Abstract
Purpose.
The choroid plays a vital role in the health of the outer retina. While measurements of choroid using optical coherence tomography show altered thickness in aging and macular disease, detailed histopathologic and proteomic analyses are lacking. In this study we sought to evaluate biochemical differences in human donor eyes between very thin and thick choroids.
Methods.
One hundred forty-one eyes from 104 donors (mean age ± standard deviation, 81.5 ± 12.2) were studied. Macular sections were collected, and the distance between Bruch's membrane and the inner surface of the sclera was measured in control, early/dry age-related macular degeneration (AMD), neovascular AMD, and geographic atrophy eyes. Proteins from the RPE-choroid of eyes with thick and thin choroids were analyzed using two-dimensional electrophoresis and/or mass spectrometry. Two proteins with altered abundance were confirmed using Western blot analysis.
Results.
Donor eyes showed a normal distribution of thicknesses. Eyes with geographic atrophy had significantly thinner choroids than age-matched controls or early AMD eyes. Proteomic analysis showed higher levels of the serine protease SERPINA3 in thick choroids and increased levels of tissue inhibitor of metalloproteinases-3 (TIMP3) in thin choroids.
Conclusions.
Consistent with clinical imaging observations, geographic atrophy was associated with choroidal thinning. Biochemical data suggest an alteration in the balance between proteases and protease inhibitors in eyes that lie at the extremes of choroidal thickness. An improved understanding of the basic mechanisms associated with choroidal thinning may guide the development of new therapies for AMD.
Keywords: choroid, age-related macular degeneration, protease
Morphometric analysis of elderly donor eyes demonstrates thinning with geographic atrophy and increased age. Alteration in the expression of protease inhibitors helps elucidate the biochemical basis for choroidal thickness.
Introduction
The choroid is the essential vascular bed that supplies the retinal pigment epithelium and the outer retina.1–4 The choroid and choriocapillaris play a critical role in diseases affecting the eye, including uveitic diseases,5–10 infections,8,11–13 and both primary14–16 and metastatic17–19 ocular tumors. More recently, there is accumulating evidence that variations in choroidal thickness may be associated with certain eye diseases. For example, in vivo spectral-domain optical coherence tomography (OCT) of the choroid consistently demonstrates a decrease in thickness with advancing age and myopia,20–24 both risk factors for central vision-disabling choroidal neovascularization (CNV). Several OCT studies of eyes with early age-related macular degeneration (AMD) without CNV show a thinning of choroid compared to that in age-matched controls.25,26 Eyes with AMD complicated by CNV show decreased subfoveal choroidal thickness after intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy.27 Choroidal thickness changes after anti-VEGF therapy have even been observed in eyes being treated for diseases of the retinal vasculature.28 On the other hand, eyes with central serous chorioretinopathy and idiopathic polypoidal choroidopathy have a significantly thicker choroid on OCT29–33 that can persist despite anti-VEGF therapy. While OCT has proven to be a valid measure for choroidal thickness with highly reproducible results34–37 and the occurrence of choroidal thinning has been described in clinical studies, the molecular basis of this phenomenon is not well understood. In the current study we sought to evaluate the most prominent molecular differences in human donor eyes with very thick and very thin choroids, and identified an imbalance between proteases and protease inhibitors in eyes with the thickest and thinnest choroids.
Methods
Human Donor Eyes
Donor eyes were obtained from the Iowa Lions Eye Bank within 8 hours of death, following informed consent of the donors' next of kin. All research adhered to the tenets of the Declaration of Helsinki; Institutional Review Board approval is not required at the University of Iowa for donors post mortem. Eyes were either fixed in toto in 4% paraformaldehyde in phosphate-buffered saline (PBS) or dissected such that macular, temporal, inferior, and nasal punches of neural retina and RPE-choroid were flash frozen in liquid nitrogen for biochemical studies. The average age of eyes used in this study was 81.5 and standard deviation was 12.2, with a range from 21 to 100 years of age. All experiments were performed in accordance with the Declaration of Helsinki.
Quantification of Macular Thickness
Supertemporal wedges that included the macula or 8-mm punches, collected from the macula and centered on the fovea centralis, were cryoprotected in sucrose solution and embedded for cryostat sectioning, as described previously.38 Cryostat sections were collected and stained with hematoxylin/eosin and photographed using a microscope (BX41; Olympus, Center Valley, PA) and a digital camera, calibrated using a stage micrometer. The distance between Bruch's membrane and the inner surface of the sclera, assessed on hematoxylin/eosin-stained sections, was determined in 141 eyes from 104 donors (Figs. 1A, 1B). Scatter plots were generated in control eyes plotting age against thickness, and statistical significance was determined by linear regression analysis using an online analysis tool (http://www.wessa.net/rwasp_linear_regression.wasp/ [in the public domain]). Ultrastructural analysis was also performed in temporal, juxtamacular choroid in four eyes with thick and four eyes with thin choroids (mean thickness 293 and 53.1 μm, respectively). Transmission electron microscopy was performed as described previously.39
Figure 1.
