Key Points
Reduced intensity and myeloablative regimen results in comparable survival after allogeneic transplantation.
Abstract
The safety and efficacy of reduced-intensity conditioning (RIC) regimens for the treatment of pediatric acute myeloid leukemia is unknown. We compared the outcome of allogeneic hematopoietic cell transplantation in children with acute myeloid leukemia using RIC regimens with those receiving myeloablative-conditioning (MAC) regimens. A total of 180 patients were evaluated (39 with RIC and 141 with MAC regimens). Results of univariate and multivariate analysis showed no significant differences in the rates of acute and chronic graft-versus-host disease, leukemia-free, and overall survival between treatment groups. The 5-year probabilities of overall survival with RIC and MAC regimens were 45% and 48%, respectively (P = .99). Moreover, relapse rates were not higher with RIC compared with MAC regimens (39% vs 39%; P = .95), and recipients of MAC regimens were not at higher risk for transplant-related mortality compared with recipients of RIC regimens (16% vs 16%; P = .73). After carefully controlled analyses, we found that in this relatively modest study population, the data supported a role for RIC regimens for acute myeloid leukemia in children undergoing allogeneic hematopoietic cell transplantation. The data also provided justification for designing a carefully controlled randomized clinical trial that examines the efficacy of regimen intensity in this population.
Introduction
Allogeneic hematopoietic cell transplantation is an established treatment of acute myelogenous leukemia (AML).1 Myeloablative-conditioning (MAC) regimens prior to allogeneic transplantation is believed to control leukemia by combining intensive preparative therapy with the benefit of the graft vs leukemia effect. Transplantation of grafts from an HLA-matched sibling has generally been considered to be the treatment of choice for children with AML in first complete remission (CR1).2 However, with the improved results now obtained with chemotherapy alone, and the known short- and long-term risks of allogeneic hematopoietic cell transplantation, this treatment option is no longer offered to all children with AML in CR1, but is reserved for those with intermediate or high-risk disease.3-5 Transplantation of grafts from unrelated donors in CR1 is being explored in children with high-risk cytogenetics6 and is widely offered for those with recurrent leukemia.7,8
In recent years, reduced-intensity conditioning (RIC) regimens for allogeneic hematopoietic cell transplantation have been demonstrated to be safe and efficacious for adult hematologic malignancies.9 Low-regimen-related toxicity and morbidity have dramatically extended the availability of allogeneic hematopoietic cell transplantation to a large and important group of patients previously ineligible by virtue of age or organ dysfunction. RIC regimens depend upon intensive immunosuppression in order to establish donor-recipient chimerism, which rapidly develops into full-donor chimerism with withdrawal of immunosuppression or donor lymphocyte infusion. Disease control is believed to be mainly by graft vs leukemia effects, and to a lesser extent by the chemotherapy administered during conditioning for transplantation. Since most RIC regimens use relatively low doses of chemotherapy, transplant-related morbidity and mortality are low. A recent summary of the European experience that compared MAC and RIC regimens for unrelated donor transplantation for adults concluded that RIC regimens were associated with higher relapse risks in patients <50 years of age with AML, and lower non-relapse mortality in those >50 years. The overall result was leukemia-free survival that was similar with both conditioning regimens, regardless of patient age.10 A North American study also showed similar outcomes.11 The results of a phase 2 trial of RIC transplantation concluded that favorable outcomes could be achieved for children and adolescents with hematologic malignancy in remission at transplantation.12 Although that trial used a uniform transplant conditioning regimen (busulfan, fludarabine, and antithymocyte globulin), patients with a variety of hematologic malignancies were eligible and enrollment was limited to 47 patients. Another recent report that examined the role of RIC regimens for malignant and nonmalignant diseases confirmed low transplant-related mortality but high graft failure in recipients of umbilical cord blood transplantation.13 While these reports confirm that RIC regimens are well tolerated, there are few reports that have compared transplant outcomes after RIC regimens to that of MAC regimens in children for specific diseases. The Center for International Blood and Marrow Transplant Research (CIBMTR) reported outcomes after RIC regimens for pediatric acute lymphoblastic leukemia.14 In that report, transplant-related mortality and relapse rates were high, with a modest 3-year leukemia-free survival rate of 30%. In the current study, also using data reported to the CIBMTR, we compared transplant-outcomes after RIC regimens to an appropriately matched population of transplant recipients who received MAC regimens for treatment of children with AML.
Patients and methods
Data collection
The CIBMTR is a voluntary working group of more than 450 transplantation centers that contribute detailed data on consecutive allogeneic and autologous transplantations to a Statistical Center at the Medical College of Wisconsin (Milwaukee, WI) or the National Marrow Donor Program (Minneapolis, MN). Participating centers are required to report all transplants consecutively and compliance is monitored by on-site audits. Patients are followed longitudinally until death or lost to follow-up. All patients provided written informed consent for data submission and research participation. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study. This study was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria
Patients <18 years who received a first allogeneic transplant for AML between 2000 and 2009, and received RIC- or MAC- regimens, were eligible. The regimen was considered “reduced intensity” if the dose of busulfan was less than 8 mg/kg orally or less than 6.4 mg/kg intravenously, melphalan was less than 150 mg/m2, total body irradiation (TBI) dose was 200 cGy or 400 cGy (administered as single fraction), or TBI dose was between 550-600 cGy (administered as fractionated doses).15 Other regimens were considered myeloablative. Patients with Fanconi anemia, Down syndrome, and those with secondary AML were excluded.
Recipients of RIC regimens (cases) were matched with recipients of MAC regimens (controls). Cases were matched for age at transplant ± 6 years, disease status, cytogenetic risk, graft type, and year of transplant ± 5 years. When matching cases on age and year of transplant, controls with the smallest difference in age and the closest in year of transplantation were selected. A total of 67 cases-controls were matched within 6 months of their ages, 28 cases-controls within 6-12 months of their ages, 21 cases-controls within 1-2 years of their ages, and the remaining 15 cases-controls within 3-5 years of their ages.
Thirty-nine recipients of RIC transplants (cases) were matched to 141 recipients of MAC transplants (controls). Thirty-one of 39 cases were matched to 4 controls, 2 cases were matched to 3 controls, 5 cases were matched to 2 controls, and 1 case was matched to 1 control. Eighty-nine transplant centers contributed patients, with most centers (n = 79) contributing fewer than 5 patients to the study population. The remaining 10 centers each contributed between 5 and 9 patients. As expected, most centers contributed only transplants that used MAC regimens (n = 60), while 13 centers contributed only transplants that used RIC regimens, and the remaining 16 centers contributed both transplants that used both RIC and MAC regimens.
Outcomes
The primary end point was leukemia-free survival, defined as being alive and in complete remission. Death from any cause or relapse was considered an event (or treatment failure). Other outcomes studied included: neutrophil recovery, defined as achieving an absolute neutrophil count ≥ 0.5 × 109/L for 3 consecutive measurements; platelets ≥ 20 × 109/L without transfusions for 7 days; grade 2-4 acute graft-versus-host disease (GVHD)16; chronic GVHD17; transplant-related mortality defined as death not attributed to relapse, and relapse defined as morphologic recurrence of leukemia. Surviving patients were censored at last follow-up and death from any cause was considered an event.
Statistical methods
The χ-square statistic was used to compare the characteristics of the 2 treatment groups. The Kaplan-Meier estimate was used to calculate the probabilities of leukemia-free and overall survival.18 The cumulative incidence estimator was used to calculate the probabilities of neutrophil and platelet recovery, acute and chronic GVHD, transplant-related mortality, and relapse.19 For neutrophil and platelet recovery, and acute and chronic GVHD, death without the event was the competing risk. For transplant-related mortality, relapse was the competing event, and for relapse, transplant-related mortality was the competing event. Stratified Cox regression models were built to test the relative efficacy of RIC regimens compared with MAC regimens.20 Cases and controls were matched on age, disease status at transplantation, cytogenetic risk, graft type, and transplant period. Other variables tested in the multivariate models included: performance score (90-100 vs <90), recipient cytomegalovirus serostatus (positive vs negative), donor type (HLA-matched sibling vs matched unrelated donor vs mismatched unrelated donor), and GVHD prophylaxis (cyclosporine-containing vs tacrolimus-containing). Variables that attained a level of significance ≤0.05 were considered significant. There were no first-order interactions. P values were 2-sided. All analyses were performed in SAS 9.3 software (Cary, NC).
