Abstract
Recent clinical evidence suggests that the neuroprotective and beneficial effects of hormone therapy may be limited by factors related to age and reproductive status. The patient's age and length of time without circulating ovarian hormones are likely to be key factors in the specific neurological outcomes of hormone therapy. However, the mechanisms underlying age-related changes in hormone efficacy have not been determined. We hypothesized that there are intrinsic changes in estrogen receptor β (ERβ) function that determine its ability to mediate the actions of 17β-estradiol (E2) in brain regions such as the ventral hippocampus. In this study, we identified and quantified a subset of ERβ protein interactions in the ventral hippocampus that were significantly altered by E2 replacement in young and aged animals, using two-dimensional differential gel electrophoresis coupled with liquid chromatography–electrospray ionization–tandem mass spectrometry. This study demonstrates quantitative changes in ERβ protein–protein interactions with E2 replacement that are dependent upon age in the ventral hippocampus and how these changes could alter processes such as transcriptional regulation. Thus, our data provide evidence that changes in ERβ protein interactions are a potential mechanism for age-related changes in E2 responsiveness in the brain after menopause.
The neuroprotective and beneficial effects of estrogens in the brain have been reported for decades, yet recent evidence from clinical trials suggests that the benefits of estrogens in postmenopausal women might not outweigh the risks (1–3). Specifically, the risk of cardiovascular disease and invasive breast cancer was significantly increased in postmenopausal women given hormone therapy as part of the largest clinical trial performed to date (Women's Health Initiative). These results sharply contradicted substantial evidence from numerous studies in animal models, prompting a reevaluation of the data from the Women's Health Initiative studies. Later it was determined that factors contributing to the observed detrimental effects of hormone therapy in the Women's Health Initiative study included advanced age, the types of synthetic estrogens and progestins used in the study, and, perhaps most important, the number of years postmenopause prior to the initiation of hormone therapy (4). However, more than 10 years after the conclusion of these studies there is little to no mechanistic explanation for how aging contributes to a change in estrogen signaling.
One possibility is that there are age-related changes in the way the brain responds to estrogens. We hypothesized that there are intrinsic changes in the function of estrogen receptors in the brain with advanced age, and estrogen receptor β (ERβ)1 in particular has been shown to be a critical regulator of many neurobiological functions. An important component of ERβ signaling is requisite associations with intracellular coregulatory proteins. Therefore, one possibility is that the protein–protein interactions required for ERβ signaling are altered with age and the bioavailability of estrogens. Previous studies have shown that ERβ can associate with traditional coregulators in the brain such as steroid coactivator-1 and estrogen receptor associated protein 140 (5–7), and that these associations are modified by multiple factors, including age. For instance, one study demonstrated decreased association between estrogen receptor associated protein 140 and ERβ in the aged hippocampus, despite an overall increase in estrogen receptor associated protein 140 expression (6). These results raise the interesting possibility that age causes intrinsic changes in the functional properties of ERβ, altering the ability of ERβ to interact with other proteins irrespective of protein availability.
Indeed, transcriptional regulation is the conventional mechanism of action for ERβ-mediated processes; however, evidence suggests that estrogen receptors have a much broader physiological role. The neuroprotective effects of estrogens are particularly important for postmenopausal women in brain regions such as the hippocampus, which is functionally subdivided into ventral and dorsal regions. The ventral hippocampus, forged by connections to the hypothalamus and amygdala, modulates affective processes such as responses to stress and emotion, whereas the dorsal hippocampus is important for mediating cognitive functions (8–11). It is not yet clear how estrogens regulate cognitive and emotional processes, and ERβ may be of particular importance for these and other functions in postmenopausal women; as the predominant estrogen receptor in the hippocampus, ERβ is largely responsible for a number of neurobiological functions ranging from gene transcription to synaptic transmission. Although there is still much to learn about ERβ-mediated transcription, there is even less known about the role of estrogen receptors in alternative splicing, mRNA processing, and a number of cytoplasmic signaling events (12–14).
Our aim in these studies was to quantitatively assess age-related changes in novel protein interactions with ERβ in the hippocampus using young (3-month-old) and aged (18-month-old) female rats. We also quantified how 17β-estradiol (E2) affected these interactions at each age in order to better understand a potential mechanism for the differential effects of hormone therapy that have been observed in postmenopausal women. Our approach was novel in that all experiments were performed in vivo in an outbred strain of rats using highly sensitive two-dimensional difference gel electrophoresis (2D-DIGE) coupled with liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) to both quantify and identify novel ERβ–protein interactions. Our results demonstrated that E2 altered the association of ERβ with a number of previously unidentified interaction partners depending on age. Some of these novel proteins included actin binding proteins, mRNA alternative splicing proteins, and multifunctional proteins. We also provide evidence to suggest that interactions with the nuclear actin binding protein gelsolin are required for ERβ-mediated transcriptional repression. As a whole, the work presented here sheds light on two important and very novel findings that further our understanding of the molecular and physiological functions of ERβ in the brain by (i) identifying novel ERβ–protein interactions that could delineate previously unknown roles for ERβ and (ii) demonstrating how age and E2 alter these protein interactions in vivo.
EXPERIMENTAL PROCEDURES
Animals
Female Fisher 344 rats (3 months old (n = 40) or 18 months old (n = 39)) were obtained from the NIH aging colony (Taconic, Hudson, NY) and allowed to acclimate for 7 days. Next, all animals were bilaterally ovariectomized and allowed to recover for 7 days. Briefly, rats were deeply anesthetized under isoflurane gas while the ovary and distal end of the uterine horn were pulled from the body cavity through a 1-cm incision made through the skin and body wall. The horn was clamped and ligated proximal to the clamp. The ovary and distal uterine horn were then removed to ensure that all ovarian sources of E2 were eliminated, thereby creating a surgically induced model of menopause. Starting on the eighth day post-ovariectomy the animals received subcutaneous injections of 2.5 μg/kg E2 or safflower oil (vehicle) once daily for three consecutive days. Animals were sacrificed by rapid decapitation 24 h after the last injection for harvesting of trunk blood and brains.
Estradiol Enzyme-linked Immunoassay
Circulating E2 was measured using an enzyme-linked immunoassay system (Cayman Chemical). Briefly, trunk blood was collected in tubes coated with 50 units of porcine heparin (Sigma, Ann Arbor, MI) per milliliter of blood collected. Blood was then centrifuged at 4000 × g for 7 min and plasma was removed and subjected to immunoassay per the manufacturer's instructions. Plasma E2 levels in vehicle-treated animals were below the limit of detection for the assay (<6.6 pg/ml). Plasma E2 levels for E2-treated animals were 53.67 (S.E. ± 7.24) pg/ml in young and 50.56 (S.E. ± 8.78) pg/ml in aged animals (data not shown), which is within the physiological range for postmenopausal patients receiving hormone replacement therapy (17–75 pg/ml) (15, 16).
Tissue Preparation
Brains were rapidly frozen using isopentane and stored at −80 °C until further processing. Frozen brains were sectioned at 200 μm on a freezing microtome, and the ventral hippocampus was microdissected using a 0.75-mm Palkovit's brain punch tool (Stoelting, Wood Dale, IL). The specificity of the microdissection was confirmed using The Rat Brain in Stereotaxic Coordinates (74) and was as follows: from bregma, −4.16 to −5.80 mm; dorsal–ventral, 6.0 to 9.0 mm; anterior–posterior, 3.0 to 6.0 mm (17). Punches were pooled (four animals per sample, from a total of 20 animals per age per treatment) and placed in CERI solution of nondenaturing NE-PER Nuclear Protein Extraction Reagents (Thermo Scientific), supplemented with 7x EDTA-free Complete Mini Protease Inhibitors (Roche). Nuclei were subjected to lysis, and insoluble material including DNA was pelleted and excluded from the soluble portion of the extracts.
Immunoprecipitation
Nuclear extracts were subjected to co-immunoprecipitation for ERβ and associated proteins (Ab288, Clone 14C8, Abcam, Cambridge, MA (1 μg/100 μg protein)) overnight. Subsequently, antibody and extracts were incubated with magnetic beads for 10 min at room temperature (Millipore Protein G, Billerica, MA), and after antibody binding, beads were washed three times with 1X PBS prior to elution with 0.2 m glycine. Two additional antibodies against ERβ were tested using the same paradigm: ligand-binding domain (LBD), a generous gift from Jan Ake Gustafsson (1 μg/100 μg protein) (18), and H-150 (1 μg/100 μg protein, Santa Cruz Biotechnology, Santa Cruz, CA). Protein spots that were common among all three antibodies were considered specific, whereas those that did not overlap were excluded from the final analysis. In addition, a control rabbit-anti-IgG antibody was used under the same experimental paradigm to identify nonspecific spot patterns. Following co-immunoprecipitation, samples were desalted and prepared for isoelectric focusing using the 2-D Cleanup system (GE Healthcare). Confirmation of interaction partners in vitro using an ERβ-expressing neuronal cell line (IVB) was accomplished by immunoprecipitating ERβ interaction partners in the presence of the homobifunctional imidoester crosslinker dimethyl 3,3′-dithiobispropionimidate (Thermo Scientific). IVB cells were grown in media containing 10% FBS before a 24-h treatment period in which cells were grown in media containing charcoal dextran-stripped media treated with 100 nm E2 or 0.001% EtOH. Following treatment, plated cells were washed three times in cold 1X PBS pH 8.0 prior to a 30-min incubation with 5 mm dimethyl 3,3′-dithiobispropionimidate (4 °C). Cells were washed three times with cold PBS again before incubation with a quenching buffer (100 mm Tris-HCl pH 8.0, 150 mm NaCl) for 10 min (4 °C). Cells were lysed (TPER, Thermo Scientific) in the presence of protease inhibitor (Roche). The resulting lysate was incubated with respective partner antibodies (GELS (Cell Signaling, Danvers, MA, #8090), VCP (Thermo Scientific, PA5–17486), GAPDH (Santa Cruz, sc-25778), or rabbit IgG control (Santa Cruz, sc-2027)) as described above. Crosslinks were reversed by incubation with 150 mm dithiothreitol for 30 min. Equal parts input from vehicle/E2-treated cells served as a positive control.
CyDye Labeling
7.5 μg from each sample was combined and aliquoted into an internal standard to correspond with each sample being compared (n = 3 for each group). Each standard (7.5 μg) and sample (7.5 μg) was reduced using 2 nmol tris(2-carboxyethyl)phosphine for 1.5 h at 37 °C in the dark. Then, all samples and standards were labeled with 4 nmol Cy5 or Cy3 DIGE Fluor saturation dyes (GE Healthcare) for 30 min at 37 °C in the dark. Saturating dyes are an advantage over minimal dyes as they label of ∼98% of cysteine sulfhydryls (compared with ∼6% of lysines), resulting in maximum sensitivity. The reaction was stopped by an equal volume of 2x rehydration buffer (UTC (7 m urea, 2 m thiourea, 4% w/v CHAPS) with Pharmalytes (2% v/v final) and DTT (130 mm final)).
Isoelectric Focusing and SDS-PAGE
Each dyed sample and corresponding standard (15 μg of protein: 7.5 μg Cy3-labeled pooled internal standard and 7.5 μg Cy5-labeled experimental group) were incorporated into a rehydration buffer (UTC with 0.5% v/v IPG buffer 3–11NL, 15 mg/ml Destreak Reagent), applied to a 24-cm 3–11NL Immobiline Drystrip, and subjected to active rehydration (10 h at 50 V) followed by an optimized run program: (i) step, 500 V for 500 Vh; (ii) gradient, 1000 V for 1000 Vh; (iii) gradient, 8000 V for 16,500 Vh; (iv) step, 8000 V for 42,000 Vh (75-μA limit at 15 °C, 61,000 total Vh). After the first dimension strips were equilibrated in 1% w/v DTT, 2.5% w/v iodoacetamide and a brief 1X SDS Running buffer wash before being resolved on 12% SDS-PAGE at 2 W per gel (limit: 500 V, 40 mA/gel) for 17 to 30 h.
Imaging and Analysis
Gels were imaged on a Typhoon 9400 (Cy5: ex: 633 nm em: 670 nm BP 30, Cy3: ex: 532 nm em: 580 nm BP 30 100 pixels, 450 PMT) prior to analysis with DeCyder Analysis software (GE). Using differential in-gel analysis, each gel was analyzed individually for processing up to 1500 spots, using standard spot exclusion for the following properties: slope > 1, area < 200, volume < 2500, and peak height < 16 or > 10,000. All gels were analyzed together using the Biological Variance Analysis (BVA) module.
Spot Analysis and Statistics
The BVA module was used to compare replicate gels and perform intergel statistical analysis. The BVA module accounts for the spots identified and confirmed in each gel's differential in-gel analysis workspace and automatically selects a master gel (the gel displaying the most confirmed spots) to match and compare each replicate gel against. Each protein spot was matched individually by examining each gel, using match vectors and creating landmark spots to affirm accurate spot matching. The standard abundance quantifies a given protein spot based upon protein spot volume, area, and background. Each spot is then normalized to its own internal standard and log transformed for statistical tests. Each gel represents the pooled internal standard (equal amounts of protein from each experimental replicate) compared with samples from young vehicle (YV), young estradiol (YE), aged vehicle (AV), and aged E2 (AE) treated animals. Within each group, samples were run in three independent experiments (i.e. Experiment 1, YV1 versus internal standard; Experiment 2, YV2 versus internal standard; etc.). Each sample (i.e. YV1, YV2, YV3) was representative of four pooled ventral hippocampus samples taken from different animals, thereby contributing to a biological variance of 12 animals per group for analytical gels (n = 3 independent experiments). Statistical significance for two-dimensional spot analysis was determined using DeCyder software by calculating an average log standard abundance for each group being tested (i.e. YV versus YE); thus the statistical significance could be determined by using one-way ANOVA (p < 0.05).
Spot Picking
After electrophoresis and analysis of analytical gels, a preparative gel representing ∼400 μg of co-immunoprecipitated protein was used to pick spots for peptide identification via tandem mass spectrometry. Gels were fixed and post-stained with Sypro Ruby and/or Coomassie G250 to visualize protein spots for excision. Although individual protein spots were analyzed through BVA, because of the small size of protein spots and low visibility of some post-stained spots, groups or “chains” of similar spots were picked and pooled. Spots from preparative gels were picked using the Ettan DIGE automated spot picker, and residual gel spots were excised using a sterile glass Pasteur pipette. Reference markers were placed at 3.5 cm and 10 cm from the edge of glass plates following fixative treatment with Bind Silane (8% ethanol (v/v), 0.002% acial acetic acid, 0.0001% Bind-Silane). Spot-picking parameters that were customized from standard settings included Jazz 1.3 mm, 50-μl aspiration volume, and 51-μl dispense volume.
In-gel Digestion of Peptides
The excised protein gel bands or gel spots were washed twice with analytical-grade water and 1:1 v/v 0.1 NH4HCO3 for 15 min with agitation. The washing solution was then removed completely and enough ACN was added to cover the gel particles. After the gel particles shrank and stuck together, the ACN was removed and the gel particles were rehydrated in 0.1 m NH4HCO3 for 10 min. An equal volume of ACN was then added to finally get 1:1 v/v 0.1 NH4HCO3:ACN. After 10 min of incubation, removal of all liquid, and drying of the gel particles in a vacuum centrifuge, proteins were reduced with 10 mm DTT and alkylated with 55 mm iodoacetamide in 0.1 m NH4HCO3. After reduction and alkylation, gel particles were washed as described above. Following tryptic digestion for 24 h at 37 °C, the peptides were recovered and extracted from the gel particles by the addition of 10 ml of 25 mm NH4HCO3 and 5% formic acid and ACN (5 ml of each). After pooling and drying down of all the extracts, the tryptic peptides were dried and resuspended in a formic acid:water:ACN mixture (0.1:95:5) in preparation for LC-ESI-MS/MS analysis.
Identification of Proteins with LC-ESI-MS/MS
The suspended tryptic peptides were introduced into the LC-ESI-MS/MS system. First, the peptides were separated on a reversed-phase column (C-18 PepMap100, LC Packings/Dionex, Sunnyvale, CA). Peak elution during the 60 min when peptides were expected to elute was accomplished with a flow rate of 200 nl/min using a gradient. Solvent A was 0.1% formic acid in 5% ACN, and solvent B was 0.1% formic acid in 95% ACN. The eluted peptides were then sprayed into the QSTAR XL mass spectrometer (Applied Biosystems and Sciex, Concord, ON, Canada) with a 2000-V electrospray voltage applied on the ESI emitter tip. This was a Q-TOF tandem mass spectrometer with reflection mode. Full MS scans in the m/z range of 200–1800 Da were followed by data-dependent acquisition of MS/MS spectra for the three most abundant ions. MS scans were used to identify candidates for fragmentation during MS/MS scans. Up to three 1.5-s MS/MS scans (65–1800 Da) were collected after each scan. The scanned ion was assigned a charge in the range of +2 to +4, and the dynamic exclusion was 40. Protein identifications were performed with PEAKS version 5.1 (Bioinformatics Solutions Inc., Waterloo, ON), which is a standard way to identify the peptides whose sequences are in a database. The following settings were used in our experiments: Trypsin was specified as the enzyme with one nonspecific missed cleavage and three maximum missed cleavages allowed in the database search. We set carbamidomethylation (mono mass 57.0215 Da) on cysteine residues as a fixed modification and oxidation (mono mass 15.9949 Da) on methionine residues as a variable modification. The mass error tolerance for precursor ions was 0.1 Da, and for fragment ions it was 0.3 Da. The search database contained sequences identified as rat in NCBI's database (June 2011, updated from NCBI website, www.ncbi.nlm.nih.gov), which was created using the FASTA filtering tool. This composite database contained ∼500,000 entries, and the quality of the peptide–spectrum matches made through database searching was determined by PEAKS score. Specifically, a PEAKS score > 80 denotes very confident matches, a score of 60 to 80 denotes good matches, and a score of <60 represents peptides with one hit or protein with two poor matches. The high-confidence peptides identified through the above steps were used to infer the proteins. Proteins identified with a PEAKS score of 60 or more are listed in Tables I and II.
Table I. Identified proteins altered by age and E2.
| Pick spot number | BVA spot number | Accession number | Molecular weight (Kda) | Estimated isoelectric point | PEAKS score | Percent coverage | I.D. | Log standard abundance |
Function | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YV | YE | AV | AE | |||||||||
| 22 | 288 | gi28373861 | 36 | 4.92 | 99 | 25.71 | Annexin V (ANXAV) spot A | 0 | 0.05 | −0.06 | 0 | Multifunctional |
| - | ↑ | - | ↑ | |||||||||
| 22 | 304 | gi28373862 | 36 | 4.92 | 99 | 25.71 | Annexin V (ANXAV) spot B | −0.26 | −0.06 | 0.04 | −0.04 | Multifunctional |
| - | ↑ | - | ↓ | |||||||||
| 22 | 343 | gi28373863 | 36 | 4.92 | 99 | 25.71 | Annexin V (ANXAV) spot C | −0.03 | 0.24 | 0.03 | −0.03 | Multifunctional |
| - | ↑ | - | - | |||||||||
| 15 | 195 | gi120538378 | 47 | 5.7 | 93.2 | 10.72 | Heterogeneous nuclear ribonucleoprotein H (HnRNPH) spot A | −0.03 | 0.23 | −0.05 | −0.01 | RNA splicing |
| - | ↑ | - | - | |||||||||
| 15 | 224 | gi120538378 | 47 | 5.7 | 93.2 | 10.72 | Heterogeneous nuclear ribonucleoprotein H (HnRNPH) spot B | −0.09 | 0.09 | 0.05 | 0.01 | RNA splicing |
| - | ↑ | - | - | |||||||||
| 15 | 186 | gi120538378 | 47 | 5.7 | 93.2 | 10.72 | Heterogeneous nuclear ribonucleoprotein H (HnRNPH) spot C | 0.01 | 0.12 | 0.12 | 0.08 | RNA splicing |
| - | ↑ | - | ↓ | |||||||||
| 15 | 200 | gi120538379 | 47 | 5.7 | 93.2 | 10.72 | Heterogeneous nuclear ribonucleoprotein H spot D | −0.15 | 0.03 | −0.04 | −0.01 | RNA splicing |
| - | ↑ | - | - | |||||||||
| 5 | 52 | gi149038929 | 80 | 5.75 | 49.4 | 6.43 | Gelsolin (GELS) spot A | −0.12 | 0.09 | −0.03 | −0.02 | Actin binding, coactivator |
| - | ↑ | - | - | |||||||||
| 5 | 54 | gi149038929 | 80 | 5.75 | 49.4 | 6.43 | Gelsolin (GELS) spot B | 0.01 | 0.14 | −0.08 | −0.04 | Actin binding, coactivator |
| - | ↑ | - | - | |||||||||
| 21 | 225 | gi62662279 | 38 | 6.97 | 99.1 | 26.01 | Annexin A1 (ANXA1) | −0.02 | 0.08 | 0.04 | 0.02 | Coactivator |
| - | ↑ | - | - | |||||||||
| 9 | 141 | gi116242506 | 74 | 5.97 | 93 | 14.58 | Heat shock protein 70 (HSP70) spot A | −0.08 | −0.03 | 0 | 0.07 | Chaperone |
| - | ↓ | - | - | |||||||||
| 9 | 145 | gi116242506 | 74 | 5.97 | 93 | 14.58 | Heat shock protein 70 (HSP70) spot B | 0.06 | −0.18 | 0.01 | 0.06 | Chaperone |
| - | ↓ | - | - | |||||||||
| 9 | 193 | gi116242507 | 75 | 5.97 | 93 | 14.58 | Heat shock protein 70 (HSP70) spot C | 0.13 | 0.07 | −0.02 | 0.05 | Chaperone |
| - | ↓ | - | ↑ | |||||||||
| 16 | 218 | gi158186649 | 47 | 6.16 | 98.6 | 19.35 | Enolase 1 α (ENO1)/Myc binding protein (MBP) | 0.06 | −0.01 | −0.18 | −0.07 | Multifunctional |
| - | ↓ | - | ↑ | |||||||||
| 2 | 12 | gi17865351 | 89 | 5.1 | 88.6 | 13.4 | Valosin containing protein (VCP) | 0.21 | 0.02 | 0.13 | 0.03 | Multifunctional |
| - | ↓ | - | - | |||||||||
| 20 | 295 | gi62662278 | 36 | 8.14 | 68.2 | 6.57 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) spot A | 0.1 | −0.12 | 0.06 | 0.05 | Coactivator/metabolism |
| - | ↓ | - | - | |||||||||
| 20 | 348 | gi62662278 | 36 | 8.14 | 68.2 | 6.57 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) spot B | 0.04 | −0.16 | −0.07 | 0.05 | Coactivator/metabolism |
| - | ↓ | - | - | |||||||||
Note: up and down arrows indicate statistically significant changes in log standard abundance (one-way ANOVA between YV versus YE and AV versus AE, p < 0.05).
Table II. Identified proteins unaltered by age and E2.
| Pick spot number | Accession number | Estimated isoelectric point | Molecular weight (Da) | PEAKS score | Percent coverage | Protein I.D. | Category |
|---|---|---|---|---|---|---|---|
| 1 | gi209862801 | 5.45 | 106,790 | 71.5 | 2.89 | Alanyl-tRNA synthetase (AARS) | Translation |
| 3 | gi84781723 | 6.56 | 80,461 | 60.8 | 1.84 | TNF receptor-associated protein 1 (TRAP1) | Chaperone |
| 4 | gi28467005 | 4.93 | 84,815 | 98.9 | 17.87 | Heat shock protein 90 (HSP90A) | Chaperone |
| 4 | gi91234898 | 4.97 | 83,282 | 98.9 | 21.13 | Heat shock protein 84 (HSP90B) | Chaperone |
| 6 | gi6981504 | 8.29 | 83,501 | 60.9 | 1.14 | Spinocerebellar ataxia 1 (AT-1) | Coactivator |
| 7 | gi54400730 | 5.96 | 57,458 | 90.8 | 5.23 | T-complex 1 (TCP1) | Chaperone |
| 7 | gi149023097 | 5.88 | 53,587 | 61.4 | 8.98 | Glucose regulated protein 58 (GRP58) | Chaperone |
| 8 | gi25742763 | 5.97 | 72,347 | 94.3 | 23.24 | Glucose regulated protein 78 (GRP78) | Chaperone |
| 10 | gi12053837 | 5.78 | 47,057 | 94.3 | 23.24 | Glucose regulated protein 75 (GRP75) | Chaperone |
| 11 | gi38328248 | 4.93 | 50,164 | 92.8 | 22.84 | α tubulin (TUBBA) | Structural |
| 11 | gi20799322 | 4.73 | 2,050,066 | 75.2 | 20.49 | Neuron-specific class III β tubulin | Structural |
| 12 | gi56383 | 5.91 | 60,966 | 69.4 | 10.47 | Heat shock protein (HSP60) | Chaperone |
| 12 | gi149028522 | 5.98 | 68,350 | 60.9 | 1.47 | Minichromosome maintenance deficient 7 (MCM7) | DNA helicase |
| 13 | gi149039794 | 5.39 | 42,801 | 78.1 | 11.4 | Heterogeneous nuclear ribonucleoprotein K (HnRNP K) | RNA processing |
| 14 | gi58865414 | 7.53 | 54,161 | 99 | 13.32 | Annexin A11 (ANXA11) | Multifunction |
| 17 | gi13592093 | 5.19 | 41,279 | 63.2 | 4.08 | Heat shock protein 70 interacting protein (HIP) | Chaperone |
| 17 | gi488838 | 5 | 47,220 | 97.7 | 12.76 | Calcium-binding protein 1 (CaBP1) | Multifunction |
| 17 | gi1374715 | 5.18 | 51,203 | 97.4 | 10.95 | ATP synthase β subunit | Metabolism |
| 17 | gi25742677 | 5.09 | 47,408 | 65.5 | 8.61 | Proteasome 26S | Proteasome |
| 18 | gi149053068 | 5.32 | 39,540 | 94 | 7.76 | Eukaryotic translation initiation factor 4A1 (EIF4A1) | RNA helicase |
| 19 | gi119959830 | 5.29 | 31,747 | 95 | 16.61 | β actin (ACTA) | Structural |
| 19 | gi149043182 | 5.23 | 34,264 | 93.8 | 7.53 | α actin (ACTB) | Structural |
| 19 | gi149036532 | 5.31 | 33,670 | 97 | 12.58 | γ actin (ACTG) | Structural |
Western Blotting
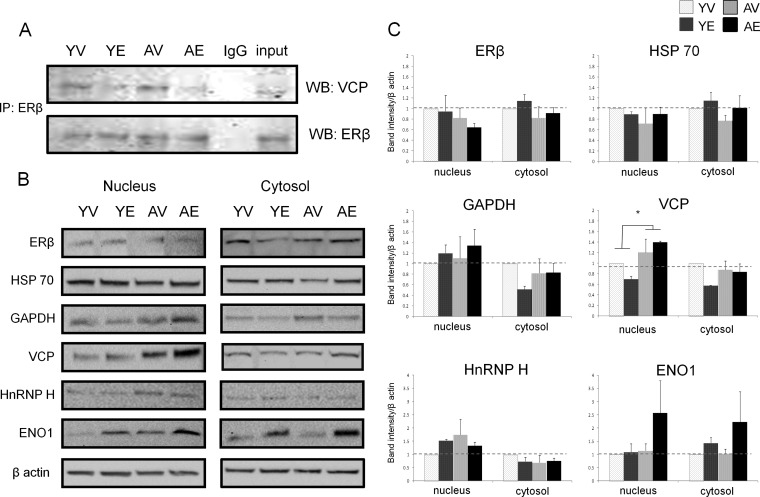
Co-immunoprecipitated proteins were obtained as described above, added to a denaturing 4X Laemmli buffer, and boiled at 95 °C for 5 min. Samples were resolved on 4%–20% SDS-PAGE gels for 1.5 h at 90 V and transferred to 0.45-μm PVDF membranes overnight at 10 mA/gel. Membranes were blocked with 5% bovine serum albumin (BSA) for 1 h before the addition of 1° antibody in TBST with 5% BSA and 0.01% NaN3 for 1.5 h. All 1° antibodies were used at a 1:1000 dilution: VCP (Pierce, PA5–17486), ERβ (Santa Cruz, Sc-8974x), ENO1 (Santa Cruz, sc-15343), GAPDH (Santa Cruz, sc-25778), GELS (Cell Signaling, #8090), HnRNPH (Santa Cruz, sc-15387), HSP70 (GenTex, GTX-104126), and β-actin (Cell Signaling, Aurora, IL, 4970S). Blots were washed three times with TBST for 5 min prior to the application of 1:4000 goat α-rabbit-HRP (1 h; Santa Cruz, sc-2004) in TBST with 5% BSA. Blots were washed three times with TBST and imaged on a Bio-Rad Chemidoc XRS+ imager using ECL Chemiluminescent substrate (Pierce). Densitometry was performed using ImageLab software. Lanes were detected manually and bands were detected using the “high sensitivity” detection limit. Lane-based background subtraction was applied using the rolling disk method utilizing a hypothetical disk with a radius of 10 mm to trace the background profile of each lane. Statistical significance (via two-way ANOVA and Tukey post hoc analysis) was calculated using an average of three or more independent blots using samples from different animals (n = 3, p < 0.05). β-Actin measurements were acquired after probing for the protein of interest by stripping blots with 6 m GnHCl, 0.2% Triton 100, 20 mm Tris-HCl pH 7.5 twice at room temperature, followed by 3 washes with TBSTr. To confirm the presence of ERβ on the two-dimensional gels, samples were labeled (Cy3) and resolved and visualized on a two-dimensional gel as described previously. Then a portion of the gel narrowed for the molecular weight and isoelectric range of ERβ (molecular weight ∼ 55 kDa, pI ∼ 8.8) was transferred onto a PVDF membrane. The membrane was imaged as described previously and then probed with 1° anti-ERβ antibody (sc-8974x) and secondary goat α-rabbit-Cy5 (GE Healthcare, PA-45011V) and imaged accordingly (supplemental Fig. S2A).
Gelsolin Knock-down and Reporter Assays
Gelsolin expression was knocked down in HEK 293T cells by transient transfection using 50 nm or 100 nm specific siRNA (or control scrambled siRNA) with Fugene 9 transfection reagent (Roche). Notably, the 100 nm concentration also significantly reduced β-actin; therefore a 50 nm concentration was used for reporter assay analysis. Briefly, cells were grown to 60% confluence and transfected with Fugene 9:GELS siRNA (3:1 ratio) and grown for 24 h prior to a second transient transfection with 150 ng rERβ1, ERE-tk-luc/AP-1-tk-luc and 5 ng Renilla-luc control promoter constructs. Cells were allowed to grow in the presence of estrogens (10% FBS) for 36 h before being assayed for luciferase activity using the Dual Luciferase Reporter Assay system (Promega, Madison, WI) as described previously (19). All constructs were transfected in replicates of six wells within each assay, and each transfection assay was repeated in a minimum of three independent experiments.
RESULTS
Global Quantification of ERβ Protein Associations as a Function of Age and E2
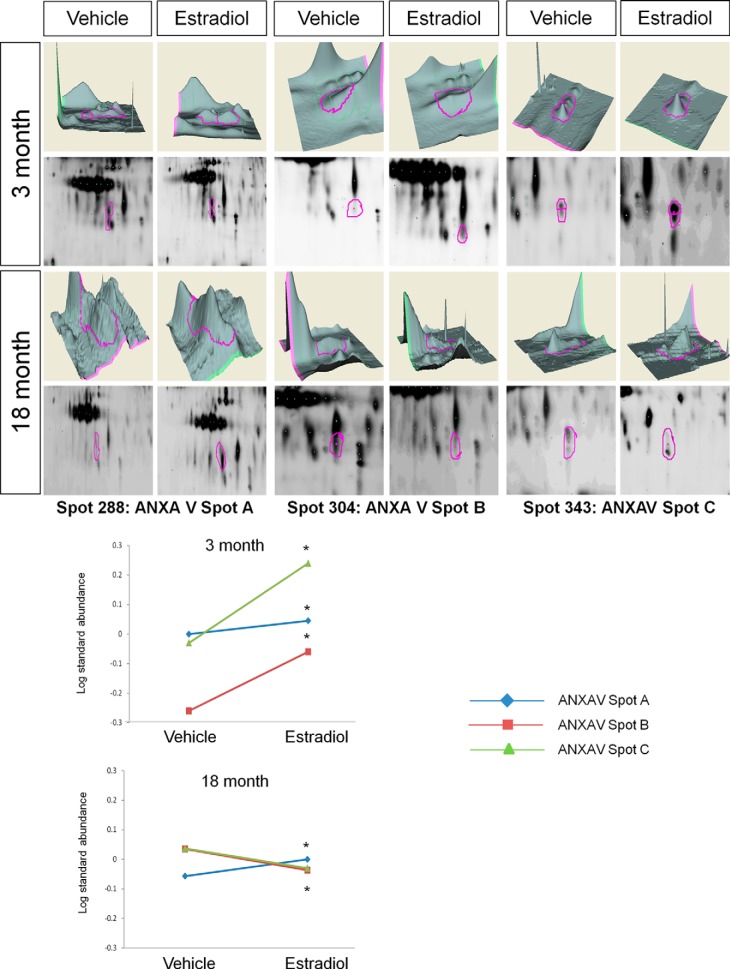
To determine global changes in protein spots co-immunoprecipitated with ERβ from the ventral hippocampus, proteins were subjected to 2D-DIGE and the protein spot patterns were analyzed using DeCyder software (GE Healthcare). There were a total of 19 protein spots that were significantly altered with E2 among all young and aged replicates examined (19/743), equivalent to 2.56% of the total protein spots examined in this paradigm. Notably, E2 treatment significantly altered ERβ association with all 19 protein spots in the young animals, yet only 5 of those 19 spots were changed in aged animals (Table I). Quantification of each age group demonstrated that E2 treatment significantly increased the log standard abundance of 7 protein spots and decreased the log standard abundance of 12 protein spots in young animals (YV to YE, Table I, Fig. 1). Interestingly, E2 treatment in aged animals failed to significantly alter the log standard abundance of the majority of proteins that were co-immunoprecipitated with ERβ. In stark contrast to the findings for young animals, E2 treatment increased only three spots in aged animals and decreased only two spots (AV to AE, Table I, Fig. 1). From the 19 spots that were altered by E2, the identities of 17 protein spots were determined using LC-ESI-MS/MS (Fig. 2A). Moreover, BVA spot 288, identified in a cluster of spots as Annexin V, was the only protein spot that E2 affected similarly (increased) in both young and old animals (Table I).
Fig. 1.
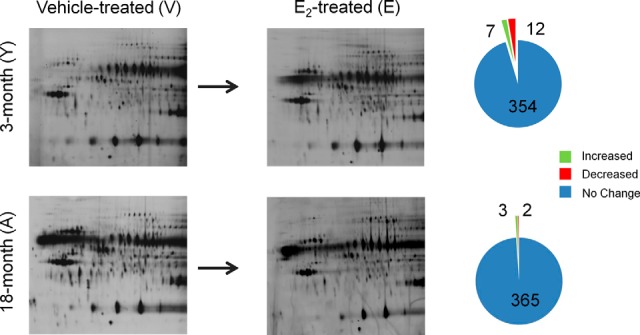
Summary of age- and E2-related global changes in ERβ–protein interactions in the rat ventral hippocampus. Representation of protein spots from each group that were significantly increased (green) or decreased (red) from vehicle control samples compared with a pooled internal standard using DeCyder Analysis software. (YV, young (3-month-old) vehicle (safflower oil)-treated animals; YE, young (3-month-old) E2-treated animals; AV, aged (18-month-old) vehicle (safflower oil)-treated animals; AE, aged (18-month-old) E2-treated animals; n = 3 for each group). Horizontal dimension = isoelectric focusing range; vertical dimension = molecular weight.
Fig. 2.
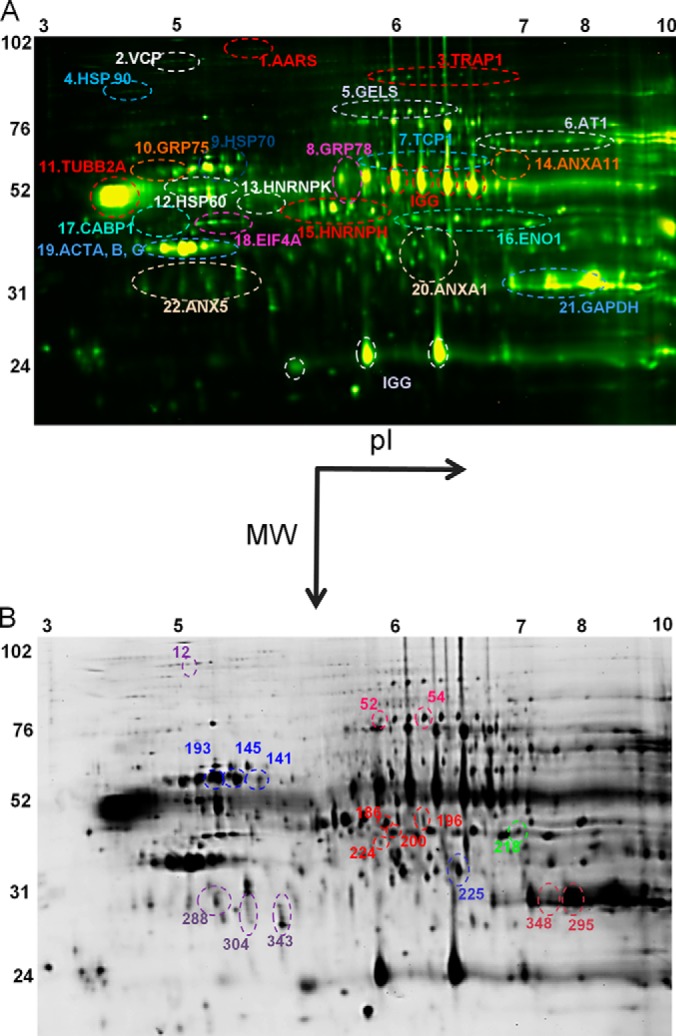
Representation of identified ERβ-interaction partners from rat ventral hippocampus. A, representative two-dimensional image of Cy-labeled proteins (and corresponding BVA spot numbers) co-immunoprecipitated with ERβ identified by tandem mass spectrometry (PEAKS score > 60) from rat ventral hippocampus. Horizontal dimension = isoelectric focusing range; vertical dimension = molecular weight. B, representative two-dimensional image of Cy-labeled proteins co-immunoprecipitated with ERβ that were significantly altered by age or E2 treatment from rat ventral hippocampus (n = 3, one-way ANOVA, p < 0.05).
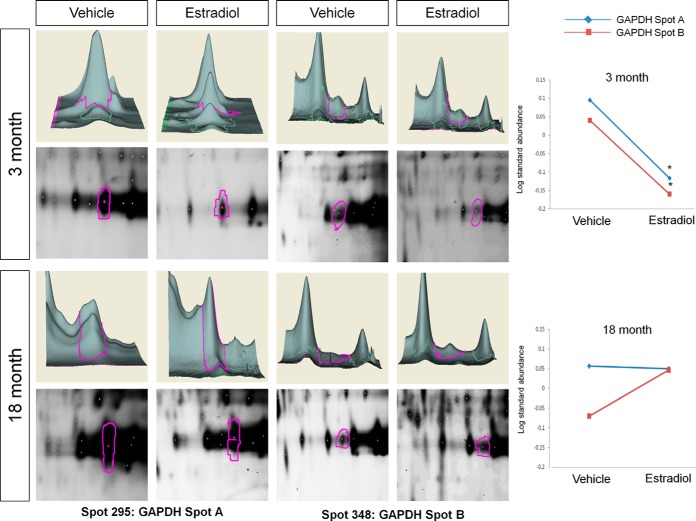
Because of the nature of quantitative 2D-DIGE experiments using scarce samples from in vivo experiments, only two groups can be reliably compared in any given gel (i.e. YV versus YE); therefore we performed a second set of experiments comparing vehicle-only-treated samples from young and aged animals. This analysis was performed to establish whether aging alone, in the absence of E2, altered the baseline of the identified protein interactions. From the 19 proteins significantly altered by E2 treatment in young and/or aged animals, only 1 (BVA spot 295 (GAPDH spot A); data not shown) was significantly changed by age alone. The log standard abundance of BVA spot 295 GAPDH spot A was significantly decreased in AV animals relative to young, suggesting that the baseline interaction between ERβ and GAPDH might decrease with age, regardless of E2 bioavailability.
As previously mentioned, only 2.56% of all the proteins that co-immunoprecipitated with ERβ in this paradigm were altered by E2 treatment dependent on age. Proteins that were not significantly altered by E2 fell into the same functional categories as those that were changed, including chaperone proteins, structural proteins, coactivators, DNA/RNA binding proteins, and multifunctional proteins (Table II, Fig. 2A).
Protein Identification and Analysis of ERβ-associated Proteins
In humans, E2 administration has dichotomous effects dependent upon age and/or menopausal status; however, there is little biochemical evidence to explain this phenomenon. Our results showed that E2 treatment differentially altered ERβ protein–protein interactions in young versus aged animals, providing evidence of a putative mechanism for age-dependent effects of E2. We identified several clusters of protein spots based on their shared isoelectric point, molecular weight, and migration pattern (Fig. 2A). Some spot clusters were pooled into a single sample for protein identification using LC-ESI-MS/MS, and the proteins with the highest PEAKS scores (>60), matching molecular weight, and isoelectric range were identified as representing the entire cluster. A protein may be seen as a horizontal chain of spots due to slight changes in charge through modifications to the protein (i.e. amidation, carbamylation, glycosylation, etc.). Also, vertical chains of protein can be the result of molecular weight modifications (i.e. cleavage, ubiquitination, etc.) that do not alter the overall charge or isoelectric point. However, results obtained were confirmed by subjecting replicate spots within a cluster for peptide identification, and all spots within a cluster were identified as the same protein. Finally, these clusters have been functionally grouped into those that had an overall increase or overall decrease in log standard abundance following E2 treatment in young animals.
Spot Clusters That Increased in E2-treated Young Animals
Annexin V (ANXAV) was identified as the most highly abundant protein in a cluster of three spots that changed following E2 treatment. Further, E2 treatment had quantitatively distinct effects on the log standard abundance of each spot within this cluster (Fig. 3, Table I). For instance, each spot increased following E2 treatment in young animals (Table I, BVA spots 288 (spot A), 304 (spot B), and 343 (spot C); Fig. 3). However, E2 treatment in aged animals increased the log standard abundance of spot A, decreased spot B, and had no significant effect on spot C (Table I, Fig. 3).
Fig. 3.
DeCyder topography, gel image analysis, and average log standard abundance of annexin V (ANXAV) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
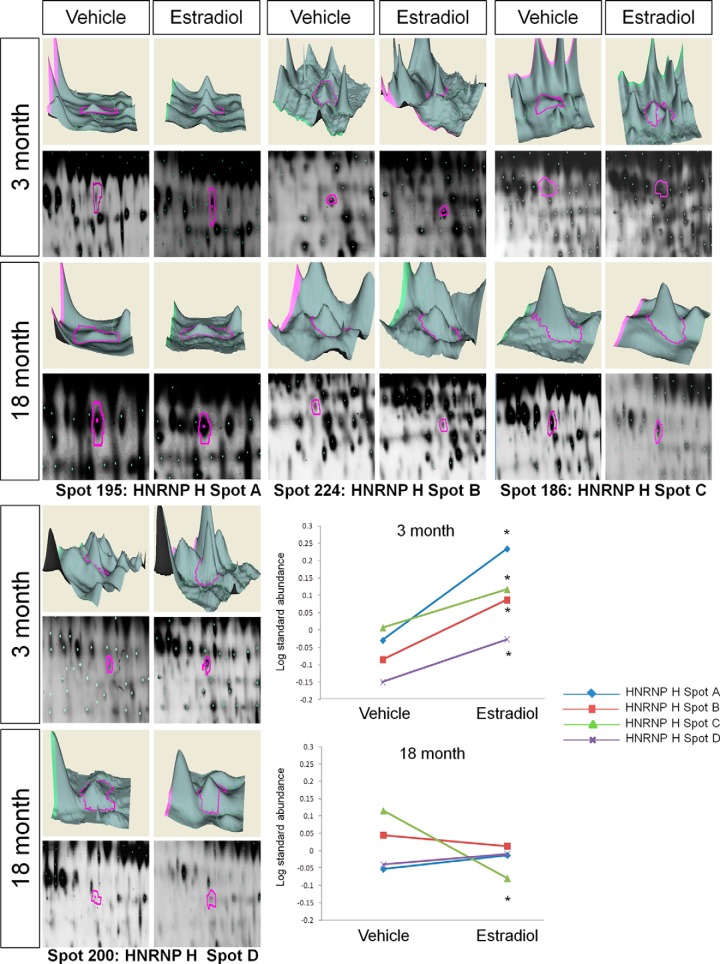
Like with the ANXAV cluster, E2 treatment significantly increased ERβ association with all four spots identified as HnRNP H in young animals (Table I, BVA spots 195 (spot A), 224 (spot B), 186 (spot C), and 200 (spot D); Fig. 4). By contrast, E2 treatment did not increase any of these spots in the aged animals; spot C was significantly decreased, and the other three were unaffected (Table I, Fig. 4).
Fig. 4.
DeCyder topography, gel image analysis, and average log standard abundance of heteronuclear riboprotein H (HnRNP H) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
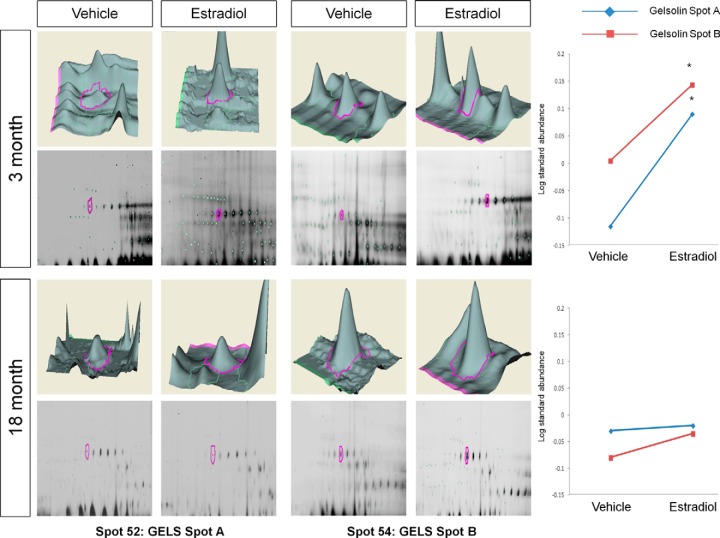
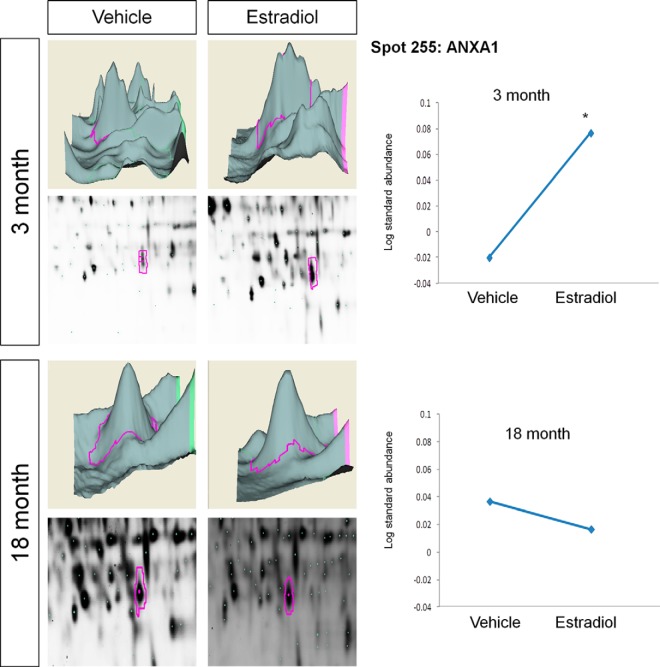
Young animals treated with E2 had a significant increase in two spots identified as a cluster of gelsolin proteins (GELS) (Table I, BVA spots 52 (spot A) and 54 (spot B); Fig. 5), yet E2 had no effect in aged animals. Notably, in this cluster GELS was the only predominant peptide match in the group of spots selected that corresponded to its approximate isoelectric point and size. Moreover, this spot cluster was split into three samples and GELS was the only protein identified, and it was observed in all three samples (spots 52, 54, and 56 (not significantly changed by E2)), despite a PEAKS score less than 60 (pick spot 5, PEAKS score of 49.5). Similar to the results from GELS, the log standard abundance of ANXA1 (Table I, BVA spot 225; Fig. 6) was significantly increased by E2 in young animals but was not significantly altered by E2 in aged animals. Taken together, these data suggest that E2 may enhance some ERβ–protein interactions in young animals but has an opposite or no effect on the same interaction in older animals.
Fig. 5.
DeCyder topography, gel image analysis, and average log standard abundance of gelsolin (GELS) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
Fig. 6.
DeCyder topography, gel image analysis, and average log standard abundance of annexin 1 (ANXA1) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
Spot Clusters That Decreased in E2-treated Young Animals
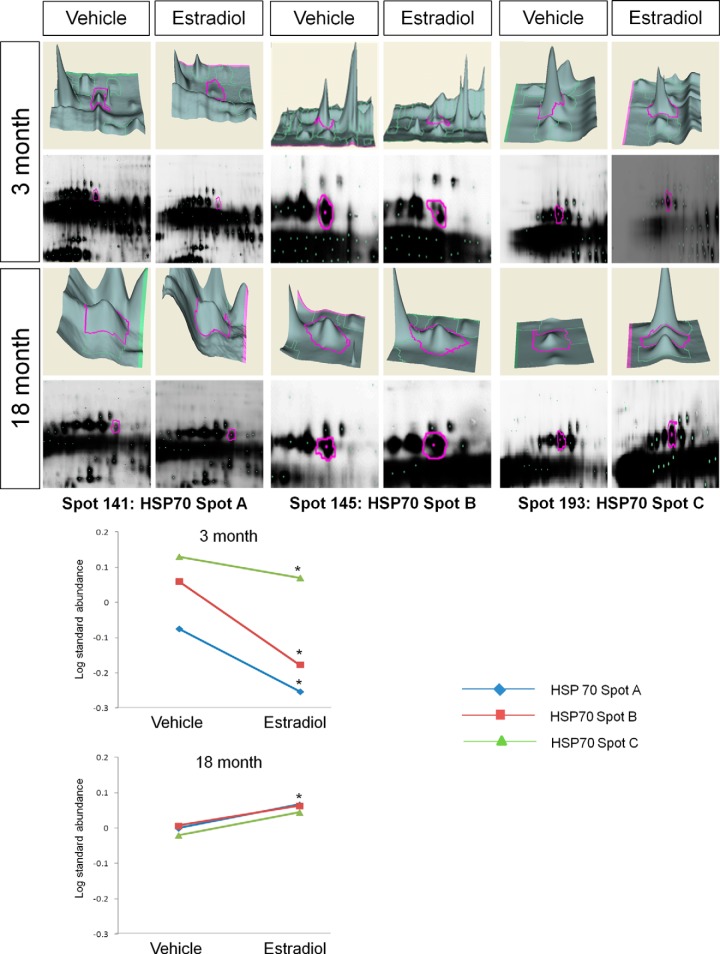
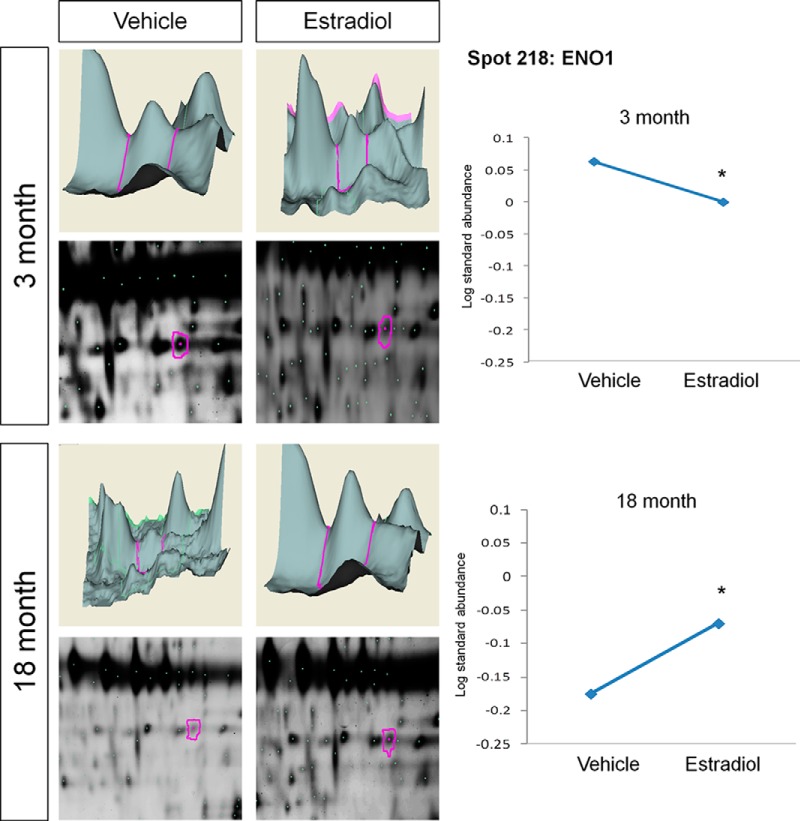
In addition to E2-induced increases in ERβ protein associations, there were also proteins that showed a significantly decreased log standard abundance with ERβ following E2 treatment. For example, BVA spots 141 (HSP70 spot A), 145 (HSP70 spot B), and 193 (HSP70 spot C) (Table I; Fig. 7), corresponding to the spot cluster identified as HSP70, were all significantly decreased with E2 treatment in young animals, yet the opposite effect was observed in aged animals for spot C, which was significantly increased (Fig. 7). HSP70 spots A and B remained unchanged following E2 treatment in aged animals. Exhibiting a similar pattern, the BVA spot identified as α-enolase (ENO1) (Table I, BVA spot 218) was also significantly decreased with ERβ in response to E2 treatment in young animals, and like HSP70 it associated more with ERβ in aged animals treated with E2 (Table I, Fig. 8).
Fig. 7.
DeCyder topography, gel image analysis, and average log standard abundance of heat shock protein 70 (HSP70) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
Fig. 8.
DeCyder topography, gel image analysis, and average log standard abundance of α-enolase (ENO1) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
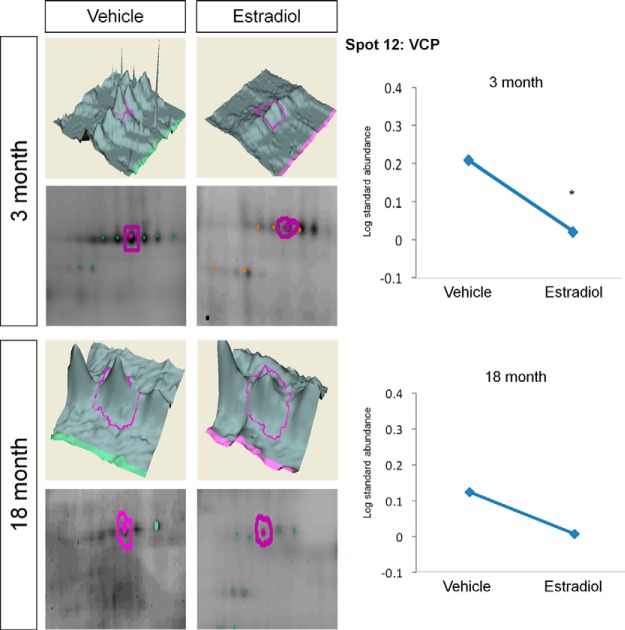
The commonly considered housekeeping protein glyceraldehyde-3-phosphate (GAPDH) was also found to be associated with ERβ in both young and aged animals. E2 treatment significantly decreased GAPDH association in young but not aged animals (Table I, Fig. 9). Alternatively, BVA spot 12, found in the group of spots identified as valosin containing protein (VCP/p97), was significantly decreased with E2 treatment in the young animals and also tended to decrease with E2 treatment in the aged animals (Table I, Fig. 10). In order to further validate the quantitative changes observed using 2D-DIGE and DeCyder analysis, we performed Western blot analysis on ERβ co-immunoprecipitated samples used for 2D-DIGE. As expected, VCP was decreased with E2 in young animals and had a tendency to decrease with E2 treatment in aged animals (Fig. 11A), confirming the sensitivity and accuracy of the 2D-DIGE system when employed with LC-ESI-MS/MS. Further reverse co-immunoprecipitation in ERβ-expressing neuronal cells confirmed novel interactions between ERβ and GAPDH/GELS/VCP in the presence and absence of E2 (supplemental Fig. S1).
Fig. 9.
DeCyder topography, gel image analysis, and average log standard abundance of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in response to E2 in young and aged animals. For each panel from top left to right, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
Fig. 10.
DeCyder topography, gel image analysis, and average log standard abundance of valosin containing protein/p97 (VCP) in response to E2 in young and aged animals. For each panel from top right to left, 3 month: YV representative topography, YE representative topography, YV representative gel image, and YE representative gel image. 18 month: AV representative topography, AE representative topography, AV representative gel image, and AE representative gel image. Graph depicts log transformed average abundance normalized to internal standard and matched to master gel. Average calculated from three independent experiments with a biological variance of four pooled animals per experiment (n = 3, BV = 12). *Significance from vehicle, p < 0.05.
Fig. 11.
Confirmation of ERβ–VCP interaction and subcellular expression of ERβ-interaction partners. A, confirmation of ERβ–VCP interaction in ventral hippocampus by immunoblot with corresponding input and nonspecific IgG control. B, representative immunoblots for nuclear and cytosolic ERβ, HSP70, GAPDH, VCP, HNRNP H, and ENO1 expression in ventral hippocampus normalized to β-actin. C, quantification of densitometric analysis of protein expression calculated from at least three independent experiments (n = 3). *Significant difference between groups (two-way ANOVA, p < 0.05).
Finally, E2 treatment in young animals significantly decreased two protein spots (Table I, BVA spots 79 and 351; supplemental Fig. S3) that were in the vicinity of the GELS cluster but were unable to be identified. These same spots were not significantly altered by E2 in aged animals (Table I, supplemental Fig. S3).
Quantification of the Effects of Age and E2 on Protein Expression
One possible explanation for changes in protein–protein interaction could be a corresponding change in relative protein expression levels of the partners in question. Therefore, we analyzed the subcellular expression levels of some of the proteins of particular interest that were identified as ERβ-interacting proteins in the ventral hippocampus. We selected VCP and GAPDH for their potential role in apoptosis and disease, HSP70 because it is known to interact with estrogen receptors, ENO1 as a novel estrogen receptor interaction partner, and HnRNP H because of its role in alternative splicing, a process known to increase with age. Further, it is also possible that a change in nuclear/cytosolic shuttling could account for a change in protein associations; therefore the cytosolic fractions were examined as well.
First, we analyzed the expression levels of ERβ in the ventral hippocampus. Some studies have reported age-related changes in ERβ expression; however, the reports are inconsistent and dependent on brain region (20–24). Our results showed no statistical difference in ERβ protein expression analyzed using a two-way ANOVA (Figs. 11B and 11C). HSP70 levels followed a similar pattern, but again there were no significant changes in HSP70 cytosolic or nuclear expression. Interestingly, VCP was the only protein that showed a statistically significant increase as a main effect of age in 18-month-old animals (Figs. 11B and 11C; F(1,8) = 0.0237, p < 0.05), but there was no effect of E2 and no statistically significant interaction. Interestingly, this change in expression did not correlate with the interaction observed via 2D-DIGE between ERβ and VCP in aged animals, which showed a trend toward decreased association with ERβ (Table I, Fig. 8). There was also a trend toward increased ENO1 expression in E2-treated aged animals, which corresponded to an observed increase in ERβ–ENO1 interaction (Figs. 8, 11B, and 11C). GAPDH and HnRNP H expression levels were unchanged by age and treatment. From these data, we can conclude that relative protein expression levels are not solely responsible for changes in ERβ–protein interactions in vivo.
Transcriptional Effect of Gelsolin Knock-down on ERβ-mediated Promoter Activity
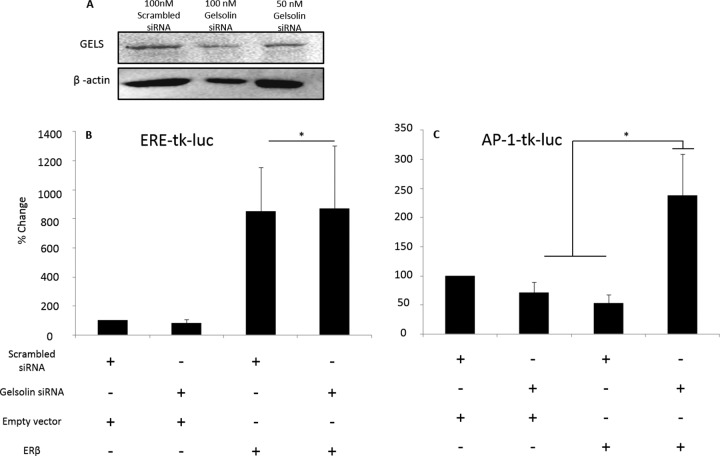
Dynamic protein–protein interactions are critical for cellular functions. In the nucleus, ERβ is well characterized as a transcription factor that regulates gene promoters by binding to specific enhancer elements. Gelsolin has recently been described as a transcriptional enhancer for nuclear receptors including ERα, but not ERβ, at an estrogen response element (ERE)-mediated minimal promoter (25). To test the functional consequence of a disruption in ERβ–gelsolin interactions, we used siRNA to knock down gelsolin in a neuronal-derived ERβ-expressing cell line. Importantly, 100 nm gelsolin siRNA reduced β-actin expression; therefore a lower concentration of siRNA was used (50 nm; Fig. 12A). Our results showed that gelsolin knockdown (50 nm) abolished ERβ-induced repression of an activator protein 1 (AP-1)-mediated promoter (Fig. 12C), but not an ERE-mediated promoter.
Fig. 12.
Effects of siRNA knock-down of gelsolin on ERβ-mediated AP-1 and ERE promoter activity. (A) HEK293T cells were transiently transfected with 50 or 100 nm gelsolin siRNA or scrambled siRNA followed by cotransfection with an expression vector containing rERβ1 (150 ng) and (B) 150 ng tk-ERE-Firefly-luciferase or (C) tk-AP1-Firefly-luciferase reporter constructs plus 5 ng Renilla-luciferase control. Data represent the percentage change in the dual luciferase ratio of relative light units (ERE or AP-1:Renilla). *Significance from empty vector control, p < 0.05.
DISCUSSION
The evidence presented herein lends support for the hypothesis that there is an intrinsic change in ERβ function upon the reintroduction of E2 with advanced age. First, we provide evidence that shows novel age- and E2-dependent interactions between ERβ and ANXA1, ANXAV, GAPDH, VCP, GELS, ENO1, and HnRNPs. Importantly, these changes do not appear to be a consequence of subcellular localization or relative protein expression levels. Second, we confirmed and identified several known and novel ERβ–protein interaction partners, and we quantified changes in these interactions as a function of advanced age and E2 treatment. Finally, these studies are the first to broadly characterize changes in ERβ protein interactions in vivo in the ventral hippocampus.
The most compelling data from these analyses are the observed changes in dynamic E2-induced ERβ protein associations with age. This is the first study to provide evidence that suggests changes in overall ERβ function as a molecular mechanism to explain dichotomous effects of estrogens as a consequence of age. Importantly, only 5 of the 19 ERβ protein interactions were altered by E2 in aged animals, which contrasted markedly with the fact that all 19 ERβ protein interactions were affected in young animals. Overall, a very small percentage (2.56%) of the confirmed protein spots changed significantly with age and E2, attesting to the specificity of these results. These data also support the hypothesis that ERβ signaling in response to E2 is drastically altered by age, as the expression levels of these proteins did not significantly correlate with changes in protein–protein interaction. In this study there were no significant changes in ERβ protein levels with age or E2 treatment, and this is consistent with a similar report that recently demonstrated no age or E2 deprivation effects on ERβ expression (24). E2 deprivation or replacement decreased ERβ expression in 24-month-old but not 18-month-old rats (20–24). Other studies demonstrated E2-mediated decreases in ERβ expression, but these reports are variable depending upon the end point (mRNA versus protein) and brain region examined. Nevertheless, our results from the ventral hippocampus clearly demonstrate that E2 significantly altered ERβ protein–protein interactions in an age-dependent manner.
The role of E2 in neuroprotection has been under investigation for some time, but delineating the exact actions that lead to a protective outcome has been difficult. The prevailing hypothesis in the field is one of a “healthy cell bias” in which the actions of E2 are protective prior to insult. E2 can be pro-apoptotic or anti-apoptotic depending upon the cellular context and estrogen receptor subtype. ERβ has been characterized as predominantly antiproliferative and pro-apoptotic in cancer models, in contrast to the proliferative and anti-apoptotic role of ERα. However, in the brain, both receptors demonstrate protection against various neurological insults such as ischemia and glutamate toxicity (26, 27). E2 can induce anti-apoptotic factors such as Bcl-2 (28–31), and overexpression of Bcl-2 can induce nuclear localization of factors such as ANXA1 (32). Here we not only identified an increased interaction between ANXA1 and ERβ with E2 administration, but we also demonstrated that this interaction was no longer affected by E2 in aged animals.
Similarly, we showed that ANXAV interactions with ERβ were also changed with age, and ANXAV could play a role in neuroprotection. The samples that we obtained from the ventral hippocampus represent a diverse and heterogeneous population of cells including supporting glial cells that can senesce. Senescence in the brain has been postulated to contribute to pathological states such as Alzheimer disease, and E2 has been shown to protect against both senescence and dementia (35–40). Translocation of ANXAV to the nucleus by various factors (33) has been thought to predict cellular senescence (34). It is not clear how E2 could protect against senescence, but we speculate that the interaction between ERβ and ANXAV could contribute to the role of E2 in senescence. Annexins including ANXA11, which was associated with ERβ but unaffected by age or E2 (Table II), have been reported to localize to the nuclear envelope and may associate with microtubules at the nuclear membrane and assist in nuclear breakdown (41). This event could potentially explain the presence of structural proteins pulled down in our results. Interestingly, E2 can also modulate the expression of members of the annexin family (42, 43), further supporting interplay between annexins and estrogen receptors. Taken together, these results suggest that protective aspects of E2 signaling could be mediated through estrogen receptor–ANXA interactions demonstrated here and in other reports (12, 44, 45), and unique to this study is an age-related change in some of these associations with ERβ.
Like annexins, the multifunctional protein GAPDH is not typically considered a nuclear protein, but the data from this and other studies suggest that there is a complex relationship connecting GAPDH, E2, and estrogen receptors. Our results showed that ERβ–GAPDH interaction decreased following E2 administration in young animals and was unaffected by E2 in aged animals. Moreover, the ERβ–GAPDH interaction was the only protein interaction that was decreased by age alone. Not only do these data indicate that there is likely a decrease in the amount of ERβ–GAPDH interaction in aged animals, but they also demonstrate that E2 is ineffective for altering this interaction in aged animals. In the initial study that determined GAPDH was an AR coactivator, GAPDH did not enhance the transcriptional activity of ERα or GR at their respective response elements (46), but it may still bind non-chromatin bound ERβ when it is translocated to the nucleus (47, 48). S-nitrosylation of GAPDH initiates apoptosis by translocating to the nucleus and interacting with Siah1 (an E3-ubiquitin ligase), also known as BAG-1. BAG-1 has been shown to interact with ERα and facilitate the down-regulation of estrogen receptors over extended periods of E2 deprivation (24). Overall, the role for a nuclear interaction between ERβ and GAPDH is not yet clear, but a change in this interaction could dysregulate the balance between E2 neuroprotection and apoptosis in aged animals.
The possibility of S-nitrosylated GAPDH and ERβ interactions underscores the probability that posttranslational modifications contributed to changes in the observed interactions in this study and warrants further investigation. Interestingly, the ERβ-selective agonist diarylproprionitrile induces S-nitrosylation proteins as a cardioprotective mechanism in the heart (49). Loss of S-nitrosylated proteins with age could ostensibly contribute to a loss of cardioprotective effects of E2 in older patients (50). Moreover, S-nitrosylation of interaction partners mediated through ERβ could result in the characteristic “chain” patterns observed in the 2D-DIGE experiments. Other possible explanations for the chain patterns are modifications to protein charge such as carbamylation of proteins, which can occur in urea-based buffers, or phosphorylation (51). Protein modifiers such as p38 and SUMO are reported to affect ERβ signaling and change with age (52–54); thus it is possible that modifications to ERβ or its interaction partners by these types of proteins could contribute to the observed effects.
Another novel finding from these results was the observed increase in nuclear VCP protein levels with age and the age-related changes in ERβ–VCP interactions. Similar to reported interactions between estrogen receptor associated protein 140 and ERβ, there was a trend toward decreased VCP–ERβ interaction with age, yet VCP nuclear expression paradoxically increased significantly as a factor of age. VCP is an AAA+ class of ATPase that has been recently implicated in diseases where polyglutamine-mediated protein accumulation is observed (55), but the mechanisms involving VCP in these diseases have yet to be elucidated. In some instances, VCP has been shown to interact with polyglutamine tract proteins in the nucleus, potentially mediating the aggregation of polyglutamine aggregates (55). VCP can interact with nuclear receptor transcriptional complexes and suppress transcription (56), but the exact function of VCP within a nonpathological nuclear protein complex is unknown (57). Although the interaction between ERβ and VCP has not been fully characterized, the neuroprotective role of E2 and the potential role of VCP in neurodegenerative diseases amount to an intriguing correlation suggesting that changes in ERβ–VCP interactions with age might have significant functional consequences. Notably, nuclear ataxin-1 (also identified as an ERβ interaction partner in this study) has been implicated in polyglutamine-induced diseases (58), but this interaction was unaltered by age or E2 treatment. Our data are consistent with another report that showed an interaction between VCP and ERα when ERα was bound to a 9x-ERE in vitro (12); however, this is the first report demonstrating an age-related change in interactions between VCP and ERβ.
The data presented herein suggest that the isolated ERβ fraction may be part of non-DNA bound complexes that can direct transcription. We observed an age-related change in the association of the actin binding protein gelsolin and ERβ. Gelsolin, also a known steroid hormone coregulator, enhances ERα-mediated transcription (25) but, prior to this study, had not been shown to alter ERβ-mediated transcription. We observed that E2 increased the ERβ–GELS interaction in young animals, but there was no significant change in this interaction in aged animals (Fig. 3C). Importantly, using reporter-gene assays we demonstrated that knocking down gelsolin abolished ERβ-induced repression of an AP-1-mediated promoter. However, the loss of gelsolin did not interrupt the activation of an ERE-mediated promoter, further validating published reports (25). Gelsolin–ERβ interactions may be important for maneuvering ERβ to other nuclear protein complexes, such as AP-1-associated proteins, to facilitate transcription. Further, these data suggest that when ERβ–gelsolin interactions change with age, E2 modulation of AP-1-mediated transcriptional activity will be changed as well.
We also identified a clear association between ERβ and the structural protein actin, but this interaction was not altered by age or E2. Importantly, an interaction between estrogen receptors and actin has been demonstrated by others (12, 44, 45). In fact, actin has an essential transcriptional role in the nucleus (60–62). Actin serves as a scaffold to assist in gene regulation and also is a key component in nuclear export, as suggested by the leucine-rich nuclear export signal within actin that has aided in nuclear export of viral RNA (63, 64). Thus, we hypothesize that the changes in interactions between ERβ and actin-associated proteins such as GELS and ENO1, which has been shown to modulate DNA methyltransferase (65), could be a result of changes in nuclear actin structure (59).
Other actin-bound nuclear proteins include the family of HnRNPs, which cooperate with actin to influence mRNA processing and splicing, and in this study we demonstrated through co-immunoprecipitation that ERβ might have a role in these processes. HnRNPs are molecular determinants of all facets of mRNA processing including alternative splicing (66). Other HnRNPs have been shown to associate with ERα, but this study is the first to report an interaction between ERβ and HnRNP H (66, 67). In this report the ERβ–HnRNP H interaction was enhanced by E2 in young animals but was decreased or unchanged by E2 in aged animals, suggesting that in aged animals the influence of E2 over the actions of an ERβ–HnRNP H complex might be altered. Estrogen receptors have been shown to participate on some level in miRNA processing (68, 69) and mRNA splicing (13), and evidence suggests that aging may lead to a global increase in alternative splicing (70). Recent findings demonstrate that ERβ is differentially spliced depending upon age and E2 treatment (71). Alternative splice variant expression could contribute to the observed changes in protein–protein interactions, as the antibodies used in these studies targeted the N terminus of ERβ but the majority of alternative splice variants identified contain C termini modifications that would not have been differentiated by these antibodies (72). Further investigation into ERβ–HnRNP interactions could help to explain E2- and age-related changes in alternative splicing.
Our intentional exclusion of chromatin and our selection of protein spots on the two-dimensional gel that were limited to those common among three separate antibodies against ERβ was a highly conservative approach. Therefore, this approach likely excluded a number of putative interaction partners for ERβ that we were unable to characterize. Moreover, changes in individual cellular populations (e.g. pyramidal CA1, CA3, interneuron, etc.) might be obscured when the entire ventral hippocampus is examined as in this study; however, the whole region was used to (a) obtain enough protein for analytical and preparative gels, Western blotting confirmation, and expression analysis and (b) gain a broad view of nuclear proteins associated with ERβ in vivo. Another exclusionary factor comes from the antibody selected for ERβ co-immunoprecipitation; the chosen antibody was selected because following pull-down, a conservative number of protein spots were visible after co-immunoprecipitation. Spots that did not exhibit significant overlap among the three antibodies tested were excluded from analysis in order to avoid false-positive interactions. However, this conservative approach might have precluded the discovery of additional ERβ–protein interaction partners, suggesting that the identified proteins in this study represent only a subset of ERβ-associated proteins. It is also important to note that the interactions described in this dataset may be direct or indirect and warrant further investigation.
The data presented here fill a knowledge gap in the field regarding (a) protein interactions with ERβ in the ventral hippocampus and (b) a possible mechanistic explanation for changes in E2-mediated processes in aged individuals. Notably, nuclear hormone receptors have been shown to associate with upward of 10,000 “coregulatory” proteins (73), yet the complete ERβ interactome has not been described. The data herein contribute to our understanding of the ERβ interactome in the brain and also quantify ERβ–protein interactions as a function of age. The protein interactions with ERβ described in this manuscript represent a novel fraction of proteins that may serve to supplement the existing role of ERβ in mediating gene expression and possibly neuroprotection in the hippocampus. Taken together, these novel ERβ–protein interactions require further in-depth study in order for the scope of ERβ functions to be fully elucidated. Moreover, determining the functional consequences of these changes with age and hormone replacement is essential in order for the neurological costs and benefits of hormone therapy to be determined.
Supplementary Material
Acknowledgments
We thank Dr. Keith Philibert for preparing samples for mass spectrometry and for his careful review of this manuscript. We also thank Dr. Jan Ake Gustafsson for the generous gift of the anti-ERβ antibody (ligand-binding domain, LBD) used for comparison in these studies.
Footnotes
Author contributions: N.N.M., M.J.G., J.L.R., and T.R.P. designed research; N.N.M., E.P., Y.S.R., M.M.P., S.A.P., C.L.S., and X.Y. performed research; N.N.M. and M.J.G. contributed new reagents or analytic tools; N.N.M., E.P., Y.S.R., X.Y., M.J.G., J.L.R., and T.R.P. analyzed data; N.N.M. and T.R.P. wrote the paper.
* This work was funded by Grant Nos. NIH R01AG033605, NIH F31 AG039931, and NIH OD010662.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- ERβ
- estrogen receptor β
- 2D-DIGE
- two-dimensional difference gel electrophoresis
- BVA
- Biological Variance Analysis
- YV
- young vehicle-treated
- YE
- young estradiol-treated
- AV
- aged vehicle-treated
- AE
- aged estradiol-treated
- ANOVA
- analysis of variance
- ACN
- acetonitrile
- ANXA
- annexin
- ENO1
- α-enolase
- ERα
- estrogen receptor α
- E2
- 17β-estradiol
- ERE
- estrogen response element
- AP-1
- activator protein 1
- GAPDH
- glyceraldehyde-3-phosphate
- GELS
- gelsolin proteins
- HNRNP
- heterogeneous nuclear riboprotein
- LC-ESI-MS/MS
- liquid chromatography–electrospray ionization–tandem mass spectrometry
- VCP
- valosin containing protein.
REFERENCES
- 1. Shumaker S. A., Legault C., Kuller L., Rapp S. R., Thal L., Lane D. S., Fillit H., Stefanick M. L., Hendrix S. L., Lewis C. E., Masaki K., Coker L. H. (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291, 2947–2958 [DOI] [PubMed] [Google Scholar]
- 2. Chlebowski R. T., Anderson G. L., Gass M., Lane D. S., Aragaki A. K., Kuller L. H., Manson J. E., Stefanick M. L., Ockene J., Sarto G. E., Johnson K. C., Wactawski-Wende J., Ravdin P. M., Schenken R., Hendrix S. L., Rajkovic A., Rohan T. E., Yasmeen S., Prentice R. L. (2010) Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 304, 1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossouw J. E., Prentice R. L., Manson J. E., Wu L., Barad D., Barnabei V. M., Ko M., LaCroix A. Z., Margolis K. L., Stefanick M. L. (2007) Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297, 1465–1477 [DOI] [PubMed] [Google Scholar]
- 4. Hogervorst E., Bandelow S. (2010) Sex steroids to maintain cognitive function in women after the menopause: a meta-analyses of treatment trials. Maturitas 66, 56–71 [DOI] [PubMed] [Google Scholar]
- 5. Greco B., Allegretto E. A., Tetel M. J., Blaustein J. D. (2001) Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology 142, 5172–5181 [DOI] [PubMed] [Google Scholar]
- 6. Paramanik V., Thakur M. K. (2010) Interaction of estrogen receptor associated protein (ERAP) 140 with ER beta decreases but its expression increases in aging mouse cerebral cortex. Cell. Mol. Neurobiol. 30, 961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shao W., Halachmi S., Brown M. (2002) ERAP140, a conserved tissue-specific nuclear receptor coactivator. Mol. Cell. Biol. 22, 3358–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan L., Zhao Z., Orr P. T., Chambers C. H., Lewis M. C., Frick K. M. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci. 30, 4390–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fanselow M. S., Dong H. W. (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hampson R. E., Simeral J. D., Deadwyler S. A. (1999) Distribution of spatial and nonspatial information in dorsal hippocampus. Nature 402, 610–614 [DOI] [PubMed] [Google Scholar]
- 11. Shumaker S. A., Legault C., Rapp S. R., Thal L., Wallace R. B., Ockene J. K., Hendrix S. L., Jones B. N., 3rd, Assaf A. R., Jackson R. D., Kotchen J. M., Wassertheil-Smoller S., Wactawski-Wende J. (2003) Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289, 2651–2662 [DOI] [PubMed] [Google Scholar]
- 12. Nalvarte I., Schwend T., Gustafsson J. A. (2010) Proteomics analysis of the estrogen receptor alpha receptosome. Mol. Cell. Proteomics 9, 1411–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masuhiro Y., Mezaki Y., Sakari M., Takeyama K., Yoshida T., Inoue K., Yanagisawa J., Hanazawa S., O'Malley B. W., Kato S. (2005) Splicing potentiation by growth factor signals via estrogen receptor phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 102, 8126–8131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ambrosino C., Tarallo R., Bamundo A., Cuomo D., Franci G., Nassa G., Paris O., Ravo M., Giovane A., Zambrano N., Lepikhova T., Janne O. A., Baumann M., Nyman T. A., Cicatiello L., Weisz A. (2010) Identification of a hormone-regulated dynamic nuclear actin network associated with estrogen receptor alpha in human breast cancer cell nuclei. Mol. Cell. Proteomics 9, 1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt G., Andersson S. B., Nordle O., Johansson C. J., Gunnarsson P. O. (1994) Release of 17-beta-oestradiol from a vaginal ring in postmenopausal women: pharmacokinetic evaluation. Gynecol. Obstet. Invest. 38, 253–260 [DOI] [PubMed] [Google Scholar]
- 16. Talboom J. S., Williams B. J., Baxley E. R., West S. G., Bimonte-Nelson H. A. (2008) Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol. Learn. Mem. 90, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banasr M., Soumier A., Hery M., Mocaer E., Daszuta A. (2006) Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol. Psychiatry 59, 1087–1096 [DOI] [PubMed] [Google Scholar]
- 18. Saji S., Jensen E. V., Nilsson S., Rylander T., Warner M., Gustafsson J. A. (2000) Estrogen receptors alpha and beta in the rodent mammary gland. Proc. Natl. Acad. Sci. U.S.A. 97, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mott N. N., Pak T. R. (2012) Characterisation of human oestrogen receptor beta (ERbeta) splice variants in neuronal cells. J. Neuroendocrinol. 24, 1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson M. E., Rosewell K. L., Kashon M. L., Shughrue P. J., Merchenthaler I., Wise P. M. (2002) Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech. Ageing Dev. 123, 593–601 [DOI] [PubMed] [Google Scholar]
- 21. Chakraborty T. R., Ng L., Gore A. C. (2003) Age-related changes in estrogen receptor beta in rat hypothalamus: a quantitative analysis. Endocrinology 144, 4164–4171 [DOI] [PubMed] [Google Scholar]
- 22. Gundlah C., Kohama S. G., Mirkes S. J., Garyfallou V. T., Urbanski H. F., Bethea C. L. (2000) Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res. Mol. Brain Res. 76, 191–204 [DOI] [PubMed] [Google Scholar]
- 23. Sharma P. K., Thakur M. K. (2006) Expression of estrogen receptor (ER) alpha and beta in mouse cerebral cortex: effect of age, sex and gonadal steroids. Neurobiol. Aging 27, 880–887 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Q. G., Han D., Wang R. M., Dong Y., Yang F., Vadlamudi R. K., Brann D. W. (2011) C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc. Natl. Acad. Sci. U.S.A. 108, E617-E624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishimura K., Ting H. J., Harada Y., Tokizane T., Nonomura N., Kang H. Y., Chang H. C., Yeh S., Miyamoto H., Shin M., Aozasa K., Okuyama A., Chang C. (2003) Modulation of androgen receptor transactivation by gelsolin: a newly identified androgen receptor coregulator. Cancer Res. 63, 4888–4894 [PubMed] [Google Scholar]
- 26. Bryant D. N., Dorsa D. M. (2010) Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuroscience 170, 1261–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubal D. B., Rau S. W., Shughrue P. J., Zhu H., Yu J., Cashion A. B., Suzuki S., Gerhold L. M., Bottner M. B., Dubal S. B., Merchanthaler I., Kindy M. S., Wise P. M. (2006) Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology 147, 3076–3084 [DOI] [PubMed] [Google Scholar]
- 28. Frasor J., Danes J. M., Komm B., Chang K. C., Lyttle C. R., Katzenellenbogen B. S. (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144, 4562–4574 [DOI] [PubMed] [Google Scholar]
- 29. Dubal D. B., Shughrue P. J., Wilson M. E., Merchenthaler I., Wise P. M. (1999) Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J. Neurosci. 19, 6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi K. C., Kang S. K., Tai C. J., Auersperg N., Leung P. C. (2001) Estradiol up-regulates antiapoptotic Bcl-2 messenger ribonucleic acid and protein in tumorigenic ovarian surface epithelium cells. Endocrinology 142, 2351–2360 [DOI] [PubMed] [Google Scholar]
- 31. Bynoe M. S., Grimaldi C. M., Diamond B. (2000) Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc. Natl. Acad. Sci. U.S.A. 97, 2703–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishido M. (2005) Overexpression of Bcl-2 inhibits nuclear localization of annexin I during tumor necrosis factor-alpha-mediated apoptosis in porcine renal LLC-PK1 cells. Regul. Pept. 124, 45–51 [DOI] [PubMed] [Google Scholar]
- 33. Imanishi T., Kobayashi K., Hano T., Nishio I. (2005) Effect of estrogen on differentiation and senescence in endothelial progenitor cells derived from bone marrow in spontaneously hypertensive rats. Hypertens. Res. 28, 763–772 [DOI] [PubMed] [Google Scholar]
- 34. Imanishi T., Tsujioka H., Akasaka T. (2010) Endothelial progenitor cell senescence—is there a role for estrogen? Ther. Adv. Cardiovasc. Dis. 4, 55–69 [DOI] [PubMed] [Google Scholar]
- 35. Imanishi T., Hano T., Nishio I. (2005) Estrogen reduces angiotensin II-induced acceleration of senescence in endothelial progenitor cells. Hypertens. Res. 28, 263–271 [DOI] [PubMed] [Google Scholar]
- 36. Imanishi T., Hano T., Nishio I. (2005) Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J. Hypertens. 23, 1699–1706 [DOI] [PubMed] [Google Scholar]
- 37. Bhat R., Crowe E. P., Bitto A., Moh M., Katsetos C. D., Garcia F. U., Johnson F. B., Trojanowski J. Q., Sell C., Torres C. (2012) Astrocyte senescence as a component of Alzheimer's disease. PLoS One 7, e45069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raina A. K., Pardo P., Rottkamp C. A., Zhu X., Pereira-Smith O. M., Smith M. A. (2001) Neurons in Alzheimer disease emerge from senescence. Mech. Ageing Dev. 123, 3–9 [DOI] [PubMed] [Google Scholar]
- 39. Mohiti J., Caswell A. M., Walker J. H. (1997) The nuclear location of annexin V in the human osteosarcoma cell line MG-63 depends on serum factors and tyrosine kinase signaling pathways. Exp. Cell Res. 234, 98–104 [DOI] [PubMed] [Google Scholar]
- 40. Klement K., Melle C., Murzik U., Diekmann S., Norgauer J., Hemmerich P. (2012) Accumulation of annexin A5 at the nuclear envelope is a biomarker of cellular aging. Mech. Ageing Dev. 133, 508–522 [DOI] [PubMed] [Google Scholar]
- 41. Tomas A., Moss S. E. (2003) Calcium- and cell cycle-dependent association of annexin 11 with the nuclear envelope. J. Biol. Chem. 278, 20210–20216 [DOI] [PubMed] [Google Scholar]
- 42. Kawaminami M., Yamaguchi K., Miyagawa S., Numazawa S., Ioka H., Kurusu S., Hashimoto I. (1998) Ovariectomy enhances the expression and nuclear translocation of annexin 5 in rat anterior pituitary gonadotrophs. Mol. Cell. Endocrinol. 141, 73–78 [DOI] [PubMed] [Google Scholar]
- 43. Castro-Caldas M., Duarte C. B., Carvalho A. R., Lopes M. C. (2001) 17beta-estradiol promotes the synthesis and the secretion of annexin I in the CCRF-CEM human cell line. Mediators Inflamm. 10, 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ivanova M., Abner S., Pierce W., Jr., Klinge C. (2011) Ligand-dependent differences in estrogen receptor beta-interacting proteins identified in lung adenocarcinoma cells corresponds to estrogenic responses. Proteome Sci. 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tarallo R., Bamundo A., Nassa G., Nola E., Paris O., Ambrosino C., Facchiano A., Baumann M., Nyman T. A., Weisz A. (2011) Identification of proteins associated with ligand-activated estrogen receptor alpha in human breast cancer cell nuclei by tandem affinity purification and nano LC-MS/MS. Proteomics 11, 172–179 [DOI] [PubMed] [Google Scholar]
- 46. Harada N., Yasunaga R., Higashimura Y., Yamaji R., Fujimoto K., Moss J., Inui H., Nakano Y. (2007) Glyceraldehyde-3-phosphate dehydrogenase enhances transcriptional activity of androgen receptor in prostate cancer cells. J. Biol. Chem. 282, 22651–22661 [DOI] [PubMed] [Google Scholar]
- 47. Sawa A., Khan A. A., Hester L. D., Snyder S. H. (1997) Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc. Natl. Acad. Sci. U.S.A. 94, 11669–11674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishitani R., Tanaka M., Sunaga K., Katsube N., Chuang D. M. (1998) Nuclear localization of overexpressed glyceraldehyde-3-phosphate dehydrogenase in cultured cerebellar neurons undergoing apoptosis. Mol. Pharmacol. 53, 701–707 [DOI] [PubMed] [Google Scholar]
- 49. Lin J., Steenbergen C., Murphy E., Sun J. (2009) Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 120, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santhanam L., Tuday E. C., Webb A. K., Dowzicky P., Kim J. H., Oh Y. J., Sikka G., Kuo M., Halushka M. K., Macgregor A. M., Dunn J., Gutbrod S., Yin D., Shoukas A., Nyhan D., Flavahan N. A., Belkin A. M., Berkowitz D. E. (2010) Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ. Res. 107, 117–125 [DOI] [PubMed] [Google Scholar]
- 51. McCarthy J., Hopwood F., Oxley D., Laver M., Castagna A., Righetti P. G., Williams K., Herbert B. (2003) Carbamylation of proteins in 2-D electrophoresis—myth or reality? J. Proteome Res. 2, 239–242 [DOI] [PubMed] [Google Scholar]
- 52. Suh Y. (2001) Age-specific changes in expression, activity, and activation of the c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinases by methyl methanesulfonate in rats. Mech. Ageing Dev. 122, 1797–1811 [DOI] [PubMed] [Google Scholar]
- 53. Li F., Zhang L., Craddock J., Bruce-Keller A. J., Dasuri K., Nguyen A., Keller J. N. (2008) Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech. Ageing Dev. 129, 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Akar C. A., Feinstein D. L. (2009) Modulation of inducible nitric oxide synthase expression by sumoylation. J. Neuroinflammation 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hirabayashi M., Inoue K., Tanaka K., Nakadate K., Ohsawa Y., Kamei Y., Popiel A. H., Sinohara A., Iwamatsu A., Kimura Y., Uchiyama Y., Hori S., Kakizuka A. (2001) VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 8, 977–984 [DOI] [PubMed] [Google Scholar]
- 56. Koike M., Fukushi J., Ichinohe Y., Higashimae N., Fujishiro M., Sasaki C., Yamaguchi M., Uchihara T., Yagishita S., Ohizumi H., Hori S., Kakizuka A. (2010) Valosin-containing protein (VCP) in novel feedback machinery between abnormal protein accumulation and transcriptional suppression. J. Biol. Chem. 285, 21736–21749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jung S. Y., Malovannaya A., Wei J., O'Malley B. W., Qin J. (2005) Proteomic analysis of steady-state nuclear hormone receptor coactivator complexes. Mol. Endocrinol. 19, 2451–2465 [DOI] [PubMed] [Google Scholar]
- 58. Klement I. A., Skinner P. J., Kaytor M. D., Yi H., Hersch S. M., Clark H. B., Zoghbi H. Y., Orr H. T. (1998) Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95, 41–53 [DOI] [PubMed] [Google Scholar]
- 59. Zheng B., Han M., Bernier M., Wen J. K. (2009) Nuclear actin and actin-binding proteins in the regulation of transcription and gene expression. FEBS J. 276, 2669–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang S. M., Huang C. J., Wang W. M., Kang J. C., Hsu W. C. (2004) The enhancement of nuclear receptor transcriptional activation by a mouse actin-binding protein, alpha actinin 2. J. Mol. Endocrinol. 32, 481–496 [DOI] [PubMed] [Google Scholar]
- 61. Hofmann W. A., Stojiljkovic L., Fuchsova B., Vargas G. M., Mavrommatis E., Philimonenko V., Kysela K., Goodrich J. A., Lessard J. L., Hope T. J., Hozak P., de Lanerolle P. (2004) Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat. Cell Biol. 6, 1094–1101 [DOI] [PubMed] [Google Scholar]
- 62. Hofmann W., Reichart B., Ewald A., Muller E., Schmitt I., Stauber R. H., Lottspeich F., Jockusch B. M., Scheer U., Hauber J., Dabauvalle M. C. (2001) Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J. Cell Biol. 152, 895–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wada A., Fukuda M., Mishima M., Nishida E. (1998) Nuclear export of actin: a novel mechanism regulating the subcellular localization of a major cytoskeletal protein. EMBO J. 17, 1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tovy A., Siman Tov R., Gaentzsch R., Helm M., Ankri S. (2010) A new nuclear function of the Entamoeba histolytica glycolytic enzyme enolase: the metabolic regulation of cytosine-5 methyltransferase 2 (Dnmt2) activity. PLoS Pathog. 6, e1000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Han X., Aenlle K. K., Bean L. A., Rani A., Semple-Rowland S. L., Kumar A., Foster T. C. (2013) Role of estrogen receptor alpha and beta in preserving hippocampal function during aging. J. Neurosci. 33, 2671–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McNally L. M., Yee L., McNally M. T. (2006) Heterogeneous nuclear ribonucleoprotein H is required for optimal U11 small nuclear ribonucleoprotein binding to a retroviral RNA-processing control element: implications for U12-dependent RNA splicing. J. Biol. Chem. 281, 2478–2488 [DOI] [PubMed] [Google Scholar]
- 67. Buratti E., Baralle M., De Conti L., Baralle D., Romano M., Ayala Y. M., Baralle F. E. (2004) hnRNP H binding at the 5′ splice site correlates with the pathological effect of two intronic mutations in the NF-1 and TSHbeta genes. Nucleic Acids Res. 32, 4224–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pak T. R., Rao Y. S., Prins S. A., Mott N. N. (2013) An emerging role for microRNAs in sexually dimorphic neurobiological systems. Pflugers Arch. 465(5), 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yamagata K., Fujiyama S., Ito S., Ueda T., Murata T., Naitou M., Takeyama K., Minami Y., O'Malley B. W., Kato S. (2009) Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol. Cell 36, 340–347 [DOI] [PubMed] [Google Scholar]
- 70. Tollervey J. R., Wang Z., Hortobagyi T., Witten J. T., Zarnack K., Kayikci M., Clark T. A., Schweitzer A. C., Rot G., Curk T., Zupan B., Rogelj B., Shaw C. E., Ule J. (2011) Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Res. 21, 1572–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J. M., Hou X., Adeosun S., Hill R., Henry S., Paul I., Irwin R. W., Ou X. M., Bigler S., Stockmeier C., Brinton R. D., Gomez-Sanchez E. (2012) A dominant negative ERbeta splice variant determines the effectiveness of early or late estrogen therapy after ovariectomy in rats. PLoS One 7, e33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mott N. N., Pak T. R. (2013) Estrogen signaling and the aging brain: context-dependent considerations for postmenopausal hormone therapy. ISRN Endocrinol. 2013, 814690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Malovannaya A., Lanz R. B., Jung S. Y., Bulynko Y., Le N. T., Chan D. W., Ding C., Shi Y., Yucer N., Krenciute G., Kim B. J., Li C., Chen R., Li W., Wang Y., O'Malley B. W., Qin J. (2011) Analysis of the human endogenous coregulator complexome. Cell 145, 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Paxinos G., Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4th Ed., Academic Press, Waltham, MA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.