Distribution of choroidal thicknesses in human donor maculae. Examples of thick (A) and thin (B) choroids in sections labeled with anti-CD45 antibody (green fluorescence) and Ulex europaeus agglutinin-I lectin (red fluorescence). The yellow lines indicate the measured distances used for donor eyes (i.e., the distance between Bruch's membrane and the inner surface of the sclera). Note the cross section of a short posterior ciliary artery in the sclera in (A) (arrow). A histogram showing the distribution of thicknesses of 141 donor eyes is illustrated in (C). Scale bar: 100 μm.
Identification of Proteins With Altered Abundance in Thin Choroids
A subset of eyes studied for morphology was also suitable for biochemical analyses. For initial experiments to identify major differences between thick and thin choroids, we prepared two pools of macular RPE–choroid protein; results were replicated in different eyes for confirmation (see below). Donors with thin (n = 2 eyes from two separate donors) and thick (n = 2 eyes from two separate donors) choroids were selected. The measured values for the thin set were 74.8 and 94.1 μm and for the thick group were 198.3 and 210.1 μm, respectively. Samples were homogenized using disposable tissue grinders (Kontes, Kimble Chase, Vineland, NJ) in an ice-cold solution consisting of 1% Triton X-100 in PBS with protease inhibitors (Complete kit; Roche, Indianapolis, IN), and two pools were generated, consisting of 100 μg total protein each.
Proteins were separated using two-dimensional (2D) gel electrophoresis (Kendrick Laboratories, Madison, WI). The conditions for the separation were 2% ampholyte in the first dimension and 10% acrylamide in the second (SDS-PAGE) dimension. Gels from the thin and thick pools were stained with mass spectrometry–compatible silver stain40 and were aligned using UnwarpJ (a plug-in for ImageJ available at http://rsb.info.nih.gov/ij/index.html [in the public domain]). Spots were selected that differed between the two pools, and mass spectrometry of tryptic peptides was performed using liquid chromotography–tandem mass spectrometry (LC-MS/MS) on a LTQ XL linear ion trap mass spectrometer (Thermo Scientific, Waltham, MA) at the University of Iowa Proteomics Core. Tryptic peptides were matched to known proteins in the Swiss Prot database using a commercial software package (Scaffold 3; http://www.proteomesoftware.com [in the public domain]).
In parallel, a shotgun proteomic experiment was conducted in which two pools were generated from two thin and two thick choroids, respectively, using the same protein samples as above. Protein profiling was performed using a commercial vendor (Bioproximity LLC, Chantilly, VA). Tryptic peptides were generated and separated using LC-MS/MS (Easy nano LC II HPLC system; Thermo Scientific). Normalization and quantification was performed using the normalized spectral abundance factor (NSAF) method.41
Proteins whose abundances varied by more than 1.5-fold between thick and thin choroids were evaluated using the DAVID functional annotation website (david.abcc.ncifcrf.gov/ [in the public domain]).42,43
Validation of Altered Expression of SERPINs and TIMP3
For assessment of levels of alpha-1-antichymotrypsin/serine protease SERPINA3 identified by 2D gel electrophoresis, additional thin and thick choroids were evaluated by Western blot analysis. Protein was extracted from juxtamacular, superior 6-mm punches of RPE-choroid, and blots were probed using antibodies directed against alpha-1-antichymotrypsin (0.7 μg/mL; Abcam, Cambridge MA) as described previously44 except that Alexa 488–conjugated secondary antibodies (Invitrogen, Carlsbad, CA) were used for detection. Blots were stained with Ponceau-S (ST-180; Boston BioProducts, Ashland, MA) to verify that differences in protein abundance were not due to grossly different amounts of protein in each well.
Replication Study
For confirmation of upregulated tissue inhibitor of metalloproteinases-3 (TIMP3) levels, protein samples of RPE-choroid from five additional eyes with thin choroids (mean thickness 61.1 μm) and five with thick choroids (mean thickness 244.1 μm) were evaluated and probed with anti-TIMP3 antibody as described previously.44 Protein loading was assessed using Ponceau-S as above.
Results
Variability of Choroidal Thickness in Human Donor Eyes
In human choroids, with or without disease, we observed a range of macular thicknesses from ∼31 to ∼385 μm (Fig. 1C). The mean thickness was 150.0 μm, and the standard deviation was 71.1 μm. These measurements were somewhat lower than those observed clinically in perfused eyes using OCT (e.g., see Ref. 22).