Results
Patients, diseases, and transplant characteristics
Patients, diseases, and transplant characteristics are shown in Table 1. Thirty-nine transplantations used RIC regimens and 141 used MAC regimens. Conditioning regimens are described in Table 2. Although a variety of regimens were used, about half of MAC transplant recipients received TBI-containing regimens, and the remaining received non-TBI regimens. Busulfan with cyclophosphamide was the most frequently used non-TBI regimen. Approximately 20% of RIC regimens included low-dose TBI, with most patients receiving regimens including alkylating agents and fludarabine. The median age of patients included in both treatment groups was 14 years. In vivo T-cell depletion was equally likely in both groups (49% for RIC regimens vs 42% for MAC regimens; P = .39). Although the characteristics of the 2 treatment groups were largely similar, there were differences. Recipients of RIC regimens were more likely to report a poor performance score at transplantation (80 or lower), and were less likely to receive allografts from HLA-matched siblings compared with recipients of MAC regimens. Additionally, recipients of RIC regimens were more likely to have had a significant fungal infection prior to transplantation, 15 (38%) vs 24 (17%) after MAC regimens (P = .01). Considering unrelated donor transplants, recipients of RIC regimens were more likely to receive allografts from HLA-matched donors compared with recipients of MAC regimens. For unrelated donor transplantation, HLA-match was considered allele-level HLA typing at HLA-A, -B, -C, and -DRB1. For umbilical cord blood transplantation, HLA-matching was considered a low-resolution match at HLA-A and -B, and allele-level at -DRB1. There were no differences in GVHD prophylaxis regimens between the 2 treatment groups, and cyclosporine-containing regimens were more commonly used than tacrolimus-containing regimens in both cases and controls. Interestingly, there were no differences in the proportion of patients who received RIC and MAC regimens by transplant period (2000-2004 vs 2005-2009; P = .37). The median follow-up of surviving patients after RIC and MAC transplants was 5 years and 4 years, respectively. In this registry study, we had limited ability to determine why a RIC or MAC regimen was selected for a particular child. We sought to assess whether certain transplant centers had a preference for one preparative regimen or the other, and using the frailty model to look for a transplant center effect, we found none (P = .49).
Table 1.
Patients, disease, and transplant characteristics
| Reduced-intensity regimens | Myeloablative regimens | P | |
|---|---|---|---|
| Number of patients | 39 | 141 | |
| Age (y) | .70 | ||
| ≤10 | 14 (36%) | 52 (37%) | |
| 11-18 | 25 (64%) | 89 (63%) | |
| Sex | .87 | ||
| Male | 19 (49%) | 73 (52%) | |
| Female | 20 (51%) | 68 (48%) | |
| Performance score | .05 | ||
| 90-100 | 24 (62%) | 112 (79%) | |
| 60-80 | 13 (33%) | 24 (17%) | |
| Not reported | 2 (5%) | 5 (4%) | |
| Recipient cytomegalovirus serostatus | .08 | ||
| Negative | 8 (21%) | 52 (37%) | |
| Positive | 31 (79%) | 89 (63%) | |
| Disease status at transplantation | .90 | ||
| CR1 | 14 (36%) | 56 (40%) | |
| 2nd complete remission | 20 (51%) | 67 (48%) | |
| Relapse or induction failure * | 5 (13%) | 18 (13%) | |
| Cytogenetic risk group† | .99 | ||
| Favorable | 6 (15%) | 21 (15%) | |
| Intermediate | 27 (70%) | 99 (70%) | |
| Poor | 6 (15%) | 21 (15%) | |
| Donor type‡ | .003 | ||
| HLA-matched sibling | 5 (13%) | 40 (28%) | |
| Matched unrelated | 18 (46%) | 28 (20%) | |
| Mismatched unrelated | 16 (41%) | 73 (52%) | |
| Graft type | .92 | ||
| Bone marrow | 14 (36%) | 55 (39%) | |
| Peripheral blood | 13 (33%) | 43 (30%) | |
| Cord blood | 12 (31%) | 43 (30%) | |
| GVHD prophylaxis | .17 | ||
| Tacrolimus containing | 14 (36%) | 35 (24%) | |
| Cyclosporine containing | 25 (64%) | 106 (75%) | |
| Median (range) follow-up (mo) | 60 (12-97) | 47 (3-121) |
Matched unrelated in RIC group: cord blood (n = 1), bone marrow (n = 7), peripheral blood (n = 10); matched unrelated in MAC group: cord blood (n = 1), bone marrow (n = 14), peripheral blood (n = 13); mismatched unrelated in RIC group: cord blood (n = 11), bone marrow (n = 3), peripheral blood (n = 2); and mismatched unrelated in MAC group: cord blood (n = 42), bone marrow (n = 18), peripheral blood (n = 13).
