Abstract
Patients with damage to the medial temporal lobe show deficits in forming new declarative memories but can still recall older memories, suggesting that the medial temporal lobe is necessary for encoding memories in the neocortex. Here, we found that cortical projection neurons in the perirhinal and entorhinal cortices were mostly immunopositive for cholecystokinin (CCK). Local infusion of CCK in the auditory cortex of anesthetized rats induced plastic changes that enabled cortical neurons to potentiate their responses or to start responding to an auditory stimulus that was paired with a tone that robustly triggered action potentials. CCK infusion also enabled auditory neurons to start responding to a light stimulus that was paired with a noise burst. In vivo intracellular recordings in the auditory cortex showed that synaptic strength was potentiated after two pairings of presynaptic and postsynaptic activity in the presence of CCK. Infusion of a CCKB antagonist in the auditory cortex prevented the formation of a visuo-auditory association in awake rats. Finally, activation of the entorhinal cortex potentiated neuronal responses in the auditory cortex, which was suppressed by infusion of a CCKB antagonist. Together, these findings suggest that the medial temporal lobe influences neocortical plasticity via CCK-positive cortical projection neurons in the entorhinal cortex.
Keywords: auditory cortex, neural plasticity, long-term potentiation, entorhinal cortex, hippocampal system, memory formation
Introduction
The hippocampal system consists of the hippocampus and adjacent entorhinal, perirhinal, and parahippocampal cortices1. Functioning as the gateway from the hippocampus to the neocortex, the entorhinal and perirhinal cortices form strong reciprocal connections with the neocortex2,3,4. Observations that patients with hippocampal damage show deficits in forming new long-term memories for facts and events5,6 have led to our current understanding that the hippocampal system is essential for establishing enduring declarative memories1. These patients, however, can still recall older memories7,8,9, suggesting that these memories are permanently stored in the neocortex10,11.
An outstanding question is whether the medial temporal lobe sends a “plasticity-enabled” signal to the neocortex to switch on the encoding of new associations. If so, which chemical is likely to serve as this signal? As neurons in the entorhinal and perirhinal cortices project to the neocortex, including the auditory cortex2,3, we expect that the candidate signal is released from their axons. Previous findings of heavy cholecystokinin (CCK) labeling in the entorhinal and perirhinal cortices12,13,14 prompted us to examine whether neurons projecting from the entorhinal and perirhinal cortices to the neocortex contain CCK.
CCK is the most abundant of all neuropeptides15. Reports that blocking CCK receptors suppresses conditioned fear16 and knocking out the CCK-B receptor gene reduces anxiety-like behavior17 in rodents suggest that CCK is associated with memory function. This possibility is further supported by findings that CCK is involved in hippocampal long-term potentiation and long-term depression18. Mice lacking the CCK gene exhibit poor performance in a passive avoidance task and impaired spatial memory19. Therefore, we examined whether CCK is involved in neocortical plasticity both within and across sensory modalities using extracellular recordings in anesthetized rats. As synaptic strength is potentiated only by multiple trains of high-frequency stimulation that evoke simultaneous presynaptic and postsynaptic activity20,21,22,23,24, we investigated the conditions required for synaptic plasticity in the presence of CCK using intracellular recordings. In the awake rat, we further tested whether preventing CCKB receptor activation disrupts the establishment of a cross-modal association, similar to what is observed after entorhinal cortex inactivation25. Finally, we examined whether activation of the entorhinal cortex potentiates neuronal responses in the auditory cortex and whether this potentiation is suppressed by local application of a CCKB antagonist in the auditory cortex.
Results
CCK-containing neurons in the medial temporal lobe project to the neocortex
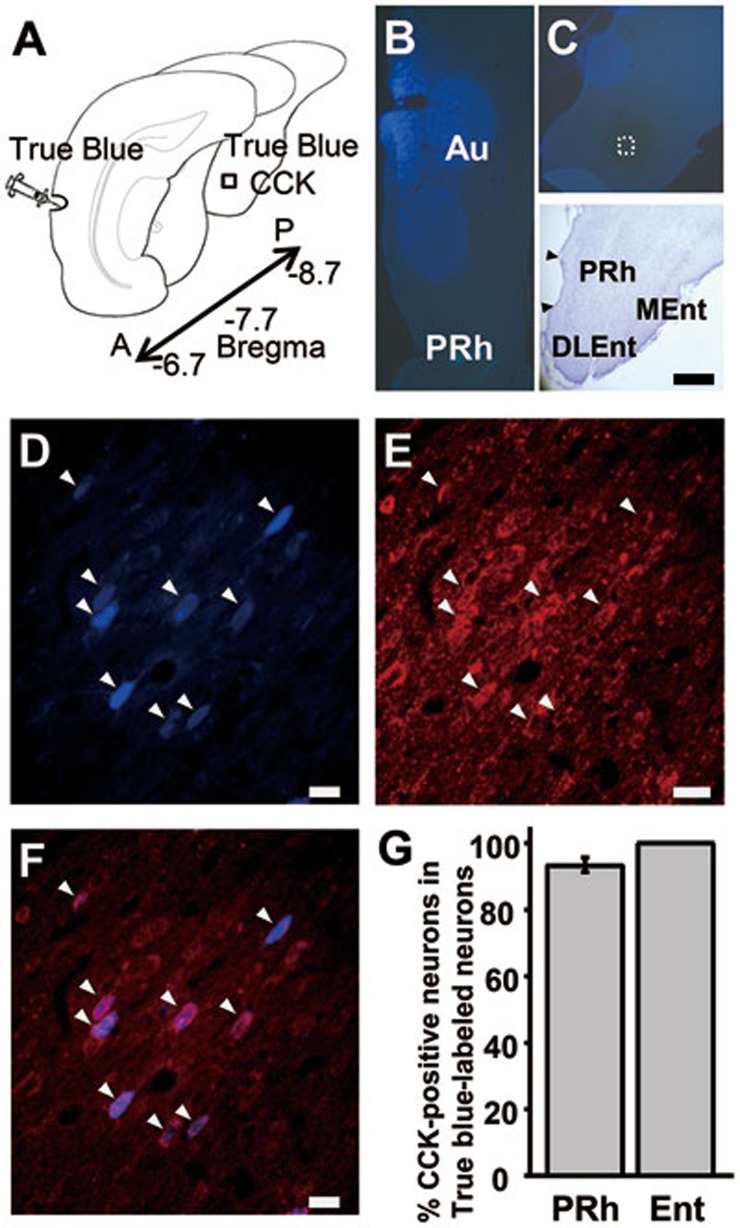
We first investigated whether CCK-containing neurons in the entorhinal and perirhinal cortices of the medial temporal lobe send axonal projections to the auditory cortex in rats. After True Blue was infused into the auditory cortex (Figure 1A and 1B), retrogradely labeled neurons in the entorhinal cortex were examined (Figure 1C). Retrogradely labeled neurons were found in both the entorhinal and perirhinal cortices after infusion of True Blue into the auditory cortex (Figure 1). The high degree of True Blue (Figure 1D) and CCK (Figure 1E) co-labeling (Figure 1F) indicates that the majority of projection neurons in the perirhinal (93.5% ± 2.15%, n = 6) and entorhinal (100%) cortices were CCK-positive (Figure 1G). True Blue-labeled neurons in the perirhinal and entorhinal cortices were mainly in layer V. These findings suggest that the medial temporal lobe may influence auditory cortex activity through CCK-containing neurons.
Figure 1.
Perirhinal and entorhinal cortices interact with the auditory cortex through CCK-containing neurons. (A) Experimental preparation. True Blue was infused into the auditory cortex, and True Blue and CCK were co-labeled in the entorhinal cortex. (B) True Blue infusion site in the auditory cortex. (C) Location of the retrogradely labeled neurons in the entorhinal cortex. (D-F) Retrograde True Blue labeling (D) and CCK immunoreactivity (E) in the entorhinal cortex after infusion of True Blue into the auditory cortex. Overlay of the two images (F). (G) Percentage of True Blue-labeled neurons also labeled with CCK in the perirhinal and entorhinal cortices. Nissl stain delineating the boundaries of the hippocampal formation. Au, auditory cortex; PRh, perirhinal cortex; Ent, entorhinal cortex; DLEnt and MEnt, dorsolateral and medial regions of the entorhinal cortex; A, anterior; P, posterior. Scale bars: 500 μm (A-C); 20 μm (D-F).
Local infusion of CCK potentiates auditory responses in anesthetized rats
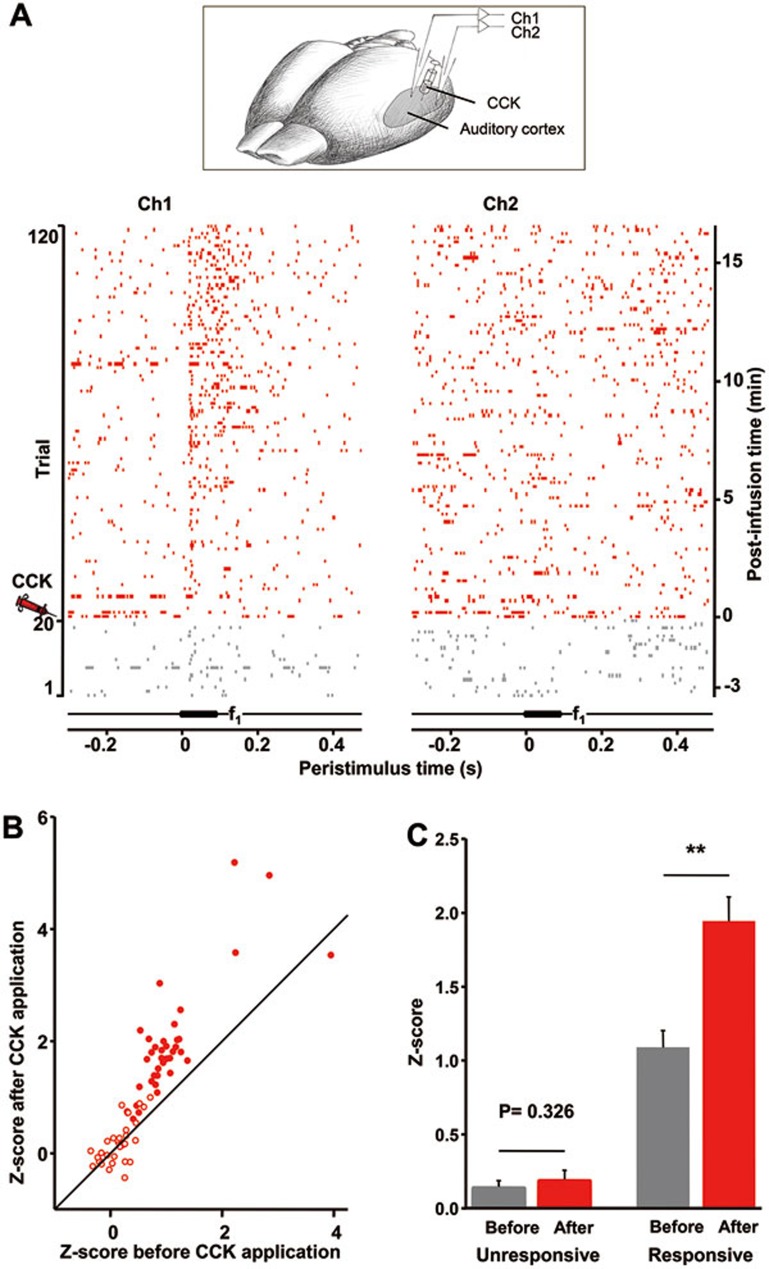
We speculate that CCK is a neuromodulator of cortical neuroplasticity. If this hypothesis is correct, we should be able to induce neuronal plasticity in anesthetized animals by local infusion of CCK. First, we attempted to potentiate neuronal responses in the auditory cortex after local infusion of CCK. As shown by the gray traces in Figure 2A, the neuron recorded in channel 1 (Ch1) responded weakly to the pure-tone stimulus f1 before infusion of CCK into the auditory cortex near the recording site. Afterward, the same auditory stimulus was repeatedly presented with an inter-stimulus interval of 10 s. Approximately 5 min after CCK infusion, the auditory response in Ch1 started to show potentiation. This potentiation grew larger at 7 min and was maintained throughout the recording period (i.e., for 17 min). The neuron recorded in channel 2 (Ch2) showed no response to the stimulus f1 either before or after CCK infusion.
Figure 2.
Enhancement of neuronal responses in the auditory cortex after local infusion of CCK in anesthetized rats. (A) Raster plots show neuronal responses to a pure-tone stimulus (f1 = 9.9 kHz) before (trials 1-20; gray) and after (trials 21-120; red) CCK was infused near the recording sites in the auditory cortex. Responses of a neuron to f1 (Ch1, left panel) increased after CCK infusion, whereas those of a neuron that was unresponsive to f1 (Ch2, right panel) did not change after CCK infusion. Experimental preparation is shown at the top panel. (B) Z-scores of neuronal responses to the auditory stimulus 15-20 min after CCK infusion plotted as a function of Z-scores before CCK infusion for all neurons that were responsive (filled circles) or unresponsive (open circles) to the auditory stimulus. Z-scores were calculated based on differences between average neuronal firing during the most responsive period after stimulus onset and spontaneous firing during an 8-s period before stimulus onset for each neuron. (C) Mean Z-scores before and after CCK infusion for unresponsive and responsive neurons in the auditory cortex. **P < 0.01, paired t-test.
In total, we recorded 69 neurons that responded to the auditory stimulus. Paired t-tests were used to compare responses of neurons to the auditory stimulus with their spontaneous firing rates. We categorized neurons with a P-value > 0.1 as unresponsive and neurons with a P-value ≤ 0.1 as responsive. Of the 32 unresponsive neurons, 21 showed minimal increases and 11 showed minimal decreases in Z-score after infusion of CCK and stimulus presentation for 20 min (open circles in Figure 2B). Of the 37 responsive neurons, 36 showed increases and 1 showed a minimal decrease in Z-score (filled circles in Figure 2B). Z-scores of unresponsive neurons did not change 20 min after CCK infusion (0.14 ± 0.05 vs 0.19 ± 0.07, n = 32, P = 0.326, paired t-test, Figure 2C), whereas Z-scores of responsive neurons significantly increased after CCK infusion (1.09 ± 0.12 vs 1.94 ± 0.17, n = 37, P < 0.001, paired t-test). These results indicate that a neuronal response to the stimulus is a prerequisite for CCK-induced potentiation in anesthetized rats.
Our next question was whether CCK infusion would allow a neuron to begin responding to a particular auditory stimulus to which it previously showed no response. We used two auditory stimuli; one was a stimulus that was too weak to evoke action potentials but presumably induced some form of presynaptic activity and the other was a stimulus that was strong enough to trigger action potentials. The strong stimulus was a pure tone at a frequency that evoked neuronal responses under anesthesia, and the weak stimulus was a pure tone at a frequency that did not evoke neuronal responses. We examined whether neurons would show altered responses to the weak stimulus after multiple pairings of strong and weak stimuli in the presence of CCK (Figure 3A). Neuronal responses to the weak stimulus were compared before and after infusion of CCK, phosphate buffered saline (PBS)/acetylcholine, or no drug at the recording site.
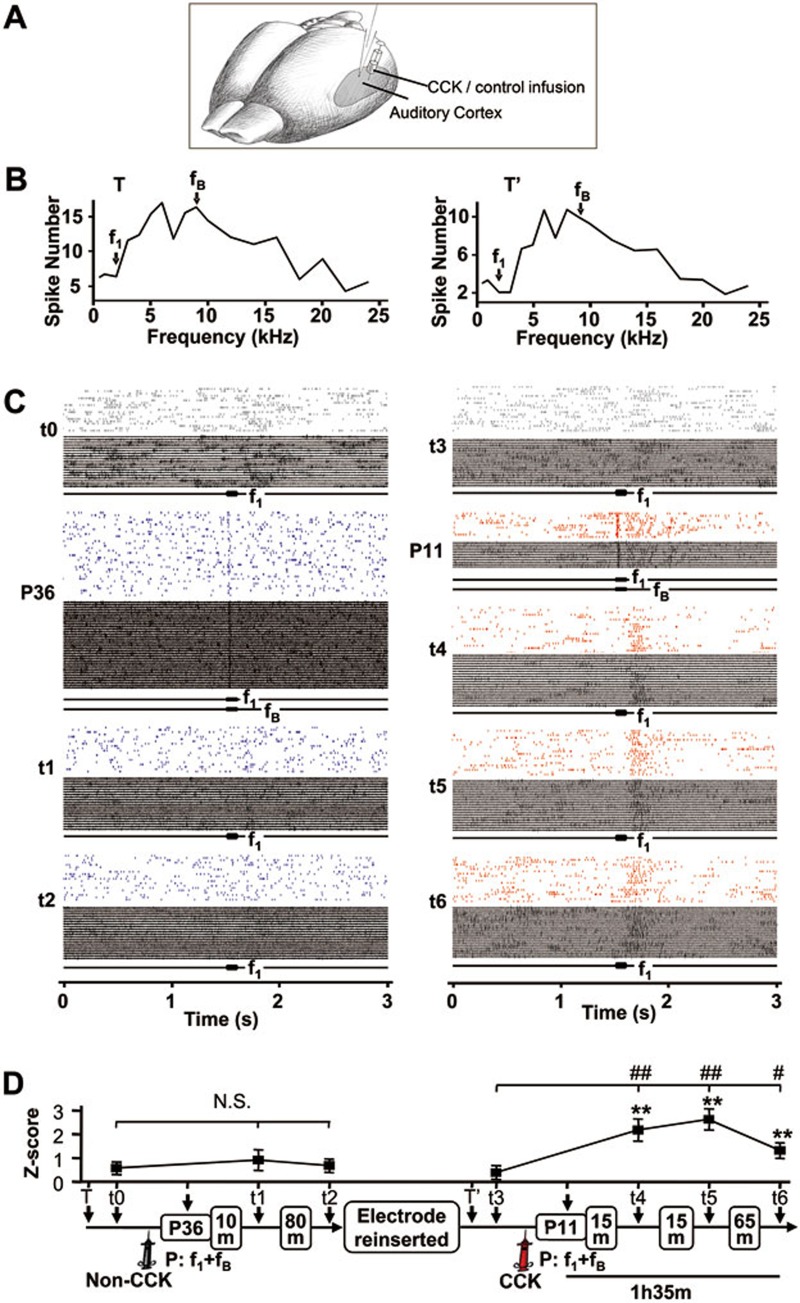
Figure 3.
Neuroplasticity induced by local infusion of CCK into the auditory cortex of anesthetized rats. (A) Experimental preparation is shown. (B) Frequency-response functions were used to identify frequencies that evoked little to no neuronal response (f1) and the best neuronal response (fB). Frequency-response functions were acquired for 18 frequencies (500 Hz-24 kHz) presented in random order. Each frequency was repeated 30 times. Neuronal response was defined as the average number of spikes between 0 and 60 ms from stimulus onset. The measured time points (T and T') were 1 h before and 4 h after the control infusion that preceded CCK infusion. (C) The left panel shows neuronal responses (raster plots and raw traces) to f1 at different times before (t0) and after the control infusion (t1-t2). The right panel shows neuronal responses after changing to a CCK infusion pipette (t3-t6). Stimulus pairings consisted of simultaneous presentation of f1 and fB for 36 (P36) and 11 trials (P11). In all raster plots and raw traces, trials are presented in bottom-up order. (D) Z-scores were calculated based on differences between average neuronal firing during a 60-ms period after stimulus onset and spontaneous firing during a 60-ms period before stimulus onset. The experimental timeline is depicted below the graph. **P < 0.01, paired t-test (stimulus-evoked vs spontaneous firing); N.S., not significant; #P < 0.05 and ##P < 0.01, one-way ANOVA.
After determining the frequency response profile of an auditory cortical neuron (Figure 3B, left panel), a frequency (f1) (t0 in Figure 3C, Z = 0.573 ± 0.263, P = 0.602, paired t-test) was selected as the weak stimulus. This weak stimulus was paired with a frequency that evoked the best neuronal response (fB, strong stimulus) for 36 trials (P36). Figure 3C (left panel) shows a representative neuron that responded to the paired stimuli (P36) but did not show significantly enhanced responses to the weak stimulus after pairings in the absence of CCK (t1, Z = 0.919 ± 0.434, P = 0.051; t2, Z = 0.678 ± 0.283, P = 0.126; Figure 3D).
After a CCK infusion pipette was used and the electrode array was reinserted into the same recording site, a neuron that was likely different from that previously recorded (Figure 3B, right panel) showed a weak response to the weak stimulus f1 (t3, Z = 0.390 ± 0.291, P = 0.292, Figure 3C and 3D). In the presence of CCK, only 11 pairings between the weak stimulus f1 and the strong stimulus fB (P11, given within 110 s) were required to significantly potentiate neuronal responses to the weak stimulus f1 (t4, Z = 2.177 ± 0.463, P < 0.001; t5, Z = 2.621 ± 0.448, P < 0.001; Figure 3C and 3D, right). This increase was maintained for at least 1 h and 35 min (t6, Z = 1.307 ± 0.327, P < 0.01; Figure 3D). The increase in Z-score after 11 pairings (P11) in the presence of CCK was statistically significant (one-way analysis of variance (ANOVA)).
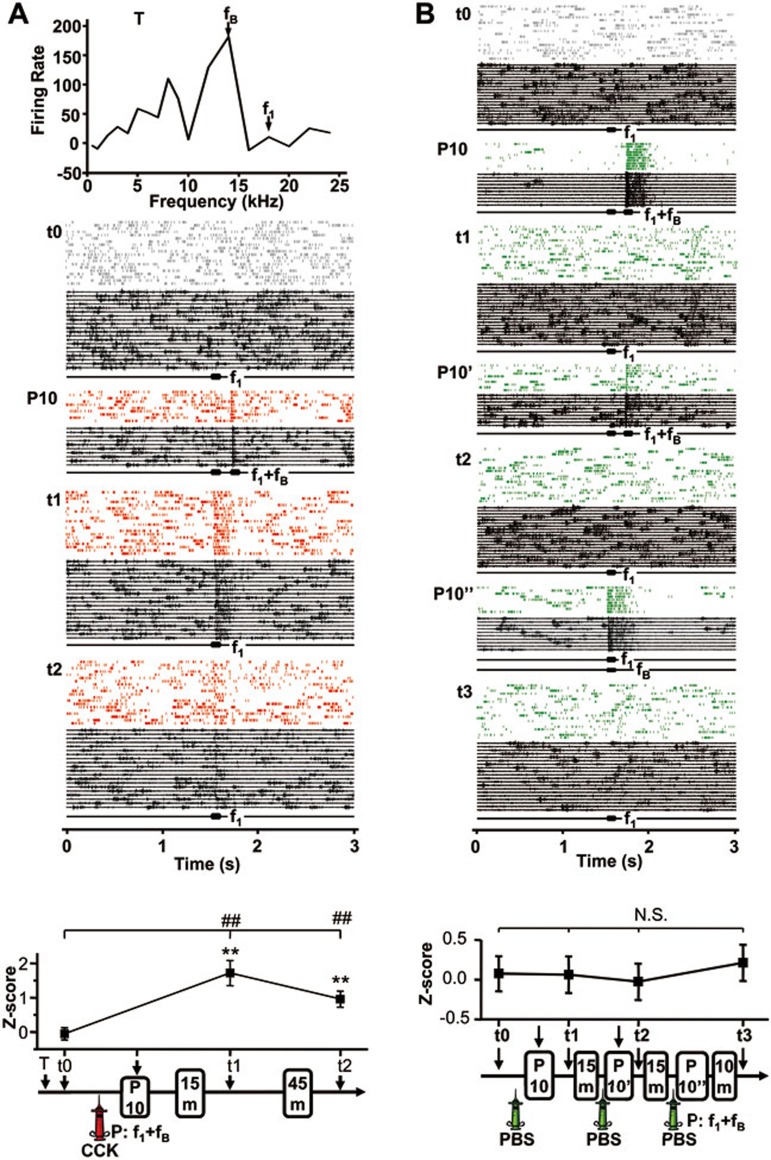
Another example of long-term plasticity involving the weak stimulus is shown in Figure 4A. Before stimulus pairings, the neuron did not respond to a weak stimulus (f1= 18 kHz, t0, Z = 0.050, P = 0.250, paired t-test). After local infusion of CCK, the weak stimulus f1 was paired with a strong stimulus at the best frequency (fB= 14 kHz, f1+fB) for 10 trials (P10; note that f1 and fB were presented in a sequential manner). As shown in the raster plots and raw traces, the neuron responded to the strong stimulus during the pairings (P10) as evidenced by the timing of action potentials. Fifteen minutes after the pairings (t1), the neuron also showed a strong response to the weak stimulus f1 (Z = 1.718, P < 0.001). This increased response was still present 1 h after the pairings (t2, Z = 0.960, P < 0.01). This change in response to the weak stimulus occurred only in rats infused with CCK (Figure 4A) and not in control rats infused with PBS (Figure 4B). With PBS infusion, the representative neuron responded to the strong stimulus (P10, P10', and P10”, Figure 4B), but no change in neuronal response was observed after pairing the weak and strong stimuli (t0 vs t1-t3, Figure 4B).
Figure 4.
Neuronal responses to a weak stimulus after pairing with a strong stimulus in the presence of CCK in anesthetized rats. (A) An unresponsive neuron in the auditory cortex became responsive after 10 pairings (P10) of f1 (18 kHz) and fB (14 kHz). Raster plots and raw traces show neuronal responses to f1before (t0) and after stimulus pairings (t1, t2). Z-scores were calculated based on differences between average neuronal firing during a 30-ms period after stimulus onset and spontaneous firing during a 30-ms period before stimulus onset. **P < 0.01, paired t-test (stimulus-evoked vs spontaneous firing); ##P < 0.01, one-way ANOVA. A frequency-response function was acquired at the beginning of the experiment (T). (B) Pairing of f1 and fB after local infusion of PBS did not induce a change in neuronal responses. Raster plots and raw traces show neuronal responses to f1 at different times before and after pairings of f1 and fB during three blocks of 10 trials (P10, P10', and P10”). Z-scores were calculated based on differences between average neuronal firing during a 90-ms period after stimulus onset and spontaneous firing during a 90-ms period before stimulus onset. N.S., not significant, one-way ANOVA.
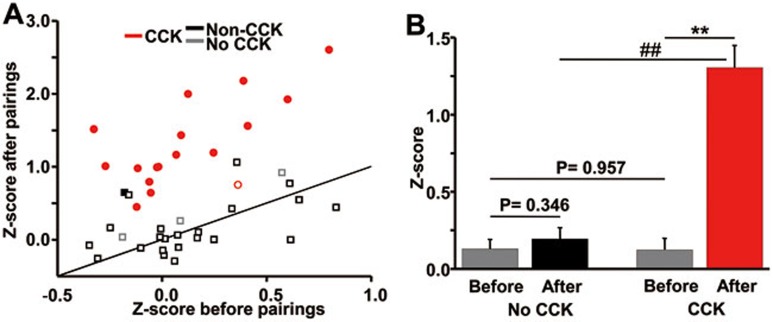
Of the 17 neurons that underwent stimulus pairings in the presence of CCK, 16 showed a significant post-pairing increase in Z-score (filled red circles, Figure 5A). This was in contrast to the control experiments, in which 25 of 26 neurons that underwent stimulus pairings without CCK showed no significant post-pairing increase in Z-score (open squares, Figure 5A). The effects of drug and stimulus pairings were analyzed using a two-way repeated measures ANOVA, which revealed a significant interaction (P < 0.001), indicating that both drug and stimulus pairings were necessary to produce changes in Z-scores. Post-hoc analyses using t-tests were further conducted. In control conditions, mean Z-scores after stimulus pairings were equivalent to those before stimulus pairings (0.195 ± 0.073 vs 0.129 ± 0.062, n = 26, P = 0.346, paired t-test, Figure 5B). In the CCK condition, however, mean Z-scores after stimulus pairings were significantly increased (1.305 ± 0.143 vs 0.124 ± 0.074, n = 17, P < 0.001, paired t-test). Z-scores for the control and CCK groups were similar before stimulus pairings but significantly different after stimulus pairings (P < 0.001, unpaired t-test, Figure 5B).
Figure 5.
Post-pairing changes in neuronal responses with and without CCK infusion in the auditory cortex of anesthetized rats. (A) Post-pairing Z-scores were plotted as a function of pre-pairing Z-scores for all neurons in the experimental condition (i.e., infusion of CCK) and control conditions (No CCK, no infusion; Non-CCK, infusion of PBS and infusion of PBS/acetylcholine). Neurons showing a significant change in Z-score after stimulus pairings are shown as filled symbols, otherwise they are shown as open symbols. Different colors indicate different conditions. (B) Mean Z-scores before and after stimulus pairings with or without the presence of CCK. All three control conditions were considered as the no CCK condition. **P < 0.01, paired t-test; ##P < 0.01, unpaired t-test. Post-hoc t-tests were performed after two-way repeated measures ANOVA.
CCK enables visual responses of auditory neurons in anesthetized rats
In a separate experiment, we successfully produced a cross-modal association between a light stimulus and the electrical stimulation of the auditory cortex using classical fear conditioning in behaving rats. Auditory cortical neurons around the stimulation site started to respond to the light stimulus after 20 or 30 trials25. The involvement of the medial temporal lobe was essential for the formation of this cross-modal association. In line with our hypothesis that the medial temporal lobe influences the auditory cortex through CCK, we predict that the same cross-modal association should be produced in anesthetized rats with local infusion of CCK in the auditory cortex.
To test this prediction, we examined whether auditory neurons would respond to a visual stimulus after pairing the visual stimulus with a strong auditory stimulus in the presence of CCK in the anesthetized rat cortex. Responses of auditory cortical neurons to a visual stimulus were compared before and after CCK was locally infused to the recording site and a visual stimulus was paired with a noise burst or electrical stimulation of the auditory cortex. Recording electrodes and the drug infusion pipette were inserted into the auditory cortex. Two experiments with CCK infusion and two control experiments with no CCK infusion were performed in naïve rats. Seven control experiments were performed without CCK before later infusion of CCK.
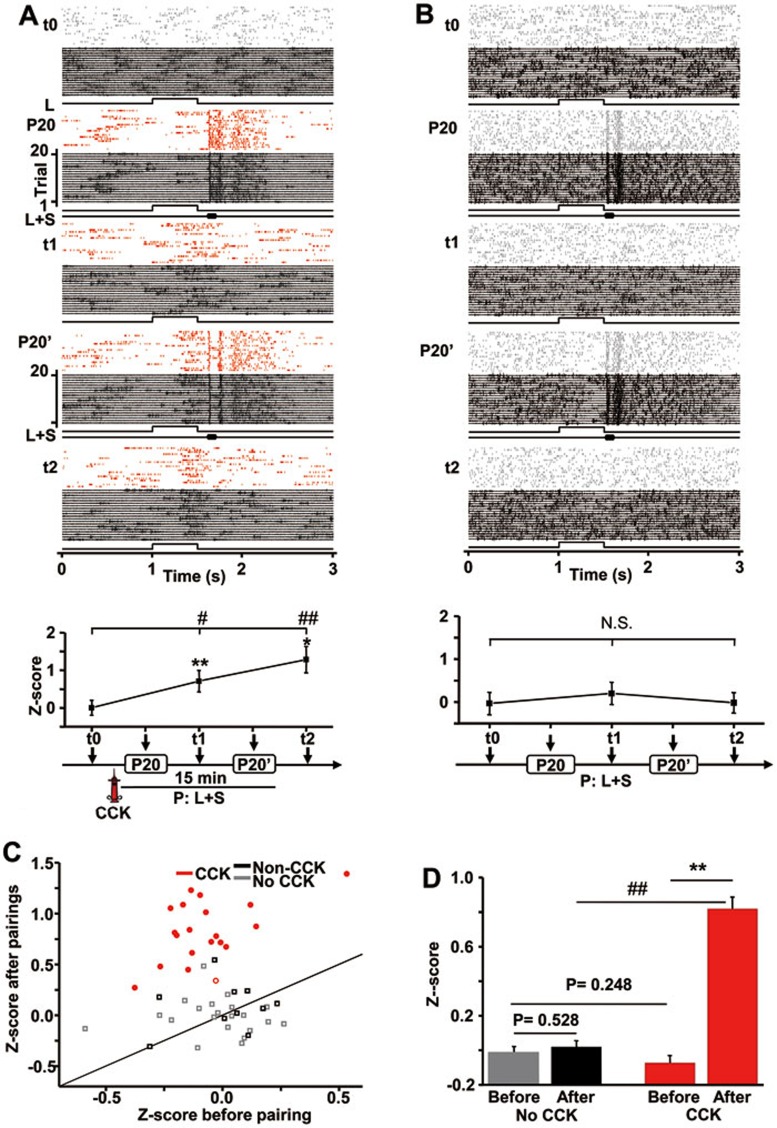
Before stimulus pairings, a representative neuron responded to the auditory stimulus (responses to noise bursts alone are not shown, but detectable at P20 during simultaneous presentation of visual and auditory stimuli, Figure 6A) but not to the visual stimulus (t0, Figure 6A). Five minutes after CCK was infused into the auditory cortex, a 500-ms light stimulus was presented, followed by a noise burst 100 ms later. The stimulus pairing was repeated for 20 trials (P20, Figure 6A). After the pairings, the neuron began to respond to the light stimulus (t1). Responses to the light stimulus were potentiated after 20 additional trials (P20' and t2; note that the neuron responded to the early light stimulus component of the paired light and sound stimuli in several trials shown in P20 and most trials shown in P20', Figure 6A). Z-scores of neuronal responses to the light stimulus increased from t0 (0.000 ± 0.202, P = 0.306, paired t-test) to t1 (0.711 ± 0.286, P < 0.001, paired t-test) and t2 (1.284 ± 0.394, P < 0.05, paired t-test) (Figure 6A).
Figure 6.
Cross-modal responses after pairing auditory and visual stimuli in the presence of CCK in anesthetized rats. (A) Top panel: Raster plots and raw traces show neuronal responses to the light stimulus (L; t0, t1, and t2) and the combined light stimulus and noise burst stimulus (L+S; P20 and P20') at different times before and after CCK infusion. Bottom panel: Z-scores were calculated based on the difference between average neuronal firing during a 200-ms period after light stimulus onset and spontaneous firing during a 200-ms period before stimulus onset. Experimental timeline is illustrated below the graph. *P < 0.05, **P < 0.01, paired t-test; #P < 0.05, ##P < 0.01, one-way ANOVA. (B) Top panel: Raster plots and raw traces show neuronal responses to the light stimulus (t0, t1, and t2) and the combined light and sound stimuli (P20 and P20') at different times in a naïve control rat with no CCK infusion (a different rat from that shown in A). Bottom panel: Z-scores were calculated based on the difference between average neuronal firing during a 120-ms period after light stimulus onset and spontaneous firing during a 120-ms period before light stimulus onset. N.S., not significant, one-way ANOVA. (C) Z-scores of neuronal responses to the light stimulus after stimulus pairings are plotted as a function of Z-scores before stimulus pairings for all neurons in the experimental condition (with CCK infusion) and control conditions (No CCK, no infusion; Non-CCK, infusion of PBS). Neurons showing a statistically significant change in Z-score after stimulus pairings are shown as filled symbols, otherwise they are shown as open symbols. (D) Mean Z-scores before and after stimulus pairings with or without the presence of CCK. **P < 0.01, paired t-test; ##P < 0.01, unpaired t-test. Post-hoc t-tests were performed after two-way repeated measures ANOVA.
Control experiments were carried out in naïve rats with no CCK infusion. A representative neuron did not respond to the light stimulus (t0; Figure 6B) but responded to the noise burst as reflected by its responses to the paired light and sound stimuli (P20 and P20'). The neuron showed no changes in its response to the light stimulus after stimulus pairings (t1 and t2). Responses to the light stimulus were not significantly different from spontaneous firing (P > 0.05, t-test), and Z-scores were not significantly different before and after stimulus pairings (P > 0.05, one-way ANOVA) (Figure 6B).
In total, 19 of the 20 recorded neurons showed a significant increase in Z-score after stimulus pairings in the presence of CCK, whereas 0 of the 9 neurons showed a change in Z-score after stimulus pairings without CCK (Figure 6C). The effects of drug and stimulus pairings were analyzed using a two-way repeated measures ANOVA, which revealed a significant interaction (P < 0.001), indicating that both drug and stimulus pairings were necessary to produce changes in Z-scores. Post-hoc analyses using t-tests were further conducted. Mean Z-scores before and after stimulus pairings were equivalent when no CCK was present (0.011 ± 0.033 vs 0.020 ± 0.036, P = 0.528, n = 31, t-test), whereas mean Z-scores significantly increased after stimulus pairings in the presence of CCK (0.073 ± 0.042 vs 0.820 ± 0.068, P < 0.001, n = 20, t-test) (Figure 6D). Z-scores in the CCK and no CCK groups were similar before stimulus pairings but significantly different after stimulus pairings (P < 0.001, unpaired t-test, Figure 6D).
Potentiation of synaptic strength after local infusion of CCK in anesthetized guinea pigs
The current understanding is that synaptic strength of cortical and hippocampal neurons is potentiated only by multiple trains of high-frequency stimulation that evoke simultaneous presynaptic and postsynaptic activity20,21,22,23,24. However, in line with our hypothesis that CCK is involved in neuronal plasticity in the cortex, we predict that synaptic strength can be changed after only a few pairings of presynaptic and postsynaptic co-activity in the presence of CCK.
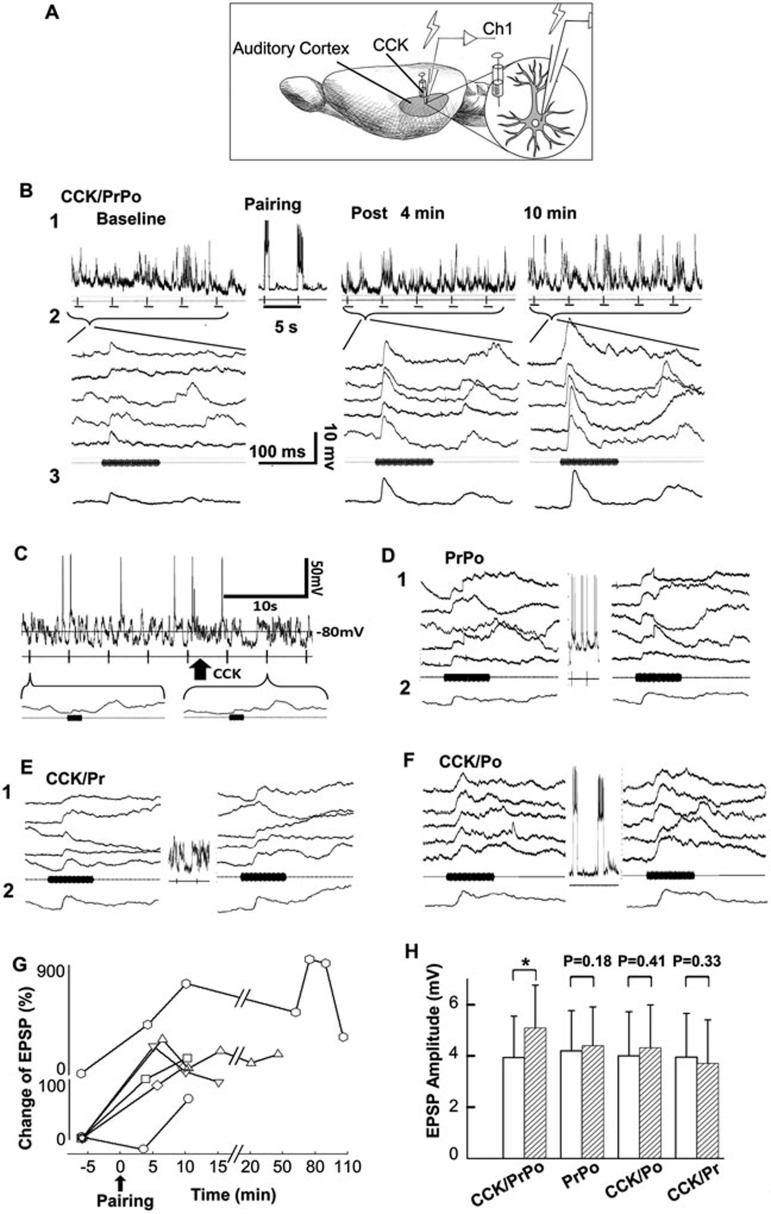
Using in vivo intracellular recordings in anesthetized guinea pigs, we tested whether synaptic strength in the auditory cortex could be changed by only two pairings of presynaptic and postsynaptic activity in the presence of CCK (Figure 7A). We used an auditory stimulus to provide presynaptic input to the neuron and depolarizing current injection to produce postsynaptic neuronal firing. A representative neuron responded to the auditory stimulus with an excitatory postsynaptic potential (EPSP) (Figure 7B). Reponses of 5 individual neurons and the averaged trace are shown before, 4 min after, and 10 min after two pairings of presynaptic and postsynaptic activity in the presence of CCK (CCK/Pr/Po, Figure 7B). The auditory stimulus triggered presynaptic neurotransmitter release, and current injection into the recorded neuron induced postsynaptic firing. Compared with baseline, EPSP amplitude was potentiated 4 min after the pairings (Figure 7B) and became progressively larger 10 min after the pairings (average EPSP amplitude: baseline, 4.3 mV; 4 min, 8.6 mV; 10 min, 12.9 mV; Figure 7B).
Figure 7.
In vivo intracellular recordings of potentiated synaptic strength induced by two pairings of presynaptic and postsynaptic activity after CCK infusion in the auditory cortex of anesthetized guinea pigs. (A) Experimental preparation is shown. (B) Neuronal responses to repeated auditory stimuli before (baseline) and after local infusion of CCK and stimulus pairings (1: raw traces, 2: 5 consecutive individual trials, and 3: averaged traces). Pairings consisted of depolarization of the recorded neuron (producing action potentials) for 400-600 ms during presentation of the auditory stimulus. (C) Raw data show neuronal responses to auditory stimuli before, during, and after CCK infusion. (D-F) Neuronal responses to repeated auditory stimuli before (1: five consecutive individual trials, and 2: averaged traces) and after stimulus pairings in different conditions. (D) Depolarization of the recorded neuron (producing action potentials) during two presentations of the auditory stimulus (PrPo); (E) Two presentations of the auditory stimulus after CCK infusion (CCK/Pr); (F) Two depolarizations of the recorded neuron after CCK infusion (CCK/Po). (G) Responses of 6 different neurons to the auditory stimulus were examined at different times after stimulus pairings. (H) Means and standard errors of EPSP amplitude before (open bars) and after (hashed bars) experimental and control procedures. Experimental procedures involved the presence of CCK, presynaptic input (Pr, auditory stimulus), and postsynaptic activity (Po, current injection). EPSP responses to the auditory stimuli were compared before and after four different sessions: (1) PrPo (n = 11); (2) CCK/Pr (n = 14); (3) CCK/Po (n = 12); and (4) depolarization of the recorded neuron during two presentations of the auditory stimulus after CCK infusion (CCK/PrPo; n = 14). *P < 0.05, paired t-test.
This potentiation of EPSP amplitude was statistically significant 4 min after the two pairings of presynaptic input and postsynaptic firing in the presence of CCK (3.9 ± 1.6 vs 5.1 ± 1.7 mV, P < 0.01, n = 14, paired t-test, Figure 7H). This potentiation was maintained in each neuron as long as the recordings lasted: 10 min (n = 6), 15 min (n = 3), 50 min (n = 2), or 110 min (n = 1) (Figure 7G).
In one control experiment, local infusion of CCK did not cause direct changes in membrane potential of a recorded neuron (Figure 7C). Presynaptic input (Pr, reflected in EPSP to the auditory stimulus), postsynaptic action potentials (Po), and the presence of CCK were three factors that were necessary to produce changes in membrane potential; any combination of two factors (PrPo, CCK/Pr, or CCK/Po for two trials) failed to produce changes in membrane potential (Figure 7D-7F). Statistically, no synaptic strengthening was observed after simultaneous presynaptic and postsynaptic activity without local infusion of CCK in the auditory cortex (PrPo, 4.2 ± 1.6 vs 4.4 ± 1.5 mV, n = 11, P = 0.178, t-test, Figure 7H), when there was only presynaptic activity in the presence of CCK (CCK/Pr, 4.0 ± 1.7 mV vs 3.7 ± 1.7 mV, n = 14, P = 0.331, t-test, Figure 7H), or when there was only postsynaptic firing in the presence of CCK (CCK/Po, 4.0 ± 1.7 mV vs 4.3 ± 1.7 mV, n = 12, P = 0.412, t-test, Figure 7H).
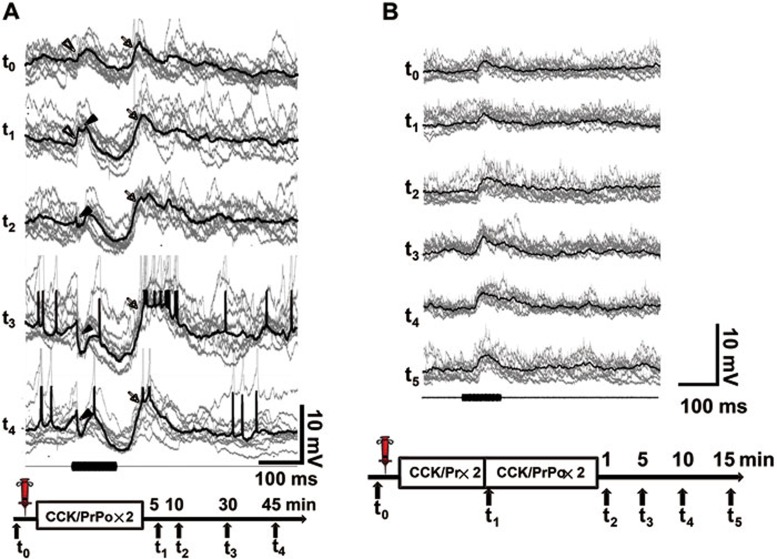
In the presence of CCK, the auditory cortex was capable of undergoing progressive plastic changes. A representative neuron showed an increase in the initial EPSP component at the first measurement after the pairings (open arrowheads, t1 vs t0, Figure 8A). Furthermore, an inhibitory component appeared at the first measurement after the pairings (filled arrowhead, t1) and intensified over time (filled arrowheads, t2-t4), and the second EPSP component also increased over time after the pairings (arrows, t0-t4, Figure 8A). In another representative neuron, there was no change in the first EPSP component after presentation of only the auditory stimulus (t1 vs t0, Figure 8B), but potentiation was induced after pairings of presynaptic and postsynaptic activity in the presence of CCK (t2 vs t1, Figure 8B). In summary, the auditory cortical neurons underwent synaptic changes reflecting combined effect of excitatory and inhibitory synapses.
Figure 8.
Time course of evoked membrane potential after CCK infusion in the auditory cortex of anesthetized guinea pigs. (A) Progressive changes after CCK infusion in the auditory cortex of an anesthetized guinea pig. Intracellular recordings show averaged responses (thick trace) and individual trial responses (thin trace) to repeated auditory stimuli before (t0) and at different times (t1-t4) after infusion of CCK and two pairings of auditory stimulus and current injection (CCK/PrPo×2). Open arrowheads show the initial EPSP component before and after the pairings (t0 and t1). Filled arrowheads show an early inhibitory component that appeared after the pairings (t1-t4). Arrows show the second EPSP component before and after the pairings (t0-t4). Scale bars apply to all traces. Experimental timeline is illustrated below the traces. (B) Change in neuronal responses after the pairings of presynaptic and postsynaptic activity in the presence of CCK. Traces show neuronal responses (averaged, thick; individual trials, thin) to the auditory stimulus before (t0) and after local infusion of CCK (t1-t5). The t1 measurement was carried out after two presentations of the auditory stimulus alone (CCK/Pr×2), and the t2-t5 measurements were carried out at different times after two pairings of the auditory stimulus and depolarization (CCK/PrPo×2) as shown in the below experimental timeline.
CCKB antagonist prevents the establishment of a cross-modal association in awake rats
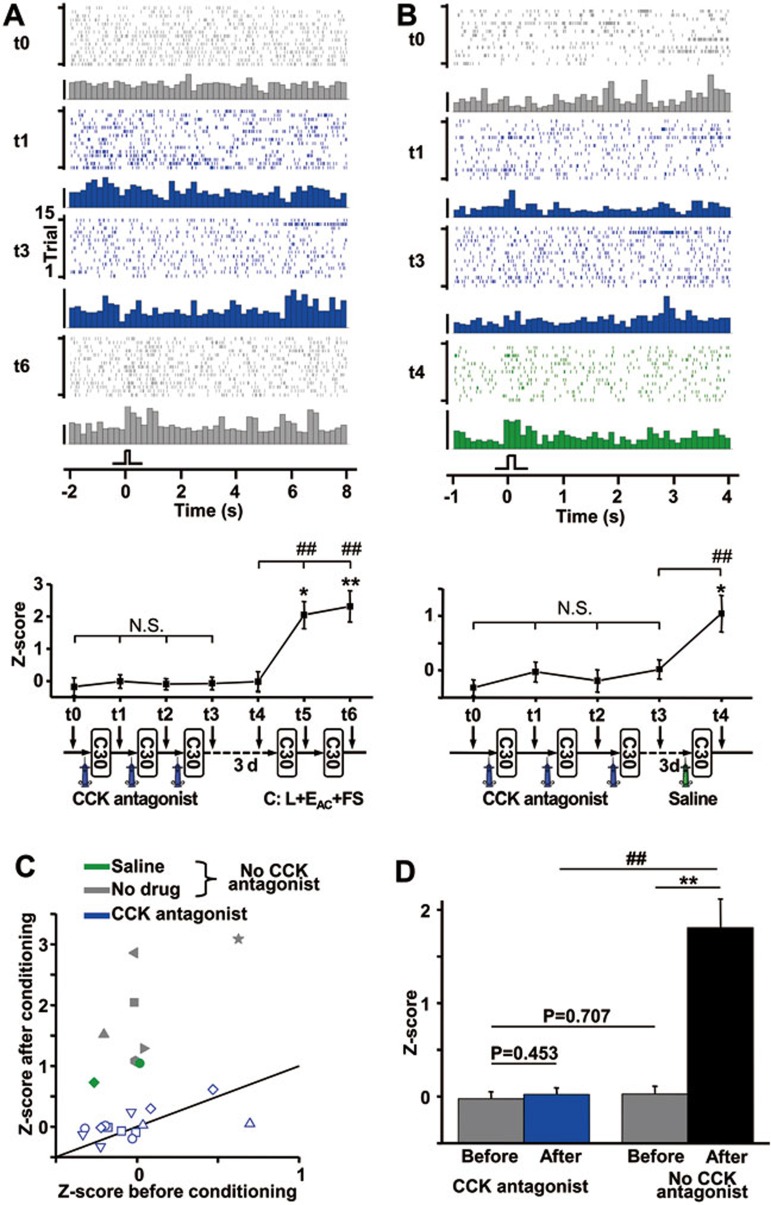
In the next experiment, we tested our hypothesis that CCK enables neuronal plasticity in the neocortex of awake rats. This experiment was designed to examine whether abolishing CCK receptor activation disrupts the establishment of artificial visuo-auditory associations, similar to what occurs after entorhinal cortex inactivation25. If a new association cannot be established in the auditory cortex with local infusion of a CCKB antagonist, this would suggest that CCK is the communication signal between the medial temporal lobe and the neocortex. In a separate experiment, we found that fewer than 30 trials of aversive conditioning involving the sequential presentation of a light stimulus and electrical stimulation of the auditory cortex were necessary to induce a visuo-auditory association in the auditory cortex of awake rats. Neurons that were initially unresponsive to the light stimulus became responsive to the stimulus after conditioning, providing evidence of the establishment of a visuo-auditory association25.
