible factor,30 erythropoietin,31 or 2 soluble factors of the transforming growth factor-β family, growth differentiation factor 15 and twisted gastrulation protein-1 (TWSG1).32,33 More recently, inhibition of hepcidin messenger RNA (mRNA) expression was also linked to growth factors and testosterone.34-36
Here we report data from a genome-wide screen that provides the first comprehensive list of putative hepatic hepcidin regulators. In secondary analyses of our screening hits, we uncover links between hepcidin suppression and the Ras/RAF MAPK and mTOR pathways, which are known to promote hepatocyte proliferation and cell growth, as well as play roles in the control of liver nutrient homeostasis.
Materials and methods
Cell culture
The human hepatocarcinoma cell lines Huh7 and Hep3B were obtained from ATCC (Wesel, Germany). Human liver tissue was obtained according to institutional guidelines (Charité University Medicine, Berlin) from liver resections of tumor patients. Written informed consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki. Primary human hepatocytes (PHHs) were isolated by a 2-step collagenase perfusion technique.37 Primary murine hepatocytes were isolated from the livers of C57/BL-6 male mice and plated on collagen-coated tissue culture plates as described before.38 Culture conditions for all cell models used in the study are described in the supplemental data, available on the Blood Web site.
Genome-wide siRNA screening
To conduct the genome-wide RNA interference (RNAi) screen, the small interfering RNA (siRNA) library ThermoFisher siGenome siARRAY SMARTpool (Dharmacon, Lafayette, CO) was used. As positive controls, we included siRNAs against SMAD4, STAT3, and SMAD7, as well as a scrambled siRNA pool (Dharmacon) as a negative control (supplemental Table 1). The screen was performed as previously reported.29 The bioinformatic analyses using DAVID and STRING tools are described in the supplemental data.
Hepcidin reporter constructs
Luciferase reporter constructs that contain the full-length 2762-bp hepcidin promoter (WT_2.7 kb) or its mutant derivatives (STAT_BS_2.7 kb and BMP_RE1_BMP_RE2_2.7 kb), as well as the murine ferroportin promoter or the human DAP kinase promoter reporters, were described previously.14,18,39,40
Transfection of siRNAs, plasmid DNA, and luciferase assay
For secondary assays, we reverse-transfected Huh7 cells (24 000 cells/well) with 10 pmol siRNA (pooled or individual duplexes; Dharmacon; supplemental Table 1) using 0.2 μL Dharmafect1 in a 96-well format. PHHs (450 000 cells/well, 12-well plates) were transfected with 40 pmol of siRNA using 2 μL RNAiMAX (Invitrogen). After 72 hours, cells were collected to extract total RNA.
For the transfection of hepcidin reporter plasmids and siRNAs, Huh7 cells were initially transfected with pooled siRNAs (Dharmacon; supplemental Table 1) as described before. After 24 hours, the reporter plasmids (150 ng of hepcidin/ferroportin/DAP kinase promoter constructs and 20 ng of CMV-Renilla control plasmid) were transfected using 0.3 μL of TransIT (Mirus). After 48 hours, luciferase activity was measured as described.29
For the analysis of hepcidin-stimulating conditions, Huh7 cells were transfected in 12-well plates with 40 pmol of siRNA using 2 μL Lipofectamine2000 (Invitrogen). Cells were starved from serum for 48 hours, treated with interleukin-6 (IL-6) (20 ng/mL; R&D Systems, Wiesbaden, Germany) or BMP-6 (10 ng/mL, R&D Systems), and collected for total RNA isolation after 15 hours.
Expression vectors (200 ng) (supplemental Table 2) were transfected into Huh7 cells (in a 24-well format) together with 180 ng of hepcidin reporter constructs and 40 ng of CMV-Renilla plasmid using 0.8 μL of TransIT (Mirus). After 48 hours, luciferase activity was measured using the Dual-Luciferase-Reporter assay system (Promega) and a Centro LB 960 luminometer (Berthold Technologies).
Preparation of total RNA, RT, and real-time qPCR analysis
Isolation of total RNA and the protocols for reverse transcription and real-time quantitative polymerase chain reaction (qPCR) were described previously.18 Total RNA extraction from mouse livers was performed using Trizol (Invitrogen). Total RNA extraction for the secondary assays was performed using the QuickExtract RNA Extraction Kit (Epicentre Biotechnologies, Madison, WI) according to the manufacturer’s instructions (see supplemental data for details). Sequences of the quantitative PCR primers are shown in supplemental Table 3. Expression levels of human glyceraldehyde-3-phosphate-dehydrogenase or murine actin or Rpl19 were used as normalization controls.
Treatment of Huh7 cells with small molecules
Huh7 or Hep3B cells were plated, and 24 hours later the indicated concentrations of Wortmannin (4 hours; Calbiochem), Akt inhibitor VIII (9 hours, Sigma), rapamycin (15 hours, Sigma), sorafenib (9 hours, Nexavar; Bayer), or metformin (15 hours, Sigma) were added. Conditions for treatments of murine primary hepatocytes are described in the supplemental data.
Reverse-phase protein arrays (RPPAs)
Huh7 cells (400 000 cells/well, 6-well plates) were reverse-transfected with 200 pmol siRNA using 6 μL Dharmafect1. After 24 hours or 48 hours, cells were harvested for protein isolation using a nondenaturating lysis buffer. A detailed protocol for RPPA, description of data analysis, and the list of validated antibodies (supplemental Table 4) used for targeted proteome profiling are available in the supplemental data.
Rasa1 knockout mice
The generation of inducible Rasa1 knockout mice was described previously (see supplemental data).41 Seven- to eight-week-old Rasa1-floxed female mice with and without engineered Ert2Cre transgene were administered with tamoxifen (MP biochemicals) to induce nuclear translocation of the Ert2Cre protein and disruption of the Rasa1 gene. Mice were analyzed 6 weeks after tamoxifen injection. Plasma iron measurement using the Iron Kit (Thermo Electron GmbH, Dreieich, Germany) and determination of nonheme tissue iron content were performed as described previously.42 Experiments were approved by the University of Michigan Committee on the Use and Care of Animals.
Statistical analysis
For analysis of the screening data, the cellHTS2 package (Bioconductor)43 was used as described previously.29 Throughout the study, the data are expressed as a mean of at least 3 independent experiments ± standard deviation (SD). The Student t test was used for estimation of statistical significance.
