Dear Editor,
Golden Syrian hamsters are small rodents, but they display many features that resemble the physiology and metabolism of humans. Hamsters have been widely used in many research areas, including carcinogenesis1, reproduction2, virology3, diabetes4 and cardiovascular diseases5. With respect to lipid and glucose metabolism, hamsters, like humans, exhibit high levels of cholesteryl ester transport protein (CETP), intestinal-only ApoB editing, low levels of hepatic low-density lipoprotein (LDL) receptor activity6 and a high glycemic response to dietary fructose7, all of which are not observed in other rodents such as mice and rats. Consequently, hamsters, like humans, exhibit enhanced susceptibility to atherosclerosis (AS) and diabetes8, which led to the widespread use of hamsters in studies on AS and diabetes.
In the past 2-3 decades, due to the fast development of transgenic and knockout mice, hamsters were gradually replaced by these mouse models. However, due to multiple differences between mice and humans with respect to physiology and metabolism, the use of gene-manipulated mice has limited value in disease modeling and pathophysiological studies. Extensive literature search has revealed an absence of reports on genetically manipulated hamster models. To capitalize on the special metabolic features of hamsters, we aim to generate gene-manipulated hamsters as an alternate rodent model for general applications. As the initial step to create a genetically manipulated hamster, we utilized a highly efficient lentiviral vector to generate transgenic hamsters expressing enhanced green fluorescent protein (eGFP).
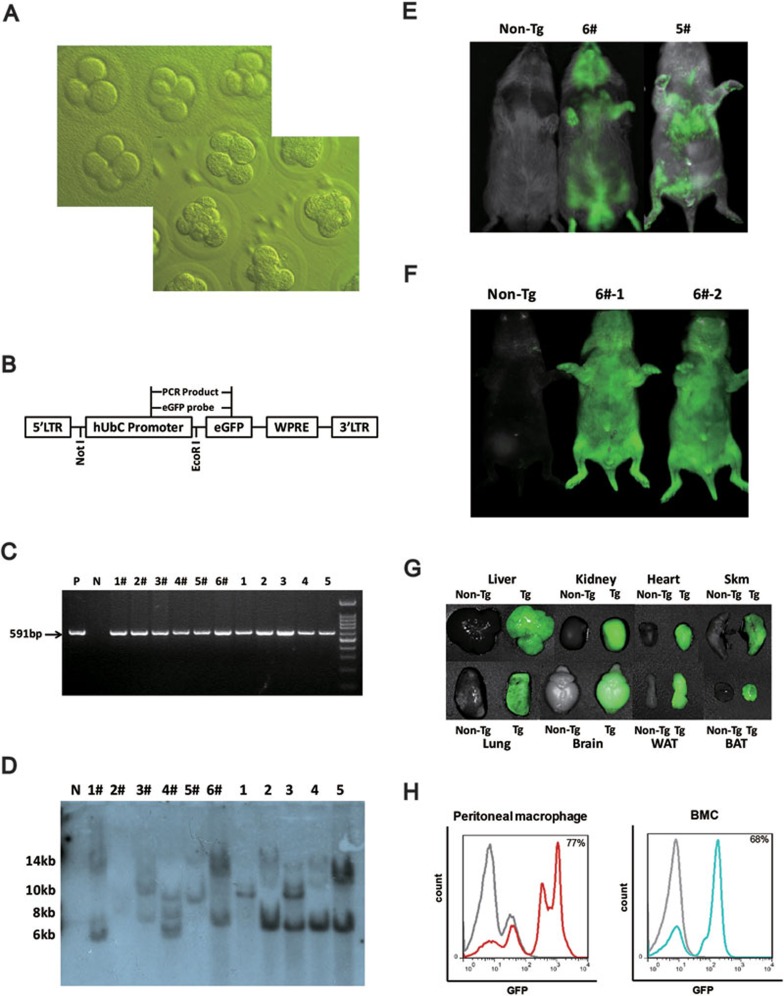
By modifying and optimizing the protocols for producing transgenic mice and rabbits in our laboratory9,10, we developed a specific procedure for hamster superovulation, fertilized egg harvesting, perivitelline space microinjection and embryo transfer. After the successful culture of fertilized hamster eggs that developed into 4- and 8-cell embryos in vitro (Figure 1A), we implanted these embryos into pseudopregnant females. We obtained 7-10 pups/litter in 4 out of 7 surrogate mothers. Next, we microinjected 50-100 picoliters of a lentiviral eGFP vector (Figure 1B) at a titer of 2 × 109 titer units/ml into the perivitelline space of the fertilized eggs to generate transgenic hamsters that express eGFP.
Figure 1.
Generation of transgenic golden Syrian hamsters. (A) Fertilized hamster eggs cultured in HECM-10 medium developed into 4- and 8-cell embryos in vitro. (B) Diagram of the lentiviral pLOV-UbiC-EGFP vector used to generate transgenic hamsters. The vector consists of an LTR (long terminal repeat), the human ubiquitin C promoter, and a sequence encoding eGFP and WPRE (woodchuck posttranscriptional regulatory element). The EcoRI restriction site was used for Southern blot analysis. (C) eGFP PCR in the founders (F0, lanes 1# - 6#) and in first generation (F1, lanes 1-5) animals generated from founder 6#. Genomic DNA was extracted from the ears of the hamsters. “P” indicates the positive control PCR obtained using the eGFP plasmid, and “N” indicates the negative control PCR from a normal hamster. The size of the PCR product is 591 bp. (D) Southern blot of eGFP in the founders (F0, lanes 1# - 6#) and in the F1s (lanes 1-5) generated from founder 6#. Thirty micrograms (for F0 hamsters) or 10 μg (for F1 hamsters) of genomic DNA were digested with EcoRI. The transgene was detected using a 591-bp DIG-labeled probe against the eGFP sequence. (E) Direct fluorescence imaging of the founders (F0). The non-transgenic hamster is on the left with 2 eGFP-transgenic hamsters in the middle and on the right. (F) Direct fluorescence imaging of the F1 hamsters. The non-transgenic hamster is on the left with 2 eGFP-transgenic hamsters in the middle and on the right. (G) Direct fluorescence imaging of different organs from F1 hamsters, including the liver, kidney, heart, skeletal muscle (Skm), lung, brain, white adipose tissue (WAT) and brown adipose tissue (BAT). Each pair of organs is arranged with the non-transgenic animal on the left and the eGFP-transgenic animal on the right. (H) Flow cytometry analysis of peritoneal macrophages (left) and bone marrow cells (right) from one F1 hamster. The results for non-transgenic animals are depicted in gray, and the results for eGFP-transgenic animals are depicted with red or blue lines.