In the present experiment, the infusion cannula was effective in delivering the drug, evidenced by the abolishment of spontaneous firing after the infusion of a glutamate receptor antagonist, DNQX (Figure 9). A representative neuron in the stimulation site of the auditory cortex initially showed no response to the light stimulus (t0, Figure 10A). After a CCKB antagonist (L-365, 260) was infused into the stimulation site, no obvious responses to the visual stimulus were observed even after three blocks of 30 conditioning trials (using the same experimental paradigm of our previous study25) (t1-t3, Figure 10A). By contrast, a visuo-auditory association was readily established after conditioning in the absence of the CCKB antagonist, with significant neuronal responses to the light stimulus observed after one or two sessions of 30 conditioning trials (t5 and t6, Figure 10A). In a control experiment, saline infusion failed to prevent encoding of the association, excluding the possibility of a non-specific action (t4, Figure 10B). Collectively, a visuo-auditory association was not established after three sessions of 30 conditioning trials in the presence of the CCKB antagonist (t1-t3), but was observed after only a single session of 30 conditioning trials in the presence of saline (Figure 10B).
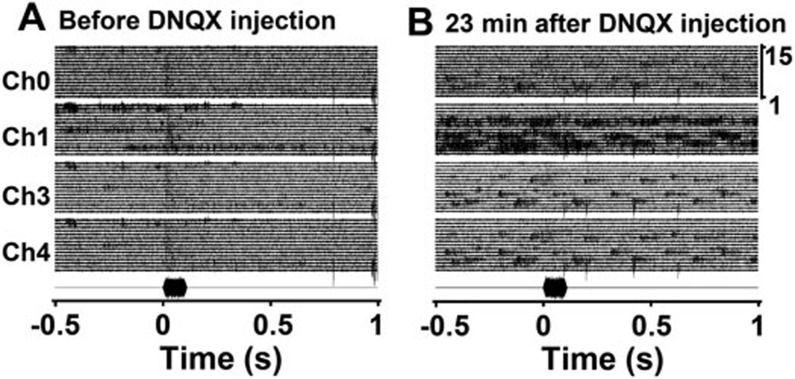
Figure 9.
Effectiveness of the implanted infusion cannula was tested using a glutamate receptor antagonist. Raw traces show neuronal responses of four channels to repeated auditory stimuli recorded by four separate electrodes in the auditory cortex. (A) Neuronal responses before DNQX injection. (B) Neuronal responses 23 min after DNQX injection. Noise recorded in the right panel was likely generated by animal movements.
Figure 10.
A CCK antagonist blocked the encoding of an associative memory in the auditory cortex of awake rats. (A) Raster plots and peristimulus time histograms (PSTHs) show neuronal responses to the light stimulus before (t0, gray) and after (t1 and t3, blue) local infusion of a CCK receptor antagonist in the auditory cortex as well as after (t6, gray) conditioning without the CCK receptor antagonist. For both A and B, each conditioning trial consisted of sequential presentation of a light stimulus (L), electrical stimulation of the auditory cortex (EAC), and foot shock (FS). Conditioning sessions consisted of 30 trials (C30). Scale bar for PSTHs: 25 spikes/bin; bin: 200 ms. Z-scores were calculated based on the differences between average neuronal firing during a 1.2-s period after the onset of a light stimulus and spontaneous firing during the same duration before stimulus onset. Experimental timelines are shown below the graphs. *P < 0.05, **P < 0.01, paired t-test; N.S., not significant, ##P < 0.01, one-way ANOVA. (B) Raster plots show neuronal responses to the light stimulus before (t0, gray) and after local infusion of a CCK receptor antagonist (t1 and t3, blue) or saline (t4, green) and conditioning. Scale bar for PSTHs: 25 spikes/bin; bin: 100 ms. Z-scores were calculated based on the differences between average neuronal firing during a 1-s period after the onset of a light stimulus and spontaneous firing during the same duration before stimulus onset. Experimental timelines are shown below the graphs. *P < 0.05, paired t-test; N.S., not significant, ##P < 0.01, one-way ANOVA. (C) Z-scores of neuronal responses to the light stimulus after conditioning are plotted as a function of Z-scores before conditioning for all neurons in both the experimental condition (with infusion of CCK receptor antagonist) and control conditions (without infusion of CCK antagonist, i.e., no injection or injection of saline). Neurons showing statistically significant changes in Z-score after conditioning are shown as filled symbols, otherwise they are shown as open symbols. Different control conditions are indicated by different colors. (D) Mean Z-scores before and after conditioning with or without the CCK antagonist in the auditory cortex. **P < 0.01, paired t-test; ##P < 0.01, unpaired t-test. Post-hoc t-tests were performed after two-way repeated measures ANOVA.
As a summary of experiments in awake rats, Z-scores of neuronal responses to the visual stimulus after conditioning were plotted as a function of Z-scores before conditioning (Figure 10C). No neurons showed a significant increase in Z-score after conditioning in the presence of the CCKB antagonist, whereas all neurons in 8 rats showed a significant increase when no antagonist was present. The effects of drug and conditioning were analyzed using two-way repeated measures ANOVA. There was a significant main effect for both factors (P < 0.001) and a significant interaction between the two factors (P < 0.001). Post-hoc analyses using t-tests were further conducted. Mean Z-scores did not change after conditioning in the presence of the CCKB antagonist (0.026 ± 0.062 vs 0.026 ± 0.078, n = 14, P = 0.455, paired t-test), but increased significantly (0.022 ± 0.095 vs 1.708 ± 0.310, P < 0.001, n = 8, paired t-test) after conditioning without the CCKB antagonist (Figure 10D). Z-scores from the CCKB antagonist and no CCKB antagonist groups were equivalent before conditioning but significantly different after conditioning (P < 0.001, unpaired t-test, Figure 10D).
Activation of the entorhinal cortex potentiates responses in the auditory cortex in anesthetized rats
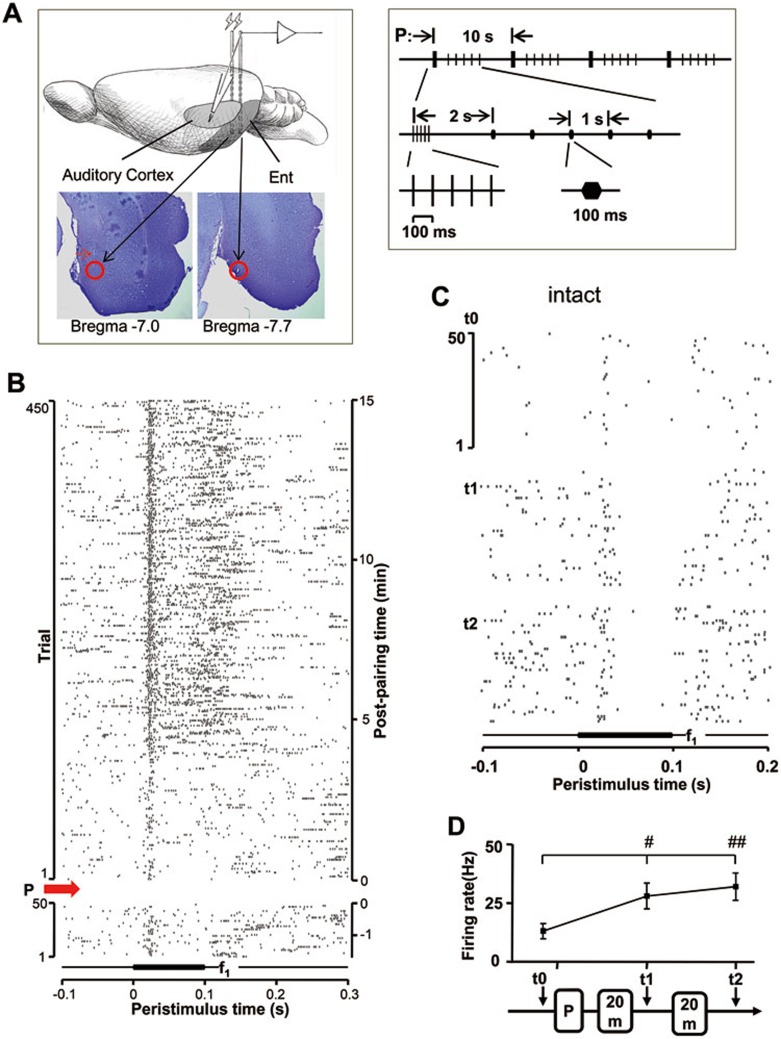
To examine whether activation of neocortical projection neurons in the entorhinal cortex of the medial temporal lobe enables neural plasticity in anesthetized rats, we examined neuronal responses in the auditory cortex after pairings of entorhinal cortex activation and auditory stimuli (pairing session, P: Figure 11A, right; experimental preparation and stimulation sites in the entorhinal cortex shown in Nissl sections: Figure 11A, left). As shown in Figure 11B, the response of a neuron to the same auditory stimulus increased ∼5 min after stimulus pairings (marked by P and a red arrow). This change was maintained throughout the recording period, lasting at least 15 min after stimulus pairings. In another example shown in Figure 11C, the response of a neuron to the same auditory stimulus increased 20 min after stimulus pairings and further increased 40 min after stimulus pairings. Compared to baseline responses before stimulus pairings (13.0 ± 3.13 Hz, t0), neuronal responses significantly increased 20 min (28.0 ± 5.56 Hz, t1, P < 0.05, t0 vs t1, one-way ANOVA) and 40 min (32.0 ± 5.85 Hz, t2, P < 0.01, t0 vs t2, one-way ANOVA) after stimulus pairings (Figure 11D). These findings indicate that pairings of entorhinal cortex activation and auditory stimuli could potentiate responses in the auditory cortex in anesthetized rats.
Figure 11.
Enhanced auditory response after electrical stimulation of the entorhinal cortex in anesthetized rats. (A) Experimental preparation (left panel) and details of the stimulus pairings (P) (right panel). (B) Raster plots showing neuronal responses in the auditory cortex before and after stimulus pairings. Pairing sessions started at 0 min indicated on the right vertical axis (marked by a red arrow at the left vertical axis). An auditory stimulus (100-ms duration) was repeatedly delivered at time 0 in the horizontal axis with an inter-stimulus interval of 2 s. Only partial raster data of 400 ms is shown here. Two stimulating electrodes simultaneously activated two sites of the entorhinal cortex to provide a larger opportunity to activate auditory cortex projection neurons. Lesions were made at the end of the experiment (following the method described in64), shown by red circles in the Nissl-stained sections. The truck of the other stimulation electrode was identified in the left panel marked by the red arrow. (C) Raster plots showing auditory responses of another neuron before (t0) and after (t1 and t2) stimulus pairings. (D) Neuronal responses to the auditory stimulus before and 20 and 40 min after stimulus pairings for the neuron shown in C. Firing rates after stimulus pairings were compared with that before stimulus pairings. #P < 0.05 and ##P < 0.01, one-way ANOVA.
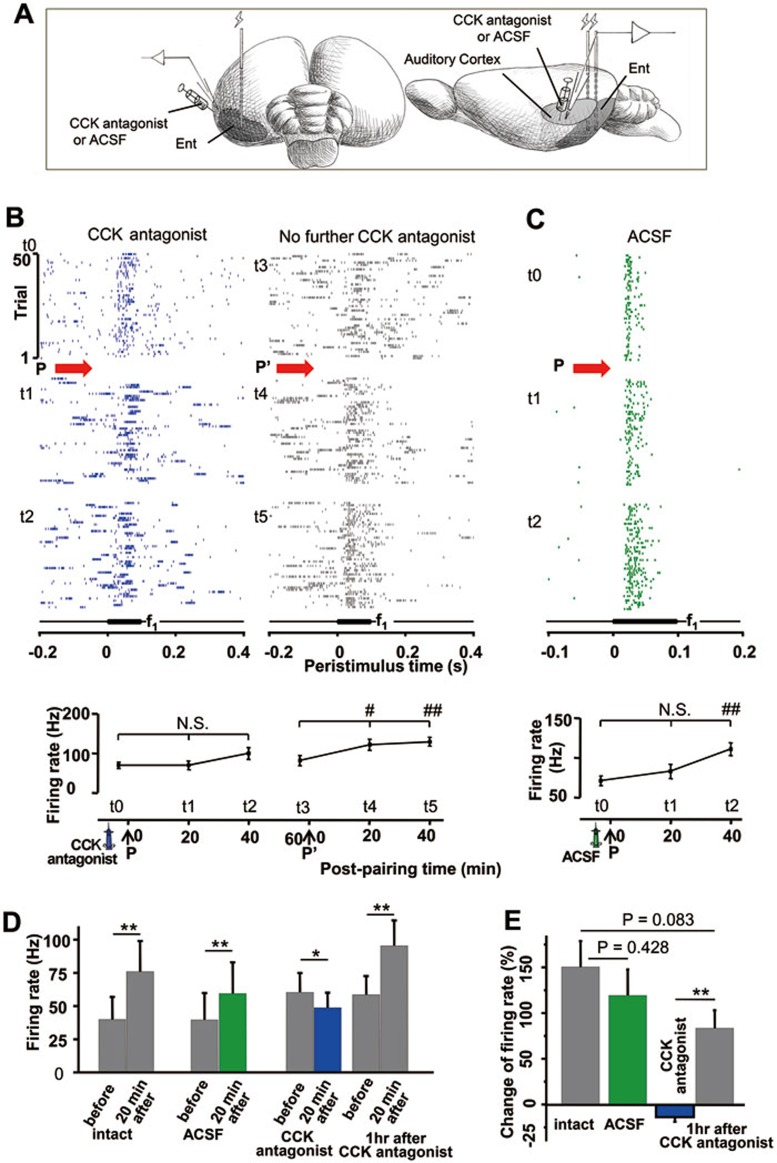
CCKB antagonist suppresses entorhinal cortex-potentiated responses in the auditory cortex in anesthetized rats
In the final experiment, we tested our hypothesis that the entorhinal cortex enables neural plasticity in the neocortex through the action of CCK. If our hypothesis is true, then an infusion of CCKB antagonist in the auditory cortex should suppress neuroplasticity in the auditory cortex after pairings of entorhinal cortex activation and auditory stimuli (Figure 12A). A representative neuron shown in Figure 12B responded to the auditory stimulus (69.6 ± 7.76 Hz) before stimulus pairings (t0, baseline) and showed no significant change in firing rate 20 min (t1, 69.6 ± 11.0 Hz, P = 1, t0 vs t1, one-way ANOVA) or 40 min (t2, 100 ± 14.8 Hz, P = 0.064, t0 vs t2, one-way ANOVA) after stimulus pairings in the presence of the CCKB antagonist. However, this neuron showed a significant increase in firing rate 20 min (122 ± 14.0 Hz, P < 0.05, t3 vs t4, one-way ANOVA) and 40 min (130 ± 11.0 Hz, P < 0.01, t3 vs t5, one-way ANOVA) after further pairings of entorhinal cortex activation and auditory stimuli without further infusion of CCKB antagonist (P', 82.4 ± 12.8 Hz, t3).
Figure 12.
Application of CCKB antagonist in the auditory cortex suppressed entorhinal cortex activation-induced potentiation of neuronal responses in the auditory cortex. (A) Experimental preparation is shown. (B) Raster plots showing neuronal responses before (t0) and after (t1 and t2) stimulus pairing. CCKB antagonist was infused into the auditory cortex before t0. Pairings (P) of electrical activation of the entorhinal cortex and presentation of auditory stimuli were performed immediately after t0 at time 0 in the horizontal axis. Sixty minutes after the first pairing session, when the CCKB antagonist was perfused away by CSF circulation, another pairing session (P') was performed. Neuronal responses in the auditory cortex were examined at 20-min intervals as shown in the right (t3, before P'; t4 and t5, 20 and 40 min after P', respectively (i.e., 80 and 100 min after P)). Lower panel shows neuronal responses to the auditory stimulus before and after stimulus pairing (t0-t5). Firing rates after stimulus pairing were compared with that before stimulus pairings. N. S., not significant, #P < 0.05 and ##P < 0.01, one-way ANOVA. (C) Raster plots show auditory responses before (t0) and after (t1 and t2) stimulus pairings (P) with an infusion of ACSF as a vehicle control. ACSF was infused before t0. The pairing session (P) was performed immediately after t0 at time 0 in the horizontal axis. Neuronal responses in the auditory cortex were examined at 20-min intervals. Lower panel shows neuronal responses to the auditory stimulus before and after stimulus pairings (t0-t2). Firing rates after stimulus pairings were compared with that before stimulus pairings. N. S., not significant, ##P < 0.01, one-way ANOVA. (D) Bar graphs show means and SEMs of neuronal responses before and 20 min after stimulus pairings in the following conditions: intact (no infusion), infusion of ACSF, infusion of CCKB antagonist, and 1 h after infusion of CCKB antagonist. *P < 0.05 and **P < 0.01, paired t-test. (E) Bar graphs show means and SEMs of changes (%) in neuronal response before and 20 min after stimulus pairings. **P < 0.01, paired t-test (CCK antagonist group vs 1 h after CCK antagonist group).
As shown in Figure 12C, infusion of a control solution did not suppress neuroplasticity. Firing rate did not change 20 min after stimulus pairings (70.7 ± 6.22 vs 83.3 ± 8.58 Hz, P = 0.239, t0 vs t1, one-way ANOVA), but increased 40 min after stimulus pairings (111 ± 7.71 Hz, t0 vs t2, P < 0.001, one-way ANOVA).
All control neurons showed significantly increased responses after pairings of entorhinal cortex activation and auditory stimuli (no infusion: 39.9 ± 17.0 vs 75.9 ± 23.1 Hz, P < 0.01, n = 7, paired t-test; infusion of vehicle: 39.7 ± 20.0 vs 59.5 ± 23.6 Hz, P < 0.01, n = 10, paired t-test; no further infusion 60 min after infusion of CCKB antagonist: 58.7 ± 14.0 vs 95.5 ± 19.0 Hz, P < 0.01, n = 12, paired t-test; Figure 12D). By contrast, in the presence of the CCKB antagonist, neuronal responses showed a slight but statistically significant decrease after stimulus pairings (60.5 ± 14.7 vs 48.9 ± 11.4 Hz, P < 0.05, n = 12, paired t-test; Figure 12D).
We observed a slight but non-significant difference between neuronal responses from intact control (i.e., no infusion) and vehicle control groups (151% ± 28.7% vs 119% ± 28.4%, Figure 12E), indicating that infusion may have had a small suppressive effect. This might explain the slight suppression observed after infusion of the CCKB antagonist (13.5% ± 5.23%). The mean change in neuronal response after the last stimulus pairings 1 h after infusion of CCKB antagonist was 83.5% ± 19.8%, which was smaller than that for the intact control group, suggesting that the CCKB antagonist may have had a lasting effect even 1 h after infusion.
Summarizing the results of these final two experiments, we found that activation of the entorhinal cortex induced neuronal plasticity in the auditory cortex, leading to a potentiation of neuronal responses to an auditory stimulus. Local infusion of a CCKB antagonist into the auditory cortex abolished this entorhinal cortex-induced potentiation.
Discussion
The present study demonstrates that cortical projection neurons in the perirhinal and entorhinal cortices contain CCK. Infusion of CCK in the auditory cortex of anesthetized rats induced neural plasticity, potentiating neuronal responses in the auditory cortex and enabling cortical neurons to start responding to an auditory stimulus that was paired with a tone that robustly triggered action potentials. CCK infusion also enabled neurons in the auditory cortex to start responding to a light stimulus that was paired with a noise burst or electrical stimulation of the auditory cortex. Furthermore, in vivo intracellular recordings in guinea pigs showed that EPSP amplitude was potentiated after two pairings of auditory stimulus-evoked presynaptic activity and current injection-evoked postsynaptic activity in the presence of CCK. This change in synaptic strength required the presence of CCK as well as simultaneous presynaptic and postsynaptic activity. In the awake rat, local infusion of a CCKB antagonist into the auditory cortex prevented the formation of a visuo-auditory association in the auditory cortex even after 90 conditioning trials, in contrast to the successful formation of the association after only 20-30 trials without the CCKB antagonist. In the anesthetized rat, neuronal responses in the auditory cortex were potentiated after pairing electrical stimulation of the entorhinal cortex with auditory stimuli. Local infusion of a CCKB antagonist in the auditory cortex suppressed this potentiation. Together, these findings suggest that the medial temporal lobe influences cortical plasticity through CCK.
In a recent study, an artificial association between a visual stimulus and electrical stimulation of the auditory cortex was established through classical conditioning25. After 20 conditioning trials, the visual stimulus activated auditory cortical neurons near the stimulation site. Inactivation of the entorhinal cortex blocked the encoding of this association. These results seem to mimic symptoms of the patient H.M., for whom no new declarative memories could be formed after bilateral surgical removal of the temporal lobe. Previous experiments in rats have shown that inactivation of the perirhinal cortex disrupts the encoding, retrieval, and consolidation of object recognition memory26, whereas deep brain stimulation in the medial temporal lobe of human patients enhances memory performance27. These findings are consistent with our current understanding that the formation of associative memories in the neocortex requires interactions with the hippocampal system5,6. Here, we propose a model in which the medial temporal lobe sends a plasticity-enabled signal through the entorhinal and perirhinal cortices to the neocortex to reinforce neural plasticity. We hypothesize that this plasticity-enabled switch is the neuromodulator CCK.
CCK is the most abundant of all neuropeptides15. Earlier studies have shown that CCK modulates social and exploratory behaviors in rodents17,28,29. A link between CCK and memory function was suggested by the discovery of CCK-positive neurons in the hippocampus18. Heavy CCK labeling, however, was also observed in the perirhinal and entorhinal cortices12. In the present study, neurons in the entorhinal and perirhinal cortices that project to the auditory cortex were found to contain CCK and therefore may fulfill the plasticity-enabled signal.
Previous studies show that activation of CCKB receptors in the amygdala potentiates the acoustic startle response30,31, and application of a CCKB antagonist attenuates fear-potentiated startle32. Also, blocking CCK receptors suppresses conditioned fear16, and knocking out the CCKB receptor gene reduces anxiety-like behavior17. Based on the findings in the present study, this suppressed conditioned fear and reduced anxiety-like behavior may result from disrupted learning in the absence of a functional CCK pathway. Evidence that mice lacking the CCK gene show poor performance in a passive avoidance task and impaired spatial memory19 supports this possibility.
Experience enables plastic changes in the auditory cortex33,34,35. For instance, pairing a weakly responsive sound with a sound that evokes strong responses with a short inter-stimulus interval induces a shift in auditory cortical frequency tuning in ferrets36, and activation of the cholinergic system promotes this plasticity37. In the present study in anesthetized rats, repetitive presentations of an auditory stimulus after local infusion of CCK near the recorded auditory cortical neuron potentiated the neuronal responses. After pairing weak and strong stimuli during 11 trials in the presence of CCK, auditory cortical neurons started to respond strongly to the weak stimuli, which initially evoked no or weak responses. In other words, auditory cortical plasticity normally observed only in behaving rats appeared after repetitive presentation of the stimulus or after 11 pairing trials (over a period of 110 s) in the presence of CCK in anesthetized rats. However, the shift in frequency tuning in anesthetized ferrets after pairing two sounds was maintained for less than 12 min36 and thus, was most likely not CCK-dependent.
Previous studies suggest that stimuli in one sensory modality can influence the activity of cortical areas associated with other sensory modalities. In humans, an auditory stimulus can activate the visual cortex after paired auditory-visual stimuli38,39,40. Visual stimuli, such as silent lip-reading and light, can activate the auditory cortex41,42,43, and auditory stimuli can activate the visual cortex39,44,45. In trained monkeys, somatosensory neurons46,47 and prefrontal cortical neurons48,49,50 respond to both auditory and visual stimuli. In awake rats, the co-occurrence of auditory and visual stimuli with foot shock causes neurons in the auditory cortex to begin responding to the visual stimulus25. The strong and direct cortical projections from the visual cortex to the auditory cortex in other studies51 provide an anatomical substrate for the ready establishment of visuo-auditory associations. In the present study using anesthetized rats, pairing a visual stimulus with a strong auditory stimulus for 20 trials in the presence of CCK enabled auditory cortical neurons to respond to the visual stimulus.
High-frequency or theta-burst stimulation is widely used to produce changes in the synaptic strength of cortical and hippocampal neurons in anesthetized animals, cultured neurons, and slice preparations21,22,23,24. In the presence of CCK, no high-frequency stimulation was required to enhance synaptic efficacy. However, simultaneous presynaptic input and postsynaptic firing were still prerequisites for a change in synaptic strength. It was striking that only two trials of coordinated presynaptic and postsynaptic activity were sufficient to produce a strong synaptic change in the presence of CCK. It will be interesting to investigate possible links between high-frequency stimulation and CCK in long-term potentiation.
Infusion of CCK in the auditory cortex induced complex changes in EPSPS, including alterations in the initial component as well as changes in the inhibitory and secondary components. These alterations were probably caused by changes in the surrounding neural circuitry, including GABAergic neurons. Although beyond the scope of the current study, early studies show that many GABAergic neurons are CCK-immunopositive and that all CCK-containing neurons in the neocortex are GABAergic52. A more recent study, however, shows that many CCK mRNA-labeled neurons in the neocortex are excitatory53.