Results
Genome-wide RNAi screening identifies a large number of putative hepcidin activators enriched for signal transducers, transcription regulators, and inflammatory genes
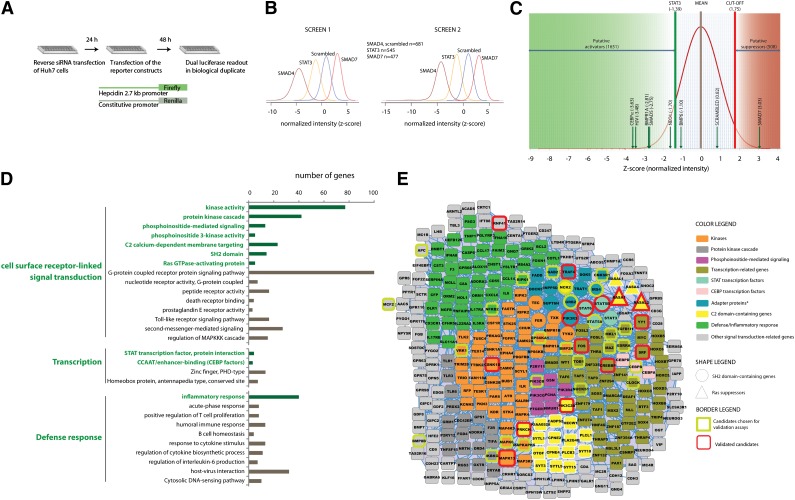
To identify activators and repressors of hepcidin expression on a genome-wide scale, we extended our previously established screening approach29 to target 19 599 human genes. We chose Huh7 cells (despite a known mutation in the HFE gene) because this cell line fulfills critical prerequisites for RNAi screening: fast cell proliferation, good transfection efficiency, relatively high hepcidin mRNA expression, and well-preserved hepcidin-regulating pathways.14,18,21,29 In the screening assay, Huh7 cells were reverse-transfected with a pool of 4 siRNAs and with a hepcidin promoter containing firefly luciferase reporter together with a SV40 promoter-containing Renilla luciferase control vector (Figure 1A). Screening data are shown as mean z-scores from 2 biological replicates and represent suppression (low-negative z-score) or activation (high-positive z-score) of the hepcidin promoter after the knockdown of individual target genes. SMAD4, STAT3, and SMAD7 siRNAs were used as positive controls, and their effects were clearly distinguished from the scrambled negative siRNA control (Figure 1B).
Figure 1.
Genome-wide RNAi screen identifies a large number of putative hepcidin activators enriched for signal transducers, transcription regulators, and inflammatory genes. (A) Schematic representation of the high-throughput RNAi screening strategy. (B) Normalized luciferase intensities obtained upon RNAi of target genes were calculated as z-scores and indicate suppression (low-negative z-scores) or activation (high-positive z-scores) of the hepcidin promoter. (B) Distribution of z-scores after knockdown of the positive control genes SMAD4, STAT3, and SMAD7 is well separated from the negative scrambled siRNA control. (C) Distribution of the mean z-scores for 19 599 screened target genes. Indicated are the z-scores obtained for the siRNA-mediated knockdown of STAT3 (for the identification of 1651 putative hepcidin activators), the z-score of 1.75 (for the identification of 508 putative hepcidin suppressors), and the z-scores obtained for the known hepcidin regulators included in the siRNA library. (D) Functional enrichment analysis using DAVID software identifies genes involved in signal transduction, transcription regulation, and defense/inflammatory response to be overrepresented among putative hepcidin activators. (E) Enriched categories that are marked in green in (D) are the focus of the STRING interaction networks of the positive putative hepcidin regulators. The interaction network is visualized using the software tool Cytoscape. *The category “adapter protein” was by itself not enriched among the putative activators but was visualized as an interesting functional subgroup that also contains overrepresented SH2 domain proteins.
We next plotted the distribution of the mean z-scores for each gene in the library, excluding the control siRNAs (Figure 1C). Known regulators of hepcidin promoter activity, such as CEBPα, HJV, BMPR1A, SMAD5, neogenin (NEO1), and BMP-6, are indicated. The cutoff value for putative hepcidin activators was defined based on hepcidin promoter activity in response to the knockdown of STAT3, a well-studied activator of hepcidin transcription.14 Because hepcidin suppressors are less well-defined, we selected an arbitrary cutoff (z-score 1.75). Application of these cutoff values in our data set included a large number of novel candidate hepcidin regulators (1651 activator and 508 suppressor candidates; Figure 1C) for further bioinformatic analysis. The annotated screening data and the hit lists are provided in the supplemental data (supplemental File 1).
To search for functional relationships among the putative hepcidin regulators, we performed bioinformatic analyses using the software tools DAVID44 and STRING.45 DAVID analysis identified 193 enriched functional terms among the putative hepcidin activators (supplemental Table 5 and supplemental File 2). From these categories, approximately 50% are nonredundant and enriched for genes involved in signal transduction, transcriptional regulation, and defense/inflammatory responses (Figure1D; supplemental Table 7). Within these overrepresented broader functional categories, DAVID analysis further distinguishes proteins that function as protein kinases, SH2-domain proteins, Ras suppressors or regulators involved in phosphoinositide-mediated signaling, and STAT and CEBP transcription factors. In parallel, STRING analysis additionally revealed that 967 (59%) of the potential positive regulators of hepcidin expression are interconnected. Integration of the data output of DAVID and STRING using Cytoscape46 yielded the selected enriched functional categories depicted in the interaction network (Figure 1E). Similar bioinformatic analysis performed for putative hepcidin suppressors is presented as supplementary material because none of the selected inhibitory genes could be validated in the secondary assays (supplemental Tables 6 and 7, supplemental File 3, and supplemental Figure 1).
Secondary assays validate 15 novel functionally diverse hepcidin activators
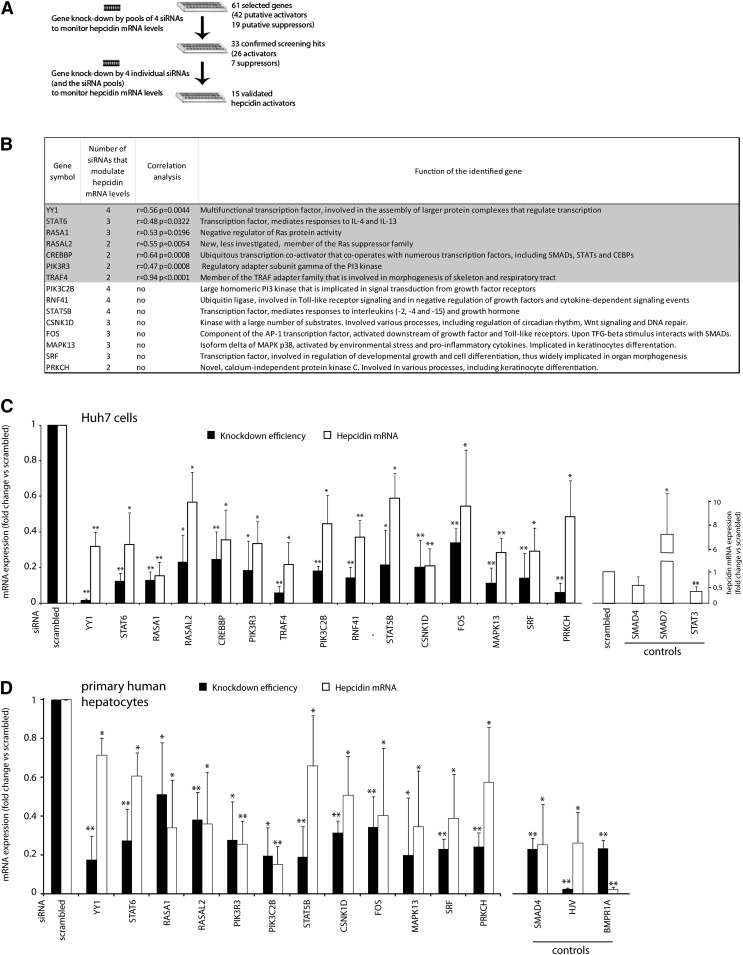
DAVID and STRING analyses supported by literature mining guided the selection of 61 putative hepcidin regulators for secondary assays (Figure 1E; supplemental Table 8). Literature searches aimed to identify candidate modifiers linked to known hepcidin-regulating pathways and to conditions that affect systemic iron homeostasis (eg, inflammation or hypoxia). We further selected genes with links to putative novel modes of hepcidin regulation, such as an involvement of Ras, NF-κB, or β-catenin signaling; circadian regulatory mechanisms; and proteins with adapter functions or transcription factors. In the validation assays, each candidate gene was targeted by a siRNA pool to assess whether its knockdown affects endogenous hepcidin mRNA expression similarly to the hepcidin luciferase reporter construct (Figure 2A; supplemental Table 8). For 33 genes (54%) confirmed by this approach, we performed RNAi using 4 individual siRNAs (Figure 2A). Fifteen of the candidate genes displayed significantly changed hepcidin mRNA expression in response to at least 2 siRNAs (Figure 2B-C; supplemental Figures 2 and 3) and were considered as validated. Within this group, 7 genes showed alterations of hepcidin mRNA levels that correlated significantly with the knockdown efficiency of each single siRNA (Figure 2B). In addition, 12 of 15 novel hepcidin modifiers tested control hepcidin mRNA expression in a qualitatively similar manner in human primary hepatocytes (Figure 2D). All validated hepcidin activators are novel and implicated in diverse cellular processes, such as responses to growth stimuli, inflammation, the assembly of transcriptional complexes, cell differentiation, and morphogenesis, and they comprise kinases, transcription factors, protein adapters, and Ras protein suppressors (Figure 2B).
Figure 2.
Secondary validation assays identify 15 novel hepcidin activators. (A) Workflow of the secondary assays. To validate the selected hepcidin regulators, we applied siRNA pools and 4 individual siRNAs to knockdown the genes of interest. (B) Shown are 15 genes that were validated by the experimental workflow outlined in (A). Genes were considered as validated if at least 2 of 4 siRNAs caused a significant change in hepcidin mRNA expression. In addition, we assessed whether the knockdown efficiency of each single siRNA correlated significantly with the observed changes in hepcidin mRNA expression (the correlation coefficient [r] and its significance [P value] were calculated for all obtained data points). The indicated protein functions are based on information available in the SwissProt database. (C-D) Shown are mRNA levels of hepcidin and target genes after RNAi in (C) Huh7 cells or (D) PHHs. Data for siRNA pools are shown (results for individual siRNAs are shown in supplemental Figures 2 and 3). The positive controls are indicated. Levels of mRNA expression were determined by real-time qPCR. Results are presented as fold change compared with samples transfected with scrambled siRNA. The mean of at least 3 independent experiments is shown. Significant changes are marked by *(P < .05) or **(P < .001).
Novel hepcidin activators control steady-state hepcidin transcription in a BMP-RE–dependent manner and contribute to the hepcidin responses to IL-6 and BMP-6
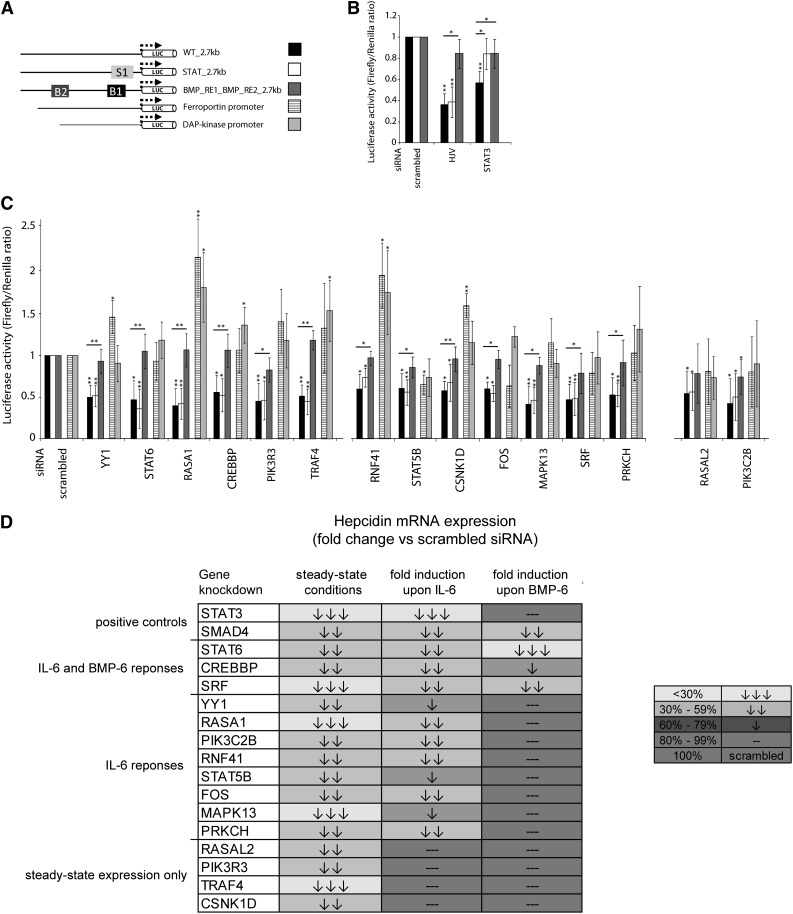
Given the functional diversity of the 15 novel hepcidin regulators, we next investigated whether they operate via the well-characterized hepcidin-activating pathways. To test this, we first analyzed the responses of hepcidin promoter constructs with mutations in the IL-6 (STAT-BS) or BMP/SMAD-responsive elements (BMP-RE1 and/or BMP-RE2) (Figure 3A). Throughout the experiments, the siRNA-mediated knockdowns of HJV and STAT3 served as positive controls (Figure 3B). As specificity controls, we included luciferase reporters driven either by the ferroportin or the DAP-kinase promoter.39,40 We found that none of the newly identified hepcidin regulators control hepcidin promoter activity in a STAT-BS–dependent manner, as we had observed for the positive control STAT3.14 However, the knockdown of 13 genes reduced hepcidin promoter activity in a manner that requires functional BMP-REs, similar to both positive controls HJV and STAT3 (Figure 3C).18,21 The siRNA-mediated depletion of only 2 novel activators, PIK3C2B and RASAL2, did not significantly depend on the BMPREs. Taken together, these data suggest that the validated hepcidin activators broadly connect cellular signaling with the regulation of hepcidin steady-state expression in a BMP-REs–dependent manner. This further implies that the BMP-REs are not only required for the hepcidin responses to the previously known stimuli but also confer broader promoter functionality.
Figure 3.
Novel hepcidin activators control steady-state hepcidin transcription in a BMP-REs–dependent manner and contribute to the hepcidin responses to IL-6 and BMP-6. (A) The name of each hepcidin promoter reporter construct refers to the elements that have been mutated within the wild-type hepcidin 2.7-kb promoter. B1 and B2 indicate the BMP-RE1 and the BMP-RE2, respectively, whereas S1 indicates the STAT-BS. Reporters driven by the ferroportin or the DAP-kinase promoters were included as specificity controls. (B-C) Shown are the responses of the wild-type and mutated hepcidin promoter to knockdowns of HJV and STAT3 (B), and to knockdowns of the identified hepcidin regulators (C). Results are presented as ratios between the luciferase activity (± SD of Firefly/Renilla) obtained from samples transfected with specific siRNA and samples transfected with control siRNA. Results represent a mean of 5 independent experiments. Statistically significant changes are marked by *(P < .05) or **(P< .005). (D) Novel hepcidin activators are partially involved in the hepcidin responses to IL-6, whereas BMP signals override the requirement for most of the identified modifiers. Huh7 cells were transfected with the indicated siRNAs, starved from serum for 48 hours, and then treated for 15 hours with IL-6 (20 ng/mL) or BMP-6 (10 ng/mL) (supplemental Figure 4). Hepcidin mRNA levels were determined by real-time qPCR analysis. Hepcidin steady-state mRNA level (as shown in Figure 2C) or fold induction of hepcidin mRNA expression in response to stimuli are set to 100% for cells transfected with scrambled siRNA control. The mean of at least 3 independent experiments is shown. Arrows indicate significant changes (P < .05) compared with scrambled siRNA.
We next examined whether the newly identified regulators of hepcidin steady-state levels are also required for its induction by IL-6 or BMP-6 (Figure 3D; supplemental Figure 4). As positive controls for these studies, we used siRNA-mediated knockdown of SMAD4 and STAT3. Consistent with previous studies,14,18 we observed that STAT3 is required only for IL-6–driven hepcidin induction, whereas SMAD4 expression is critical to control the hepcidin mRNA increase under both stimulatory conditions tested. Our data show that although the IL-6 response depends on several of the newly identified factors, strikingly, the BMP-6–driven hepcidin induction seems to override the requirements for most of the modifiers and involves only 3 transcription factors: STAT6, CREBBP, and SRF. Finally, some of the new BMP-REs–dependent regulators (eg, TRAF4 or PIK3R3) are not required for the hepcidin responses under stimulatory conditions.
siRNA-mediated depletion of the novel hepcidin activators alters the cellular signaling network
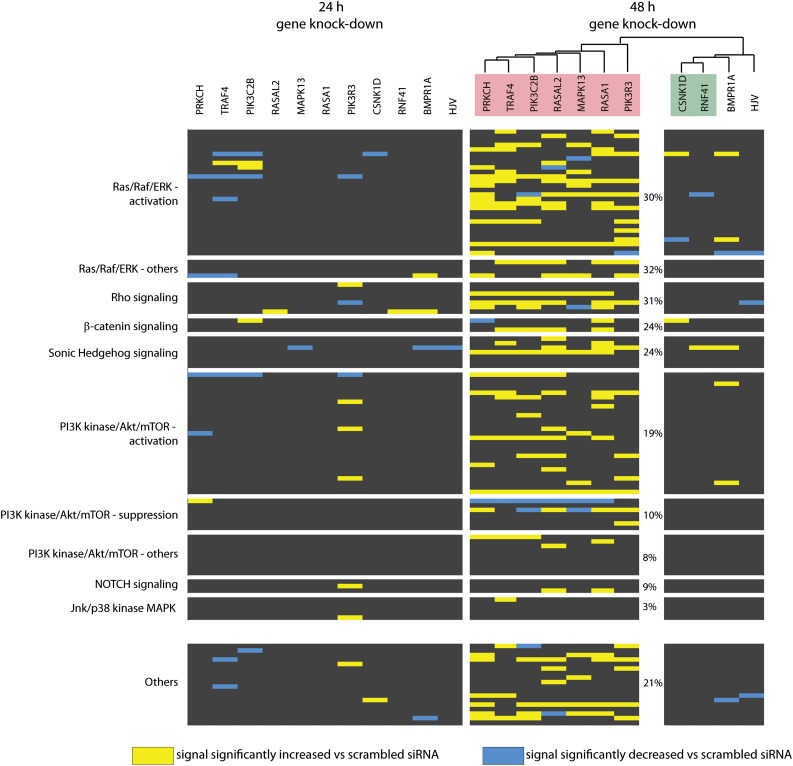
Because our secondary analysis suggested that some of the novel hepcidin modifiers may operate independently of JAK-STAT or BMP/SMAD signaling, we next explored whether the identified genes can affect other signaling pathways. We performed siRNA-mediated knockdown of 9 novel hepcidin regulators (transcription factors were excluded from the analysis) and assessed changes of the cell signaling network using RPPAs 24 hours and 48 hours after siRNA transfection. The arrays were designed to monitor 117 signals that inform about absolute expression levels and/or the phosphorylation status of 82 proteins linked to different signaling pathways (Figure 4; supplemental Figure 5).47 Several of the pathways probed by RPPAs, such as Ras/MAPK, PI3K/Akt/mTOR, and β-catenin signaling, are interconnected (Figure 5A) and play crucial roles in liver processes such as regeneration, response to injury, carcinogenesis, or nutrient homeostasis.48-50
Figure 4.
RPPA analysis identifies alterations of the cellular signaling network upon siRNA-mediated depletion of the novel hepcidin activators. Huh7 cells were transfected with the indicated siRNAs and protein extracts were collected 24 or 48 hours later. RNAi of HJV and BMPR1A was used as a positive control. Equal protein amounts were analyzed by RPPAs using antibodies that recognize total levels and/or phosphorylation abundance of 82 proteins involved in different signaling pathways. Because proteins involved in PI3K/Akt/mTOR and Ras/MAPK signaling are highly represented on the arrays, we further distinguished those signals that could serve as markers for the pathway activation and/or whose altered levels may exert their activation or suppression (supplemental Figure 5). Protein signals that were significantly altered compared with scrambled siRNA transfection are marked by a color code. Clustering of the obtained signal signatures at the 48-hour time point divides the analyzed hepcidin regulators into 2 groups, with either stronger (pink) or minor (green) effects on cellular signaling. On the right side, we further indicated how many protein signals (%) from each category were increased upon overall depletion of this group of hepcidin modifiers (pink) that confer strong alteration of the signaling signature.
Figure 5.
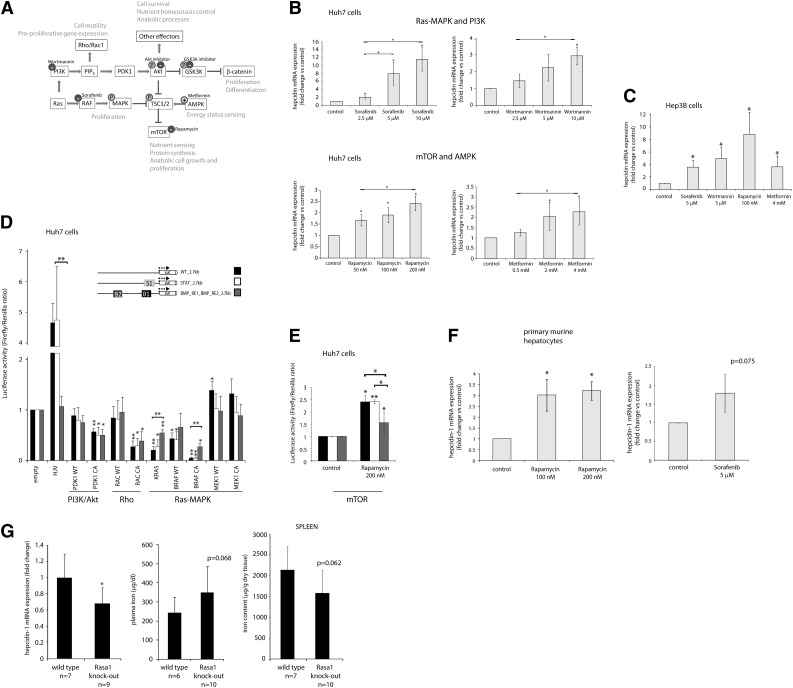
Identification of Ras/RAF MAPK and mTOR signaling as critical pathways for hepcidin suppression. (A) The interconnected signaling network, which involves Ras/MAPK, PI3K/Akt/mTOR, and β-catenin signaling, plays a crucial role in key cell processes such as proliferation, anabolic growth, nutrient homeostasis, survival, and motility. Small molecules used to modulate pathway activity are shown. (B,C,F) (B) Huh7 cells, (C) Hep3B cells, or (F) murine primary hepatocytes were treated with the indicated concentrations of small molecules: Wortmannin (4 hours), rapamycin (15 hours), sorafenib (9 hours), or metformin (15 hours). Levels of hepcidin mRNA expression were determined by real-time qPCR analysis and presented as fold change compared with untreated cells. (D-E) Huh7 cells were transfected with the wild-type and mutated hepcidin promoter reporter constructs. (D) The cells were cotransfected with expression vectors containing complementary DNA sequences of the indicated signaling genes. The clones were designed to encode wild-type (WT) and constitutively active (CA) protein variants (supplemental Table 2). Forced expression of HJV served as a positive control. (E) Cells were treated with rapamycin for 15 hours. The results are presented as fold change of luciferase activity (±SD of Firefly/Renilla) compared with overexpression of empty vector control (D) or untreated cells (E). (G) Shown are relative hepcidin mRNA, plasma, and splenic iron levels in mice 6 weeks after tamoxifen-induced systemic loss of Rasa1. The efficiency of Rasa1 deletion at the protein level in liver and spleen tissue is shown in supplemental Figure 6. Results represent a mean of at least 3 (B,F), 4 (C-D), or 5 (E) independent experiments. Statistically significant changes are marked by *(P < .05) or **(P < .001).
Significant changes were mainly observed after 48 hours of RNAi (Figure 4; supplemental Figure 5). Hierarchical clustering of RPPA data divided the analyzed genes into 2 groups: those that trigger stronger and those that trigger minor changes of cellular signaling. The second group contains the controls HJV and BMPR1A, as well as 2 novel hepcidin activators, RNF41 and CSNK1D, suggesting that their mode of action is different compared with the other analyzed hepcidin modifiers. Within the first group of hepcidin regulators (PRKCH, TRAF4, PIK3C2B, RASAL2, MAPK13, RASA1, and PIK3R3), we detected the most pronounced alterations controlling the Ras/MAPK and Rho signaling pathways. The PI3K/Akt/mTOR, GSK3K/β-catenin, or Sonic Hedgehog signaling pathways were more mildly affected, whereas NOTCH or Jnk/p38 signaling remained basically unaltered. Taken together, a combination of individual signal alterations may sum up to a suppression of hepcidin mRNA expression after the siRNA-mediated knockdown of the novel hepcidin activators.
Identification of Ras/RAF MAPK and mTOR signaling as critical pathways for hepcidin suppression
Guided by the information obtained from the RPPA data, we next interrogated the implicated cellular signaling networks by small molecules. As depicted in Figure 5A, the G-protein Ras plays a central role within the analyzed network and signals through both the RAF MAPK and PI3K signaling pathways. PI3K activates Rho signaling and, via PDK1, the Akt kinase. In turn, the Akt kinase induces diverse downstream pathways, including GSK3K/β-catenin and mTOR signaling. The activation of mTOR is also mediated by RAF MAPK signaling, whereas its suppression can be achieved by increased AMP kinase activity.50
Our results indicate that hepcidin mRNA expression is strongly activated in a dose-dependent manner upon inhibition of the RAF MAP kinase (by sorafenib) and, to a lesser extent, by suppression of the PI3K (by Wortmannin) and the mTOR kinase (by rapamycin), as well as by activation of the AMP kinase (by metformin) (Figure 5B). Similar hepcidin responses to these small molecule inhibitors were observed in Hep3B cells (Figure 5C). By contrast, inhibition of the Akt and GSK3K kinases did not affect hepcidin mRNA levels (data not shown).
In a complementary approach, we stimulated Ras/RAF MAPK, Rho, and PI3K signaling pathways by overexpression of the critical regulators of these pathways and assessed hepcidin promoter activity (Figure 5D). In addition to the wild-type versions of these signaling factors, we alternatively transfected constitutively active (CA) isoforms (supplemental Table 2); we also used mutated, in addition to the wild-type, hepcidin reporters. Forced expression of HJV was used as a positive control, and the expected increase in reporter expression was observed from the wild-type and STAT-BS–mutated hepcidin promoter, but not from the BMP-REs mutant version. Strikingly, our data show that induction of Ras- and BRAF-dependent signals by overexpression of their oncogenic, constitutively active mutants potently suppresses hepcidin promoter–driven transcription in a BMP-REs–dependent manner. By contrast, the promoter activity remains largely unaltered upon the forced expression of MEK1, a kinase that is located further downstream in the MAPK signaling pathway. Stimulation of the Rho protein RAC1, as well as forced expression of the PI3K effector PDK1 kinase, inhibit activity of the wild-type and mutated hepcidin promoters (Figure 5D) to a similar extent as we observed for the specificity control, the ferroportin promoter (data not shown). In addition, we found that induction of the hepcidin promoter by rapamycin also requires BMP-REs (Figure 5E). Altogether, our combined approaches uncover the Ras/RAF MAPK and mTOR signaling pathways as key effectors for hepcidin suppression. We propose that these responses link systemic iron availability with proliferation, cell growth, and bioenergetic metabolic adjustments in response to mitogenic signals and the nutrient status.48,49
We next tested whether the hepcidin response to the mTOR and Ras/RAF MAPK signaling pathways is preserved in primary hepatocytes or mice. We show that mTOR and, to a lesser extent, RAF inhibitors induce hepcidin mRNA expression in murine primary hepatocytes, consistent with our data obtained in hepatocarcinoma cell lines (Figure 5F). We further analyzed iron-related parameters in inducible knockout mice for the Ras suppressor Rasa1, a mouse model that develops a lymphatic vessel disorder.51 We show that systemic disruption of Rasa1 expression, including liver and spleen tissues (supplemental Figure 6), for 6 weeks reduces liver hepcidin mRNA expression. Consistently, we observe a tendency for an increase of plasma iron and a decrease of spleen iron levels (Figure 5G), whereas liver iron levels are not significantly changed between wild-type and Rasa1 knockout mice (data not shown). These findings suggest that the mTOR and Ras/RAF MAPK signaling pathways contribute to the hepcidin control in primary cells and mice.
Discussion
In this study we aimed to define molecular processes and signaling pathways that modify hepcidin transcription. Initially, we conducted an unbiased, genome-wide RNAi screen and followed up with extensive analyses to gain insight into networks that control hepcidin expression.
Analysis of our genome-wide screening data demonstrates that the established RNAi approach accurately detects most hepcidin activators previously identified by genetic studies (Figure 1). We further identified the hepcidin suppressor SMAD729 but not other known hepcidin inhibitors, such as growth differentiation factor 15 or TWSG1, that are typically produced by the erythron,32,33 or hypoxia-related regulators (eg, hypoxia-inducible factor transcription factors30) that may only become active upon hypoxia. The siRNA pool targeting TMPRSS6 may not have been reliable because it was replaced in the next generation of the siRNA library.
The identification of enriched functional categories among the screening hits indicates coherence and high quality of the screen. Notably, the JAK-STAT signaling pathway and genes involved in bone development were found to be enriched among putative hepcidin activators (supplemental Table 7). From 61 candidate genes that were enriched for genes involved in signal transduction and transcriptional regulation, we validated 15 (25%) novel hepcidin activators in 2 rounds of stringent validation assays in Huh7 cells. Twelve of these genes were further shown to control hepcidin mRNA levels in a qualitatively similar manner in human primary hepatocytes, clearly demonstrating that their role in the control of hepcidin expression is not restricted to a hepatocarcinoma cell line (Figure 2).
The unbiased identification of novel hepatocytic hepcidin regulators may have important clinical implications because it may aid in explaining the causes of iron-related disorders of unknown genetic origin and may reveal modifiers of disease severity in patients with HH. To this end, the list of putative hepcidin regulators generated in this study may serve as a resource, which could be integrated with data from genome-wide association, quantitative trait loci, and deep sequencing studies to identify new genetic modifiers of iron homeostasis.9,52-54 Future work based on the data from this RNAi screen is also expected to shed light on thus far unidentified links between HFE/TfR2 and BMP/HJV-dependent signaling.24,25 Our current analysis has validated 4 proteins with adapter function and SH2 domains, which typically mediate protein-protein interactions involved in signal transduction. In addition, we identified 4 proteins that harbor the lipid membrane–targeting C2 domain (Figure 1E). Future studies will address the role of these and other hits from our screen in the formation of the iron-sensing complex in hepatocytes.
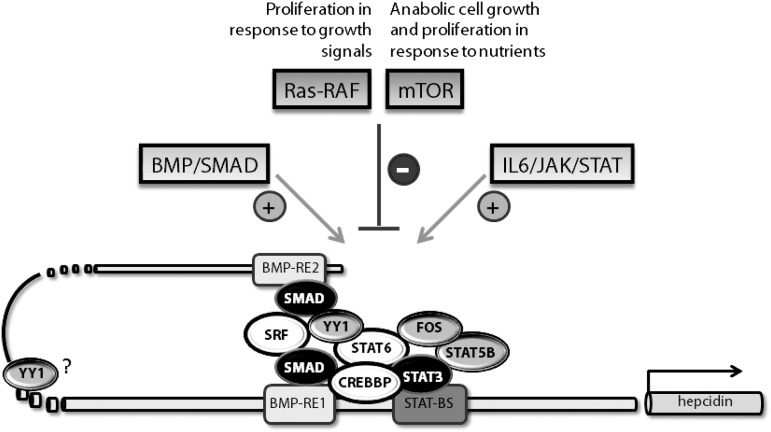
Our work further shows that neither of the hepcidin responses depends solely on the transcription factors SMAD4 or STAT3, suggesting a more complex transcriptional machinery that controls hepcidin promoter activity (Figures 3D and 6 and supplemental Figure 4). STAT6, CREBBP, and SRF mimic SMAD4 requirements for hepcidin induction in response to IL-6 and BMP-6, suggesting that they may directly cooperate with the SMAD factors to form a core transcriptional complex at the hepcidin promoter. In addition, STAT6 that was postulated to form heterodimers with STAT355—as well as the transcription coactivator CREBBP, which can bind STATs and SMADs56—represent very promising candidates for the molecular bridging between the IL-6 and BMP-6–triggered hepcidin responses. Furthermore, YY1, which binds to transcription complexes involving CREBBP, SRF, and SMADs, may physically bend DNA and thus organize the DNA-protein complex on target promoters.57-59 Altogether, the identified transcription regulators exhibit scaffolding properties, positively interact with each other and/or with SMADs, and therefore may confer a transcriptional architecture for the regulation of the hepcidin promoter. The finding that the validated transcription factors regulate hepcidin transcription in a BMP-REs–dependent manner supports these hypotheses (Figure 3C).
Figure 6.
In addition to the known pathways that activate hepcidin expression, we show that Ras/RAF-triggered signals and the mTOR pathway are involved in hepcidin suppression. This finding links the control of iron homeostasis with enhanced cell proliferation and anabolic growth under nutrient-rich and growth-stimulating conditions. Furthermore, we identify new components of the transcriptional machinery that control steady-state levels of hepcidin expression and hepcidin induction upon IL-6 stimulation. Among these, 3 factors (STAT6, CREBBP, and SRF) are additionally required for the BMP-triggered hepcidin response.
The 15 identified hepcidin activators represent a wide spectrum of proteins involved in signal transduction and transcription regulation that appear not to converge on one particular pathway (Figure 2B). Only subsets of the identified hepcidin activators are required for the hepcidin responses under stimulatory conditions, suggesting that modulation of hepcidin steady-state mRNA levels may involve other signaling pathways (Figure 3D). The combination of protein array analysis (Figure 4), small molecule treatment of hepatoma cell lines and primary hepatocytes, and protein overexpression studies revealed that Ras/RAF-triggered and mTOR signaling specifically suppress hepcidin expression (Figures 5 and 6). Thus these pathways may operate downstream of the identified hepcidin regulators. In line with this, PRKCH suppresses ERK MAPK signaling and activates the novel hepcidin modulator MAPK13.60 Importantly, two of the identified hepcidin modifiers, RASA1 and RASAL2, directly connect to Ras protein inactivation.41,61,62 Our observation that systemic loss of Rasa1 in the mouse affects liver hepcidin expression further indicates that regulators of Ras activity may function as modifiers of iron homeostasis in vivo. Of note, 2 similar syndromes hallmarked by vascular malformations are associated with mutations in SMAD4 or the Ras suppressor RASA1,63,64 further corroborating a link between Ras-dependent signaling events and the SMAD pathway.
Recent reports demonstrated that growth factors (eg, epidermal growth factor) that act via the MAPK and the PI3K pathways, as well as testosterone that induces epidermal growth factor signaling, suppress hepcidin expression.34,36 Our independent data extend these findings by showing that upstream Ras/RAF-dependent signals, rather than downstream MEK1/ERK signaling, inhibit hepcidin mRNA expression. Together with the previous observation that ERK kinases activate hepcidin mRNA expression in response to holotransferrin,65 these results suggest that other noncanonical signals initiated by Ras/RAF are involved in hepcidin inhibition. This is consistent with the finding that among the genes that positively regulate hepcidin transcription, the Ras suppressors but not other components of the MAPK pathway constitute an enriched functional group. Consistent with the fact that IL-6 not only activates the JAK/STAT but also the Ras/MAPK pathway,66 we observe that full hepcidin induction upon IL-6 treatment depends on the Ras suppressor RASA1, RNF41, and PRKCH, among others, which all inhibit growth factor–triggered signaling.60,67 Importantly, our results also identify the nutrient-sensing mTOR pathway as a mediator of hepcidin inhibition. Thus hepcidin suppression and the subsequent increase of systemic iron availability are associated with pathways that are not only implicated in hepatocyte proliferation upon a growth stimulus but also in anabolic cell growth and metabolic switches in response to elevated nutrient levels.48,49
Finally, the pathways identified in our study are widely implicated in liver physiology and disease. Growth factors and cytokines trigger the Ras/MAPK and mTOR pathways to promote hepatocyte proliferation and growth and thus drive liver regeneration in various models of liver injury.48,68 Ras/MAPK and mTOR signaling also represent the major pathways that are aberrantly activated in hepatocellular carcinoma (HCC), the most detrimental consequence of several chronic liver diseases.50,69 We thus propose that decreased hepcidin expression, which is observed in liver stress conditions including viral70 and alcohol hepatitis,71 and ultimately in HCC,72 may involve activation of the Ras/MAPK and mTOR pathways (Figures 5 and 6). Importantly, the RAF inhibitor sorafenib is applied for combined therapies of HCC.73 Our work indicates that, apart from interfering with cancer cell proliferation and angiogenesis, administration of sorafenib may mediate iron restriction for the growing tumor by inducing hepcidin expression.
The mTOR pathway functions as a key sensor of the nutritional status and thus adjusts cellular metabolism to environmental growth conditions. In the liver it plays a critical role for the maintenance of glucose and lipid homeostasis.49 Because of the nutrient overload associated with obesity and type 2 diabetes, the activity of mTOR signaling is elevated. This in turn promotes overproduction of lipids, which can further exacerbate the metabolic syndrome. An increasing body of evidence underscores the role of elevated body iron status in the pathogenesis of insulin-resistant diabetes,12 which suggests aberrant hepcidin regulation under such clinical conditions. One study suggests that hepcidin levels are elevated in patients diagnosed with metabolic syndrome and correlate with the increased levels of ferritin.11 However, more recent findings indicate hepcidin deficiency in patients with type 2 but not type 1 diabetes, suggesting that hepcidin suppression is not attributed to insulin deficiency or hyperglycemia but may be linked to insulin resistance and nutrient overload.74 It still remains to be elucidated how hepcidin expression is affected in relatively healthy but obese persons. Interestingly, alteration of iron homeostasis has been proposed as a possible underlying cause for the development of microcytic anemia in patients treated with the mTOR inhibitor rapamycin. This hypothesis was largely based on the observation that the anemic state can be corrected by intravenous iron delivery but remains refractory to oral iron administration, suggesting hepcidin-mediated inhibition of dietary iron absorption.75 Our finding that rapamycin induces hepcidin expression may offer an explanation for these clinical findings in rapamycin-treated patients.
Supplementary Material
Acknowledgments
The authors thank Dr Daniel Seehofer for performing surgical resection of human liver tissue, and Sandra Bonefas, Katharina Waldow, and Svantje Braun for technical assistance.
This study is supported by eRARE HMAIRON (BMBF), the Dietmar Hopp Stiftung, the Deutsche Forschungsgemeinschaft (SFB1036), and the Virtual Liver funding initiatives of the BMBF (M.U.M.); a ERASysBio+ grant “LivSysiPS” (0315717C) by the BMBF (U. Korf); the BMBF within the Virtual Liver Network (0315745) (L.A.D., U. Klingmüller); a Marie-Curie Excellence Team Grant and the Helmholtz Alliance for Systems Biology (M.B.); and the National Institutes of Health (RO1HL096498) (P.D.K.).
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.M.-S. designed research, performed experiments and wrote the manuscript; F.R., A.R.d.S., D.C., F.D., A.R., R.U., P.E.L., and P.D.K. performed experiments and analyzed data; G.D., C.O., L.A.D., and U. Klingmüller contributed relevant reagents or vital model systems; M.B and U. Korf supervised experiments and edited the manuscript; and M.W.H. and M.U.M. designed the project, supervised research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martina U. Muckenthaler, University of Heidelberg, Department of Pediatric Oncology, Hematology and Immunology, Im Neuenheimer Feld 153, 69120 Heidelberg, Germany; e-mail: martina.muckenthaler@med.uni-heidelberg.de; Matthias W. Hentze, European Molecular Biology Laboratory (EMBL), Meyerhofstrasse 1, 69117 Heidelberg, Germany; e-mail: hentze@embl.de.