Effects of Aging and AMD
Maculas from eyes without AMD were evaluated as a function of age. Hematoxylin-eosin–stained sections from 22 normal eyes, ages 57 to 93, were measured as described above. Eyes under 50 years of age were excluded for this comparison since they could not be designated as AMD or non-AMD. We observed decreasing thickness with increasing age (coefficient of determination = 0.19) that was statistically significant (t-statistic −2.7, P < 0.05).
In addition to these 22 control eyes, we also studied eyes with early/dry AMD (n = 21), neovascular AMD (n = 6), and geographic atrophy (n = 12), which were selected from our research eye bank collection as described previously.45 There was no significant difference in choroidal thickness between age-matched donors and early AMD or eyes with CNV. In contrast, eyes with geographic atrophy showed significantly thinner choroids than any of the other categories (P < 0.01 for control versus geographic atrophy, P < 0.05 for dry AMD versus geographic atrophy, Fig. 2).
Figure 2.
Effects of age and AMD on choroidal thickness. (A) Scatter plot of age (x-axis) and choroidal thickness (y-axis) of non-AMD controls used in the study. Among samples included in this study, age showed a statistically significant negative relationship to thickness (slope P < 0.05), although the correlation was weak (r2 = 0.19). (B) Donor maculae were categorized as controls (CTL), early/dry AMD (ARM), neovascular AMD (CNV), or geographic atrophy (GA). Eyes with geographic atrophy showed significant choroidal thinning compared to the other categories (P < 0.05). Diamonds indicate individuals, and filled squares indicate averages for each category.
Ultrastructural Evaluation
Transmission electron microscopy was performed on a subset of eyes (thick and thin, four each). The most salient difference between the stroma of thick and thin choroids was the dense and abundant fibrillar collagen in the latter. While thick choroids showed a considerable network of bundled collagen, loosely packed and separated by fibroblast processes and other cellular elements, as well as ground substance, thin choroids showed a much higher density of collagen and elastin (Fig. 3).
Figure 3.
Examples of ultrastructure of thick (A, B) and thin (C, D) choroidal stroma. Collagen fibrils (f) in thick choroids were present in bundles that were loosely packed, with considerable intervening ground substance and fibrocyte processes (fp). Thin choroids showed much higher density of fibrillar collagen, without appreciable ground substance (electron-lucent material in [A] and [B]). Note the large coarse collagen fibrils (cf) in (C). ec, endothelial cell; m, melanocytes; v, vascular lumen. Scale bar: 2 μm.
Biochemical Detection of Differentially Expressed Proteins in Thick and Thin Choroids
Pools of thick and thin choroid proteins were prepared and separated using 2D gel electrophoresis. The overall pattern of protein spots was remarkably similar between thin and thick choroids (Fig. 4). Positive matches from differential spots included SERPINA3/alpha-1-antichymotrypsin (α-1-ACT) (spot series A), vimentin (spot B), and SERPINA1/alpha-1-antitrypsin (α-1-AT) (spot C).
Figure 4.
Comparison of thick and thin choroids by 2D gel electrophoresis. Pools of thick (left) and thin (right) RPE-choroids were evaluated. Three differences that were also observed with mass spectrometry are indicated by arrows (A, alpha-1-antichymotrypsin; B, vimentin; C, alpha-1-antitrypsin).
Proteins with attenuated expression in thin choroids were validated by Western blot, using the original pools (Supplementary Fig. S1) on an additional five thin and five thick RPE–choroid punches (Fig. 5). The decreased abundance of α-1-antichymotrypsin/SERPINA3 was confirmed in both pools and in additional, unpooled, individual samples.
Figure 5.
Western blot showing elevated alpha-1 antichymotrypsin/SERPINA3 in thick choroids. Left: Ponceau S protein stain; right: same blot probed with antibodies directed against SERPINA3/alpha-1-antichymotrypsin.
In a shotgun proteomics experiment, the protein with the largest increase in thick choroid samples, based on fold change, was another member of the serine protease inhibitor family (SERPINB1), an inhibitor of neutrophil elastase (Table 1). Thick choroid samples also showed higher levels of retinal proteins, which we assume is due to retinal contamination of one or both of the thick samples during dissection. In addition, thick choroid contained more C-reactive protein and macrophage-associated CD163. Proteins with at least 1.5-fold higher abundance in thick choroids were assessed using the DAVID analysis platform and were found to be most enriched in translational elongation/ribosome categories (Benjamini P values < 10−12). Plasma proteins albumin and immunoglobulin heavy and light chains were similar between thick and thin pools (log2 ratio between 0 and 0.8), indicating that differences in plasma proteins are not a major source of difference between thick and thin pools in this experiment.