Disease status at transplant was primary induction failure for 1 patient in the RIC group and 7 patients in the MAC group.
Cytogenetic risk group: favorable risk group included the t(8;21), t(15;17), and inv(16); high risk was defined by the presence of -7, -5, del (5q), abnormalities of the long arm of chromosome 3, or complex karyotype that was defined as more than 4 abnormalities; all other AML karyotypes were classified as intermediate risk.
For unrelated donor transplantation, an HLA-match was considered allele-level HLA typing at HLA-A, -B, -C, and -DRB1. For umbilical cord blood transplantation, HLA-matching was considered a low-resolution match at HLA-A and -B, and allele level at -DRB1.
Table 2.
Conditioning regimens
| Number | |
|---|---|
| Reduced-intensity regimen | |
| TBI + cyclophosphamide + fludarabine | 7 |
| TBI + other chemotherapeutic agents | 4 |
| Busulfan + fludarabine | 8 |
| Melphalan + fludarabine | 13 |
| Cyclophosphamide + fludarabine | 4 |
| Cyclophosphamide + melphalan | 2 |
| Cyclophosphamide + etopside | 1 |
| Myeloablative regimen | |
| TBI + cyclophosphamide | 63 |
| TBI + other chemotherapeutic agents | 8 |
| Busulfan + cyclophosphamide | 58 |
| Busulfan + melphalan | 6 |
| Busulfan + fludarabine | 6 |
Outcomes
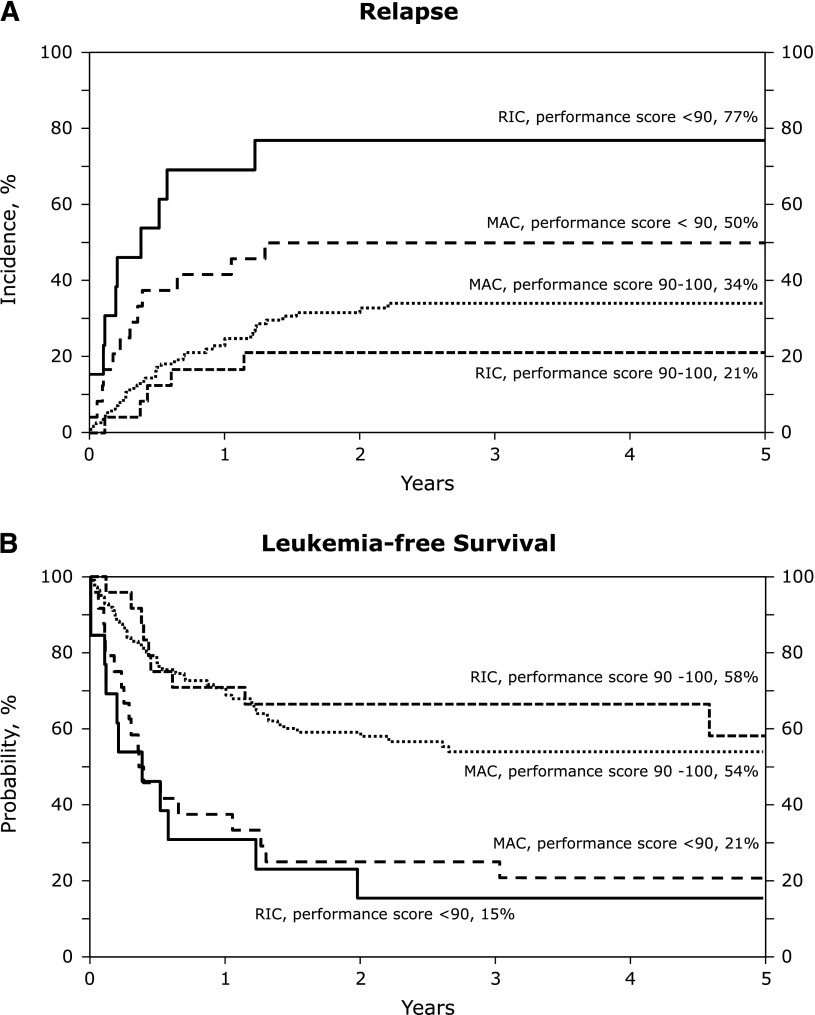
The unadjusted probabilities of transplant-outcomes are shown in Table 3. There were no significant differences between treatment groups in the probabilities of hematopoietic recovery, acute and chronic GVHD, transplant-related mortality, relapse, and leukemia-free and overall survival after RIC and MAC transplants. These observations were confirmed in multivariate analysis and the results are presented in Table 4. After adjusting for patient age, disease status at transplantation, cytogenetic risk, graft type, and transplant period, the only factor associated with relapse, leukemia-free and overall survival was the performance score. The 5-year probabilities of relapse after RIC and MAC regimens in patients with good performance scores were 21% (95% CI; 8-39) and 34% (95% CI; 25-43), respectively (P = .17). The corresponding probabilities of relapse in patients with poor performance scores were 77% (95% CI; 44-92) and 50% (95% CI; 29-68), respectively (P = .08) (Figure 1A). The 5-year probabilities of leukemia-free survival in patients with good performance scores were 58% (95% CI; 33-77) after RIC regimens and 54% (95% CI; 44-63) after MAC regimens (P = .74). The corresponding probabilities of leukemia-free survival in those with poor performance scores were 15% (95% CI; 2-39) and 21% (95% CI; 8-39) (P = .67) (Figure 1B).