References
- 1.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 3.Bridle KR, Frazer DM, Wilkins SJ, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361(9358):669–673. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 5.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 6.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33(1):21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 7.Rochette J, Le Gac G, Lassoued K, Férec C, Robson KJ. Factors influencing disease phenotype and penetrance in HFE haemochromatosis. Hum Genet. 2010;128(3):233–248. doi: 10.1007/s00439-010-0852-1. [DOI] [PubMed] [Google Scholar]
- 8.Fleming RE, Holden CC, Tomatsu S, et al. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 2001;98(5):2707–2711. doi: 10.1073/pnas.051630898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensaid M, Fruchon S, Mazères C, Bahram S, Roth MP, Coppin H. Multigenic control of hepatic iron loading in a murine model of hemochromatosis. Gastroenterology. 2004;126(5):1400–1408. doi: 10.1053/j.gastro.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Papanikolaou G, Tzilianos M, Christakis JI, et al. Hepcidin in iron overload disorders. Blood. 2005;105(10):4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinelli N, Traglia M, Campostrini N, et al. Increased serum hepcidin levels in subjects with the metabolic syndrome: a population study. PLoS ONE. 2012;7(10):e48250. doi: 10.1371/journal.pone.0048250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta. 2009;1790(7):671–681. doi: 10.1016/j.bbagen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 15.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16(3):291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl) 2009;87(5):471–480. doi: 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 19.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 21.Verga Falzacappa MV, Casanovas G, Hentze MW, Muckenthaler MU. A bone morphogenetic protein (BMP)-responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J Mol Med (Berl) 2008;86(5):531–540. doi: 10.1007/s00109-008-0313-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Ryan JD, Ryan E, Fabre A, Lawless MW, Crowe J. Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis. Hepatology. 2010;52(4):1266–1273. doi: 10.1002/hep.23814. [DOI] [PubMed] [Google Scholar]
- 24.Kautz L, Meynard D, Besson-Fournier C, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114(12):2515–2520. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 25.Corradini E, Garuti C, Montosi G, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137(4):1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50(6):1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 27.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mleczko-Sanecka K, Casanovas G, Ragab A, et al. SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood. 2010;115(13):2657–2665. doi: 10.1182/blood-2009-09-238105. [DOI] [PubMed] [Google Scholar]
- 30.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117(7):1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto JP, Ribeiro S, Pontes H, et al. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPalpha. Blood. 2008;111(12):5727–5733. doi: 10.1182/blood-2007-08-106195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 33.Tanno T, Porayette P, Sripichai O, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114(1):181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodnough JB, Ramos E, Nemeth E, Ganz T. Inhibition of hepcidin transcription by growth factors. Hepatology. 2012;56(1):291–299. doi: 10.1002/hep.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12(2):280–291. doi: 10.1111/acel.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latour C, Kautz L, Besson-Fournier C, et al. Testosterone perturbs systemic iron balance through activation of EGFR signaling in the liver and repression of hepcidin [published online ahead of print August 1, 2013]. Hepatology. 2013 doi: 10.1002/hep.26648. doi: 10.1002/hep.26648. [DOI] [PubMed] [Google Scholar]
- 37.Godoy P, Hewitt NJ, Albrecht U, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87(8):1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huard J, Mueller S, Gilles ED, Klingmüller U, Klamt S. An integrative model links multiple inputs and signaling pathways to the onset of DNA synthesis in hepatocytes. FEBS J. 2012;279(18):3290–3313. doi: 10.1111/j.1742-4658.2012.08572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 40.Raval A, Tanner SM, Byrd JC, et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell. 2007;129(5):879–890. doi: 10.1016/j.cell.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapinski PE, Bauler TJ, Brown EJ, Hughes ED, Saunders TL, King PD. Generation of mice with a conditional allele of the p120 Ras GTPase-activating protein. Genesis. 2007;45(12):762–767. doi: 10.1002/dvg.20354. [DOI] [PubMed] [Google Scholar]
- 42.Vujić Spasić M, Kiss J, Herrmann T, et al. Hfe acts in hepatocytes to prevent hemochromatosis. Cell Metab. 2008;7(2):173–178. doi: 10.1016/j.cmet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Boutros M, Brás LP, Huber W. Analysis of cell-based RNAi screens. Genome Biol. 2006;7(7):R66. doi: 10.1186/gb-2006-7-7-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 45.Szklarczyk D, Franceschini A, Kuhn M, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39(Database issue):D561–D568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cline MS, Smoot M, Cerami E, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2(10):2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loebke C, Sueltmann H, Schmidt C, et al. Infrared-based protein detection arrays for quantitative proteomics. Proteomics. 2007;7(4):558–564. doi: 10.1002/pmic.200600757. [DOI] [PubMed] [Google Scholar]
- 48.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 49.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dufour J-F, Clavien P-A. Signaling pathways in liver diseases. 2nd ed. New York, NY: Springer-Verlag; 2009. [Google Scholar]
- 51.Lapinski PE, Kwon S, Lubeck BA, et al. RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Invest. 2012;122(2):733–747. doi: 10.1172/JCI46116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pichler I, Minelli C, Sanna S, et al. Identification of a common variant in the TFR2 gene implicated in the physiological regulation of serum iron levels. Hum Mol Genet. 2011;20(6):1232–1240. doi: 10.1093/hmg/ddq552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grant GR, Robinson SW, Edwards RE, et al. Multiple polymorphic loci determine basal hepatic and splenic iron status in mice. Hepatology. 2006;44(1):174–185. doi: 10.1002/hep.21233. [DOI] [PubMed] [Google Scholar]
- 54.Jones BC, Beard JL, Gibson JN, et al. Systems genetic analysis of peripheral iron parameters in the mouse. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R116–R124. doi: 10.1152/ajpregu.00608.2006. [DOI] [PubMed] [Google Scholar]
- 55.Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci USA. 1998;95(10):5590–5594. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–1577. [PubMed] [Google Scholar]
- 57.Natesan S, Gilman M. YY1 facilitates the association of serum response factor with the c-fos serum response element. Mol Cell Biol. 1995;15(11):5975–5982. doi: 10.1128/mcb.15.11.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25(8):1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 59.Lee KH, Evans S, Ruan TY, Lassar AB. SMAD-mediated modulation of YY1 activity regulates the BMP response and cardiac-specific expression of a GATA4/5/6-dependent chick Nkx2.5 enhancer. Development. 2004;131(19):4709–4723. doi: 10.1242/dev.01344. [DOI] [PubMed] [Google Scholar]
- 60.Efimova T, Deucher A, Kuroki T, Ohba M, Eckert RL. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 delta mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277(35):31753–31760. doi: 10.1074/jbc.M205098200. [DOI] [PubMed] [Google Scholar]
- 61.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603(2):47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 62.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129(5):865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Boon LM, Mulliken JB, Vikkula M. RASA1: variable phenotype with capillary and arteriovenous malformations. Curr Opin Genet Dev. 2005;15(3):265–269. doi: 10.1016/j.gde.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Gallione CJ, Repetto GM, Legius E, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004;363(9412):852–859. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- 65.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94(6):765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang CH, Chen CF, Chen WM, Fong YC. IL-6 increases MMP-13 expression and motility in human chondrosarcoma cells. J Biol Chem. 2011;286(13):11056–11066. doi: 10.1074/jbc.M110.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu XB, Goldberg AL. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc Natl Acad Sci USA. 2002;99(23):14843–14848. doi: 10.1073/pnas.232580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Espeillac C, Mitchell C, Celton-Morizur S, et al. S6 kinase 1 is required for rapamycin-sensitive liver proliferation after mouse hepatectomy. J Clin Invest. 2011;121(7):2821–2832. doi: 10.1172/JCI44203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48(6):2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 70.Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48(5):1420–1429. doi: 10.1002/hep.22486. [DOI] [PubMed] [Google Scholar]
- 71.Darshan D, Frazer DM, Anderson GJ. Molecular basis of iron-loading disorders. Expert Rev Mol Med. 2010;12:e36. doi: 10.1017/S1462399410001687. [DOI] [PubMed] [Google Scholar]
- 72.Maegdefrau U, Arndt S, Kivorski G, Hellerbrand C, Bosserhoff AK. Downregulation of hemojuvelin prevents inhibitory effects of bone morphogenetic proteins on iron metabolism in hepatocellular carcinoma. Lab Invest. 2011;91(11):1615–1623. doi: 10.1038/labinvest.2011.123. [DOI] [PubMed] [Google Scholar]
- 73.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 74.Sam AH, Busbridge M, Amin A, et al. Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabet Med. 2013;30(12):1495–1499. doi: 10.1111/dme.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sofroniadou S, Kassimatis T, Goldsmith D. Anaemia, microcytosis and sirolimus—is iron the missing link? Nephrol Dial Transplant. 2010;25(5):1667–1675. doi: 10.1093/ndt/gfp674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.