Table 1.
Proteins More Abundant in Thick RPE-Choroid
|
ENSEMBL Protein Name |
NSAF Thin |
NSAF Thick |
Base 2 Log Ratio |
Gene Name |
Description |
| ENSP00000370115 | 2.83 | 71.75 | −4.67 | SERPINB1 | Serpin peptidase inhibitor, clade B (ovalbumin), member 1 |
| ENSP00000255030 | 4.78 | 117.22 | −4.62 | CRP | C-reactive protein, pentraxin-related |
| ENSP00000278833 | 3.05 | 66.79 | −4.45 | ROM1 | Retinal outer segment membrane protein 1 |
| ENSP00000354513 | 1.99 | 43.58 | −4.45 | CALD1 | Caldesmon 1 |
| ENSP00000230381 | 3.10 | 48.79 | −3.98 | PRPH2 | Peripherin 2 (retinal degeneration, slow) |
| ENSP00000255266 | 1.25 | 19.63 | −3.98 | PDE6A | Phosphodiesterase 6A, cGMP-specific, rod, alpha |
| ENSP00000352071 | 1.85 | 26.77 | −3.85 | CD163 | CD163 molecule |
| ENSP00000255945 | 3.26 | 42.76 | −3.72 | GIMAP4 | GTPase, IMAP family member 4 |
| ENSP00000307705 | 4.89 | 64.23 | −3.72 | HIST1H1E | Histone cluster 1, H1e |
| ENSP00000319690 | 3.50 | 45.97 | −3.72 | HNRNPC | Heterogeneous nuclear ribonucleoprotein C (C1/C2) |
| ENSP00000369741 | 23.54 | 298.85 | −3.67 | RPS6 | Ribosomal protein S6 |
| ENSP00000274364 | 0.68 | 7.74 | −3.51 | IQGAP2 | IQ motif containing GTPase activating protein 2 |
| ENSP00000345156 | 4.98 | 56.70 | −3.51 | RPL14 | Ribosomal protein L14 |
| ENSP00000239761 | 0.74 | 7.73 | −3.39 | MRC1 | Mannose receptor, C type 1 |
| ENSP00000264605 | 1.79 | 18.76 | −3.39 | MLPH | Melanophilin |
| ENSP00000354791 | 0.84 | 8.81 | −3.39 | DCTN1 | Dynactin 1 |
| ENSP00000334876 | 1.90 | 18.32 | −3.27 | GRK1 | G protein-coupled receptor kinase 1 |
| ENSP00000354739 | 6.49 | 62.52 | −3.27 | RPL12 | Ribosomal protein L12 |
| ENSP00000359345 | 7.21 | 66.31 | −3.20 | RPL5 | Ribosomal protein L5 |
| ENSP00000371393 | 1.75 | 15.32 | −3.13 | PGM2 | Phosphoglucomutase 2 |
In the same analysis, TIMP3 was found to be more abundant in thin choroids (Table 2), in contrast to other protease inhibitors. Bioinformatic analysis of proteins that were more abundant in thin choroids (≥1.5-fold enriched) using the DAVID tool showed enrichment of proteins associated with oxidation-reduction and fatty acid metabolism (Benjamini corrected P values = 2.2 × 10−4 and P = 7.2 × 10−5, respectively).
Table 2.
Proteins More Abundant in Thin RPE-Choroid
|
ENSEMBL Protein Name |
NSAF Thin |
NSAF Thick |
Base 2 Log Ratio |
Gene Name |
Description |
| ENSP00000266085 | 416.25 | 4.44 | 6.55 | TIMP3 | TIMP metallopeptidase inhibitor 3 |
| ENSP00000343577 | 105.16 | 3.41 | 4.95 | TPSAB1 | Tryptase alpha/beta 1 |
| ENSP00000332256 | 43.93 | 1.83 | 4.58 | ALDH1A3 | Aldehyde dehydrogenase 1 family, member A3 |
| ENSP00000311489 | 8.96 | 0.39 | 4.51 | SPTBN2 | Spectrin, beta, non-erythrocytic 2 |
| ENSP00000313420 | 3.11 | 0.23 | 3.78 | PRKDC | Protein kinase, DNA-activated, catalytic polypeptide |
| ENSP00000222573 | 13.93 | 1.22 | 3.51 | ITGB8 | Integrin, beta 8 |
| ENSP00000231887 | 14.81 | 1.30 | 3.51 | EHHADH | Enoyl-CoA, hydratase/3-hydroxyacyl CoA dehydrogenase |
| ENSP00000273784 | 52.39 | 5.10 | 3.36 | AHSG | Alpha-2-HS-glycoprotein |
| ENSP00000347665 | 6.35 | 0.62 | 3.36 | COL18A1 | Collagen, type XVIII, alpha 1 |
| ENSP00000265631 | 12.69 | 1.39 | 3.19 | SLC25A13 | Solute carrier family 25 (aspartate/glutamate carrier), member 13 |
| ENSP00000268251 | 17.14 | 1.88 | 3.19 | ABAT | 4-Aminobutyrate aminotransferase |
| ENSP00000348762 | 11.71 | 1.28 | 3.19 | LSS | Lanosterol synthase (2,3-oxidosqualene-lanosterol cyclase) |
| ENSP00000296046 | 17.98 | 2.25 | 3.00 | CPA3 | Carboxypeptidase A3 (mast cell) |
| ENSP00000321988 | 25.42 | 3.18 | 3.00 | SULT1A1 | Sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1 |
| ENSP00000369927 | 46.42 | 5.81 | 3.00 | AKR1C3 | Aldo-keto reductase family 1, member C3 |
| ENSP00000221283 | 10.84 | 1.58 | 2.78 | STXBP2 | Syntaxin binding protein 2 |
| ENSP00000225275 | 8.63 | 1.26 | 2.78 | MPO | Myeloperoxidase |
| ENSP00000262211 | 12.96 | 1.89 | 2.78 | SGK3 | Serum/glucocorticoid regulated kinase family, member 3 |
| ENSP00000363639 | 75.61 | 11.03 | 2.78 | TXN | Thioredoxin |
| ENSP00000367281 | 12.90 | 1.88 | 2.78 | BEST1 | Bestrophin 1 |
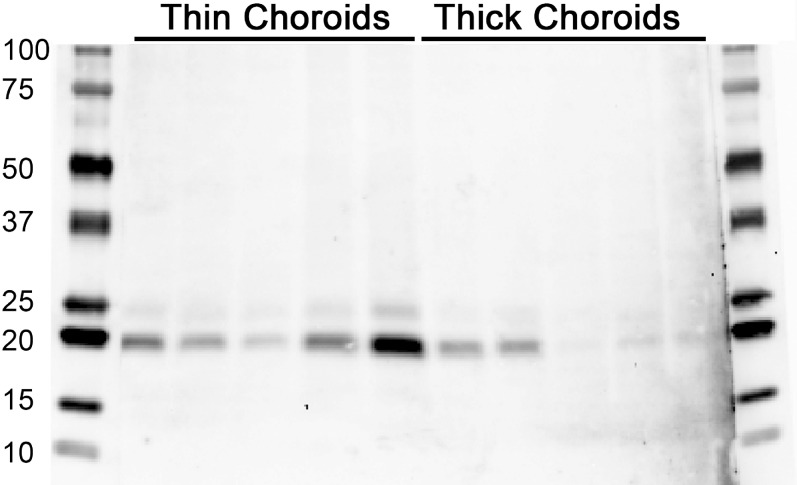
Validation of TIMP3 levels was performed using an additional five thin and five thick samples, which confirmed an overall increase of TIMP3 protein in eyes with thin choroids (Fig. 6).
Figure 6.
Western blot showing abundance of TIMP3 in thin (left lanes) compared to thick (right lanes) choroids. Each lane contains 15 μg total protein from a separate RPE–choroid punch and from a different donor. Blots were probed with anti-TIMP3 antibody. As a group, TIMP3 abundance was notably higher in thin choroids.
Discussion
The choroid provides oxygen and other nutrients to the highly metabolic outer retina as well as to the RPE. With the widespread application of enhanced depth imaging (EDI)-OCT, clinicians and scientists can now routinely visualize the choroid, qualitatively and quantitatively. One outcome of these studies in living patients is that the macular choroid has been found to vary in thickness by anatomic location, age, and, to a lesser degree, pharmacologic exposures. While there is not universal agreement on the importance of choroidal thickness in eye disease, choroidal atrophy is a recognized component of some forms of AMD where it is accompanied by subretinal drusenoid deposits.46,47 In this study we sought to characterize the anatomical and molecular bases of choroidal thickness in the maculas of human eye donors.
We found that donor eyes displayed a normal distribution of thickness (Fig. 1). The measurements made in the current study were overall somewhat thinner than those observed using OCT. This may be due to the nonlinearity of the OCT, which assesses the reflectivity of different interfaces, or it may simply reflect the lack of choroidal perfusion in a deceased individual. The latter possibility is supported by findings that drugs that increase blood flow, such as sildenafil citrate, induce choroidal swelling that is measurable by EDI-OCT.48
Ramrattan and colleagues49 previously characterized changes in several morphometric parameters including choroidal thickness in aging in donor eyes. One interesting finding in their study was that choroidal thickness appears to decline with age, with most of the trend over the first five decades of life. Our findings reveal a statistically significant but numerically modest age-related decline; however, it should be noted that only three eyes under age 50 were included in the current study. It is also noteworthy that our thickness measurements made using frozen sections were in good agreement with those obtained by Ramrattan et al.49 using paraffin sections.
To identify factors associated with the extremes of choroidal thickness, we performed an experiment to evaluate differences in proteins between the thickest and thinnest samples. We found that eyes with thin choroids have reduced levels of vimentin, a cytoskeletal protein expressed by choroidal fibroblasts,50 which may be consistent with loss of cellularity in thin choroids. Thin choroids also showed reduced levels of serine protease inhibitors (SERPINs) compared to those with thick choroids. In contrast, the metalloprotease inhibitor TIMP3 was more abundant in thin choroids than in thick choroids. These findings were validated by Western blot experiments using unpooled samples (n = 5 human eyes/category).
The SERPINs alpha-1-antitrypsin and alpha-1-antichymotrypsin inhibit a range of serine proteases and elastases involved in tissue remodeling, including neutrophil elastases.51,52 A reduction in the activity of SERPINs, observed in the thin choroid group, might be expected to allow increased proteolysis by enzymes such as cathepsin G, which has been linked to degradation of proteoglycans present in ground substance of connective tissues.53 Increased degradation of extracellular ground substance components may lead to collapse of the choroid and choroidal thinning. Thus, the general finding that SERPINs were less abundant in thin choroids seems mechanistically logical and straightforward.
In contrast, the relative abundance of TIMP3 in thin choroids seems counterintuitive, since TIMP3 inhibits several metalloproteases and, at least simplistically, would be expected to prevent matrix breakdown. However, mutations in the TIMP3 gene are responsible for Sorsby fundus dystrophy, an autosomal dominant maculopathy characterized by extracellular matrix imbalance in Bruch's membrane54,55 and a dramatic thickening of the latter structure. Also, mice lacking TIMP3 develop an abnormal, dilated choroidal vasculature,56 and TIMP3 itself interferes with VEGF–VEGF receptor interactions.57 It is therefore plausible that an increased level of TIMP3 might inhibit normal physiological vascular repair and turnover in the choroid, resulting in eventual choroidal thinning. Consistent with this notion, induction of TIMP3 overexpression in tumors was recently shown to be associated with a high density of endothelial cells, but without feeder vessels or pericytes, and with reduced VE-cadherin expression.58 Thus, at least in the abnormal tumor microenvironment, TIMP3 may suppress endothelial cell differentiation. A similar pathway in choroid could affect its thickness. It will be of interest to determine whether the choroidal endothelial cells show altered differentiation in cases of choroidal thinning.
In a large study combining RT-PCR and morphometry, Bailey and colleagues59 evaluated the relationship between TIMP3 expression and Bruch's membrane thickness, and found that TIMP3 RNA was more abundant in eyes with a thin Bruch's membrane. Our findings on TIMP3 protein and choroidal thickness are consistent with these findings. Although Bruch's membrane thickness was contained within the measurements in the current study, it represented less than 5% of the measured choroidal thickness, and differences at the extremes of choroidal thickness were therefore not due to differences at the level of Bruch's membrane.
In summary, we have identified altered abundance of different classes of protease inhibitors in thick and thin human choroids. In view of the altered choroidal thickness observed in macular diseases, especially central serous chorioretinopathy and geographic atrophy, an understanding of the basic mechanisms associated with choroidal thinning will provide better insight into pathogenesis of these diseases and guide the development of novel therapies.
Acknowledgments
The authors thank Grefachew Workalemahu, Miles Flamme-Wiese, and Megan Riker for excellent technical assistance.
Portions of this research were presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Seattle, Washington, May 5–9, 2013.
Supported in part by National Institutes of Health Grants EY017451 (RFM), EY016822 (EMS), and 1-DP2-OD007483-01 (BAT); Howard Hughes Medical Institute (EMS); Elmer and Sylvia Sramek Charitable Foundation (RFM/BAT); the Stephen A. Wynn Foundation; and the Hansjoerg EJW Kolder MD, PhD Professorship for Best Disease (RFM).
Disclosure: E.H. Sohn, None; A. Khanna, None; B.A. Tucker, None; M.D. Abràmoff, None; E.M. Stone, None; R.F. Mullins, None
References
- 1. Hayreh SS. Segmental nature of the choroidal vasculature. Br J Ophthalmol. 1975; 59: 631–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castro-Correia J. Understanding the choroid. Int Ophthalmol. 1995; 19: 135–147 [DOI] [PubMed] [Google Scholar]
- 3. McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994; 35: 3799–3811 [PubMed] [Google Scholar]
- 4. Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012; 31: 377–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watzke RC, Packer AJ, Folk JC, Benson WE, Burgess D, Ober RR. Punctate inner choroidopathy. Am J Ophthalmol. 1984; 98: 572–584 [DOI] [PubMed] [Google Scholar]
- 6. Rao NA. Mechanisms of inflammatory response in sympathetic ophthalmia and VKH syndrome. Eye (Lond). 1997; 11 (pt 2): 213–216 [DOI] [PubMed] [Google Scholar]
- 7. Vasconcelos-Santos DV, Sohn EH, Sadda S, Rao NA. Retinal pigment epithelial changes in chronic Vogt-Koyanagi-Harada disease: fundus autofluorescence and spectral domain-optical coherence tomography findings. Retina. 2010; 30: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasconcelos-Santos DV, Rao PK, Davies JB, Sohn EH, Rao NA. Clinical features of tuberculous serpiginouslike choroiditis in contrast to classic serpiginous choroiditis. Arch Ophthalmol. 2010; 128: 853–858 [DOI] [PubMed] [Google Scholar]
- 9. Brown J, Folk JC, Reddy CV, Kimura AE. Visual prognosis of multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ophthalmology. 1996; 103: 1100–1105 [DOI] [PubMed] [Google Scholar]
- 10. Gaudio PA, Kaye DB, Crawford JB. Histopathology of birdshot retinochoroidopathy. Br J Ophthalmol. 2002; 86: 1439–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffin JR, Pettit TH, Fishman LS, Foos RY. Blood-borne Candida endophthalmitis. A clinical and pathologic study of 21 cases. Arch Ophthalmol. 1973; 89: 450–456 [DOI] [PubMed] [Google Scholar]
- 12. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990; 97: 1288–1297 [DOI] [PubMed] [Google Scholar]
- 13. Sherman MD, Nozik RA. Other infections of the choroid and retina. Toxoplasmosis, histoplasmosis, Lyme disease, syphilis, tuberculosis, and ocular toxocariasis. Infect Dis Clin North Am. 1992; 6: 893–908 [PubMed] [Google Scholar]
- 14. McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983; 96: 502–509 [DOI] [PubMed] [Google Scholar]
- 15. Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976; 20: 415–431 [DOI] [PubMed] [Google Scholar]
- 16. Shields CL, Shields JA, Augsburger JJ. Choroidal osteoma. Surv Ophthalmol. 1988; 33: 17–27 [DOI] [PubMed] [Google Scholar]
- 17. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997; 104: 1265–1276 [DOI] [PubMed] [Google Scholar]
- 18. Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983; 27: 211–232 [DOI] [PubMed] [Google Scholar]
- 19. Chambers JD, Mosher ML. Intraocular involvement in systemic lymphoma. Surv Ophthalmol. 1966; 11: 562–564 [PubMed] [Google Scholar]
- 20. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147: 811–815 [DOI] [PubMed] [Google Scholar]
- 21. Nishida Y, Fujiwara T, Imamura Y, Lima LH, Kurosaka D, Spaide RF. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012; 32: 1229–1236 [DOI] [PubMed] [Google Scholar]
- 22. Switzer DW, Mendonça LS, Saito M, Zweifel SA, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012; 32: 1265–1271 [DOI] [PubMed] [Google Scholar]
- 23. Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013; 120: 175–180 [DOI] [PubMed] [Google Scholar]
- 24. Ho M, Liu DTL, Chan VCK, Lam DSC. Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology. 2013; 120: 1909–1914 [DOI] [PubMed] [Google Scholar]
- 25. Hu Z, Wu X, Ouyang Y, Ouyang Y, Sadda SR. Semiautomated segmentation of the choroid in spectral-domain optical coherence tomography volume scans. Invest Ophthalmol Vis Sci. 2013; 54: 1722–1729 [DOI] [PubMed] [Google Scholar]
- 26. Ko A, Cao S, Pakzad-Vaezi K, et al. Optical coherence tomography-based correlation between choroidal thickness and drusen load in dry age-related macular degeneration. Retina. 2013; 33: 1005–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012; 119: 1621–1627 [DOI] [PubMed] [Google Scholar]
- 28. Tsuiki E, Suzuma K, Ueki R, Maekawa Y, Kitaoka T. Enhanced depth imaging optical coherence tomography of the choroid in central retinal vein occlusion. Am J Ophthalmol. 2013; 156: 543–547 [DOI] [PubMed] [Google Scholar]
- 29. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118: 840–845 [DOI] [PubMed] [Google Scholar]
- 30. Kim S-W, Oh J, Kwon S-S, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011; 31: 1904–1911 [DOI] [PubMed] [Google Scholar]
- 31. Jirarattanasopa P, Ooto S, Tsujikawa A, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012; 119: 1666–1678 [DOI] [PubMed] [Google Scholar]
- 32. Yang L, Jonas JB, Wei W. Optical coherence tomography-assisted enhanced depth imaging of central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2013; 54: 4659–4665 [DOI] [PubMed] [Google Scholar]
- 33. Kuroda S, Ikuno Y, Yasuno Y, et al. Choroidal thickness in central serous chorioretinopathy. Retina. 2013; 33: 302–308 [DOI] [PubMed] [Google Scholar]
- 34. Branchini L, Regatieri CV, Flores-Moreno I, Baumann B, Fujimoto JG, Duker JS. Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology. 2012; 119: 119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chhablani J, Barteselli G, Wang H, et al. Repeatability and reproducibility of manual choroidal volume measurements using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 2274–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang L, Lee K, Niemeijer M, Mullins RF, Sonka M, Abramoff MD. Automated segmentation of the choroid from clinical SD-OCT. Invest Ophthalmol Vis Sci. 2012; 53: 7510–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahman W, Chen FK, Yeoh J, Patel P, Tufail A, Da Cruz L. Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 2267–2271 [DOI] [PubMed] [Google Scholar]
- 38. Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990; 38: 1383–1388 [DOI] [PubMed] [Google Scholar]
- 39. Skeie JM, Hernandez J, Hinek A, Mullins RF. Molecular responses of choroidal endothelial cells to elastin derived peptides through the elastin-binding protein (GLB1). Matrix Biol. 2012; 31: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O'Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997; 18: 349–359 [DOI] [PubMed] [Google Scholar]
- 41. Paoletti AC, Parmely TJ, Tomomori-Sato C, et al. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc Natl Acad Sci U S A. 2006; 103: 18928–18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009; 4: 44–57 [DOI] [PubMed] [Google Scholar]
- 43. Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009; 37: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mullins RF, Skeie JM, Malone EA, Kuehn MH. Macular and peripheral distribution of ICAM-1 in the human choriocapillaris and retina. Mol Vis. 2006; 12: 224–235 [PubMed] [Google Scholar]
- 45. Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009; 147: 801–810 [DOI] [PubMed] [Google Scholar]
- 47. Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013; 33: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vance SK, Imamura Y, Freund KB. The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina. 2011; 31: 332–335 [DOI] [PubMed] [Google Scholar]
- 49. Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994; 35: 2857–2864 [PubMed] [Google Scholar]
- 50. Cao J, Zhao L, Li Y, et al. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest Ophthalmol Vis Sci. 2010; 51: 6009–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baker C, Belbin O, Kalsheker N, Morgan K. SERPINA3 (aka alpha-1-antichymotrypsin). Front Biosci. 2007; 12: 2821–2835 [DOI] [PubMed] [Google Scholar]
- 52. Abboud RT, Nelson TN, Jung B, Mattman A. Alpha1-antitrypsin deficiency: a clinical-genetic overview. Appl Clin Genet. 2011; 4: 55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sommerhoff CP, Nadel JA, Basbaum CB, Caughey GH. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Invest. 1990; 85: 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weber BH, Vogt G, Pruett RC, Stohr H, Felbor U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet. 1994; 8: 352–356 [DOI] [PubMed] [Google Scholar]
- 55. Fariss RN, Apte SS, Luthert PJ, Bird AC, Milam AH. Accumulation of tissue inhibitor of metalloproteinases-3 in human eyes with Sorsby's fundus dystrophy or retinitis pigmentosa. Br J Ophthalmol. 1998; 82: 1329–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Janssen A, Hoellenriegel J, Fogarasi M, et al. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci. 2008; 49: 2812–2822 [DOI] [PubMed] [Google Scholar]
- 57. Qi JH, Ebrahem Q, Moore N, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003; 9: 407–415 [DOI] [PubMed] [Google Scholar]
- 58. Spurbeck WW, Ng CYC, Strom TS, Vanin EF, Davidoff AM. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002; 100: 3361–3368 [DOI] [PubMed] [Google Scholar]
- 59. Bailey TA, Alexander RA, Dubovy SR, Luthert PJ, Chong NH. Measurement of TIMP-3 expression and Bruch's membrane thickness in human macula. Exp Eye Res. 2001; 73: 851–858 [DOI] [PubMed] [Google Scholar]