Table 3.
Univariate analyses
| Outcomes | Reduced-intensity regimens | Myeloablative regimens | P | ||
|---|---|---|---|---|---|
| Events/Evaluable cases | Probability (95% CI), % | Events/Evaluable cases | Probability (95% CI), % | ||
| At day 28 | |||||
| Neutrophil recovery | 37/39 | 87 (75-96) | 130/139 | 84 (78-90) | .11 |
| At day 100 | |||||
| Platelet recovery | 34/38 | 89 (78-97) | 117/139 | 82 (76-88) | .11 |
| At day 100 | |||||
| Grades 2-4 acute GVHD | 12/39 | 28 (15-43) | 57/141 | 39 (31-47) | .22 |
| At 5 y | |||||
| Chronic GVHD | 17/38 | 46 (30-62) | 50/140 | 37 (29-46) | .38 |
| Transplant-related mortality | 5/39 | 16 (5-31) | 20/141 | 16 (10-22) | .73 |
| Relapse | 15/39 | 39 (24-54) | 54/141 | 39 (30-47) | .95 |
| Leukemia-free survival | 20/39 | 46 (29-63) | 74/141 | 46 (37-55) | .82 |
| Overall survival | 20/39 | 45 (29-62) | 69/141 | 48 (39-56) | .99 |
Table 4.
Multivariate analyses
| Hazard ratio (95% CI) | P | |
|---|---|---|
| Grade 2-4 acute GVHD | ||
| Reduced-intensity regimen | 1.00 | — |
| Myeloablative regimen | 1.62 (0.87-3.04) | .13 |
| Donor type | ||
| HLA-matched sibling | 1.00 | — |
| Unrelated donor | 2.45 (1.24-4.90) | .01 |
| Chronic GVHD | ||
| Reduced-intensity regimen | 1.00 | — |
| Myeloablative regimen | 0.89 (0.49-1.61) | .71 |
| T-cell depletion | ||
| Yes | 1.00 | — |
| None | 1.91 (1.11-3.26) | .02 |
| Donor type | ||
| HLA-matched sibling | 1.00 | — |
| Unrelated donor | 2.30 (1.14-4.65) | .02 |
| Transplant-related mortality | ||
| Reduced-intensity regimen | 1.00 | — |
| Myeloablative regimen | 1.53 (0.54-4.35) | .42 |
| T-cell depletion | ||
| None | 1.00 | — |
| Yes | 2.84 (1.26-6.37) | .02 |
| Relapse | ||
| Reduced-intensity regimen | 1.00 | — |
| Myeloablative regimen | 1.26 (0.69-2.31) | .45 |
| Performance score | ||
| 90-100 | 1.00 | — |
| <90 | 3.00 (1.75-5.13) | <.0001 |
| Treatment failure | ||
| Reduced-intensity regimen | 1.00 | — |
| Myeloablative regimen | 1.29 (0.77-2.16) | .33 |
| Performance score | ||
| 90-100 | 1.00 | — |
| <90 | 2.98 (1.86-4.70) | <.0001 |
| Overall survival | ||
| Reduced-intensity regimen | 1.00 | — |
| Myeloablative regimen | 1.24 (0.74-2.08) | .41 |
| Performance score | ||
| 90-100 | 1.00 | — |
| <90 | 3.22 (2.01-5.16) | <.0001 |
| Prior significant fungal infection | ||
| None | 1.00 | — |
| Yes | 1.83 (1.12-2.98) | .02 |
Figure 1.
The 5-year probabilities of relapse and leukemia-free survival of patients after RIC and MAC regimens. (A) The cumulative incidence of relapse by conditioning regiment intensity and performance score at transplant. (B) The probabilities of leukemia-free survival by conditioning regimen intensity and performance score at transplant.
Overall, 20 of 39 recipients with RIC transplantation and 69 of 141 recipients with MAC transplantation did not survive. In both groups, recurrent leukemia was the predominant cause of death, accounting for 75% of deaths in the RIC group and 65% of deaths in the MAC group. Death from infections was slightly more prevalent after MAC regimens compared with RIC regimens (19% vs 10%). In contrast to the MAC group where 2 patients died of GVHD, there were no deaths attributed to GVHD in the RIC group.
Discussion
In this study, we describe and compare transplant outcomes for patients <18 years of age with AML who received either RIC or MAC regimens for their allogeneic hematopoietic cell transplantation. Notably, we show that relapse rates are not higher after RIC regimens compared with MAC regimens, and that MAC regimens are not associated with higher transplant-related mortality compared with RIC regimens. Currently, MAC regimens are considered the standard of care for allogeneic hematopoietic cell transplantation in children and adolescents with AML.1,2,7 Yet, the current study, albeit in a carefully controlled but nonrandomized analysis of a modest number of patients, suggest that both transplant conditioning strategies are acceptable. Previously, the use of high-dose chemotherapy was said to provide a potential benefit to disease ablation, raising the concern that relapse will be more frequent if less intense transplant conditioning regimens were used. When RIC regimens were introduced more than a decade ago, studies demonstrated less peri-transplant morbidity and mortality with these regimens, allowing for transplantation in older and sicker patients.21,22 Some studies in adults have confirmed higher relapse rates with RIC regimens, but lower transplant-related mortality that offsets this disadvantage.10,11 In children, transplant-related mortality is generally lower after allogeneic transplantation, potentially reducing the advantage of a RIC regimen should relapse rates be higher with this approach. In this study, we saw no increase in relapse rates with the use of RIC regimens and no change in rates of transplant-related mortality, perhaps reflecting the already low transplant-related mortality seen with MAC regimens in this population.
An important limitation of this registry study is the lack of information regarding the rationale for the selection of a RIC regimen. We can only speculate that patients who received RIC were either treated on an institutional protocol or judged to be at high risk for transplant-related mortality by the treating physician. Indeed, in this study, patients receiving RIC regimens had significantly worse performance scores, which may partly explain the selection of the RIC regimen. Moreover, prior fungal infections were more common in recipients, supporting this possibility. It is important to also recognize that the sample size of the RIC cohort was small, and the preparative strategy varied, likely limiting the power to see smaller differences in outcomes. Interestingly, RIC regimens were selected for some patients in CR1, perhaps due to low performance scores and comorbidities, and also for patients with relapse or induction failure. We performed a controlled analysis, matching recipients of RIC transplantation with those who received MAC transplantation on age, disease status, cytogenetic risk, graft type, and transplant period known prognostic factors, to attempt to reduce the impact of this heterogeneity on the analysis.
In adults, RIC regimens are generally associated with lower transplant-related mortality relative to dose-intensive conditioning.21,22 However, we observed comparable transplant-related mortality rates after MAC regimens in our cohort, implying regimens with higher intensity are well tolerated in young patients. There are some important limitations to our study and caution must be used in interpreting this data based on a small sample size of retrospective data. We were unable to test for the comorbidity index of these patients, as the data were not collected consistently during the study period. Others have shown that the presence of comorbidities can have an adverse effect on survival in the pediatric population, and there is no reason to believe that this is not applicable to this study as well.23,24 In the current study, we used the performance score as a surrogate for coexisting morbidities and confirm that scores <90 are associated with higher relapse risks, and consequently, lower leukemia-free and overall survival. An additional benefit of the use of a RIC-preparative regimen might be the preservation of fertility and the reduced risk of secondary malignant neoplasms. Our follow-up is short, and we are unable to address these important issues in this study.
Our observations support embarking on a carefully controlled randomized trial to establish the role of RIC regimens in this population. In fact, in adults, the results of observational studies comparing RIC and MAC regimens for AML and myelodysplastic syndrome has led to a national trial in the US, through the Blood and Marrow Transplant Clinical Trials Network.
Acknowledgments
This study was funded by Public Health Service grant (U24-CA76518) from the National Cancer Institute, the National Heart Lung and Blood Institute, the National Institute of Allergy and Infectious Diseases, and the Health Resources and Services Administration (HHSH234200637051C).
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health or any other agency of the United States government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.B., W.H., M.-J.Z., and S.M.D. designed the study; W.H. and M.-J.Z. analyzed and interpreted the data; M.B. and S.M.D. drafted the manuscript; and H.A.-A., M.F.A., B.B., P.A.C., M.S.C., M.A.D., J.T.H., S.J., C.L.K., K.R.S., M.K., K.A.K., L.E.L., P.A.M., N.S., M.A.P., T.P., A.S., S.S., A.E.W., and L.C.Y. critically reviewed the manuscript. All authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stella M. Davies, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; e-mail: stella.davies@cchmc.org.
References
- 1.Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116(13):2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- 2.Neudorf S, Sanders J, Kobrinsky N, et al. Allogeneic bone marrow transplantation for children with acute myelocytic leukemia in first remission demonstrates a role for graft versus leukemia in the maintenance of disease-free survival. Blood. 2004;103(10):3655–3661. doi: 10.1182/blood-2003-08-2705. [DOI] [PubMed] [Google Scholar]
- 3.Chen AR, Alonzo TA, Woods WG, Arceci RJ. Current controversies: which patients with acute myeloid leukaemia should receive a bone marrow transplantation?—an American view. Br J Haematol. 2002;118(2):378–384. doi: 10.1046/j.1365-2141.2002.03701.x. [DOI] [PubMed] [Google Scholar]
- 4.Creutzig U, Reinhardt D. Current controversies: which patients with acute myeloid leukaemia should receive a bone marrow transplantation?—a European view. Br J Haematol. 2002;118(2):365–377. doi: 10.1046/j.1365-2141.2002.03697.x. [DOI] [PubMed] [Google Scholar]
- 5.Baker KS, Bresters D, Sande JE. The burden of cure: long-term side effects following hematopoietic stem cell transplantation (HSCT) in children. Pediatr Clin North Am. 2010;57(1):323–342. doi: 10.1016/j.pcl.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Kelly MJ, Horan JT, Alonzo TA, et al. Comparable survival for pediatric acute myeloid leukemia with poor-risk cytogenetics following chemotherapy, matched related donor, or unrelated donor transplantation. Pediatr Blood Cancer. 2014;61(2):269–275. doi: 10.1002/pbc.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson BE, Webb DK, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K United Kingdom Childhood Leukaemia Working Group and the Dutch Childhood Oncology Group. Results of a randomized trial in children with acute myeloid leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155(3):366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29(3):310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- 9.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 10.Ringdén O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 11.Luger SM, Ringdén O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pulsipher MA, Boucher KM, Wall D, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114(7):1429–1436. doi: 10.1182/blood-2009-01-196303. [DOI] [PubMed] [Google Scholar]
- 13.Satwani P, Jin Z, Duffy D, et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19(4):552–561. doi: 10.1016/j.bbmt.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Verneris MR, Eapen M, Duerst R, et al. Reduced-intensity conditioning regimens for allogeneic transplantation in children with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2010;16(9):1237–1244. doi: 10.1016/j.bbmt.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Concensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 17.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13(5):1091–1112, viii-ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol. 1972;34(2):187–200. [Google Scholar]
- 21.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20(1):128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 22.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28(17):2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 24.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117(9):2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]