After a local infusion of a glutamate antagonist into the entorhinal cortex of behaving rats, classical conditioning failed to produce a visuo-auditory association, which was otherwise readily produced in the absence of the antagonist25. Here, we replicated these results using local infusion of a CCKB antagonist into the auditory cortex instead of local infusion of a glutamate antagonist in the entorhinal cortex. In other words, inactivation of the CCKB receptor in the auditory cortex had a similar effect as inactivation of the entorhinal cortex in suppressing neuronal plasticity in the auditory cortex. Moreover, our final two experiments support our hypothesis that the medial temporal lobe enables neuronal plasticity in the neocortex via CCK-containing neurons in the entorhinal cortex. That is, pairing electrical stimulation of the entorhinal cortex with auditory stimuli potentiated neuronal responses in the auditory cortex, and this potentiation was suppressed by local infusion of a CCKB antagonist.
We speculate that the mechanism on memory enhancement by deep brain stimulation of the entorhinal cortex27 is related to the activation of CCK neurons. Putting together the separated anatomical evidences of neocortical projection of the entorhinal cortex and layer distribution of CCKB receptors54,55,56, layer VI neurons of the association auditory cortex are likely the target cells of entorhinal CCK neurons. A further anatomical investigation is required to address the question. Upon the activation of CCKB receptors, the induction of activity-dependent plasticity would occur in the postsynaptic neuron in the auditory cortex.
In summary, long-term plasticity in the auditory cortex was induced by pairing different stimuli in the presence of CCK. This plasticity occurred within the auditory sensory modality as well as across visual and auditory sensory modalities. The presence of a CCK antagonist in the auditory cortex blocked the encoding of visuo-auditory associations. Neuronal responses in the auditory cortex were potentiated after pairing electrical stimulation of the entorhinal cortex and auditory stimuli, and local infusion of a CCKB antagonist into the auditory cortex resulted in the suppression of this potentiation. We conclude that the medial temporal lobe enables cortical plasticity through the release of CCK from neurons in the perirhinal and entorhinal cortices.
Materials and Methods
Animals
Sprague-Dawley rats were used for immunohistochemistry, in vivo extracellular recordings, and behavioral experiments. Guinea pigs were used for in vivo intracellular recordings. Animals were confirmed to have clean external ears and normal hearing. All experimental procedures were approved by the Animal Subjects Ethics Sub-Committees of The Hong Kong Polytechnic University.
Auditory and visual stimuli
Auditory stimuli, comprised of pure tones and noise bursts, were digitally generated using a computer-controlled Tucher-Davis Technologies (TDT, Alachua, FL, USA) auditory workstation and delivered through a coupled electrostatic speaker (ED1, TDT). Auditory stimuli were presented via the speaker placed at about 40 cm above the animal or to the ear contralateral to the implanted electrodes via a hollow ear bar for the anesthetized animal. Sound pressure level was calibrated with a condenser microphone (Center Technology, Taipei). The visual stimulus, comprised of white light, was generated by light-emitting diodes placed 40 cm above the experimental chamber. When the light was on, the illumination at the bottom of the chamber was 26 lux.
True Blue and CCK co-labeling
True Blue (5%, 1 μl, Invitrogen) in 0.1 M PBS was stereotaxically infused into the auditory cortex. All rats were sacrificed 8 days after infusion and perfused transcardially with 200 ml 0.9% NaCl followed by 300 ml 4% paraformaldehyde/0.1 M phosphate buffer containing 0.1% glutaraldehyde. Brains were removed and stored in 4% paraformaldehyde/0.1 M phosphate buffer containing 0.1% glutaraldehyde at 4 °C for 90 min. Brain blocks were soaked in 30% sucrose/0.1 M phosphate buffer until they sank, and 20-μm sections were cut on a cryostat (Leica, Germany). Sections were divided into 15 sets. One of these sets was incubated with 0.3% hydrogen peroxide in PBS for 30 min; blocked with antibody solution (PBS containing 10% normal donkey serum, 3% bovine serum albumin, 0.3% Triton X-100, and 0.02% sodium azide) for 2 h; incubated with rabbit polyclonal CCK antibody (20078; ImmunoStar; 1:200) in the antibody solution overnight; and finally incubated with 4 μg/ml AlexaFluor488-conjugated antibody against rabbit IgG (A11008; Invitrogen) in the antibody solution. All procedures were conducted at room temperature and followed by three 5-min washes in PBS. Control experiments to test the specificity of the reactions included preadsorption of CCK rabbit primary antiserum with 50 g/mL sulfated CCK and omission of the primary antiserum. No staining was observed in these control experiments.
Images of stained cells were acquired using an epifluorescence microscope equipped with appropriate filters (AlexaFluor488, excitation: 488 nm, emission: 505-530 nm; True Blue, excitation: 365 nm, emission: 420 nm). Images were processed and superimposed to identify double-labeled neurons using Photoshop software (Version CS5.1). One set of sections from each rat was stained using the Nissl method.
In vivo extracellular recordings in anesthetized rats
Anesthesia was induced with urethane sodium (2 g/kg, i.p.) and maintained throughout surgery and neuronal recordings with periodic supplements. Atropine sulfate (0.05 mg/kg, s.c.) was administered 15 min before induction of anesthesia to inhibit tracheal secretions. A local anesthetic (xylocaine, 2%) was liberally applied to the incision site. Animals were prepared for surgery as previously described57,58. Briefly, rats were mounted in a stereotaxic device, and a midline incision was made in the scalp. A craniotomy was performed at the temporal lobe (∼3.0–5.0 mm posterior and ∼2.5-∼6.5 mm ventral to bregma) to access the auditory cortex, and the dura mater was opened.
Four tungsten microelectrodes with impedances of 1-3 MΩ (Frederick Haer & Co., Bowdoinham, ME, USA) and an attached drug infusion pipette (50-μm tip size) were used to record neuronal responses in the auditory cortex. Electrodes were remotely controlled by a stepping motor microdrive outside the soundproofed room. Neuronal signals recorded by the electrode, together with the auditory and light stimuli, were amplified and stored using TDT (OpenEX, TDT) and Axoscope softwares (Axon Instruments, Sunnyvale, CA, USA). Single-unit spikes were distinguished using spike sorting software (OpenSorter, TDT; SPKtool, an open-source MATLAB code, Mathworks, Inc., Natick, MA, USA). A threshold of 3 standard deviations (SDs) above baseline was used to distinguish spikes. A template matching method in SPKtool or a K-means clustering method in OpenSorter was used to sort single-unit spikes. The unit with the highest amplitude and a normal overlaid spike profile was chosen from each electrode. Another inclusion criterion was that the number of spikes with an inter-spike interval of < 2 ms in the histogram should be < 0.2% of the total number of spikes. The latency of spike occurrence relative to stimulus delivery was calculated using Matlab (Mathworks, Inc.).
In the first experiment, we examined whether repeated presentations of auditory stimuli after infusion of CCK induces neuronal plasticity in the auditory cortex. Recorded neurons were confirmed to be auditory neurons by showing responses to auditory stimuli. A pure-tone stimulus of non-best-frequency (f1) was selected as the testing stimulus. CCK octapeptide (CCK-8, abbreviated as CCK, 0.4 μl, 10 ng/μl, Tocris) was infused into the auditory cortex through the implanted pipette. Neuronal responses to the stimulus f1 were monitored for at least 15 min.
In the next experiment, neuronal responses to pure tones of different frequencies were recorded (i.e., frequency-response functions). For each rat, an f1 stimulus was chosen that met two conditions: (1) it evoked no neuronal response, and (2) it was in close temporal proximity to the best frequency (designated as “fB”). CCK (0.2 μl, 10 ng/μl) was infused into the auditory cortex through the implanted pipette. The weak and strong stimuli with frequencies of f1 and fB were presented either simultaneously or sequentially with a 100-ms inter-stimulus interval. The sound intensity of f1+fB was adjusted to between 60 and 80 dB, which was strong enough to trigger action potentials in the neurons of interest. After the pairings of weak and strong stimuli, neuronal responses to the weak stimulus were examined at different time delays. In control experiments, rats received infusions with PBS and acetylcholine (1 μl, 10 nM), infusions with PBS, or no infusions.
For visuo-auditory association experiments, the weak stimulus was changed to a visual light stimulus. Neurons that responded to the auditory stimulus but not the light stimulus were selected (n = 20). After infusion of CCK (1 μl, 0.2 μl/min), the light stimulus was paired with a strong auditory stimulus (noise burst) or electrical stimulation of the auditory cortex that evoked neuronal responses. The light stimulus (L) preceded the auditory stimulus (S) by 100 ms. Neuronal responses to the light stimulus were compared before and after pairings of the light stimulus with the strong auditory stimulus (L+S).
One single-unit spike was isolated from each recording electrode. Single-unit spikes were distinguished using the same method as previously described. We adopted an analysis of Z-scores (mean ± SE (standard error)) to examine neuronal responses to the light stimulus59. The Z-score of the neuronal response to the light stimulus was calculated against the mean neuronal spontaneous firing rate before stimulus onset. The duration of sampled recordings was set after examining the neuronal responses to the stimulus and was kept the same for each unit. Spontaneous neuronal firing was calculated before stimulus onset using the same sample duration. Z-scores represent the distance between stimulus-evoked neuronal responses and average spontaneous firing in units of SD (i.e., Z = (x-μ)/δ where x is the stimulus-evoked neuronal response of each trial, and μ and δ are the mean and SD of spontaneous firing rates of all trials). Higher Z-scores generally indicate larger neuronal responses to the stimulus but also depend on the number of testing trials. Mean Z-scores were calculated over repeated trials. Comparisons between stimulus-evoked neuronal responses and spontaneous firing over repeated trials were performed using t-tests. Changes in Z-score after each pairing session were used to assess the effectiveness of the pairing in inducing neuronal plasticity. Post-pairing Z-scores were plotted as a function of pre-pairing Z-scores for the same units. Comparisons of mean Z-scores before and after each pairing session within neurons were performed using paired t-tests, and comparisons between groups were performed using one-way ANOVA. Statistical significance was set at P < 0.05.
In vivo intracellular recordings in anesthetized guinea pigs
As CCK is conserved between rats and guinea pigs60, and our lab has optimized intracellular recording techniques in guinea pigs, we used guinea pigs (n = 17) for intracellular recordings as previously described61,62. Cerebrospinal fluid (CSF) was released at the level of the medulla through an opening at the back of the neck, and animals were artificially ventilated. The recording electrode was a glass pipette filled with 3.0 M KAc or 0.5 M KCl (pH 7.6, 0.05 M Tris HCl buffer) with a resistance between 60 and 150 MΩ. The electrode was advanced perpendicular to the surface of the auditory cortex using a stepping motor. After the electrode reached the surface of the neocortex, the opening of the skull was covered by low temperature-melting paraffin. The electrode was then advanced 4 μm per step.
A negative potential was detected upon penetrating the membrane of a cell. After amplification, the membrane potential and the auditory stimulus were stored using Axoscope software. The direct current level of the recording electrode was frequently checked and set to zero, and its value after each recording was used to adjust the recorded membrane potential for some neurons. Neurons showing a resting membrane potential of ≤ 50 mV and spontaneous spikes (if any) at > 50 mV were included.
The auditory stimuli were noise bursts or pure tones between 40 and 80 dB that triggered EPSPs in the recorded neurons. Neurons with an EPSP onset of less than 40 ms after auditory stimuli were included. To elicit postsynaptic neuronal firing, a depolarizing current lasting 200-600 ms was injected into the recorded neurons across the entire auditory stimulus presentation period. CCK (0.2 μl) was infused through a glass pipette with a 15-μm tip placed ∼300 μm away from the recording electrode.
EPSP responses to the auditory stimuli were compared before and after 4 different sessions: (1) depolarization of the recorded neuron (strong enough to elicit action potentials) during two presentations of the auditory stimulus (i.e., pairing session); (2) two presentations of the auditory stimulus after CCK infusion; (3) two depolarizations of the recorded neuron after CCK infusion; and (4) depolarization of the recorded neuron during two presentations of the auditory stimulus after CCK infusion. EPSP amplitude in response to the noise burst was averaged over 10 trials before and after the pairings. Group data were expressed as mean ± SE. Only neurons showing EPSP amplitudes 0.5 mV and spontaneous firing rates < 2 Hz were included. High spontaneously firing neurons were excluded to minimize the possibility of including neuronal plasticity caused by spontaneous firing in the presence of CCK. Statistical differences were evaluated using paired Student's t-tests, and statistical significance was set at P < 0.05.
Stimulating, recording and drug infusion in awake rats
Rats were prepared for surgery in the same manner as described for in vivo extracellular recordings in anesthetized rats. A home-made electrode array normally consisting of 6 electrodes (2 stimulating electrodes, < 100 kΩ, stainless steel wire; 4 recording electrodes, 0.5–1.0 MΩ, tungsten wire; A-M system) was implanted into the left auditory cortex58,63. The electrode array was advanced using a micromanipulator. After the array reached a position where neurons showed good auditory responses, the opening of the skull was covered by a layer of silicone. Electrodes were connected to a 20-pin socket that was cemented to the skull.
To examine the influence of CCK on encoding of new associative memories, a cannula guide was also implanted in the auditory cortex. The guide was cemented to the skull along with the electrode array. Animals recovered from surgery for 3 days. When not in use, the guide was blocked with a dummy cannula extending 0.2 mm below the guide. During infusion, a Hamilton syringe was connected to the infusion needle, which extended 0.5 mm below the guide. Drug microinfusion was performed using a Quintessential Stereotaxic Injector (Stoelting Co., Wood Dale, IL, USA). After infusion, the needle remained in place for 5 min before being withdrawn.
Classical conditioning was performed 15 min after infusion of either CCK-B receptor antagonist (L-365,260, 50 μg/ml, 2 μl, 0.2 μl/min, Tocris; n = 5) or saline (0.9% NaCl; n = 4). During the conditioning trials, a light stimulus (100 ms) was followed by electrical stimulation of the auditory cortex 200 ms later, which was followed by a foot shock 300 ms later (0.5–0.9 mA; 600-ms duration). Therefore, the foot shock was presented 500 ms after the onset of the light stimulus. Two electrical pulses (30-200 μA) were used to activate the auditory cortex. Each session consisted of 30 trials. Later, some rat underwent one or two control sessions with no infusion (n = 2) or a saline infusion (n = 2). A 30-min rest occurred between sessions. Test trials were conducted after each conditioning session to measure neuronal responses to the conditioned visual stimulus. In some animals, a water-soluble glutamate AMPA receptor antagonist (DNQX, 15 mM, 2 μl, 0.2 μl/min, Tocris) was infused to test the effectiveness of the infusion.
Stimulation of the entorhinal cortex and local application of CCKB antagonist
The final experiments were carried out using anesthetized rats. Two stimulation electrodes were inserted in parallel at anteroposterior direction into the entorhinal cortex (bregma: ∼7.0–8.0 mm, mediolateral: 5.5 mm). Another recording electrode was inserted into the auditory cortex. After obtaining a frequency-response function for a recorded neuron, baseline neuronal responses to an auditory f1 stimulus were recorded for 200 trials. Next, electrical stimulation of the entorhinal cortex was paired with presentation of the f1 stimulus. Pairings consisted of a 5-pulse burst of electrical stimulation at 10 Hz. Two seconds after electrical stimulation, f1 (100-ms duration) was presented 5 times with a 1-s inter-stimulus interval. Pairings were repeated 4 times with an inter-pairing interval of 10 s. Neuronal responses to the f1 stimulus were recorded for 20 min or longer after stimulus pairings.
In the next experiment, a glass pipette was inserted to infuse CCKB antagonist (L-365,260 in 50% artificial cerebrospinal fluid (ACSF) + 50% dimethyl sulfoxide (DMSO)) or vehicle (50% ACSF + 50% DMSO) near the recording site in the auditory cortex. Neuroplasticity following pairings of electrical stimulation and auditory stimuli was examined after infusion of CCKB antagonist or vehicle into the auditory cortex. Neuroplasticity was examined again 1 h after antagonist infusion, when the antagonist should be perfused away by CSF circulation. Neuronal responses were sampled and compared immediately before (baseline), 20 min after, and 40 min after stimulus pairings. Comparisons of firing rates before and after each pairing within neurons were performed using paired t-tests, and comparisons between neurons were performed using ANOVA. Statistical significance was set at P < 0.05.
After the physiological experiment, an electrical current was applied to the posterior stimulation electrode in the entorhinal cortex to mark the location of the electrodes. The brain was sectioned and stained using Nissl method.
Acknowledgments
We thank Ray Guillery and Manuel Malmierca for critical comments and Eduardo Lau for administrative and technical assistance. This work was supported by the Hong Kong Research Grants Council (CRF09/9, 561410, T13-607/12R, 561009, 561111, 561212), the National Key Basic Research Program of China (2012CB966300, 2013CB530900), and the National Natural Science Foundation of China (31200852).
References
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us. Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kohler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 1986;6:3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus. Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in HM. Semin Neurol. 1984;4:249–259. [Google Scholar]
- Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- Wang SH, Teixeira CM, Wheeler AL, Frankland PW. The precision of remote context memories does not require the hippocampus. Nat Neurosci. 2009;12:253–255. doi: 10.1038/nn.2263. [DOI] [PubMed] [Google Scholar]
- Lesburgueres E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331:924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- Graham KS, Hodges JR. Differentiating the roles of the hippocampal complex and the neocortex in long-term memory storage: evidence from the study of semantic dementia and Alzheimer's disease. Neuropsychology. 1997;11:77–89. doi: 10.1037//0894-4105.11.1.77. [DOI] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Innis RB, Correa FM, Uhl GR, Schneider B, Snyder SH. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci USA. 1979;76:521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood RS, Godar SE, Reaves TA, Jr, Hayward JN. Cholecystokinin in hippocampal pathways. J Comp Neurol. 1981;203:335–350. doi: 10.1002/cne.902030303. [DOI] [PubMed] [Google Scholar]
- Köhler C, Chan-Palay V. The distribution of cholecystokinin-like immunoreactive neurons and nerve terminals in the retrohippocampal region in the rat and guinea pig. J Comp Neurol. 1982;210:136–146. doi: 10.1002/cne.902100204. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978;253:4022–4030. [PubMed] [Google Scholar]
- Tsutsumi T, Akiyoshi J, Isogawa K, Kohno Y, Hikichi T, Nagayama H. Suppression of conditioned fear by administration of CCKB receptor antagonist PD135158. Neuropeptides. 1999;33:483–486. doi: 10.1054/npep.1999.0766. [DOI] [PubMed] [Google Scholar]
- Horinouchi Y, Akiyoshi J, Nagata A, et al. Reduced anxious behavior in mice lacking the CCK2 receptor gene. Eur Neuro-Psychopharmacol. 2004;14:157–161. doi: 10.1016/S0924-977X(03)00103-2. [DOI] [PubMed] [Google Scholar]
- Dahl D, Li J. Long-lasting potentiation and depression by novel isoproterenol and cholecystokinin 8-S interactions in the dentate gyrus. Exp Brain Res. 1994;100:155–159. doi: 10.1007/BF00227288. [DOI] [PubMed] [Google Scholar]
- Lo CM, Samuelson LC, Chambers JB, et al. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2008;294:R803–R810. doi: 10.1152/ajpregu.00682.2007. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Keller A, Iriki A, Asanuma H. Identification of neurons producing long-term potentiation in the cat motor cortex: intracellular recordings and labeling. J Comp Neurol. 1990;300:47–60. doi: 10.1002/cne.903000105. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation ― a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Chen X, Guo Y, Feng J, et al. Encoding and retrieval of artificial visuo-auditory memory traces in the auditory cortex requires the entorhinal cortex. J Neurosci. 2013;33:9963–9974. doi: 10.1523/JNEUROSCI.4078-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana N, Haneef Z, Stern J, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B, Davies A, Dickinson A. Cholecystokinin attenuates incentive learning in rats. Behav Neurosci. 1995;109:312–319. doi: 10.1037//0735-7044.109.2.312. [DOI] [PubMed] [Google Scholar]
- Matto V, Harro J, Allikmets L. The effects of cholecystokinin A and B receptor antagonists on exploratory behaviour in the elevated zero-maze in rat. Neuropharmacology. 1997;36:389–396. doi: 10.1016/s0028-3908(97)00011-7. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Bradwejn J, Vaccarino FJ, Yeomans JS. Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. J Neurosci. 1997;17:1838–1847. doi: 10.1523/JNEUROSCI.17-05-01838.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Kock M, Kungel M, Schnizler HU. Cholecystokinin enhances the acoustic startle response in rats. NeuroReport. 1995;6:2081–2084. doi: 10.1097/00001756-199510010-00030. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Frankland PW, Patrisano S, Bush DEA, Yeomans JS, Vaccarino FJ. The CCKB antagonist, L-365,260, attenuates fear-potentiated startle. Peptides. 1995;7:1313–1315. doi: 10.1016/0196-9781(95)02013-m. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci. 2008;28:13629–13639. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Cabeza RE, Lobaugh NJ. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. J Neurophysiol. 1998;80:2790–2796. doi: 10.1152/jn.1998.80.5.2790. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Large-scale functional connectivity in associative learning: interrelations of the rat auditory, visual, and limbic systems. J Neurophysiol. 1998;80:3148–3162. doi: 10.1152/jn.1998.80.6.3148. [DOI] [PubMed] [Google Scholar]
- Zangenehpour S, Zatorre RJ. Crossmodal recruitment of primary visual cortex following brief exposure to bimodal audiovisual stimuli. Neuropsychologia. 2010;48:591–600. doi: 10.1016/j.neuropsychologia.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 2005;25:6797–6806. doi: 10.1523/JNEUROSCI.1571-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. Formation of associations in auditory cortex by slow changes of tonic firing. Hear Res. 2011;271:66–73. doi: 10.1016/j.heares.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Bullmore ET, Brammer MJ, et al. Activation of auditory cortex during silent lipreading. Science. 1997;276:593–596. doi: 10.1126/science.276.5312.593. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. NeuroImage. 2004;21:340–351. doi: 10.1016/j.neuroimage.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, Murray MM, Michel CM, et al. Multisensory interactions within human primary cortices revealed by BOLD dynamics. Cereb Cortex. 2007;17:1672–1679. doi: 10.1093/cercor/bhl077. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Visuo-tactile cross-modal associations in cortical somatosensory cells. Proc Natl Acad Sci USA. 2000;97:9777–9782. doi: 10.1073/pnas.97.17.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Exp Brain Res. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Buonomano DV. Timing of neural responses in cortical organotypic slices. Proc Natl Acad Sci USA. 2003;100:4897–4902. doi: 10.1073/pnas.0736909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM. Representation and integration of auditory and visual stimuli in the primate ventral lateral prefrontal cortex. Cereb Cortex. 2007;17 Suppl 1:i61–i69. doi: 10.1093/cercor/bhm099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Bajo VM, et al. Physiological and anatomical evidence for multisensory interactions in auditory cortex. Cereb Cortex. 2007;17:2172–2189. doi: 10.1093/cercor/bhl128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, DeFelipe J, Schmechel D, Brandon C, Emson PC. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc Natl Acad Sci USA. 1984;81:6526–6530. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Hirokawa J, Ichinohe N, et al. Area-specific substratification of deep-layer neurons in the rat cortex. J Comp Neurol. 2012;520:3553–3573. doi: 10.1002/cne.23160. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Innis RB, Goldman-Rakic PS. Regional distribution of cholecystokinin receptors in primate cerebral cortex determined by in vitro receptor autoradiography. J Comp Neurol. 1987;263:418–435. doi: 10.1002/cne.902630308. [DOI] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, Witter MP. Entorhinal cortex of the rat: cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus. 1997;7:146–183. doi: 10.1002/(SICI)1098-1063(1997)7:2<146::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Van Djik A, Richards JG, Trzeciak A, Gillessen D, Mohler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin8 as redioligand. J Neurosci. 1983;4:1021–1033. doi: 10.1523/JNEUROSCI.04-04-01021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, He S, He J. Change detection by thalamic reticular neurons. Nat Neurosci. 2009;12:1165–1170. doi: 10.1038/nn.2373. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Xu XX, Chen X, He S, He J. Slow recovery from excitation of thalamic reticular nucleus neurons. J Neurophysiol. 2009;101:980–987. doi: 10.1152/jn.91130.2008. [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Lignon MF, Galas MC, Amblard M, Martinez J. Cyclic cholecystokinin analogues that are highly selective for rat and guinea pig central cholecystokinin receptors. Mol Pharmacol. 1990;38:333–341. [PubMed] [Google Scholar]
- Yu YQ, Xiong Y, Chan YS, He J. Corticofugal gating of auditory information in the thalamus: an in vivo intracellular recording study. J Neurosci. 2004;24:3060–3069. doi: 10.1523/JNEUROSCI.4897-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu CH, Yu YQ, Fujimoto K, Chan YS, He J. Corticofugal projection inhibits the auditory thalamus through the thalamic reticular nucleus. J Neurophysiol. 2008;99:2938–2945. doi: 10.1152/jn.00002.2008. [DOI] [PubMed] [Google Scholar]
- Gao L, Meng XK, Ye CQ, et al. Entrainment of slow oscillations of auditory thalamic neurons by repetitive sound stimuli. J Neurosci. 2009;29:6013–6021. doi: 10.1523/JNEUROSCI.5733-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. ON and OFF pathways segregated at the auditory thalamus of the guinea pig. J Neurosci. 2001;21:8672–8679. doi: 10.1523/JNEUROSCI.21-21-08672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]