A total of 6 out of 32 live-born pups from 5 surrogate mothers each receiving 30-40 microinjected eggs were identified as being eGFP-positive by PCR genotyping, and 5 of the pups were further validated by Southern blot analysis (Figure 1C and 1D). Of the 5 positive pups validated by Southern blotting, 2 of them (males) expressed eGFP in the exposed skin area as determined by direct fluorescence imaging (Figure 1E). Transgenic lines were then established by breeding the 2 founders (F0) with non-transgenic females. Among the progenies from 3 litters, 21% of the animals were eGFP-positive as determined by direct fluorescence imaging. Representative fluorescence images of the first generation (F1) pups from one of the 3 litters are shown in Figure 1F. All the examined organs from one eGFP-positive F1 pup showed strong-to-moderate levels of green fluorescence, which include the liver, kidney, heart, skeletal muscle, lung, brain, white/brown adipose tissues, adrenal glands and eyes; however, no fluorescence was observed in these organs of the littermate control (Figure 1G and data not shown). The peritoneal macrophages and bone marrow cells from another eGFP-transgenic F1 hamster also demonstrated an approximately 70% eGFP-positive cell population as analyzed by FACS (Figure 1H).
With the establishment of transgenic hamster model in the present study, it is now possible to generate small rodent disease models that recapitulate human pathogenesis. For example, generation of a transgenic hamster overexpressing proprotein convertase subtilisin/kexin type 9 (PCSK9), a protein involved in cholesterol homeostasis by inducing LDL receptor degradation, will be highly desirable. Although mice and pigs overexpressing PCSK9 developed hypercholesterolemia and AS11, these animals, unlike humans, do not express CETP, a critical factor involved in lipid transport and AS development.
Along with the recent development of genome-editing methods such as TALENs (transcription activator-like effector nucleases) and the CRISPR/Cas system (clustered regularly interspaced short palindromic repeats/CRISPR-associated system)12, the generation of gene-targeted hamster models is thus warranted in conjunction with the hamster embryo manipulation method optimized in the present study. These hamster models will be utilized extensively in studies for metabolic cardiovascular diseases and will become the candidate models when genomics and proteomics tools are fully developed for this species in the future. Genetically altered hamsters may potentially replace mice as the mainstream animal model for metabolic cardiovascular research. Therefore, the present study will promote the use of genetically engineered hamsters as disease models. These models might recapitulate many features of human metabolic disorders while simultaneously retaining the ease of handling, the simplicity of management and the cost efficiency of small rodents as compared to genetically engineered large animals such as mini-pigs13 or non-human primates14.
Detailed methods are described in the Supplementary information, Data S1.
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB503900 and 2012CB517505) and the National Natural Science Foundation of China to G Liu (30930037 and 81121061).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.)
Supplementary Information
Materials and Methods
References
- Mishima T, Tajima Y, Kuroki T, et al. Carcinogenesis. 2009. pp. 1763–1767. [DOI] [PubMed]
- Ancel C, Bentsen AH, Sebert ME, et al. Endocrinology. 2012. pp. 1352–1363. [DOI] [PubMed]
- Rohwer RG. Science. 1984. pp. 600–602. [DOI] [PubMed]
- Hein GJ, Baker C, Hsieh J, et al. Diabetes. 2013. pp. 373–381. [DOI] [PMC free article] [PubMed]
- Ikeda Y, Gu Y, Iwanaga Y, et al. Circulation. 2002. pp. 502–508. [DOI] [PubMed]
- Briand F. Curr Opin Investig Drugs. 2010. pp. 289–297. [PubMed]
- Naples M, Baker C, Lino M, et al. Am J Physiol Gastrointest Liver Physio. 2012. pp. G1043–G1052. [DOI] [PMC free article] [PubMed]
- Jove M, Pamplona R, Prat J, et al. Nutr Metab Cardiovasc Dis. 2013. pp. 84–93. [DOI] [PubMed]
- Cui X, Wang Y, Meng L, et al. Am J Physiol Endocrinol Metab. 2012. pp. E705–E713. [DOI] [PubMed]
- Ding Y, Wang Y, Zhu H, et al. Transgenic Res. 2011. pp. 867–875. [DOI] [PubMed]
- Al-Mashhadi RH, Sorensen CB, Kragh PM, et al. Sci Transl Med. 2013. p. 166ra1. [DOI] [PubMed]
- Song J, Zhong J, Guo X, et al. Cell Res. 2013. pp. 1059–1062. [DOI] [PMC free article] [PubMed]
- Wei J, Ouyang H, Wang Y, et al. FEBS J. 2012. pp. 91–99. [DOI] [PubMed]
- Yang SH, Cheng PH, Banta H, et al. Nature. 2008. pp. 921–924. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods