Abstract
A recent study published in Immunity shows that foreign antigens elicit all-or-nothing T cell responses and that a single antigen is enough to trigger this digital cytokine secretion.
Mammalian cells sense extracellular stimuli through cell surface receptors. The contact between receptor and ligand is the starting point of signaling pathways that ultimately result in a cellular response. Measured at a population level, these responses (e.g., effector molecule production) typically increase with ligand concentrations. There is increasing evidence that population-wide measurements do not necessarily reflect single-cell behavior. When measured at the single-cell level, various signaling pathways show digital responses, i.e., they respond to stimuli by activating key molecules in an all-or-nothing manner. This was recently shown to be the case for nuclear factor κB (NF-κB)1 and nuclear factor of activated T cells 1 (NFAT1)2. In cells stimulated with TNF-α (for NF-κB) or Leukotriene C4 (for NFAT1), these transcription factors showed either high levels of nuclear translocation and subsequent gene expression or none at all. While the level of translocated transcription factors in activated cells remained constant, increasing stimuli led to a higher percentage of activated cells. Thus, the population shows a gradually increasing activation while single cells show either “on” or “off”. While digital signaling is known to convert multiple inputs into all-or-nothing action potentials in neurons3, the function that it might have in some, if not all, non-excitable cell types remains to be elucidated. Emerging evidence reveals that remarkable traits of T cell responses might be due to digital signaling. Motivated by this exciting idea, we would like to highlight a paper by Huang et al.4, which studied T cell activation at single-molecule and single-cell level and demonstrated that T cells undergo digital immune response upon antigen stimulation.
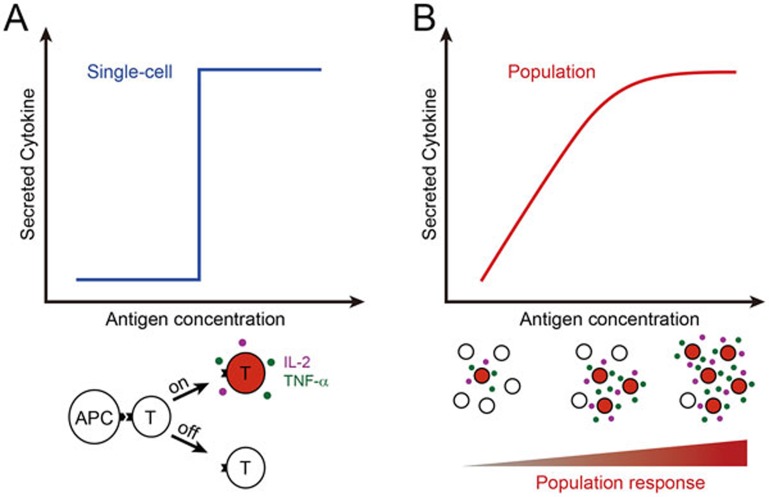
The adaptive immune response against invading pathogens starts from the specific engagement of the T-cell receptor (TCR) on the T cell surface with the antigenic peptides displayed by MHC molecules (peptide-MHC complex, pMHC) on the surface of antigen-presenting cells (APCs). TCR discriminates foreign from self antigens and initiates signaling pathways to activate T cells. For CD4+ T cells, antigen engagement turns the cells into effectors cells that help other immune cells via cytokines or cell-cell contacts and thus elicit a concerted immune response against pathogens. Huang et al.4 imaged CD4+ T cells in contact with APCs using 3D microscopy. They developed techniques to count the number of pMHC present in the immunological synapse while simultaneously quantify the secretion of IL-2 and TNF-α by T cells. These two cytokines are important regulators of the adaptive immune response and their secretion can be considered as indicator for CD4+ T cell effector function. This approach is novel because it quantifies the initial input stimulus during the natural interaction between APC and T cell and then measures the final output of signal processing. They first exposed APCs to a peptide that is known to be a strong antigen for the T cell clone under study. Biotin was attached to the peptide's N-terminus via a linker and streptavidin-conjugated quantum dots were used to reliably label pMHC. D-amino acids were used in the linker to prevent proteolysis. The employed quantum dots were bright enough for single-molecule detection and gave constant non-bleaching fluorescence. Secreted IL-2 and TNF-α were kept on the surface of T cells by capturing reagents (an antibody linking IL-2 to CD45 and a small molecule inhibiting TNF-α-releasing metalloprotease). Secreted cytokines accumulated at the cell exterior and were quantified using fluorescent antibodies. The authors found that some cells were stimulated by only a single pMHC, showing the absolute sensitivity of CD4+ T cells. Furthermore, increasing ligand number only increased the percentage of responding cells, but not cytokine secretion by a single cell. Thus, the cytokine secretion by a T cell population increases with foreign antigen concentrations, but is comprised of digital single-cell responses (Figure 1).
Figure 1.
Digital T cell response to antigen stimulation. After the engagement with an APC, T cell either switches “on” (red filling) with cytokine production or remains “off” (white filling) (A). Higher antigen concentration does not increase individual T cell cytokine release (blue graph), but the proportion of responding cells is higher. On average, this results in a gradually increasing response to higher antigen concentrations, as illustrated by the red graph and the schematic below (B).
Furthermore, the authors compared cytokine secretion by naïve T cells, memory T cells and T cell blasts. Intriguingly, even some of the naïve T cells were activated by a single pMHC, though the proportion of responding cells was low and cells responded slowly. T cell blasts and memory T cells showed similar high levels of cytokine secretion. For all three T cell types, the single-cell cytokine secretion was not enhanced by higher numbers of pMHC, demonstrating the digital nature of T cell response.
The major finding on the digital effector function of T cells reported by Huang et al. can be explained by the digital nature of T cell signaling pathways. Previous studies have reported all-or-nothing activation of signaling molecules that control cytokine gene expression, such as NF-κB5, NFAT16, Ras7 and ERK8. Ras and ERK have been suggested to acquire digital behavior by positive feedback regulation7,9. In short, when a sufficient stimulus has activated a critical amount of signaling molecules, the feedback leads to self-accelerating activation, which rapidly switches the cell activation state from “off” to “on”. Another mechanism for analog-to-digital conversion might exist for NFAT1, which sets an activation threshold by its dephosphorylation level2. The upstream Ca2+ signal accumulates to a level that is either sufficient or not to lead to a critical level of NFAT1 dephosphorylation, triggering all-or-nothing NFAT1 nuclear translocation. It remains to be elucidated how these digital signaling molecules act together in T cells and whether their behavior is different for antigens with lower TCR affinity. Future studies might show whether digital responses are reserved for strong antigens or whether weak antigens could also induce digital T cell response, but with different digital “signaling programs”. Finally, it is tempting to speculate that digital signaling is a general concept in biology. It has been found for NF-κB in fibroblasts1 and NFAT1 in mast cells2, indicating that T cells are not the only non-excitable cell types with binary responses.
References
- Tay S, Hughey JJ, Lee TK, et al. Nature. 2010. pp. 267–271. [DOI] [PMC free article] [PubMed]
- Kar P, Nelson C, Parekh AB. Curr Biol. 2012. pp. 242–247. [DOI] [PubMed]
- Clark B, Häusser M. Curr Biol. 2006. pp. R585–R588. [DOI] [PubMed]
- Huang J, Brameshuber M, Zeng X, et al. Immunity. 2013. pp. 846–857. [DOI] [PMC free article] [PubMed]
- Kingeter LM, Paul S, Maynard SK, et al. J Immunol. 2010. pp. 4520–4524. [DOI] [PMC free article] [PubMed]
- Podtschaske M, Benary U, Zwinger S, et al. PLoS one. 2007. p. e935. [DOI] [PMC free article] [PubMed]
- Das J, Ho M, Zikherman J, et al. Cell. 2009. pp. 337–351. [DOI] [PMC free article] [PubMed]
- Altan-Bonnet G, Germain RN. PLoS Biol. 2005. p. e356. [DOI] [PMC free article] [PubMed]
- Stefanová I, Hemmer B, Vergelli M, et al. Nat Immunol. 2003. pp. 248–254. [DOI] [PubMed]